Abstract
To investigate the possible influences of various factors on tumor response to radiation, regression speeds and long‐term local control rates of primary adenocarcinoma and squamous cell carcinoma of the lung after stereotactic body radiotherapy were evaluated. Ninety‐one patients (65 men and 26 women) with a median age of 76 years were serially examined using computed tomography at 2, 4 and 6 months after treatment. Tumor histology was adenocarcinoma in 62 patients and squamous cell carcinoma in 29 patients. The prescribed dose was 48 Gy in four fractions given twice a week for T1 tumors (≤3 cm) and 52 Gy in four fractions given twice a week for T2 tumors (3–5 cm). Tumor shrinkage speed and 3‐year local control rates were similar between T1 and T2 tumors and between patients with normal pulmonary function and those with impaired function. Squamous cell carcinomas shrank faster than adenocarcinomas at 2 and 4 months after radiation, but mean relative tumor size at 6 months and local control rates at 3 years did not differ significantly between the two histologies. Tumors in patients with a higher hemoglobin level tended to shrink faster but the control rates were not different. It is concluded that, although squamous cell carcinoma shrinks faster than adenocarcinoma, the two types of lung cancer are of similar radiosensitivity in terms of long‐term control rates. Radiosensitivity should not be evaluated by early tumor response. (Cancer Sci 2013; 104: 130–134)
The radiosensitivity of tumors varies with various tumor‐ and patient‐related factors. These include histology, grade of differentiation and size of tumors, and hemoglobin levels in patients. It is well known that small‐cell carcinoma of the lung responds more quickly to radiation than other histological subtypes of lung cancer. In uterine cervical cancer treated with radiation, squamous cell carcinoma (SCC) is generally associated with a better prognosis than adenocarcinoma (AD);1, 2 such observations led many oncologists to assume that SCC is more radiosensitive than AD.3, 4 In addition, smaller tumors are generally more radiosensitive than larger ones.5, 6 Furthermore, extensive clinical data show that patients with a high hemoglobin level have a better prognosis than those with a low level following definitive radiotherapy of various cancers,7, 8, 9, 10, 11 indicating that tumors in patients with a high hemoglobin level might be more radiosensitive than tumors in those with a low hemoglobin level. However, these observations mostly come from retrospective analyses of clinical data and have not been properly evaluated in a prospective study.
Stereotactic body radiotherapy (SBRT) was introduced in the mid‐1990s and has been performed in many institutions as a new treatment modality for stage I primary lung cancer.12, 13, 14 Stereotactic body radiotherapy usually uses a few sessions of relatively high‐dose irradiation. Therefore, if imaging studies are carried out serially after SBRT, shrinkage patterns and local control of irradiated tumors following SBRT can be readily evaluated. Namely, regression curves of human tumors can be obtained, as are often generated for murine tumors in laboratory studies as a growth delay assay. In addition, such patients survive for a relatively long time, so long‐term local control rates can be evaluated. In our previous study,15 we investigated regression curves and local control rates of various brain metastases after gamma knife treatment, and found that small‐cell carcinoma and SCC shrank faster than AD; however, local control rates at 6 months did not differ significantly. One major drawback of that study was that local control was evaluable only at 6 months because patients with brain metastases do not live long enough to evaluate long‐term local control.
Therefore, following that study we attempted to evaluate and compare regression curves and local control rates at ≥3 years of non‐small‐cell lung cancer (NSCLC) after SBRT. In the present study, we investigated possible differences in regression curves and local control rates due to tumor size and histology, and hemoglobin levels and pulmonary function of patients with NSCLC undergoing SBRT. We assumed that, while long‐term local control rates represent the radiosensitivity of tumors, speed of regression might be misleading in terms of radiosensitivity evaluation. It was hypothesized that SCC would regress more rapidly than AD, but local control rates might not differ. Regarding the influence of tumor size, hemoglobin level and pulmonary function, little is known about the association between regression speeds and local control rates. The present study was carried out to clarify these issues.
Materials and Methods
Study design and eligibility criteria
This was a subsidiary analysis of prospective protocol‐based studies of SBRT for stage I NSCLC; details of the SBRT studies were described previously.16, 17 In the studies, evaluation of tumor response using computed tomography (CT) was scheduled at 2, 4 and 6 months following SBRT. All patients underwent a pulmonary function test before SBRT. The major eligibility criteria for the SBRT studies were as follows: histologically confirmed NSCLC diagnosed as T1aN0M0, T1bN0M0 or T2aN0M0 stage according to the International Union Against Cancer (UICC) 2010 system18 using CT of the chest and upper abdomen, bone scintigraphy and brain magnetic resonance imaging and a World Health Organization performance status ≤2. Patients who had previously undergone radiotherapy or chemotherapy were excluded. All patients consented to the treatment after they had been informed of the method and rationale of the study.
Eligibility criteria for entry into this analysis were as follows: (i) histological diagnosis of either AD or SCC; (ii) no chemotherapy before and after SBRT until recurrence; (iii) strict compliance with the prescription dose of 48 Gy in four fractions for T1 tumors and 52 Gy in four fractions for T2a tumors; (iv) fully available CT images taken before and at 2, 4 and 6 months (±4 days) after SBRT; and (v) fully available pretreatment hemoglobin levels and pulmonary function test data using a spirometer.
Patient characteristics
From October 2004 to February 2011, 91 patients with stage I NSCLC treated with SBRT alone were included in the present study. There were 65 men and 26 women, and their median age was 76 years (age range, 61–86 years). Histology was AD in 62 patients and SCC in 29. Fifteen patients had a T1a tumor, 45 had a T1b tumor and 31 had a T2 tumor, according to the 7th UICC classification.18 The patient characteristics are summarized in Table 1. Although only patients with histologically proven AD or SCC were included in the present study, it was our policy to treat patients with suspected NSCLC when the diagnosis could not be confirmed by transbronchial lung biopsy or CT‐guided biopsy, or taking a biopsy was judged to be very difficult, provided that positron emission tomography with 18fluorodeoxyglucose showed a positive finding and the tumor size increased during the observation period.
Table 1.
Patient characteristics
| Characteristics | Patient number | T1/T2 | AD/SCC | Hb: ≥/< 13.1g/dL | Pulmonary function: normal/impaired |
|---|---|---|---|---|---|
| Age (years) | |||||
| ≥76 | 50 | 32/18 | 32/18 | 25/25 | 13/27 |
| <76 | 41 | 28/13 | 30/11 | 21/20 | 17/34 |
| Gender | |||||
| Male | 65 | 39/26 | 39/26 | 39/26 | 16/49 |
| Female | 26 | 21/5 | 23/3 | 7/19 | 14/12 |
| Performance status | |||||
| 0 | 38 | 27/11 | 29/9 | 18/20 | 14/24 |
| 1 | 45 | 27/18 | 29/16 | 23/22 | 15/30 |
| 2 | 8 | 6/2 | 4/4 | 5/3 | 1/7 |
| T stage | |||||
| T1 | 60 | – | 42/18 | 29/31 | 21/39 |
| T2 | 31 | – | 20/11 | 17/14 | 9/22 |
| Histology | |||||
| AD | 62 | 42/20 | – | 29/33 | 22/40 |
| SCC | 29 | 18/11 | – | 17/12 | 8/21 |
| Hb (g/dL) | |||||
| Median (range) | 13.1 (9.1–18.3) | ||||
| ≥13.1 | 46 | 29/17 | 29/17 | – | 9/37 |
| <13.1 | 45 | 31/14 | 33/12 | – | 21/24 |
| Pulmonary function | |||||
| Normal | 30 | 21/9 | 24/6 | 9/21 | – |
| Impaired | 61 | 39/22 | 38/23 | 37/24 | – |
AD, adenocarcinoma; Hb, hemoglobin; SCC, squamous cell carcinoma;
–, not applicable.
Stereotactic body radiotherapy methods
Our methods of SBRT have been described in detail previously.16, 17 Briefly, CT images for treatment planning were obtained under normal breathing, and with breath holding during the expiratory and inspiratory phases. The clinical target volume (CTV) was defined as the visible gross tumor volume. The CTV on CT during the three phases were superimposed on 3‐D radiation treatment planning systems (Eclipse Version 7.5.14.3; Varian Medical Systems, Palo Alto, CA, USA, or BRAINSCAN ver. 5.31; BrainLAB, Feldkirchen, Germany) to represent the internal target volume (ITV). We defined the planning target volume (PTV) margin for the ITV as 5 mm in the lateral and anteroposterior directions and 10 mm in the craniocaudal direction. Three coplanar and four noncoplanar static ports were used. Stereotactic body radiotherapy was delivered by a linear accelerator (CLINAC 23EX; Varian Medical Systems, or Novalis image‐guided system, BrainLAB) with 6‐MV photons. The treatment was performed twice a week. The median treatment period was 11 days.
The prescribed dose at isocenter was 48 Gy in four fractions for tumors with a maximum diameter of ≤3 cm, and 52 Gy in four fractions for those with a maximum diameter greater than 3 cm. For Novalis treatment, pencil beam convolution with Batho power law correction was used throughout as the dose calculation algorithm. For CLINAC 23EX treatment, pencil beam convolution with Batho power law correction was used from October 2004 to November 2008, but then it was changed to the analytical anisotropic algorithm. The change of the dose calculation algorithm could influence the dose distribution, but in a recent analysis the dose distribution within the PTV did not seem to be significantly influenced by the algorithm (Shinya Otsuka, unpublished data, 2012). Therefore, the two groups were combined. The prescribed dose represented that delivered to the isocenter, and it was ensured that 95% of the PTV received at least 80% of the prescribed dose.
Evaluation
For follow up after SBRT, chest CT was performed before and at 2, 4 and 6 months after completion of SBRT in all patients using 16‐ or 64‐row multidetector scanners. Slice thickness was 5 mm in all patients. At 2 months, all patients were evaluable. In a considerable proportion of patients, tumor size became unevaluable at 4 or 6 months because of radiation pneumonitis, and they were excluded from analysis at that point. The criterion for exclusion from tumor size evaluation was that radiation pneumonitis and tumor shadows merged so that the tumor margin became hard to delineate. This was judged by at least two radiation oncologists. Contrast materials were used on demand, but for this analysis only unenhanced images were used with pulmonary window settings.
The tumor size (maximum tumor area) was defined as π/4 × product of cross‐sectional diameters on a slice of the largest tumor size, and the relative tumor size was obtained using the ratio of the tumor size at each follow up to the pretreatment size. The pretreatment tumor size ranged from 49.5 to 1806 mm2 (median, 352 mm2). Local recurrence was defined by regrowth of the irradiated tumors and marginal recurrence was not included. Local tumor regrowth was judged using serial enlargement of fibrotic scar‐like tissues or mass‐like shadows on CT coupled with positive positron emission tomography findings (standardized uptake ratio ≥5). Biopsy was not necessarily performed. The diagnosis of local recurrence was rendered by agreement of at least three radiation oncologists. Patients were divided into two groups according to stage, histology, pretreatment hemoglobin (Hb) level and pulmonary function. The normal pulmonary function group was defined by pretreatment percentage vital capacity ≥80 and the ratio of forced expiratory volume in 1 s to forced vital capacity ≥70% and the other patients were classified as the impaired pulmonary function group.
Statistical analysis
Differences in mean relative tumor size between pairs of groups were examined using t‐test. The local control rates were calculated using the Kaplan–Meier method from the start of SBRT and differences in the rates were examined using the log‐rank test. All statistical analyses were carried out using StatView 5.0 (SAS Institute Inc., Cary, NC, USA). A P value less than 0.05 was defined as significant.
Results
All 91 patients were evaluable at 2 months, and 58 and 31 patients were evaluable at 4 and 6 months, respectively. The median follow‐up period for living patients was 39 months. The 3‐ and 4‐year local control rates are summarized in Table 2. Local recurrence developed in 13 patients (nine of the 60 T1 patients and four of the 31 T2 patients; nine of the 62 AD patients and four of the 29 SCC patients). Regional lymph node relapse occurred in 13 patients (six of the 60 T1 patients and seven of the 31 T2 patients; six of the 62 AD patients and seven of the 29 SCC patients). Distant metastases developed in 19 patients (12 of the 60 T1 patients and seven of the 31 T2 patients; 15 of the 62 AD patients and four of the 29 SCC patients).
Table 2.
Local control rates at 3 and 4 years according to T stage, histology, hemoglobin level and pulmonary function
| Local control rates at 3 and 4 years (%) | P | ||
|---|---|---|---|
| T stage | T1 | 83 | 0.89 |
| T2 | 84 | ||
| Histology | AD | 84 | 0.98 |
| SCC | 83 | ||
| Hemoglobin (g/dL) | ≥13.1 | 86 | 0.59 |
| <13.1 | 82 | ||
| Pulmonary function | Normal | 75 | 0.20 |
| Impaired | 87 |
AD, adenocarcinoma; SCC, squamous cell carcinoma.
Responses by tumor size: T1 versus T2
Figure 1 shows relative tumor sizes according to T‐stage at 2, 4 and 6 months after SBRT. No significant difference was seen at 2, 4 and 6 months between stage T1 and T2 patients. The local control rates at 3 and 4 years also did not differ significantly between T1 and T2 patients (Table 2).
Figure 1.
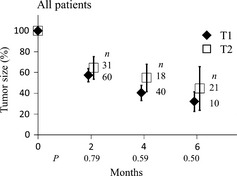
Change in relative tumor size after stereotactic body radiotherapy according to T stage. Bars represent 95% confidence intervals.
Responses by histology: AD versus SCC
Figure 2 shows relative sizes of AD and SCC at 2–6 months. Relative sizes of SCC were apparently smaller than those of AD at 2 and 4 months after SBRT (P < 0.0001 and = 0.0081), but at 6 months the mean relative tumor size did not differ significantly. Similar trends were observed when the T1 and T2 groups were analyzed separately. The local control rates did not differ significantly between AD and SCC (Table 2).
Figure 2.
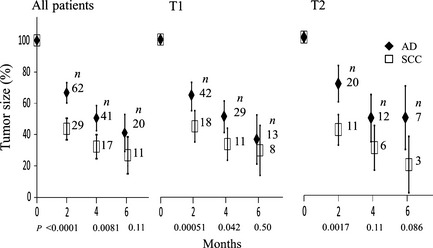
Change in relative tumor size after stereotactic body radiotherapy according to histology of lung cancer. Bars represent 95% confidence intervals. AD, adenocarcinoma; SCC, squamous cell carcinoma.
Responses by hemoglobin level
The median Hb level before SBRT for all patients was 13.1 g/dL (range, 9.1–18.3), therefore patients were classified into two groups: Hb ≥ 13.1 and < 13.1 g/dL. Figure 3 shows changes of relative tumor sizes according to the level of pretreatment hemoglobin. The high Hb group showed faster tumor shrinkage than the low Hb group at 2, 4 and 6 months after SBRT (P = 0.013, 0.034 and 0.032, respectively). This trend was also observed at 2 months in both the AD and SCC subgroups. However, the 3‐ and 4‐year local control rates did not differ significantly between the high and low Hb groups.
Figure 3.
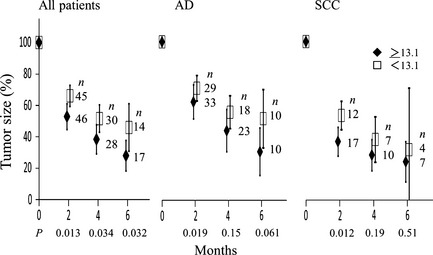
Change in relative tumor size after stereotactic body radiotherapy according to the serum hemoglobin level before treatment. Patients were divided into two groups by the median hemoglobin level. Bars represent 95% confidence intervals. AD, adenocarcinoma; SCC, squamous cell carcinoma.
Patients were also divided by the hemoglobin levels of 12, 11 and 10 g/dL. A similar trend was observed in regression curves when they were divided at 12 g/dL, but 3‐year local control rates did not differ significantly. The number of patients with Hb levels < 11 g/dL was 11, and those with Hb < 10 g/dL numbered only six, therefore no definite differences could be found in both regression curves and local control rates between the higher Hb and lower Hb groups.
Responses by pulmonary function
Figure 4 shows changes in relative tumor size according to pulmonary function. No significant difference was seen at 2, 4 and 6 months between the normal function group and the impaired function group. The local control rates also did not differ significantly between the two groups.
Figure 4.
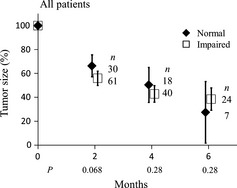
Change in relative tumor size after stereotactic body radiotherapy according to pulmonary function. Bars represent 95% confidence intervals.
Discussion
The association between tumor histology and radiosensitivity has long been discussed. In many Japanese publications, it is stated that SCC is generally more radiosensitive than AD.19, 20 In contrast, substantial laboratory and clinical data suggest similar radiosensitivity between the two using radiobiological end‐points.21, 22, 23 In the present study, SCC regressed more rapidly than AD (especially at 2 and 4 months after SBRT), but relative tumor sizes at 6 months and the 3‐ and 4‐year local control rates were similar. Because of the faster regression, it seems that SCC has been considered to be more radiosensitive than AD by many oncologists. However, it now seems clear that this is a misunderstanding, at least for lung cancer treated with SBRT. Radiosensitivity should not be judged by early responses of the tumors. However, the effects of SBRT could differ from those of conventional fractionation; the doses used in SBRT are considered to be biologically higher than the doses of conventional radiation, so further studies are encouraged for NSCLC treated with conventional fractionation to evaluate possible differences between SBRT and conventional radiotherapy. The present study also suggests that suspected NSCLC and NSCLC with no further histological subclassification might be treated with the same SBRT policy.
Our previous study on brain metastases treated with gamma knife suggests a similar trend regarding the difference between AD and SCC, despite the possible difference due to the tumor bed. However, it was not convincing because only 6‐month local control rates were evaluable in that study.15 The present study more clearly shows the difference in early response and similarity in local control between AD and SCC. However, it should be noted that this observation was obtained for lung cancer; it has now become clear that colorectal AD metastases are more difficult to control than other metastases.24, 25 Therefore, the radiosensitivity of AD might differ with the primary tumor site. Another implication of the present study is that reevaluation of whether small‐cell carcinoma is really more radiosensitive than SCC and AD is warranted. Furthermore, sarcomas have been regarded as radioresistant tumors because of the slow or limited shrinkage after radiation therapy, but the radiosensitivity of sarcomas should also be reevaluated in terms of long‐term control rates. These are topics for future studies.
It is well known that smaller tumors are generally more radiosensitive than larger ones, but in the present study there were no differences in both tumor shrinkage speeds and local control rates between T1 and T2 tumors. In our previous study on brain metastases, smaller tumors showed more rapid shrinkage and higher local control rates at 6 months.15 One of the reasons for this discrepancy might be that a higher dose (52 Gy) was used for T2 tumors than for T1 tumors (48 Gy). Another reason might be the reoxygenation phenomenon. Because gamma knife treatment was given in one fraction, the radioresistance of larger tumors might have been more clearly manifested due to an increase in the hypoxic fraction.6 In contrast, during fractionated SBRT, this radioresistance might be ameliorated by way of the reoxygenation phenomenon.26, 27 This observation would highlight the importance of fractionation in treating larger tumors.
In the present study we could not detect an influence of serum Hb level on local control rates, although tumors in patients with a higher Hb level shrank faster. In contrast to many studies reporting the negative influences of low Hb levels on local control, the lack of such an association in the present study might be related to the subjects investigated. Namely, all patients had stage I NSCLC and 91% of patients had a performance status of 0 or 1. In addition, there were very few patients with anemia as defined by Hb level < 10 or 11 g/dL. Consequently, 3‐year local control rates were over 80% in the entire population. It might be difficult to prove the influence of Hb levels on local control rates in such patient groups with a favorable prognosis mostly consisting of non‐anemic patients. This issue should be investigated further in advanced head and neck cancer and uterine cervical cancer. The discrepancy between the regression speed and local control rates due to the Hb level might be rather difficult to explain, but it was also observed in our previous study with brain metastases.15 It might be partly explained by the observation that 37% of the higher Hb level group had SCC while 27% in the lower Hb group had SCC.
Many studies have indicated that medically inoperable patients have a worse prognosis than operable patients.12, 13, 14, 16, 17 Poor pulmonary function is a cause of medical inoperability, but we could not detect the influences of pulmonary function. Many other conditions are causes of medical inoperability. Overall survival is generally lower in medically inoperable patients, but with respect to local control rates, medical inoperability might not necessarily have a negative influence.17 Pulmonary function could be related to oxygenation status, but for unknown reasons there were more patients with higher Hb levels in the impaired pulmonary function group (Table 1, P = 0.0076 by Fisher's exact test). The absence of an influence of pulmonary function might be partly explained by this observation.
All patients were evaluable at 2 months, but tumor size became unevaluable in a considerable proportion of patients at 4 and 6 months because of radiation pneumonitis. Therefore, reliability of the data at 4 and 6 months is lower than the data at 2 months; this is a limitation of the present study. However, tumor response at 2 months and local control could be evaluated in all 91 patients, and the difference in early response between AD and SCC at 2 months and similar local control rates at 3 and 4 years appear to be convincing.
In summary, the present study clearly showed that SCC of the lung shows faster shrinkage after SBRT than AD of the lung, although 3‐ and 4‐year local control rates did not differ significantly. Radiosensitivity should not be assessed by early response of the tumor. The common assertion that SCC is more radiosensitive than AD seems to have arisen from the observation of early tumor responses. This is not true, at least for NSCLC treated with SBRT.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
The authors thank Drs Fumiya Baba, Yoshimasa Mori, Takeshi Yanagi, Hiroyuki Ogino, Rumi Murata, Chikao Sugie, Shiho Ayakawa, Hiromitsu Iwata, Shinya Otsuka, Aiko Nagai, Taro Murai, Shinya Takemoto and Yoshihiko Manabe for their valuable contributions to the SBRT studies.
(Cancer Sci, doi: 10.1111/cas.12048, 2012)
References
- 1. Barillot I, Horriot JC, Pigneux J et al Carcinoma of the intact uterine cervix treated with radiotherapy alone: a French cooperative study: update and multivariate analysis of prognostic factors. Int J Radiat Oncol Biol Phys 1997; 38: 969–78. [DOI] [PubMed] [Google Scholar]
- 2. Yalman D, Aras AB, Ozkök S et al Prognostic factors in definitive radiotherapy of uterine cervical cancer. Eur J Gynaecol Oncol 2003; 24: 309–14. [PubMed] [Google Scholar]
- 3. Munzenrider JE. Recent advances in radiotherapy. Rev Interam Radiol 1977; 2: 123–33. [PubMed] [Google Scholar]
- 4. Milsom I, Friberg LG. Primary adenocarcinoma of the uterine cervix. A clinical study. Cancer 1983; 52: 942–7. [DOI] [PubMed] [Google Scholar]
- 5. Stanley JA, Shipley WU, Steel GG. Influence of tumour size on hypoxic fraction and therapeutic sensitivity of Lewis lung tumour. Br J Cancer 1977; 36: 105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shibamoto Y, Yukawa Y, Tsutsui K, Takahashi M, Abe M. Variation in the hypoxic fraction among mouse tumors of different types, sizes, and sites. Jpn J Cancer Res 1986; 77: 908–15. [PubMed] [Google Scholar]
- 7. Overgaard J, Hansen HS, Andersen AP et al Misonidazole combined with split‐course radiotherapy in the treatment of invasive carcinoma of larynx and pharynx: report from the DAHANCA 2 study. Int J Radiat Oncol Biol Phys 1989; 16: 1065–8. [DOI] [PubMed] [Google Scholar]
- 8. Grogan M, Thomas GM, Melamed I et al The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer 1999; 86: 1528–36. [DOI] [PubMed] [Google Scholar]
- 9. Henke M, Sindlinger F, Ikenberg H, Gerds T, Schumacher M. Blood hemoglobin level and treatment outcome of early breast cancer. Strahlenther Onkol 2004; 180: 45–51. [DOI] [PubMed] [Google Scholar]
- 10. Hoff CM, Hansen HS, Overgaard M et al The importance of haemoglobin level and effect of transfusion in HNSCC patients treated with radiotherapy: results from the randomized DAHANCA 5 study. Radiother Oncol 2011; 98: 23–33. [DOI] [PubMed] [Google Scholar]
- 11. Mortensen LS, Busk M, Nordmark M et al Accessing radiation response using hypoxia PET imaging and oxygen sensitive electrodes: a preclinical study. Radiother Oncol 2011; 99: 418–23. [DOI] [PubMed] [Google Scholar]
- 12. Uematsu M, Shioda A, Suda A et al Computed tomography‐guided frameless stereotactic radiotherapy for stage I non‐small‐cell lung cancer: a 5‐year experience. Int J Radiat Oncol Biol Phys 2001; 51: 666–70. [DOI] [PubMed] [Google Scholar]
- 13. Onishi H, Kuriyama K, Komiyama T et al Clinical outcomes of stereotactic radiotherapy for stage I non‐small cell lung cancer using a novel irradiation technique: patient self‐controlled breath‐hold and beam switching using a combination of linear accelerator and CT scanner. Lung Cancer 2004; 45: 45–55. [DOI] [PubMed] [Google Scholar]
- 14. Nagata Y, Wulf J, Lax I et al Stereotactic radiotherapy of primary lung cancer and other targets: results of consultant meeting of the International Atomic Energy Agency. Int J Radiat Oncol Biol Phys 2011; 79: 660–9. [DOI] [PubMed] [Google Scholar]
- 15. Kosaki K, Shibamoto Y, Hirai T et al Regression curves of brain metastases after gamma knife irradiation: difference by tumor and patient characteristics. Cancer Sci 2012; 103: 1967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baba F, Shibamoto Y, Ogino H et al Clinical outcomes of stereotactic body radiotherapy for stage I non‐small cell lung cancer using different doses depending on tumor size. Radiat Oncol 2010; 5: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shibamoto Y, Hashizume C, Baba F et al Stereotactic body radiotherapy using a radiobiology‐based regimen for stage I nonsmall cell lung cancer: a multicenter study. Cancer 2012; 118: 2078–84. [DOI] [PubMed] [Google Scholar]
- 18. Sobin LH, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Maligant Tumors, 7th edn (UICC International Union Against Cancer). New York: Wiley‐Blackwell, 2009. [Google Scholar]
- 19. Watanabe H, Kanamori I, Ohno K et al Tumor biology In: Watanabe H, Kanamori I, eds. Introduction to Radiotherapy, 2nd edn. Tokyo: Iryo Kagaku Sha, 2008; 1–30 [in Japanese]. [Google Scholar]
- 20. Tsukahara Y, Sakai Y, Noguchi H, Iwai S, Fukuta T. A study on radiosensitivity and prognostic factors of cervical adenocarcinoma. Acta Obst Gynaec Jpn 1980; 32: 1609–14. [PubMed] [Google Scholar]
- 21. Shibamoto Y, Shibata T, Miyatake S et al Assessment of the proliferative activity and radiosensitivity of human tumours using the cytokinesis‐block micronucleus assay. Br J Cancer 1994; 70: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. West CM, Davidson SE, Burt PA, Hunter RD. The intrinsic radiosensitivity of cervical carcinoma: correlations with clinical data. Int J Radiat Oncol Biol Phys 1995; 31: 841–6. [DOI] [PubMed] [Google Scholar]
- 23. Shibamoto Y, Ike O, Mizuno H, Fukase T, Hitomi S, Takahashi M. Proliferative activity and micronucleus frequency after radiation of lung cancer cells as assessed by the cytokinesis‐block method and their relationship to clinical outcome. Clin Cancer Res 1998; 4: 677–82. [PubMed] [Google Scholar]
- 24. Takeda A, Kunieda E, Ohashi T, Aoki Y, Koike N, Takeda T. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol 2011; 101: 255–9. [DOI] [PubMed] [Google Scholar]
- 25. Iwata H, Murai T, Shibamoto Y. Radiation response of the normal lung tissue and lung tumors In: Jeremic B, ed. Advances in Radiation Oncology in Lung Cancer, 2nd edn. Berlin Heidelberg: Springer–Verlag, 2011; 119–28. [Google Scholar]
- 26. Shibamoto Y, Kitakabu Y, Murata R et al Reoxygenation in the SCCVII tumor after KU‐2285 sensitization plus single or fractionated irradiation. Int J Radiat Oncol Biol Phys 1994; 29: 583–6. [DOI] [PubMed] [Google Scholar]
- 27. Murata R, Shibamoto Y, Sasai K et al Reoxygenation after single irradiation in rodent tumors of different types and sizes. Int J Radiat Oncol Biol Phys 1996; 34: 859–65. [DOI] [PubMed] [Google Scholar]


