Abstract
Survivin, a member of the inhibitor of apoptosis protein (IAP) family containing a single baculovirus IAP repeat domain, is highly expressed in cancerous tissues but not in normal counterparts. Our group identified an HLA‐A24‐restricted antigenic peptide, survivin‐2B80–88 (AYACNTSTL), that is recognized by CD8 + CTLs and functions as an immunogenic molecule in patients with cancers of various histological origins such as colon, breast, lung, oral, and urogenital malignancies. Subsequent clinical trials with this epitope peptide alone resulted in clinical and immunological responses. However, these were not strong enough for routine clinical use as a therapeutic cancer vaccine, and our previous study of colon cancer patients indicated that treatment with a vaccination protocol of survivin‐2B80–88 plus incomplete Freund's adjuvant (IFA) and α‐interferon (IFNα) conferred overt clinical improvement and enhanced the immunological responses of patients. In the current study, we further investigated whether this vaccination protocol could efficiently provide not only improved immune responses but also better clinical outcomes for advanced pancreatic cancers. Tetramer and enzyme‐linked immunosorbent spot analysis data indicated that more than 50% of the patients had positive clinical and immunological responses. In contrast, assessment of treatment with IFNα only to another group of cancer patients resulted in no obvious increase in the frequency of survivin‐2B80‐88 peptide‐specific CTLs. Taken together, our data clearly indicate that a vaccination protocol of survivin‐2B80‐88 plus IFA and IFNα is very effective and useful in immunotherapy for this type of poor‐prognosis neoplasm. This trial was registered with the UMIN Clinical Trials Registry, no. UMIN000000905. (Cancer Sci 2013; 104: 124–129)
Recent progress in human tumor immunology research has presented us with the possibility that immunotherapy could be established as an effective cancer therapy in the very near future.1, 2, 3, 4, 5, 6 Indeed, since the first discovery of a human tumor antigen in 1992,7 many clinical trials for cancer vaccines have been carried out, and these studies have suggested that active immunization using HLA class I restricted tumor antigenic peptides and the whole or part of the tumor antigenic protein could work as activators of antigen‐specific CTLs, at least in some cancer patients.8, 9, 10, 11, 12, 13, 14, 15, 16 However, even in effective cases, vaccination with these molecules alone is not sufficient to evoke a potent and stable immune response and subsequent strong clinical effect. Thus, it is crucial to develop various methods for enhancing the immunological efficacy of tumor antigens.
We have studied how tumor antigenicity can be efficiently enhanced in cancer patients since 2003. In our studies, the HLA‐A24‐restricted peptide survivin‐2B80‐88 was given s.c. to patients six times or more at biweekly intervals for colon, breast, lung, oral cavity, and urinary bladder cancers, and lymphomas. Clinically, certain patients with colon, lung, and urinary bladder cancers showed reductions in tumor markers and growth arrest as assessed by computed tomography (CT).8, 9, 10, 11, 12 These effects, however, were not strong enough for the clinical requirements as decided by the criteria for cancer chemotherapy. When assessed with the Response Evaluation Criteria in Solid Tumors, which requires more than 30% regression of tumors on CT, only one patient each of 15 with colon cancers and three with urinary bladder cancers had a positive clinical response, indicating that the therapeutic potential was obviously not strong enough for routine clinical use as a cancer treatment.
In a previous study,8 to determine if the immunogenicity of the survivin‐2B80‐88 peptide could be enhanced with other vaccination protocols, we carried out and compared clinical trials in advanced colon cancer patients with two vaccination protocols: (i) survivin‐2B80‐88 plus incomplete Freund's adjuvant (IFA); and (ii) survivin‐2B80‐88 plus IFA and a type‐I interferon (IFN), IFNα. Our data clearly indicated that, although the effect of survivin‐2B80‐88 plus IFA was not significantly different from that with survivin‐2B80‐88 alone, treatment with survivin‐2B80‐88 plus IFA and IFNα resulted in clear clinical improvement and enhanced the immunological responses of patients. We also analyzed CTLs of these patients by single‐cell sorting, and found that each CTL clone from vaccinated patients was indeed not only peptide‐specific but also cytotoxic against human cancer cells in the context of the expression of both HLA‐A24 and survivin molecules.
Pancreatic cancer is still one of most difficult malignant neoplasms to treat, so in the current study we investigated whether the most effective protocol for colon cancer patients, namely survivin‐2B80‐88 plus IFA and IFNα, could work similarly in pancreatic cancers as in colon cancers. Furthermore, we carried out frequency monitoring of survivin‐2B80‐88 peptide‐specific CTL in cases of cancer patients treated with IFNα alone, and found no overt increase of these CTLs. Once the survivin‐2B80‐88 peptide was administered with IFNα, patients showed strong clinical and immunological responses as assessed by tetramer and enzyme‐linked immunosorbent spot (ELISPOT) analyses. Taken together, our current data strongly suggest that vaccination using survivin‐2B80‐88 plus IFA and IFNα is actually very effective in patients with advanced pancreatic cancers from both the clinical and immunological points of view.
Materials and Methods
Patients
Patient selection was done as reported in our previously published work. The study protocol was approved by the Clinic Institutional Ethical Review Board of the Medical Institute of Bioregulation, Sapporo Medical University (Sapporo, Japan).8, 9, 10, 11, 12 All patients gave informed consent before being enrolled. Patients who participated in this study were required to: (i) have histologically confirmed pancreatic cancer; (ii) be HLA‐A*2402 positive; (iii) have survivin‐positive carcinomatous lesions by immunohistochemistry; (iv) be between 20 and 85 years old; (v) have unresectable advanced cancer or recurrent cancer; and (vi) have Eastern Cooperative Oncology Group performance status between 0 and 2. Exclusion criteria included: (i) prior cancer therapy such as chemotherapy, radiation therapy, steroid therapy, or other immunotherapy within the past 4 weeks; (ii) the presence of other cancers that might influence the prognosis; (iii) immunodeficiency or a history of splenectomy; (iv) severe cardiac insufficiency, acute infection, or hematopoietic failure; (v) use of anticoagulants; and (vi) unsuitability for the trial based on clinical judgment. This study was carried out at the Department of Surgery, in the Sapporo Medical University Primary Hospital from December 2005 through to November 2010.
Peptide, IFA, and IFNα preparation
The peptide, survivin‐2B80‐88 with the sequence AYACNTSTL, was prepared under good manufacturing practice conditions by Multiple Peptide Systems (San Diego, CA, USA).8, 9, 10, 12 The identity of the peptide was confirmed by mass spectrometry analysis, and the purity was shown to be more than 98% as assessed by HPLC analysis. The peptide was supplied as a freeze‐dried, sterile white powder. It was dissolved in 1.0 mL physiological saline (Otsuka Pharmaceutical, Tokyo, Japan) and stored at −80°C until just before use. Montanide ISA 51 (Seppic, Paris, France) was used as IFA. Human IFNα was purchased from Dainippon‐Sumitomo Pharmaceutical (Osaka, Japan).
Patient treatment
In this clinical study, we used the protocol illustrated in Fig. 1, with the survivin‐2B80‐88 peptide plus IFA and IFNα. In this trial, the primary endpoint was safety. The second endpoint was investigation of the antitumor effects and clinical and immunological monitoring.
Figure 1.
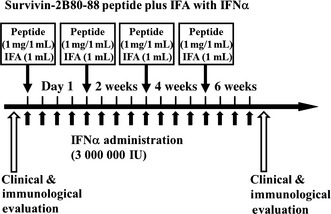
Clinical protocol of study. Survivin‐2B80‐88 and incomplete Freund's adjuvant (IFA) were mixed immediately before vaccination. The patients were then vaccinated s.c. four times at 14‐day intervals. In addition, α‐interferon (IFNα) was given twice a week close to the site of vaccination. For this, IFNα was mixed with the peptide and IFA immediately before vaccination and given at the time of peptide and IFA biweekly vaccination.
In this protocol, survivin‐2B80‐88 at a dose of 1 mg/1 mL plus IFA at a dose of 1 mL were mixed immediately before vaccination. The patients were then vaccinated s.c. four times at 14‐day intervals. In addition, IFNα at a dose of 3 000 000 IU was given s.c. twice a week close to the site of vaccination. For this, IFNα was mixed with the peptide and IFA immediately before vaccination and given at the time of peptide and IFA biweekly vaccination (Fig. 1).
Toxicity evaluation
Patients were examined closely for signs of toxicity during and after vaccination. Adverse events were recorded using the National Cancer Institute Common Toxicity Criteria.8, 9, 10
Clinical response evaluation
Physical examinations and hematological examinations were carried out before and after each vaccination.8, 9, 10 A tumor marker (Ca19‐9) was examined. Changes in the tumor marker levels were evaluated by comparison of the serum level before the first vaccination and that after the fourth vaccination. Immunohistochemical study of the HLA class I expression in patients' primary pancreatic cancer tissues was done with anti‐HLA class I heavy chain mAb EMR‐8‐513 (Funakoshi, Tokyo, Japan).
Tumor size was evaluated by CT scans or MRI by comparing the size before the first vaccination with that after the fourth vaccination. A complete response (CR) was defined as complete disappearance of all measurable and evaluable disease. A partial response was defined as a ≥30% decrease from the baseline in the size of all measurable lesions (sum of maximal diameters). Progressive disease (PD) was defined as an increase in the sum of maximal diameters by at least 20% or the appearance of new lesions. Stable disease (SD) was defined as the absence of criteria matching those for complete response, partial response, or PD.8, 9, 10 Patients who received fewer than four vaccinations were excluded from all evaluations in this study.
In vitro stimulation of PBMC, tetramer staining, and ELISPOT assay
The samples for tetramer analysis and ELISPOT analysis were simultaneously obtained at the time of the hematological examination before and after each vaccination. These experiments were carried out as in our previous report. The PBMCs were isolated from blood samples by Ficoll–Conray density gradient centrifugation. Then they were frozen and stored at −80°C. As needed, frozen PBMCs were thawed and incubated in the presence of 30 μg/mL survivin‐2B80‐88 in AIM V (Life Technologies Corp, Grand Island, NY, USA) medium containing 10% human serum at room temperature. Next, interleukin‐2 was added at a final concentration of 50 U/mL 1 h, 2 days, 4 days, and 6 days after the addition of the peptide. On day 7 of culture, the PBMCs were analyzed by tetramer staining and ELISPOT assay.
The FITC‐labeled HLA‐A*2402‐HIV peptide (RYLRDQQLL) and phycoerythrin (PE)‐labeled HLA‐A*2402‐survivin‐2B8‐88 peptide tetramers were purchased from Medical and Biological Laboratories (MBL) Co., Ltd (Nagoya, Japan). For flow cytometric analysis, PBMCs, stimulated in vitro as above, were stained with the PE‐labeled tetramer at 37°C for 20 min, followed by staining with a PE‐Cy5‐conjugated anti‐CD8 mAb (BD Biosciences, San Jose, CA, USA) at 4°C for 30 min. Cells were washed twice with PBS before fixation in 1% formaldehyde. Flow cytometric analysis was carried out using FACSCalibur and CellQuest software (BD Biosciences). The frequency of CTL precursors was calculated as the number of tetramer‐positive cells divided by the number of CD8‐positive cells.8, 10, 12
The ELISPOT plates were coated overnight in a sterile environment with an IFNγ capture antibody (BD Biosciences) at 4°C. The plates were then washed once and blocked with AIM V medium containing 10% human serum for 2 h at room temperature. CD8‐positive T cells separated from patients' PBMCs (5 × 103 cells/well) that were stimulated in vitro as above were then added to each well along with HLA‐A24‐transfected T2 cells (T2‐A24) (5 × 104 cells/well) that had been preincubated with or without survivin‐2B80‐88 (10 mg/mL) or with an HIV peptide as a negative control. After incubation in a 5% CO2 humidified chamber at 37°C for 24 h, the wells were washed vigorously five times with PBS and incubated with a biotinylated anti‐human IFNγ antibody and HRP‐conjugated avidin. Spots were visualized and analyzed using KS ELISPOT (Carl Zeiss, Oberkochen, Germany). In this study, positive (+) ELISPOT represents a more than twofold increase of survivin‐2B80‐88 peptide‐specific CD8 T cell IFNγ‐postive spots as compared with HIV peptide‐specific CD8 T cell spots, whereas negative (−) means a less than twofold increase.
Single‐cell cloning and functional assessment of tetramer‐positive CTLs
Survivin‐2B80‐88 peptide tetramer‐positive CTLs were sorted and subsequently cloned to single cells using FACS (Aria II Special Order; BD Biosciences). The peptide‐specific cytotoxicity of each of these CTLs was determined by pulsing T2A24 cells8, 17 with survivin‐2B80‐88 or HLA‐A*2402 HIV (RYLRDQQLL) peptides, as previously described.
Results
Patient profiles, safety, and clinical responses
In the present protocol with the survivin‐2B80‐88 peptide plus IFA and IFNα, six patients were enrolled in the study (Table 1). None dropped out because of adverse events due to the vaccination. They consisted of three men and three women, whose age range was 50–80 years.
Table 1.
Profiles of patients with advanced pancreatic cancer enrolled in the study and their clinical and immunological responses to vaccination with survivin‐2B80‐88 peptide, incomplete Freund's adjuvant and IFNα
| Patient no. | Age/sex | Adverse effects | Tumor markers pre/post (CA19‐9 U/mL) | CT eval. | Tetramer staininga | ELISPOTb | ||
|---|---|---|---|---|---|---|---|---|
| Pre/post | % Increase | Pre/post | % Increase | |||||
| 1 | 62/F | Induration | 136.5/31.4 | SD | 23/246 | 1069.6 | 27/294 | 1088.9 |
| 2 | 61/F | Induration | 63.6/60.6 | SD | 1/157 | 15700.0 | 25/71 | 284.0 |
| Fever | ||||||||
| 3 | 56/M | Induration | 171.4/978.8 | PD | 22/19 | 86.3 | 19/525 | 2763.2 |
| Fever | ||||||||
| Thrombopenia | ||||||||
| 4 | 80/F | Induration | 30.0/22.7 | SD | 9/1030 | 11444.4 | 1/101 | 10100.0 |
| Fever | ||||||||
| 5 | 58/M | Induration | 436.0/2885.0 | PD | 3/0 | 0.0 | 34/20 | 58.8 |
| Fever | ||||||||
| 6 | 50/M | Induration | 4389.0/4295.0 | SD | 2/7 | 350.0 | 27/85 | 314.8 |
Cytotoxic T‐lymphocyte frequency of prevaccinated (pre) and postvaccinated (post) patients was assessed with an HLA‐A24‐restricted survivin‐2B80‐88 (AYACNTSTL) peptide tetramer. HLA‐A24‐restricted HIV peptide (RYLRDQQLL) tetramer was used as a negative control. The numbers of survivin‐2B80‐88 peptide tetramer‐positive but HIV peptide‐negative CTLs in 104 × CD8 T cells are shown.
γ‐Interferon (IFNγ) secretion of pre‐ and postvaccinated patients' CD8 T cells was assesed with enzyme‐linked immunosorbent spot (ELISPOT) assay using T2‐A24 cells pulsed with survivin‐2B80‐88 peptide. The numbers of spots in 5 × 103 CD8 T cells are shown.
CT eval., evaluation by computed tomography; IFNα, α‐interferon; PD, progressive disease; SD, stable disease.
With respect to the safety, vaccination was well tolerated in all patients. Four patients had fever reaching nearly 39°C after the vaccination, possibly due to the action of IFNα. No other severe adverse events were observed during or after vaccination except for induration at the injection site, which was conduced by IFA.
The clinical outcomes for the six patients treated with survivin‐2B80‐88 plus IFA and IFNα are summarized in Table 1. In some patients, particularly No. 1, the postvaccination Ca19‐9 value was clearly decreased as compared with prevaccination, and was within the normal limit. Other patients (Nos. 2, 4, and 6) also had decreased or stable postvaccination levels of Ca19‐9, although not as large. As for tumor size evaluated by CT, four patients (Nos. 1, 2, 4, and 6) were considered to have SD, but the other two patients (Nos. 3 and 5) had PD. Consequently, it appeared that there was a close correlation between clinical SD outcomes and a reduced or stable Ca19‐9 level.
Immune responses, single‐cell cloning, and subsequent functional assessment of tetramer‐positive CTLs
As in our previous study with colon cancer patients, we determined if the survivin‐2B80‐88 peptide vaccination could actually induce specific immune responses in the patients enrolled. The peptide‐specific CTL frequency was analyzed using the HLA‐A24/peptide tetramer. The CTL frequencies before the first vaccination (prevaccination) and after the last vaccination (postvaccination) were assessed with an HLA‐A24‐restricted survivin‐2B80‐88 (AYACNTSTL) peptide tetramer, compared with an HLA‐A24‐restricted HIV peptide (RYLRDQQLL) tetramer as a negative tetramer control. The number of survivin‐2B80‐88 peptide tetramer‐positive but HIV peptide‐negative CD8 T cells in 104 CD8 T cells was determined. In the current study, ELISPOT was also carried out using these peptides.
As summarized in Table 1, four of the six patients (Nos. 1, 2, 4, and 6) had enhanced frequency with a more than 200% increase. It was also interesting that all four of these patients were also positive in the ELISPOT study, and all four had SD by CT evaluation, suggesting that immune responses might appropriately reflect clinical responses with the current vaccination protocol.
As in our previous work, we also analyzed tetramer‐positive CD8 T cells at the single‐cell level, and determined whether these T cells had specificity for the survivin‐2B80‐88 peptide and cytotoxic potential against live survivin‐2B‐positive tumor cells in the context of HLA‐A*2402. As shown in Fig. 2, patient No. 1 (62 years old, female) had a reduced serum Ca19‐9 level, and obvious immune responses as assessed by the survivin‐2B80‐88 ELISPOT and tetramer analyses (Fig. 3) after vaccination.
Figure 2.
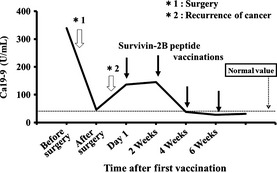
Representative illustration of the clinical effect in patient No. 1 as assessed by the serum Ca19‐9 level. Arrows indicate vaccinations with survivin‐2B80‐88 plus incomplete Freund's adjuvant with α‐interferon (IFNα).
Figure 3.
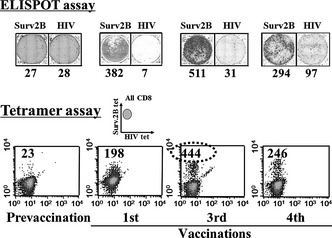
Immunological analysis of CTL responses against HLA‐A24 restricted survivin‐2B80‐88 peptide (surv2B) before and after vaccinations as assessed by enzyme‐linked immunosorbent spot (ELISPOT) and tetramer (tet) analyses. Numbers in the ELISPOT assay indicate γ‐interferon (IFNγ) secretion against survivin2B80‐88 or HIV peptide pulsed T2‐A24 cells in 104× CD8+ T cells. Numbers in tetramer analysis indicate survivin‐2B80‐88 peptide‐specific CD8+ T cells among 104× CD8+ T cells.
Subsequently, CD8 T cells of the tetramer‐positive fraction were sorted by FACS, then cultured with 1, 3, and 10 cells/well for 7–10 days. Almost all growing T cells were survivin‐2B peptide‐specific T cells (data not shown), and we next assessed peptide‐specific cytotoxicity by using these T cells. As Fig. 4 clearly shows, all T cells had very high peptide‐specific cytotoxic potential. Taken together, these data clearly indicated that the vaccination protocol with survivin‐2B80‐88 plus IFA and IFNα was capable of inducing a strong CTL response and for some pancreatic cancer patients might result in clinical effectiveness.
Figure 4.
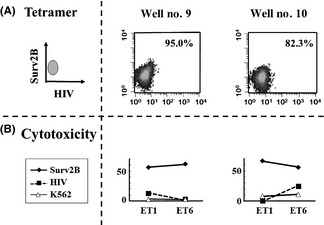
Single‐cell analysis of survivin‐2B80‐88 peptide tetramer‐positive CD8 CTL cells). Survivin‐2B80‐88 peptide tetramer‐positive CD8 T cells in Fig. 3 (circled) were sorted and cultured at 1, 3, and 10 cells/well for 7–10 days. Subsequently, clonal CTL cells were examined for their reactivity to the survivin‐2B80‐88 peptide tetramer (Surv2B) (A) and against T2A24 target cells pulsed with the survivin‐2B80‐88 peptide and HIV peptide and against control K562 cells (B). ET, effector/target ratio.
Assessment of treatment effect with IFNα alone
The above data strongly suggested that the current vaccination protocol with the survivin‐2B80‐88 peptide plus IFA and IFNα could work as a potential therapeutic regimen in pancreatic cancers. However, it remained to be clarified if IFNα alone without the peptide could function in a similar manner, at least to some extent, as this cytokine is considered to be the most potent for the activation and maturation of dendritic cells (DCs) as well as upregulation of HLA class I in tumor cells. To this end, we studied this profile in three patients with colon cancer, not pancreatic cancer, whose condition was similar to those in this study, that is, patients with unresectable advanced or recurrent cancer. This was done because patients with the latter cancer had highly advanced clinical cases, making this type of study impossible. As shown Table 2, all three patients showed no obvious increases, but rather reductions, in the frequency of survivin‐2B peptide‐specific T cells as assessed by tetramer analysis before and after two to four treatments with IFNα alone. Furthermore, this was also true for ELISPOT analysis. These data supported the idea that IFNα alone did not actively participate in the activation of survivin‐2B peptide‐specific T cells.
Table 2.
Frequency monitoring of the number of survivin‐2B80‐88 peptide tetramer‐positive CTLs in cancer patients treated with IFNα alone
| Patient no. | Tumor | Age/sex | Number of treatment | Tetramer staininga | ELISPOTb | ||
|---|---|---|---|---|---|---|---|
| Pre/post | % Increase | Pre/post | % Increase | ||||
| 1 | Colon | 60/M | 3 | 1/0 | 0.0 | 111/75 | 67.6 |
| 2 | Colon | 63/M | 4 | 11/9 | 81.8 | 44/20 | 45.5 |
| 3 | Colon | 77/F | 2 | 13/3 | 23.1 | 26/40 | 153.8 |
CTL frequency before and after treatment with IFNα alone in patients was assessed with an HLA‐A24‐restricted survivin‐2B80‐88 (AYACNTSTL) peptide tetramer. An HLA‐A24‐restricted HIV peptide (RYLRDQQLL) tetramer was used as a negative control. The number of survivin‐2B80‐88 peptide tetramer‐positive but HIV peptide‐negative CTLs in 104 CD8 T cells is shown.
γ‐Interferon (IFNγ) secretion of pre and post IFNα treatmnet were assesed with ELISPOT assay using T2‐A24 cells pulsed with survivin2B80‐88 peptide. The number of spots in 5 × 103 CD8 T cells are shown. IFNα, α‐interferon.
Discussion
Our group previously showed that the vaccination protocol of survivin‐2B80‐88 plus IFA and IFNα could work as a potent immunotherapeutic regimen in colon cancers.8 In addition to colon cancer, survivin2B protein is expressed in most tumor cells of various tissue origins, such as those in the gastrointestinal and biliary tracts and pancreas, therefore, there is a possibility that the survivin2B peptide could work as a potential therapeutic tumor vaccine in cancer patients with these neoplasms.
In this present study, we assessed whether the vaccination protocol using survivin‐2B80‐88 plus IFA and IFNα could be effective in pancreatic cancer patients from immunological and clinical points of views. Consequently, our data strongly suggested that this protocol was very effective and useful in immunotherapy for advanced pancreatic cancers as in colon cancers. Actually it was shown that more than 50% of patients with pancreatic cancers showed positive clinical and immunological responses in tetramer and ELISPOT analyses. In some cases, the immunological response of survivin‐2B80‐88 peptide‐specific CTLs was elucidated at the single‐cell level. Taken together, the current data implied that our vaccination protocol was very useful in immunotherapy for pancreatic cancers.
As shown in Fig. 3, the number of tetramer‐positive populations and IFNγ‐positive spots in the ELISPOT assay was reduced from the third to the fourth vaccination. We speculate that there could be various reasons for this reduction. One might be immune escape by the downregulation of HLA expression, cytokines, or regulatory T cells. Another might be an activity of the stored samples, or differences between the environment of the peripheral circulation and the tumor. In other words, the peptide‐specific CTL responses were reduced in immunological monitoring in the peripheral circulation, but maintained in the local cancer environment. In this case, the clinical responses, such as tumor marker (CA19‐9) level and tumor size evaluated by CT, had been maintained also after that, even though the number of tetramer‐positive populations and IFNγ‐positive spots in the ELISPOT assay was reduced between the third and fourth vaccinations. Therefore, CA19‐9 levels had been kept within normal limits and new cancer lesions had not appeared.
We evaluated immunological monitoring of this clinical protocol by tetramer staining and IFNγ ELISPOT assay. Tetramer staining recognizes the structure of the T cell receptor, and detects naive T cells, memory T cells, and activated CTLs. The ELISPOT assay detects more the functional aspects of T cells by IFNγ release, therefore, ELISPOT detects memory T cells and CTLs. In this study, the tetramer‐positive cases are also positive in the ELISPOT study. Therefore, these results indicate that memory T cells and CTLs can be effectively induced by this peptide vaccination protocol.
In this present study, we also assessed evidence concerning the extent to which peptide‐specific CTL responses in pancreatic cancer patients treated with peptide vaccines could occur at the single‐cell level. To assess this, CTLs of patients were sorted to the single‐cell level, and we confirmed that each CTL obtained from vaccinated patients was indeed peptide‐specific in the context of the expression of HLA‐A24.
Type‐I interferons such as IFNα are known to work in various immunological manners to activate T cell responses.18, 19, 20, 21, 22, 23, 24, 25 The maturation of DCs and their effect on the expression of HLA molecules seems to be the main action of this cytokine. Although we could not actually compare these features of patients' DCs and primary pancreatic tumor tissues before and after treatment with IFNα, the obvious enhancement of CTL responses and improvement of clinical responses in our previous and current studies favors the two main actions described above. These observations strongly suggest that the action of IFNα is remarkable from the aspect of being an immunogenic enhancer for human cancer peptide vaccines.
It is widely known that IFNα is involved in DC maturation and activation.18, 21 This particular cytokine is also potent for increasing the expression of MHC class I molecules.26, 27, 28, 29 Indeed, our previous study of the expression of HLA class I molecules in pancreatic cancer indicated that many tumor tissues heterogeneously expressed such molecules, with some tumor cells showing high expression, whereas others had only weak expression. Interferon‐α is presumed to actually enhance their expression even in those tumor tissues with weak expression. Moreover, because tumor patients generally show overt expression of survivin protein in their tumor tissues and, although in small numbers, survivin‐2B peptide‐specific T cells in peripheral blood, it is considered that IFNα alone may increase the frequency of these T cells in peripheral blood as well. These features of this particular cytokine lead to the possibility that treatment with IFNα alone could result in, at least to some extent, certain immunological and clinical effects of survivin‐2B peptide‐specific T cells in tumor‐bearing patients. However, we analyzed three colon cancer patients, and our data strongly suggested that there was no increase of these T cells as assessed by tetramer and ELISPOT analyses.
Taken together, our results highly suggest that the vaccination protocol with survivin‐2B80‐88 plus IFA and IFNα is very effective for pancreatic and colon cancers, and that this protocol might be useful as a standard, general immunotherapy modality for human cancers. However, further clinical studies involving many patients are necessary in order to consolidate the immunotherapeutic benefit of this vaccination protocol.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant nos 16209013, 17016061, and 15659097), and for Practical Application Research from the Japan Science and Technology Agency and for Cancer Research (15‐17 and 19‐14) from the Ministry of Health, Labor and Welfare of Japan. We are also very grateful for grants from the Akiyama Life Science Foundation and the Ono Foundation, and for continuous support providing study samples by the Hokkaido Red Cross Blood Center.
(Cancer Sci, doi: 10.1111/cas.12046, 2012)
References
- 1. Hirohashi Y, Torigoe T, Inoda S et al The functioning antigens; beyond just as the immunologic targets. Cancer Sci 2009; 100: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sato N, Hirohashi Y, Tsukahara T et al Molecular pathologic approaches to human tumor immunology. Pathol Int 2009; 59: 205–17. [DOI] [PubMed] [Google Scholar]
- 3. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nature Med 2004; 10: 909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsukahara T, Torigoe T, Tamura Y, Kawaguchi S, Wada T, Sato N. Antigenic peptide vaccination: provoking immune response and clinical benefit for cancer. Curr Immunol Rev 2008; 4: 235–41. [Google Scholar]
- 5. Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity 1999; 10: 281–7. [DOI] [PubMed] [Google Scholar]
- 6. Andersen MH, Becker JC, Straten P. Regulators of apoptosis: suitable targets for immune therapy of cancer. Nat Rev Drug Discov 2005; 4: 399–409. [DOI] [PubMed] [Google Scholar]
- 7. Van der Bruggen P, Traversari C, Chomez P et al A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991; 254: 1643–7. [DOI] [PubMed] [Google Scholar]
- 8. Kameshima H, Tsuruma T, Torigoe T et al Immunogenic enhancement and clinical effect by type‐I interferon of anti‐apoptotic protein, survivin‐derived peptide vaccine, in advanced colorectal cancer patients. Cancer Sci 2011; 102: 1181–7. [DOI] [PubMed] [Google Scholar]
- 9. Tsuruma T, Hata F, Torigoe T et al PhaseI clinical study of anti‐apoptosis protein, survivin‐derived peptide vaccine therapy for patients with advanced or recurrent colorectal cancer. J Transl Med 2004; 2: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsuruma T, Iwayama Y, Ohmura T et al Clinical and immunological evaluation of anti‐apoptosis protein, survivin‐derived peptide vaccine in phase I clinical study for patients with advanced or recurrent breast cancer. J Transl Med 2008; 6: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawaguchi S, Wada T, Ida K et al Phase Ivaccination trial of SYT‐SSX junction peptide in patients with disseminated synovial sarcoma. J Transl Med 2005; 3: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Honma I, Kitamura H, Torigoe H et al Phase I clinical study of anti‐apoptosis protein survivin‐derived peptide vaccination for patients with advanced or recurrent urothelial cancer. Cancer Immunol Immunother 2009; 58: 1801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Torigoe T, Asanuma H, Nakazawa E et al Establishment of a monoclonal anti‐pan HLA class I antibody suitable for immunostaining of formalin‐fixed tissue: unusually high frequency of down‐regulation in breast cancer tissues. Pathol Int 2012; 62: 303–8. [DOI] [PubMed] [Google Scholar]
- 14. Coulie PG, Karanikas V, Lurquin C. Cytolytic T‐cell response of cancer patients vaccinated with a MAGE antigen. Immunol Rev 2002; 188: 33–42. [DOI] [PubMed] [Google Scholar]
- 15. Nagaraj S, Pisarev V, Kinarsky L et al Dendritic cell‐based full‐length survivin vaccine in treatment of experimental tumors. J Immunother 2007; 30: 169–79. [DOI] [PubMed] [Google Scholar]
- 16. Hirohashi Y, Torigoe T, Maeda A et al An HLA‐A24‐restricted cytotoxic T lymphocyte epitope of a tumor‐associated protein, survivin. Clin Cancer Res 2002; 8: 1731–9. [PubMed] [Google Scholar]
- 17. Idenoue S, Hirohashi Y, Torigoe T et al A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res 2005; 11: 1474–82. [DOI] [PubMed] [Google Scholar]
- 18. Le Bon A, Etchart N, Rossmann C et al Cross‐priming of CD8+ T cells stimulated by virus‐induced type I interferon. Nat Immunol 2003; 4: 1009–15. [DOI] [PubMed] [Google Scholar]
- 19. Dikopoulos N, Bertoletti A, Kroeger A, Hauser H, Schirmbeck R, Reimann J. Type I IFN negatively regulates CD8+ T cell responses through IL‐10‐producing CD4+ T regulatory 1 cells. J Immunol 2005; 174: 99–109. [DOI] [PubMed] [Google Scholar]
- 20. Di Pucchio T, Pilla L, Capone I et al Immunization of stage IV melanoma patients with Melan‐A/MART‐1 and gp100 peptides plus IFN‐α results in the activation of specific CD8+ T cells and monocyte/dendritic cell precursors. Cancer Res 2006; 66: 4943–51. [DOI] [PubMed] [Google Scholar]
- 21. Gigante M, Mandic M, Wesa AK et al Interferon‐alpha (IFN‐alpha)‐conditioned DC preferentially stimulate type‐1 and limit Treg‐type in vitro T‐cell responses from RCC patients. J Immunother 2008; 31: 254–62. [DOI] [PubMed] [Google Scholar]
- 22. Schwaab T, Schwarzer A, Wolf B et al Clinical and immunologic effect of intranodal autologous tumor lysate‐dendritic cell vaccine with Aldesleukim (interleukin 2) and IFN‐α2a therapy in metastatic renal cell carcinoma patients. Clin Cancer Res 2009; 15: 4986–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trepiakas R, Pedersen AE, Met O, Svane IM. Addition of interferon‐alpha to a standard maturation cocktail induces CD38 up‐regulation and increases dendritic cell function. Vaccine 2009; 27: 2213–9. [DOI] [PubMed] [Google Scholar]
- 24. Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S. Cross‐presentation of glycolipid from tumor cells loaded with α‐galactsylceramide leads to potent and long‐lived T cell mediated immunity via dendritic cells. J Exp Med 2007; 204: 2641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Badovinac VP, Messingham KN, Jabbari A, Haring JS, Harty JT. Accelerated CD8+ T‐cell memory and prime‐boost response after dendritic‐cell vaccination. Nature Med 2005; 11: 748–56. [DOI] [PubMed] [Google Scholar]
- 26. Spadaro F, Lapenta C, Donati S et al IFN−α enhances cross‐presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood 2012; 119: 1407–17. [DOI] [PubMed] [Google Scholar]
- 27. Truong P, Heydari S, Garidou L, McGavern DB. Persistent viral infection elevates central nervous system MHC class I through chronic production of interferons. J Immunol. 2009; 183: 3895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garrido F, Cabrera T, Aptsiauri N. Hard and soft lesions underlying the HLA class I alterations in cancer cells; implications for immunotherapy. Int J Cancer 2010; 127: 249–56. [DOI] [PubMed] [Google Scholar]
- 29. Khallouf H, Marten A, Serba S et al 5‐Fluorouracil and interferon‐α immunotherapy enhances immunogenicity of murine pancreatic cancer through upregulation of NKG2S ligands and MHC class I. J Immunother 2012; 35: 245–53. [DOI] [PubMed] [Google Scholar]


