Abstract
Serum tumor markers, including α‐fetoprotein (AFP) and des‐γ‐carboxy prothrombin (DCP), are currently used in the diagnosis of hepatocellular carcinoma (HCC). There is, however, an aberrant increase in serum DCP in patients with obstructive jaundice, vitamin K deficiency or who are taking warfarin, resulting from a problem with the current methodology for measurement of this marker. This study aimed to elucidate the utility of a new biomarker, NX‐PVKA, for early diagnosis of HCC. A total of 96 patients were included in the HCC group. The control group included 138 liver cirrhosis (LC) patients without HCC. Serum concentrations of conventional DCP, AFP, AFP‐L3 and NX‐PVKA were measured. The NX‐PVKA ratio was calculated by dividing DCP by NX‐PVKA. In patients not taking warfarin, the area under the curve values of DCP, NX‐PVKA ratio, AFP and AFP‐L3 were 0.715, 0.690, 0.737 and 0.654, respectively, confirming the clinical utility of these markers in detecting HCC. In cases with DCP > 35 mAU/mL in particular, a significant increase in the NX‐PVKA ratio was observed in patients with HCC. In those cases, the cut‐off value for the NX‐PVKA ratio that was optimized by the receiver operating characteristic (ROC) curve was 1.15. In addition, the sensitivity and specificity for diagnosing HCC were 69.2% and 75.9%, respectively. Patients with HCC had higher NX‐PVKA ratios compared to patients with LC taking warfarin (P = 0.063). These results suggest that, when used in combination with DCP, the NX‐PVKA ratio is a promising novel marker for the detection of HCC.
Hepatocellular carcinoma (HCC), one of the most common cancers worldwide, is the fourth leading cause of cancer mortality in Japan.1 The most important risk factors for HCC are chronic liver injury caused by hepatitis B virus, hepatitis C virus, non‐alcoholic fatty liver disease (NAFLD) and alcohol‐induced liver disease (ALD).2, 3 It is recommended that patients who are at high risk, based on the presence of these factors, be screened for HCC with periodical imaging studies, such as ultrasonography (US), contrast‐enhanced computed tomography (CECT), or MRI. In addition, serum tumor markers including α‐fetoprotein (AFP), Lens culinaris agglutinin A‐reactive fraction of AFP (AFP‐L3), and des‐γ‐carboxy prothrombin (DCP) are currently used in the diagnosis of HCC.4, 5, 6 Although the utility of these tumor markers for the detection of HCC has become widely accepted, both the sensitivity and specificity of these markers require improvement for more accurate and rapid diagnosis.
Des‐γ‐carboxy prothrombin, also called prothrombin induced by vitamin K absence‐II (PIVKA‐II), is an abnormal prothrombin that has no coagulability. It has been widely used as a tumor marker of HCC since its efficacy was first reported by Liebman et al. in 1984.7 The precursor of prothrombin, which has 10 glutamic acid (Glu) residues in the N‐terminal domain, is converted to prothrombin by the vitamin K‐dependent enzymatic reaction of γ‐glutamyl carboxylase. Under normal conditions, all 10 of the Glu residues are completely converted to γ‐carboxylated glutamic acid by this enzymatic process. When this reaction is disturbed, however, DCP that carries some Glu residues is produced. Several mechanisms for DCP production in HCC have been proposed, including impaired activity of γ‐glutamyl carboxylase in HCC cells, a decline in the availability of vitamin K resulting from abnormal vitamin K metabolism, and overexpression of the prothrombin precursor in HCC.8
In previous studies, the sensitivity and specificity of DCP was reported to be 40–54% and 81–98%, respectively.9, 10, 11, 12, 13, 14, 15, 16, 17 Based on problems with the current methodology of measurement, however, DCP levels are reported to be aberrantly increased in the serum of patients with obstructive jaundice, vitamin K deficiency and those who are taking warfarin. Serum DCP levels are measured by using the MU‐3 antibody that reacts with DCP that has 7–9 Glu residues, which is produced in HCC.18 Des‐γ‐carboxy prothrombin induced by vitamin K deficiency, however, carries 2–9 Glu residues and also reacts partially with the MU‐3 antibody, which results in the detection of elevated DCP in non‐HCC individuals. To overcome this problem, a new technology that enables the measurement of DCP with 2–9 Glu residues (named NX‐PVKA), has been developed.19 This method uses two antibodies (P‐11 and P‐16) that are reactive mainly to DCP induced by vitamin K deficiency and less reactive to DCP produced by HCC. Toyoda et al.19 suggested that the ability to diagnose HCC in patients taking warfarin is improved by measuring the combination of NX‐PVKA and conventional DCP. The best way to combine these two markers and the utility of that combination in diagnosing HCC in patients who are not taking warfarin, however, remains unclear.
In the present study, we attempted to elucidate the clinical utility of the NX‐PVKA ratio and the optimal way to use it in diagnosing HCC in patients who are and are not taking warfarin.
Materials and Methods
Patients
Patients with chronic liver disease in whom DCP was measured for screening for HCC at Mie University Hospital from January 2011 to December 2012 were enrolled in this study. Of the individuals that were screened, 96 patients were included in the HCC group. Fifty‐five of these patients were initially diagnosed with HCC and 41 patients had a history of radical treatment for HCC and had recurrence of HCC at the time of the study period. Hepatocellular carcinoma was diagnosed based on typical findings from imaging studies, such as contrast‐enhanced computed tomography, magnetic resonance imaging, ultrasonography and abdominal angiography. The control group included 131 patients with liver cirrhosis (LC) who did not have HCC. Liver cirrhosis was diagnosed based on histological examination, laboratory examination and/or imaging studies.
The study was approved by the Institutional Review Board of Mie University Hospital. Informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
Measurements
The blood samples were centrifuged, and the sera were stored at −20°C until measurement. The concentration of conventional DCP, which is currently used in clinical settings, was measured with the Picolumi PIVKA‐II kit (Eisai, Tokyo, Japan), an electrochemiluminescent immunoassay (ECLIA) that uses MU‐3 antibody. NX‐PVKA levels were determined by the ECLIA with P‐11 and P‐16 antibodies, as previously reported by Toyoda et al.19 The NX‐PVKA ratio was calculated by dividing DCP by NX‐PVKA. Aliquots of serum samples kept at −20°C were sent to a clinical laboratory testing company (SRL, Tokyo, Japan) for the measurement of serum undercarboxylated osteocalcin (ucOC), AFP and AFP‐L3 levels.
Statistical analysis
Differences in continuous variables between the two groups were evaluated by the Mann–Whitney U‐test with IBM spss statistics 20 (IBM Japan, Tokyo, Japan). Statistical significance was defined as P < 0.05.
Results
Tumor markers in patients not taking warfarin
Des‐γ‐carboxy prothrombin was measured in a total of 1198 patients throughout the study period including 96 patients with HCC and 131 patients with LC as described above. Of these, five patients with HCC and four patients with LC were taking warfarin. The characteristics of patients who were not taking warfarin are shown in Table 1. Liver cirrhosis patients had more impaired liver function compared to HCC patients. Serum ucOC levels were not different between LC and HCC groups (Table 1) and there was no correlation between serum ucOC and DCP or NX‐PVKA (Fig. 1a,b). Figure 1(c) shows the box plots of DCP, the NX‐PVKA ratio, AFP and AFP‐L3. Des‐γ‐carboxy prothrombin levels were significantly different between the HCC group (median, 52 mAU/mL and range, 10–415 000 mAU/mL) and the LC group (median, 25 mAU/mL and range, 7–1153 mAU/mL; P < 0.01). In addition, the NX‐PVKA ratio was significantly different between the HCC group (median, 1.19 and range, 0.52–48.2) and LC group (median, 0.95 and range, 0.25–6.68; P < 0.01). Moreover, AFP levels (median, 10 ng/mL and range, 1.0–167 466 ng/mL for HCC group) and (median, 4.0 ng/mL and range, 1.0–138 ng/mL for LC group; P < 0.01), and AFP‐L3 levels (median, 0.5% and range 0–80.2% for HCC group) and (median, 0% and range 0–13.6% for LC group) were significantly different between the two groups.
Table 1.
Characteristics of patients not taking warfarin
| HCC n = 91 | LC n = 127 | P‐value | |
|---|---|---|---|
| Age (years) | 72 (36–87) | 64 (28–89) | <0.010 |
| Sex (male/female) | 73/18 | 78/56 | <0.010 |
| Etiology (HBV/HCV/alcohol/other) | 13/55/10/13 | 14/48/33/32 | <0.010 |
| Total bilirubin (mg/dL) | 0.7 (0.2–2.9) | 0.9 (0.2–8.6) | <0.010 |
| Albumin (g/dL) | 3.9 (2.6–5.0) | 3.8 (1.7–5.0) | 0.092 |
| ALT (IU/dL) | 32 (7–208) | 24 (5–129) | 0.047 |
| Platelet (×104) | 12.5 (2.7–32.5) | 9.0 (1.2–31.6) | <0.010 |
| PT‐INR | 1.03 (0.86–1.47) | 1.13 (0.9–2.1) | <0.010 |
| DCP (mAU/mL) | 52 (10–415 000) | 25 (7–1153) | <0.010 |
| NX‐PVKA ratio | 1.19 (0.52–48.2) | 0.95 (0.25–6.68) | <0.010 |
| AFP (ng/mL) | 10 (1–167 466) | 4 (1–138) | <0.010 |
| AFP L3 (%) | 0.5 (0–80.2) | 0 (0–13.6) | <0.010 |
| ucOC (ng/mL) | 1.88 (0.38–10.8) | 3.23 (0.38–19.80) | 0.224 |
| Child–Pugh (A/B/C) | 79/12 | ||
| UICC Stage (I/II/III/IV) | 39/41/7/4 | ||
| Size of tumor (mm) | 22 (6–150) | ||
| Number of tumors | 2 (1–20) | ||
| Primary/Recurrence | 52/39 | ||
| VP (+/−) | 13/78 |
AFP, α‐fetoprotein; ALT, alanine aminotransferase; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LC, liver cirrhosis; PT‐INR, prothrombin time‐international normalized ratio; UICC, Union for International Cancer Control; VP, portal vein invasion.
Figure 1.
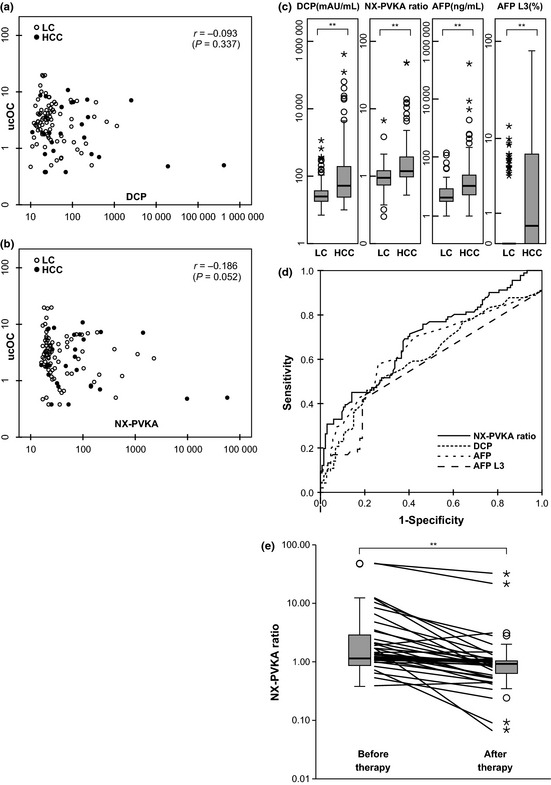
Analysis of each parameter in patients who were not taking warfarin. (a) Scatter diagram of des‐γ‐carboxy prothrombin (DCP; X axis) and undercarboxylated osteocalcin (ucOC; Y axis). (b) Scatter diagram of NX‐PVKA (X axis) and ucOC (Y axis). (c) Serum levels of DCP, NX‐PVKA ratio, α‐fetoprotein (AFP) and AFP‐L3 in patients with hepatocellular carcinoma (HCC) and liver cirrhosis (LC). Significant differences were found between patients with HCC and LC for each tumor marker (**P < 0.01). (d) Receiver operating characteristic (ROC) curves of DCP, NX‐PVKA ratio, AFP and AFP‐L3 for diagnosis of HCC. The area under the ROC curves of DCP, NX‐PVKA ratio, AFP and AFP‐L3 were 0.715, 0.690, 0.737 and 0.654, respectively. (e) Serum levels of NX‐PVKA ratio before and after therapy for HCC. Significant difference was observed (**P < 0.01).
Cut‐off values of tumor markers
Receiver operating characteristic (ROC) curves of DCP, the NX‐PVKA ratio and AFP are shown in Figure 1(d). The area under the curve (AUC) values for DCP, the NX‐PVKA ratio, AFP and AFP‐L3 were 0.715, 0.690, 0.737 and 0.654, respectively. When the cut‐off values for DCP, AFP and AFP‐L3 were set at 40 mAU/mL, 10 ng/mL and 10%, which are currently used in clinical practice, these values yielded a sensitivity and specificity for DCP, AFP and AFP‐L3 of 56.0% and 79.5%, 48.4% and 84.2% and 20.7% and 98.9%, respectively. In addition, when the cutoff level of the NX‐PVKA ratio was set at 1.5 as reported by Toyoda et al., this yielded a sensitivity value of 33.0% and specificity value of 92.9%. When the cut‐off values for DCP, the NX‐PVKA ratio, AFP and AFP‐L3 optimized by ROC curves were 35.0 mAU/mL, 1.0, 7.5 ng/mL and 5%, these values yielded a sensitivity and specificity for DCP, the NX‐PVKA ratio, AFP and AFP‐L3 of 57.1% and 77.2%, 71.4% and 59.8%, 65.9% and 74.3% and 25.6% and 87.5%, respectively. The sensitivity and specificity were 25.3% and 89.1% when we combined AFP and AFP‐L3 (Table 2).
Table 2.
Analysis of cut‐off values
| Cut‐off value | Sensitivity (%) | Specificity (%) | Accuracy (%) | |
|---|---|---|---|---|
| DCP | 35.0 (mAU/mL) | 57.1 | 77.2 | 64.2 |
| NX‐PVKA ratio | 1.0 | 71.4 | 59.8 | 56.0 |
| AFP | 7.5 (ng/mL) | 65.9 | 74.3 | 69.8 |
| AFP L3 | 5.0 (%) | 25.6 | 87.5 | 65.6 |
AFP, α‐fetoprotein; DCP, des‐γ‐carboxy prothrombin.
Change in NX‐PVKA ratio after therapy
The NX‐PVKA ratio was compared before and after treatment for HCC in 37 cases. This ratio decreased significantly post‐therapy (median 1.44, range 0.53–48.2 before therapy, median 1.00, range 0.07–32.5 after therapy; P < 0.01; Fig. 1e).
Combination of DCP and the NX‐PVKA ratio
The scatter diagram of DCP (x axis) and the NX‐PVKA ratio (y axis) is shown in Figure 2(a). The pattern of the diagram observed for DCP values <35 mAU/mL differed from that observed for DCP > 35. In cases with DCP ≤ 35, there was a strong correlation between DCP and the NX‐PVKA ratio with a correlation coefficient of 0.775 (P < 0.01; Fig. 2b). Furthermore, there were no differences in both DCP and the NX‐PVKA ratio between the LC and HCC groups (P = 0.35, P = 0.80, respectively). In cases with DCP > 35, however, the correlation between DCP and the NX‐PVKA ratio was very weak, with a correlation coefficient of 0.399 (P < 0.01; Fig. 2c). Des‐γ‐carboxy prothrombin values tended to be different between LC and HCC in this group of patients, but did not reach statistical significance (P = 0.06). On the other hand, the NX‐PVKA ratio was significantly different between the LC and HCC groups (P < 0.01; Fig. 3a). Figure 3(b) shows the ROC curve of DCP and the NX‐PVKA ratio in cases with DCP > 35. The AUC values for DCP and NX‐PVKA ratio were 0.664 and 0.796, respectively. The cut‐off value of the NX‐PVKA ratio optimized by the ROC curve was 1.15. These values yielded a sensitivity and specificity for the NX‐PVKA ratio of 69.2% and 75.9% in cases with DCP > 35, while the sensitivity and specificity were 39.6% and 94.5% for all cases. In LC patients without HCC, the majority of cases with increased DCP were included below this cut‐off level in the scatter diagram (Fig. 2c).
Figure 2.
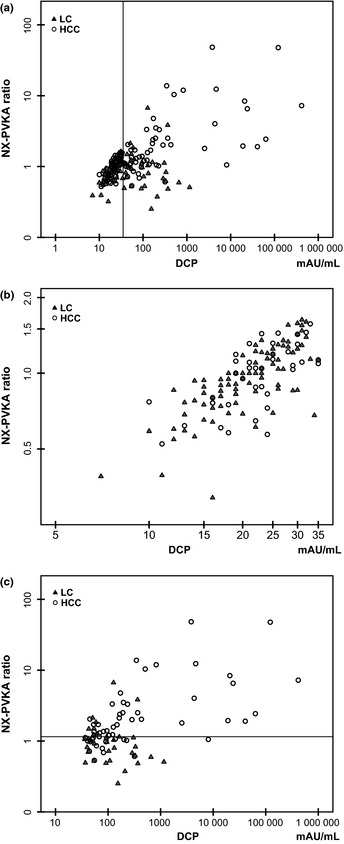
Scatter diagram of des‐γ‐carboxy prothrombin (DCP; X axis) and NX‐PVKA ratio (Y axis). (a) All cases who were not taking warfarin. The additional vertical line indicates DCP value of 35 mAU/mL. (b) Cases with DCP ≤ 35 mAU/mL. (c) Cases with DCP > 35 mAU/mL. The additional horizontal line indicates NX‐PVKA ratio of 1.15. Open circles indicate hepatocellular carcinoma (HCC) patients and closed triangles indicate liver cirrhosis (LC) patients in each panel.
Figure 3.
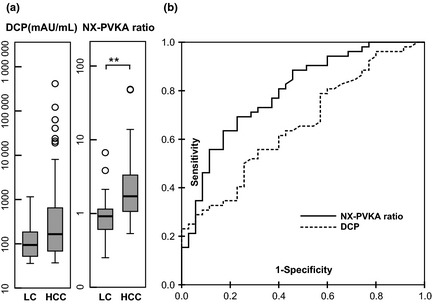
Analysis of NX‐PVKA and des‐γ‐carboxy prothrombin (DCP) in cases with DCP > 35 mAU/mL among patients not taking warfarin. (a) Significant differences were found in the NX‐PVKA ratio but not in DCP between patients with hepatocellular carcinoma (HCC) and liver cirrhosis (LC; **P < 0.01). (b) Receiver operating characteristic (ROC) curves of DCP and NX‐PVKA ratio in cases with DCP > 35 mAU/mL. The area under the ROC curves for DCP and NX‐PVKA ratio were 0.664 and 0.796, respectively.
NX‐PVKA ratio in patients with warfarin
Five patients with HCC and four patients with LC were taking warfarin. The characteristics of patients who were taking warfarin are shown in Table 3. Des‐γ‐carboxy prothrombin levels did not differ between the HCC group (median, 12 285 mAU/mL and range, 4208–29 287 mAU/mL) and the LC group (median, 11 805 mAU/mL and range, 1526–27 631 mAU/mL). Although the NX‐PVKA ratio was different between the HCC group (median, 0.64 and range, 0.39–0.83) and the LC group (median, 0.41 and range, 0.29–0.49), this difference did not reach statistical significance, probably due to the small number of study cases (P = 0.063). Similarly, AFP levels were different between the HCC group (median, 11 ng/mL and range, 2.0–132 ng/mL) and the LC group (median, 2.0 ng/mL and range, 2.0–4.0 ng/mL), but the difference did not reach statistical significance (P = 0.071; Fig. 4).
Table 3.
Characteristics of patients taking warfarin
| HCC n = 5 | LC n = 4 | P‐value | |
|---|---|---|---|
| Age (years) | 74 (63–78) | 70 (44–82) | 1.000 |
| Sex (male/female) | 4/1 | 1/3 | 0.099 |
| Etiology (HBV/HCV/alcohol/other) | 5/0/0/0 | 1/1/1/1 | 0.131 |
| Total bilirubin (mg/dL) | 0.90 (0.7–1.4) | 0.75 (0.5–1.7) | 1.000 |
| Albumin (g/dL) | 3.5 (3.1–4.3) | 4.0 (3.0–4.4) | 0.730 |
| ALT (IU/dL) | 41 (16–70) | 12 (11–36) | 0.063 |
| Platelet (×104) | 13.9 (5.4–22.2) | 14.1 (8.1–80) | 0.905 |
| PT‐INR | 1.18 (1.11–2.01) | 1.57 (1.19–2.24) | 0.286 |
| DCP (mAU/mL) | 12 285 (4208–29 287) | 11 805 (1526–27 631) | 1.000 |
| NX‐PVKA ratio | 0.64 (0.39–0.83) | 0.41 (0.29–0.49) | 0.063 |
| AFP (mAU/mL) | 11 (2–132) | 2 (2–4) | 0.071 |
| AFP L3 (%) | 0 (0–3.6) | N/A | |
| Child–Pugh (A/B/C) | 4/1/0 | ||
| UICC Stage (I/II/III/IV) | 3/1/1/0 | ||
| Size of tumor (mm) | 24 (10–51) | ||
| Number of tumors | 2 (1–5) | ||
| Primary/Recurrence | 3/2 | ||
| VP (+/−) | 1/4 |
AFP, α‐fetoprotein; ALT, alanine aminotransferase; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LC, liver cirrhosis; PT‐INR, prothrombin time‐international normalized ratio; UICC, Union for International Cancer Control; VP, portal vein invasion.
Figure 4.
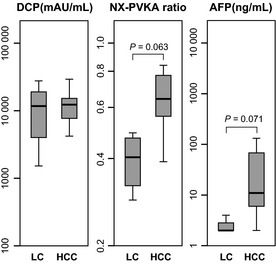
Serum levels of des‐γ‐carboxy prothrombin (DCP), NX‐PVKA ratio and α‐fetoprotein (AFP) in patients with hepatocellular carcinoma (HCC) and liver cirrhosis (LC) who were taking warfarin. Des‐γ‐carboxy prothrombin was not different between patients with HCC and LC. Both the NX‐PVKA ratio and AFP levels were higher in HCC patients compared to LC patients, though the differences did not reach statistical significance (P = 0.063 and P = 0.071, respectively).
Change in NX‐PVKA ratio after therapy
The NX‐PVKA ratio before and after therapy was measured in three patients with HCC who were taking warfarin. The ratio tended to decrease after therapy, but the difference was not statistically significant, probably due to the small number of cases (median 0.64, range 0.39–0.83 before therapy, median 0.45, range 0.34–0.45 after therapy; P = 0.285).
Discussion
In this study, we demonstrated the usefulness of the NX‐PVKA ratio in differentiating between patients with HCC and patients with LC, all of whom were not taking warfarin. The NX‐PVKA ratio was more useful particularly in patients with conventional DCP values > 35 mAU/mL. Furthermore, our results showed that the NX‐PVKA ratio is useful for predicting HCC in patients taking warfarin.
Since serum DCP is known to be elevated in vitamin K deficiency, we analyzed the correlation between DCP/NX‐PVKA and ucOC. It has been reported that ucOC is released from osteoblasts into the blood stream in vitamin K deficiency. Therefore, the serum ucOC concentration is a sensitive marker for vitamin K status.20, 21 Our results showed that serum ucOC levels did not correlate with either DCP or NX‐PVKA levels, indicating that elevations of DCP/NX‐PVKA were not due to vitamin K deficiency in these cases. Values for DCP, AFP, AFP‐L3 and the NX‐PVKA ratio were all significantly different in HCC and LC patients. The cut‐off levels of DCP, AFP, AFP‐L3 and the NX‐PVKA ratio optimized by the ROC curve, applied to all cases, were 35 mAU/mL, 7.5 ng/mL, 5% and 1.0, respectively. When these cut‐off levels were applied, the sensitivity and specificity of each of these markers were: DCP (sensitivity 57.1%, specificity 77.2%), AFP (65.9%, 74.3%), AFP‐L3 (25.6%, 87.5%) and the NX‐PVKA ratio (71.4%, 59.8%). When we combined AFP and AFP‐L3, the sensitivity and specificity were 25.3% and 89.1%, respectively. In previous studies, the sensitivity and specificity of DCP was reported to range from 40% to 54% and 81% to 98%, respectively, and that of AFP was reported to be 39–64% and 76–91% for sensitivity and specificity, respectively.22, 23, 24 In the present study, the results we obtained for DCP and AFP were consistent with previous reports. Furthermore, the sensitivity and specificity of the NX‐PVKA ratio was comparable with that of DCP and AFP, confirming the utility of the NX‐PVKA ratio for early diagnosis of HCC. NX‐PVKA ratios were decreased significantly after radical therapy for HCC, suggesting that the NX‐PVKA ratio is directly influenced by the existence of HCC and is a useful tool for following‐up on HCC.
To further explore the utility of NX‐PVKA, we examined a combination of DCP and the NX‐PVKA ratio. Since we initially hypothesized that there was a correlation between the NX‐PVKA ratio and DCP, we drew a scatter diagram of DCP and the NX‐PVKA ratio. The diagram showed two populations that had different tendencies, divided at a DCP value of 35 mAU/mL. In cases with DCP > 35 mAU/mL, there was only a weak correlation between DCP and the NX‐PVKA ratio (r = 0.399). Moreover, a significant difference in NX‐PVKA ratio was observed between HCC and LC but DCP was not different between the two groups. In these cases, the cut‐off value for the NX‐PVKA ratio that was optimized by the ROC curve was 1.15; at this cut‐off, the sensitivity and specificity for diagnosis were better than that of all cases. Also, these values were superior to those obtained by the combination of AFP and AFP‐L3 (sensitivity 39.6%, specificity 94.5% in all cases, sensitivity 69.2%, specificity 75.9% in cases with DCP > 35 by DCP and NX‐PVKA ratio versus sensitivity 25.3%, specificity 89.1% by AFP and AFP‐L3). Conversely, in cases with DCP ≤ 35 mAU/mL, DCP and the NX‐PVKA ratio were strongly correlated (r = 0.775) and there was no difference between HCC and LC patients.
One of the main findings from this study was that the NX‐PVKA ratio was very useful for detecting HCC, particularly in cases with values for DCP > 35 mAU/mL. Moreover, using the NX‐PVKA ratio, we were able to rule out the existence of HCC in most LC cases with DCP > 35 mAU/mL. The cut‐off level of conventional DCP is generally set at 40 mAU/mL in our daily clinical practice. However, we often encounter patients with liver disease who have slightly elevated DCP levels, but undetectable HCC as assessed by imaging studies. It has been reported that aberrant elevation of DCP is occasionally observed in patients with alcoholic cirrhosis or vitamin K deficiency. In our practice, we sometimes have patients who develop HCC during the course of having higher DCP levels, which were initially thought to be aberrantly elevated. We previously reported that patients who exhibit fluctuating DCP levels are more likely to develop HCC.25 For this reason, we think it is very helpful to use a combination of the NX‐PVKA ratio and DCP to ensure an early and accurate diagnosis of HCC.
Another important finding from the present study is that the NX‐PVKA ratio is also a useful marker for HCC in patients taking warfarin. Since DCP is usually remarkably elevated in patients taking warfarin, it loses utility for diagnosing HCC in these cases. In our study, DCP values did not differ between HCC and LC patients taking warfarin. Hepatocellular carcinoma patients, however, had higher NX‐PVKA ratios compared to LC patients, even if they were taking warfarin, although the difference did not reach statistical significance. Furthermore, the NX‐PVKA ratio in HCC patients taking warfarin decreased after treatment, suggesting that this ratio was useful for both detection and follow‐up of HCC. Toyoda et al.19 recommended 1.5 as the cut‐off level for the NX‐PVKA ratio in cases where warfarin is used since it had maximal accuracy at that level with sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of 60.0%, 100.0%, 100.0%, 87.5%, and 89.5%, respectively. The authors also reported, however, that when the cut‐off level was lowered to 0.65, which was optimized by the ROC, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy changed to 95.0%, 73.2%, 55.9%, 97.6%, and 78.9%, respectively. In the present study, all patients taking warfarin had lower NX‐PVKA ratios compared to cases not taking warfarin (NX‐PVKA ratio < 1 in all warfarin cases). We speculate that the reason we observed relatively lower NX‐PVKA ratios with warfarin use is that NX‐PVKA was measured with P‐11 and P‐16 antibodies that have a higher affinity to DCP with 2–9 Glu residues, which is induced by deficiency or disturbed used of vitamin K and by the effects of warfarin. This would result in relatively lower NX‐PVKA ratios since the ratio is calculated by dividing DCP by NX‐PVKA. We believe, therefore, that the cut‐off value of NX‐PVKA ratio in cases of warfarin use should be lowered. Furthermore, we propose that the number of patients taking warfarin for cardiovascular diseases will increase in the near future, as a result of aging, and the necessity for use of the NX‐PVKA ratio will increase.
However, we acknowledge the present study had a few problems. First, there were several differences in patient characteristics between the HCC and LC groups. For example, our LC patients had more impaired liver function tests, and number of alcoholic patients was greater in the LC group. Though alcohol consumption is known to increase serum DCP level,26 our HCC patients had higher levels of serum DCP/NX‐PVKA ratio indicating the elevation of DCP/NX‐PVKA ratio was derived from production by HCC rather than alcohol. Another problem in this study was that it was performed on a limited number of patients especially in warfarin cases. Further studies with more patients are therefore necessary to establish the NX‐PVKA ratio as a new and potent marker for HCC in cases taking warfarin.
In conclusion, we showed in the present study that the NX‐PVKA ratio is a novel and promising marker for the detection of HCC, especially in cases with DCP > 35 mAU/mL. The optimal cut‐off level remains to be investigated both in cases with and without warfarin. Further studies with an increased number of study cases are necessary in order to establish the diagnostic strategy for the use of this novel tumor marker.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgments
We thank Tooru Komatsu for his technical assistance.
(Cancer Sci, doi: 10.1111/cas.12149, 2013)
References
- 1. Matsuda T, Marugame T, Kamo K, Katanoda K, Ajiki W, Sobue T. Cancer incidence and incidence rates in Japan in 2006: based on data from 15 population‐based cancer registries in the monitoring of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2012; 42: 139–47. [DOI] [PubMed] [Google Scholar]
- 2. El‐Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142: 1264–73 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010; 51: 1820–32. [DOI] [PubMed] [Google Scholar]
- 4. Malaguarnera G, Giordano M, Paladina I, Berretta M, Cappellani A, Malaguarnera M. Serum markers of hepatocellular carcinoma. Dig Dis Sci 2010; 55: 2744–55. [DOI] [PubMed] [Google Scholar]
- 5. Durazo FA, Blatt LM, Corey WG et al Des‐gamma‐carboxyprothrombin, alpha‐fetoprotein and AFP‐L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol 2008; 23: 1541–8. [DOI] [PubMed] [Google Scholar]
- 6. Beppu T, Sugimoto K, Shiraki K et al Clinical significance of tumor markers in detection of recurrent hepatocellular carcinoma after radiofrequency ablation. Int J Mol Med 2010; 26: 425–33. [PubMed] [Google Scholar]
- 7. Liebman HA, Furie BC, Tong MJ et al Des‐gamma‐carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med 1984; 310: 1427–31. [DOI] [PubMed] [Google Scholar]
- 8. Inagaki Y, Tang W, Makuuchi M, Hasegawa K, Sugawara Y, Kokudo N. Clinical and molecular insights into the hepatocellular carcinoma tumour marker des‐gamma‐carboxyprothrombin. Liver Int 2011; 31: 22–35. [DOI] [PubMed] [Google Scholar]
- 9. Tsai S‐L, Huang G‐T, Yang P‐M, Sheu J‐C, Sung J‐L, Chen D‐S. Plasma des‐γ‐carboxyprothrombin in the early stage of hepatocellular carcinoma. Hepatology 1990; 11: 481–7. [DOI] [PubMed] [Google Scholar]
- 10. Takikawa Y, Suzuki K, Yamazaki K et al Plasma abnormal prothrombin (PIVKA‐π): a new and reliable marker for the detection of hepatocellular carcinoma. J Gastroenterol Hepatol 1992; 7: 1–6. [DOI] [PubMed] [Google Scholar]
- 11. Mita Y, Aoyagi Y, Yanagi M, Suda T, Suzuki Y, Asakura H. The usefulness of determining des‐γ‐carboxy prothrombin by sensitive enzyme immunoassay in the early diagnosis of patients with hepatocellular carcinoma. Cancer 1998; 82: 1643–8. [DOI] [PubMed] [Google Scholar]
- 12. Marrero JA, Su GL, Wei W et al Des‐gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology 2003; 37: 1114–21. [DOI] [PubMed] [Google Scholar]
- 13. Grazi GL, Mazziotti A, Legnani C et al The role of tumor markers in the diagnosis of hepatocellular carcinoma, with special reference to the des‐gamma‐carboxy prothrombin. Liver Transpl Surg 1995; 1: 249–55. [DOI] [PubMed] [Google Scholar]
- 14. Fujiyama S, Izuno K, Yamasaki K, Sato T, Taketa K. Determination of optimum cutoff levels of plasma des‐gamma‐carboxy prothrombin and serum alpha‐fetoprotein for the diagnosis of hepatocellular carcinoma using receiver operating characteristic curves. Tumour Biol 1992; 13: 316–23. [DOI] [PubMed] [Google Scholar]
- 15. Ishii M, Gama H, Chida N et al Simultaneous measurements of serum alpha‐fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol 2000; 95: 1036–40. [DOI] [PubMed] [Google Scholar]
- 16. Kasahara A, Hayashi N, Fusamoto H et al Clinical evaluation of plasma des‐gamma‐carboxy prothrombin as a marker protein of hepatocellular carcinoma in patients with tumors of various sizes. Dig Dis Sci 1993; 38: 2170–6. [DOI] [PubMed] [Google Scholar]
- 17. Nakagawa T, Seki T, Shiro T et al Clinicopathologic significance of protein induced vitamin K absence or antagonist II and alpha‐fetoprotein in hepatocellular carcinoma. Int J Oncol 1999; 14: 281–6. [DOI] [PubMed] [Google Scholar]
- 18. Naraki T, Kohno N, Saito H et al gamma‐Carboxyglutamic acid content of hepatocellular carcinoma‐associated des‐gamma‐carboxy prothrombin. Biochim Biophys Acta 2002; 1586: 287–98. [DOI] [PubMed] [Google Scholar]
- 19. Toyoda H, Kumada T, Osaki Y, Tada T, Kaneoka Y, Maeda A. Novel method to measure serum levels of des‐gamma‐carboxy prothrombin for hepatocellular carcinoma in patients taking warfarin: a preliminary report. Cancer Sci 2012; 103: 921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sokoll LJ, Booth SL, O'Brien ME, Davidson KW, Tsaioun KI, Sadowski JA. Changes in serum osteocalcin, plasma phylloquinone, and urinary gamma‐carboxyglutamic acid in response to altered intakes of dietary phylloquinone in human subjects. Am J Clin Nutr 1997; 65: 779–84. [DOI] [PubMed] [Google Scholar]
- 21. Sokoll LJ, Sadowski JA. Comparison of biochemical indexes for assessing vitamin K nutritional status in a healthy adult population. Am J Clin Nutr 1996; 63: 566–73. [DOI] [PubMed] [Google Scholar]
- 22. Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of α‐fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology 1994; 19: 61–6. [PubMed] [Google Scholar]
- 23. Colombo M, de Franchis R, Del Ninno E et al Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med 1991; 325: 675–80. [DOI] [PubMed] [Google Scholar]
- 24. Pateron D, Ganne N, Trinchet JC et al Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol 1994; 20: 65–71. [DOI] [PubMed] [Google Scholar]
- 25. Shimizu A, Shiraki K, Ito T et al Sequential fluctuation pattern of serum des‐gamma‐carboxy prothrombin levels detected by high‐sensitive electrochemiluminescence system as an early predictive marker for hepatocellular carcinoma in patients with cirrhosis. Int J Mol Med 2002; 9: 245–50. [PubMed] [Google Scholar]
- 26. Ohhira M, Ohtake T, Saito H et al Increase of serum des‐gamma‐carboxy prothrombin in alcoholic liver disease without hepatocellular carcinoma. Alcohol Clin Exp Res 1999; 23: 67S–70S. [DOI] [PubMed] [Google Scholar]


