Abstract
Histological vascular invasion (VI) by tumors is reportedly a risk factor influencing recurrence or survival after surgical treatment; however, few studies have evaluated which VI features affect recurrence or survival. The objective of this study was to evaluate how VI features affect recurrence in lung adenocarcinoma patients. We selected 106 patients with pathological stage I lung adenocarcinoma who showed VI and examined the properties of intravascular tumors associated with recurrence. First we investigated the relationship between the frequency of VI in a histological cross‐section and the incidence of recurrence; however, a significant impact was not observed. Microscopic examination revealed the intravascular tumors were composed of not only cancer cells but also non‐cancerous cells. To examine whether the characteristics of intravascular cancer cells and/or non‐cancerous cells have prognostic value, we examined the expression levels of epithelial–mesenchymal transition‐related markers in cancer cells and the numbers of infiltrating non‐cancerous cells, including macrophages, endothelial cells, and fibroblasts. High levels of E‐cadherin expression in the intravascular cancer cells were significant predictors of recurrence (P = 0.004), whereas the expressions of CD44, CD44 variant 6, and vimentin were not. Large numbers of intravascular CD204(+) macrophages (P = 0.016), CD34(+) microvessels (P = 0.007), and α‐smooth muscle actin (+) fibroblasts (P = 0.033) were also significant predictors of recurrence. Our results indicated VI with abundant stromal cell infiltrates might be a predictor of recurrence and suggested the tumor microenvironment created by cancer cells and stromal cells within the blood vessel may play an important role during the metastatic process.
The cause of death in most cancer patients is the development of metastases from the primary tumor. The metastatic process includes various complex steps. The process starts with the separation of cancer cells from the primary lesion, followed by the permeation of these cells into vessels. In the permeated vessels, the cancer cells overcome apoptosis (also known as anoikis), proliferate, and then transmigrate to the metastatic site.
Vascular invasion represents the early phase of metastasis.1, 2, 3 Therefore, the presence of histological intratumoral VI has been reported to be a predictor of recurrence and metastasis in surgical cases with various types of cancer.4, 5, 6 In fact, VI by testicular germ cell tumors qualifies as the local spread of tumors.7, 8 Testicular tumors localized to the testis and epididymis without VI are classified as T1, whereas they are classified as T2 when VI is present. Although VI has been reported to be a strong prognostic factor in NSCLC, the impact of VI on the revised TNM classification of NSCLC remains to be fully assessed.
Nowadays, tumor tissue is known to be composed of variable numbers of cancer cells and stromal cells, such as macrophages, endothelial cells, and fibroblasts, and cancer cells are known to interact with these surrounding stromal cells, producing a specific microenvironment that is capable of influencing tumor progression.9 Furthermore, recent studies have reported that the intravessel microenvironment has significant effects on metastasis as well as the disease at the primary site. Matsumura et al.10 reported that intralymphatic cancer cell and stromal cell phenotypes are susceptible to lymphogenic metastasis, suggesting that lymphogenic metastasis may be affected by the intralymphatic microenvironment created by these cells.
Lung cancer is the leading cause of cancer‐related deaths worldwide,11 and NSCLC accounts for the majority of lung cancers.12 Adenocarcinoma is the most frequent histological type of NSCLC, and its incidence is increasing in most countries.13 In Japan, adenocarcinoma is the most common histological type of resected lung cancer, accounting for more than 60% of all cases.14 However, although surgical resection is considered to be the most effective therapy for patients with stage I adenocarcinoma, approximately 17.3% of patients develop recurrences.15 Several studies have reported that the presence of VI has a significant impact on the recurrence of lung adenocarcinoma and the survival of patients after surgical treatment. However, few studies have investigated which VI features influence recurrence and/or survival. This issue needs to be examined to clarify the mechanisms underlying the early phase of metastasis.
The objective of the present study was to evaluate how VI features affect recurrence in lung adenocarcinoma. We therefore examined the frequencies and characteristics of intratumoral VI features and their correlations with recurrence.
Materials and Methods
Patient selection
A total of 1099 consecutive patients with pathological stage I primary lung adenocarcinoma underwent surgical resection between January 1995 and December 2007 at the National Cancer Center Hospital East, Chiba, Japan. All the surgical specimens were collected and analyzed after receiving the approval of the Ethics Committee of our institution. No patient consent was required as the research was a retrospective chart review and no personally identifiable information was included.
To examine how VI features affect recurrence, we selected 106 patients with VI excluding patients with pleural invasion (another important predictor of recurrence) from the 1099 patients (Fig. 1a). The frequency of VI was defined as the number of invaded blood vessels on the maximum cut surface of the tumor (Fig. 1b).
Figure 1.
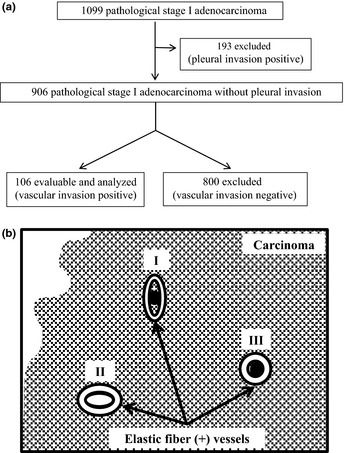
(a) Scheme showing patient selection for this study. (b) Scheme showing the definition of vascular invasion. Of the three illustrated vasculatures (numbered I–III), vascular invasion is evident in two (I and III) with the presence of cancer cells (black circles).
Histological studies
The surgical specimens had been fixed in 10% formalin or 100% methyl alcohol at the time of surgery. The specimens were sliced through the largest diameter of the primary tumor, and all the sections were embedded in paraffin. All the serial 4‐μm sections were stained using the H&E method, the Alcian blue–periodic acid–Schiff method for the detection of cytoplasmic mucin production, or the elastica van Gieson or the VVG method for the detection of elastic fibers. All the histological materials included in this series were reviewed by two pathologists (K.K. and G.I.) to ascertain the presence of VI. Intratumoral VI was defined as tumor cells existing within blood vessels with elastic fibers located inside the primary tumors (Fig. 2a,b). The pathological stage was determined based on the TNM classification of the International Union Against Cancer, 6th edition. Histological typing of the primary tumors was carried out based on the World Health Organization's classification of cell types, 6th edition. Pleural invasion was also evaluated based on whether the tumor cells had invaded the visceral pleura of the lung on elastica van Gieson‐ or VVG‐stained sections.
Figure 2.
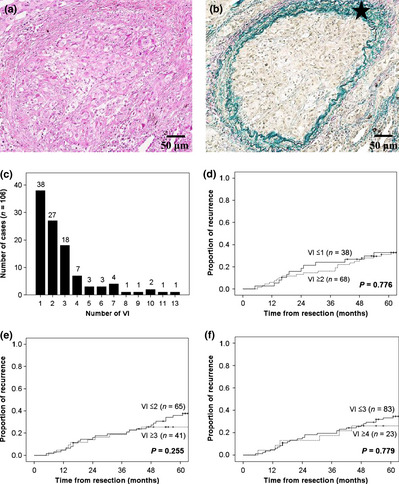
(a,b) Representative images showing vascular invasion (VI) in lung adenocarcinoma patients. Sections were stained using H&E (a) and Victoria blue van Gieson (b). The star indicates elastic fibers with positive Victoria blue van Gieson staining. (c) Distribution of cases with VI according to the frequency of VI on the maximum cut surface. (d–f) Kaplan–Meier analyses of recurrence according to a VI cut‐off level of 1 (d), 2 (e), and 3 (f).
Antibodies and immunohistochemical staining
E‐cadherin (NCH‐384; Dako Cytomation, Glostrup, Denmark), CD44 (DF1485; Novocastra, Newcastle, UK), CD44 variant 6 (VFF‐7; Acris Antibodies, Herford, Germany), and vimentin (VIM 3B4; Progen Biotechnik, Heidelberg, Germany) were used as EMT‐related markers. As for stromal cell markers, CD204 (SRA‐E5; Trans Genic, Hyogo, Japan) was used to evaluate activated macrophages, CD34 (QBEnd 10; Dako Cytomation) was used as an endothelial cell marker, and α‐SMA (1A4; Dako Cytomation) was used as a myofibroblast marker. Immunostaining was carried out using 4‐μm paraffin‐embedded tissue serial sections. The slides were deparaffinized in xylene and dehydrated in a graded ethanol series, and endogenous peroxidase was blocked with 3% hydrogen peroxide in absolute methyl alcohol. After epitope retrieval, the slides were washed with PBS and incubated overnight at 4°C using mouse anti‐human antibodies at a final dilution of 1:100 (E‐cadherin, CD44, CD44 variant 6, α‐SMA, vimentin), 1:200 (CD34), or 1:400 (CD204) in the blocking buffer. The reaction products were stained with diaminobenzidine and counterstained with hematoxylin. After the confirmation of color development, the VVG method was used to examine the immunohistochemical properties of the tumor cells and the stromal cells existing in the blood vessels.
Immunohistochemical scoring
All the stained tissue sections were semiquantitatively scored and evaluated independently under a light microscope. The labeling scores for tumor cells within intratumoral blood vessels were calculated by multiplying the percentage of positive tumor cells per lesion (0–100%) by the staining intensity level (0, negative; 1, weak; 2, strong). For CD204 and α‐SMA, the numbers of positive infiltrating cells in a hot spot were counted under a high‐power microscopic field (×400; 0.0125 mm2). For CD34, the microvessel density was calculated based on the number of CD34(+) microvessels observed under a high‐power microscopic field (×400; 0.0125 mm2), based on a protocol used in a previous study.16 We confirmed that the positive control tissues were stained by each antibody, and we also carried out negative control studies without the primary antigen for all the antibodies. When the antibody evaluations differed, the observers discussed the results, re‐examined the slides, if necessary, and debated the evaluation findings until an agreement was reached. A high score was defined as a score above the median value; a low score was defined as a score below the median value.
Statistical analysis
The length of the recurrence‐free period was calculated in months from the date of resection until the date of the first recurrence or last follow‐up. Cumulative incidences of recurrence were estimated using the Kaplan–Meier analysis, and differences in variables were evaluated using the log–rank test. The Cox proportional hazards multivariate model was used to identify independent predictors. A backward elimination stepwise procedure was used to determine independent predictors. All the variables were initially entered into a backward stepwise analysis using P‐values of 0.10 for entry and 0.05 for removal in each model. Adjusted HRs with 95% CIs were calculated for each statistically significant variable in the final stepwise model. For comparison, unadjusted HRs with 95% CIs were calculated for each candidate variable using univariate Cox analyses to assess the impact of the adjustment. Continuous variables were compared using t‐tests. The significance level was set at P < 0.05. Analyses were carried out using Dr. spss II for Windows, standard version 11.0 (SPSS Inc., Chicago, IL, USA).
Results
Risk factors for recurrence in pathological stage I adenocarcinoma patients
Table 1 shows the risk factors for recurrence according to the clinicopathological characteristics of 1099 patients who had pathological stage I adenocarcinoma. In a univariate analysis, seven variables were found to be significantly associated with recurrence (P < 0.05): male sex; smoking history; elevated serum carcinoembryonic antigen value; large tumor size (3.0 ≥ 5.0 cm), poorly differentiated carcinoma; VI; and pleural invasion. A multivariate Cox proportional hazards model indicated that the presence of VI (HR = 3.955; P < 0.001) and pleural invasion (HR = 2.136; P < 0.001) were statistically significant predictors of recurrence.
Table 1.
Univariate and multivariate analyses of risk factors for recurrence in patients with stage I adenocarcinoma
| Variable | n | Univariate analysis | P‐value† | Multivariate analysis | P‐value‡ |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||||
| Age, years | |||||
| <65 | 533 | 1 | 1 | ||
| ≥65 | 566 | 1.272 (0.954–1.697) | 0.101 | 1.158 (0.858–1.561) | 0.337 |
| Sex | |||||
| Female | 585 | 1 | 1 | ||
| Male | 514 | 1.540 (1.156–2.052) | 0.003* | 1.069 (0.711–1.607) | 0.748 |
| Smoking habits | |||||
| Non‐smoker | 558 | 1 | 1 | ||
| Ever smoker | 541 | 1.625 (1.217–2.171) | 0.001* | 1.158 (0.764–1.756) | 0.488 |
| CEA | |||||
| ≤5 | 824 | 1 | 1 | ||
| >5 | 275 | 1.681 (1.243–2.273) | 0.001* | 1.026 (0.747–1.411) | 0.872 |
| Tumor size, cm | |||||
| ≤3.0 | 860 | 1 | 1 | ||
| >3.0, ≤5.0 | 239 | 2.365 (1.758–3.182) | <0.001* | 1.372 (0.985–1.912) | 0.061 |
| Histological differentiation | |||||
| Well/mod. | 1010 | 1 | 1 | ||
| Poor | 89 | 2.010 (1.319–3.063) | 0.001* | 0.765 (0.484–1.208) | 0.250 |
| Vascular invasion | |||||
| Absent | 831 | 1 | 1 | ||
| Present | 268 | 5.566 (4.165–7.439) | <0.001* | 3.955 (2.818–5.552) | <0.001* |
| Pleural invasion | |||||
| Absent | 906 | 1 | 1 | ||
| Present | 193 | 4.152 (3.103–5.556) | <0.001* | 2.136 (1.536–2.970) | <0.001* |
*Significant. †Log–rank test. ‡Cox proportional hazard model. CEA, preoperative serum carcinoembryonic antigen level; CI, confidence interval; HR, hazard ratio; Poor, poorly differentiated carcinoma; Well/mod., well or moderately differentiated carcinoma.
Frequency of intratumoral vascular invasion is not a predictor of recurrence
To examine how VI features affect recurrence, we selected 106 patients without pleural invasion, another important predictive factor for recurrence, among 1099 patients (Fig. 1). In this cohort, age was the only variable capable of predicting recurrence.
First, we examined whether the frequency of intratumoral VI in a histological cross‐section was a predictor of recurrence (Fig. 1). Figure 2(c) shows the distribution of cases according to the frequency of VI. The group with one VI contained the largest number of cases. Figure 2(d–f) shows Kaplan–Meier analyses of the rate of recurrence according to a cut‐off level of 1, 2, and 3, respectively; however, no significant difference in the rate of recurrence between the VI‐high group and the VI‐low group was seen for each of the cut‐off levels. These results indicate that the frequency of intratumoral VI is not a predictor of recurrence.
Correlations between immunophenotypes of cancer cells in blood vessels and rate of recurrence
As shown in Figure 3(a,b), the intravascular tumor tissue was composed of not only cancer cells, but also non‐cancerous cells (arrows) that stained positive for vimentin, a marker of mesenchymal cells. We hypothesized that the microenvironment in the invaded blood vessels may be important for recurrence and examined the immunohistochemical properties of both cancer cells and non‐cancerous cells existing in the blood vessels. As EMT has been considered important for the intravasation of cancer cells, we examined the expression levels of four EMT‐related markers in cancer cells (Fig. 3c–j).17 The median staining scores were 76 for E‐cadherin, 92 for CD44, 0 for CD44 variant 6, and 0 for vimentin. The rates of recurrence in the high score (above median) and low score (below median) groups were 55.0 and 9.5 for E‐cadherin, 42.1 and 22.7 for CD44, 25.0 and 38.1 for CD44 variant 6, and 36.4 and 26.3 for vimentin, respectively. The rate of recurrence was significantly higher in the group with the high E‐cadherin score than in the group with the low E‐cadherin score (P = 0.004), but there were no significant differences in the rates of recurrence observed between the high score and low score groups for CD44, CD44 variant 6, or vimentin (P = 0.410, P = 0.229, and P = 0.846, respectively; Table 2).
Figure 3.
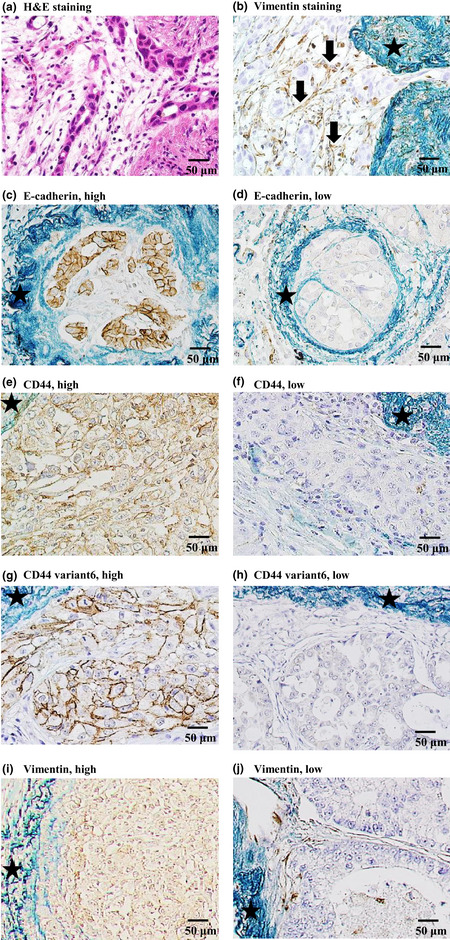
Immunohistochemical staining of tumor tissue in patients with lung adenocarcinoma with intratumoral vascular invasion. Stars indicate elastic fibers with positive Victoria blue van Gieson staining. (a,b) Intravascular stromal cells stained with (a) H&E and (b) immunohistochemical staining for vimentin. The arrows point to round or spindle‐shaped, brown‐stained, non‐cancerous cells. The staining results were negative for cancer cells. (c,d) E‐cadherin expression in cancer cells. (e,f) CD44 expression in cancer cells. (g,h) CD44 variant 6 expression in cancer cells. (i,j) Vimentin expression in cancer cells.
Table 2.
Five‐year rates of recurrence in patients with stage I adenocarcinoma according to the immunohistochemical staining scores of intravascular tumors
| No. of patients | 5‐year PR, % | P‐value† | |
|---|---|---|---|
| Cancer phenotype | |||
| E‐cadherin | |||
| High | 20 | 55.0 | |
| Low | 21 | 9.5 | 0.004* |
| CD44 | |||
| High | 19 | 42.1 | |
| Low | 22 | 22.7 | 0.410 |
| CD44 variant 6 | |||
| High | 20 | 25.0 | |
| Low | 21 | 38.1 | 0.229 |
| Vimentin | |||
| High | 22 | 36.4 | |
| Low | 19 | 26.3 | 0.846 |
| Stromal phenotype | |||
| CD204 | |||
| High | 20 | 50.0 | |
| Low | 21 | 14.3 | 0.016* |
| CD34 | |||
| High | 22 | 50.0 | |
| Low | 19 | 10.5 | 0.007* |
| α‐SMA | |||
| High | 19 | 47.4 | |
| Low | 22 | 18.2 | 0.033* |
*Significant. †Log–rank test. α‐SMA, α‐smooth muscle actin; High, greater than the median; Low, less than or equal to the median; PR, proportion of recurrence.
Correlations between number of activated macrophages, endothelial cells, and myofibroblasts in blood vessels and rate of recurrence
Next, we examined the numbers of activated macrophages (CD204+), microvessels (CD34+), and fibroblasts (α‐SMA+) within the intravascular tumor tissue (Fig. 4). The median numbers of CD204(+) macrophages, CD34(+) microvessels, and α‐SMA(+) myofibroblasts were 5.8, 1.7, and 3.6, respectively. The rates of recurrence in the high score and low score groups were 50.0 and 14.3 for CD204(+) macrophages, 50.0 and 10.5 for CD34(+) microvessels, and 47.4 and 18.2 for α‐SMA(+) myofibroblasts, respectively. The rate of recurrence was significantly associated with a high number of CD204(+) macrophages (P = 0.016), CD34(+) microvessels (P = 0.007), and α‐SMA(+) myofibroblasts (P = 0.033; Table 2).
Figure 4.
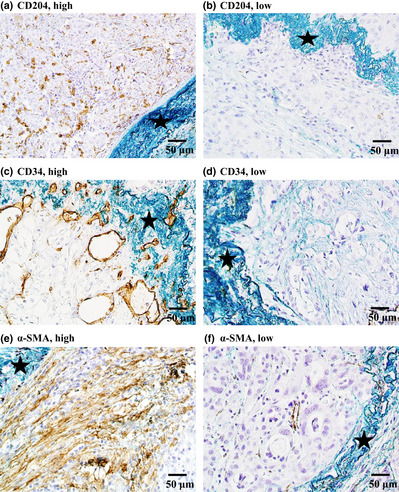
Immunohistochemical staining of non‐cancerous cells with intratumoral vascular invasion in patients with lung adenocarcinoma. (a,b) CD204(+) macrophages; (c,d) CD34(+) microvessels; (e,f) α‐smooth muscle actin (α‐SMA) (+) myofibroblasts.
Numbers of activated macrophages, endothelial cells, and myofibroblasts surrounding intravascular cancer cells with high or low scores for E‐cadherin
We compared the numbers of infiltrated activated macrophages, endothelial cells, and myofibroblasts surrounding cancer cells with high or low scores for E‐cadherin. The numbers of CD204(+) macrophages (P = 0.033) and α‐SMA(+) myofibroblasts (P = 0.011) were significantly higher in the high E‐cadherin score group than in the low E‐cadherin score group. The number of CD34(+) microvessels (P = 0.055) tended to be higher in the high E‐cadherin score group than in the low E‐cadherin score group (Fig. 5).
Figure 5.
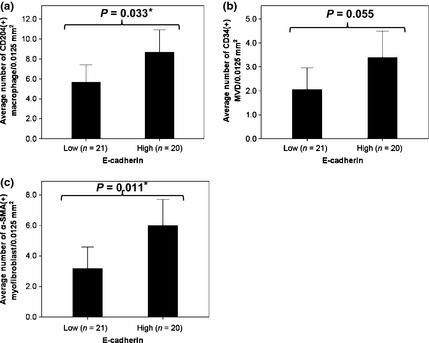
Number of intravascular non‐cancerous cells according to E‐cadherin expression in cancer cells from patients with lung adenocarcinoma. (a) CD204(+) macrophages; (b) CD34(+) microvessels; (c) α‐smooth muscle actin (α‐SMA) (+) myofibroblasts.
Discussion
Vascular invasion reportedly represents the early phase of cancer metastasis and is an independent and statistically significant predictor of recurrence in many types of cancer.4, 5, 6 However, not all patients with VI develop recurrences. First, we examined the frequency of intratumoral VI in a histological cross‐section, but this parameter was not a predictor of recurrence. Therefore, we hypothesized that the microenvironment within the invaded blood vessels is important for recurrence. In the present study, we found that various stromal cells, including macrophages, endothelial cells, and fibroblasts, infiltrate and become a constituent of the “cancer tissue” in the invaded blood vessels, similar to the primary site. Immunohistochemical staining revealed that the rate of recurrence was significantly higher in patients with a high E‐cadherin score for the cancer cells in the invaded blood vessels. In addition, the numbers of activated macrophages and fibroblasts (CD204(+) macrophages and α‐SMA(+) fibroblasts, respectively) and newly formed vessels (CD34(+) microvessels) within the intravascular tumor tissue were significantly higher among the patients with recurrences. As several studies have reported the significance of these cancer stromal cells within the primary tumor,18, 19, 20, 21 the current results imply that not only cancer cells, but also stromal cells may contribute to changes in the intravascular microenvironment and suggest that the tumor microenvironment created by cancer cells and stromal cells within the blood vessel is important for metastasis and recurrence. The present study is the first to indicate the possibility that the cancer microenvironment in invaded blood vessels has a considerable impact on recurrence.
Using animal models, Duda et al.22 reported that tumor‐associated stromal cells, such as fibroblasts, endothelial cells, or tumor‐infiltrated myeloid cells, are shed from the primary tumor together with the accompanying cancer cells and survive in the blood circulation, proliferating within metastatic nodules. These findings suggest that not only the characteristics of floating cancer cells, but also those of circulating stromal cells, may affect the metastatic process. However, using surgically resected lung adenocarcinoma cases, Matsumura et al.10 revealed that CD204(+) macrophages infiltrate within and around the cancer nests in permeated lymphatic vessels and that a large number of infiltrating CD204(+) macrophages is correlated with the presence of pulmonary metastasis. These results suggest the importance of the microenvironment in the invaded blood and or lymphatic vessels.
Epithelial–mesenchymal transition has been recognized as a phenomenon during which tumor cells in the primary lesion invade the surrounding stroma. Many researchers have postulated that the loss or repression of E‐cadherin is strongly linked with cancer invasiveness, metastasis, and patient prognosis.23, 24 However, contradictory results have also been reported. Imai et al.25 reported that E‐cadherin expression in ovarian carcinoma cells at metastatic sites of peritoneal dissemination is higher than that in the primary ovarian tumor. Moreover, the expression of E‐cadherin in intralymphatic cancer cells was significantly elevated in a group of patients that showed larger numbers of pulmonary metastases,10 consistent with the results of the present study. Although the function of E‐cadherin is essential during the metastatic process, its role may differ between cancer cells located inside and those located outside blood vessels.
The present study revealed that higher numbers of stromal cells, such as CD204(+) macrophages, CD34(+) microvessels, and α‐SMA(+) myofibroblasts, are present together with cancer cells with high levels of E‐cadherin in the tumor tissue within the invaded blood vessel. This phenomenon can be explained in two possible ways: (i) cancer cells expressing high levels of E‐cadherin may carry more stromal cells when they invade the blood vessels; and (ii) cancer cells expressing high levels of E‐cadherin may attract more stromal cell precursors in the blood circulation after vascular invasion has occurred.26 These possible molecular mechanisms will be the subjects of future examinations.
The present study examined tumor specimens from patients with lung adenocarcinoma. Whether these results are applicable to other types of cancer, such as squamous cell carcinoma or neuroendocrine carcinoma, will be the topic of future investigations.
In conclusion, this study showed that the presence of histological VI with abundant stromal cell infiltrates may predict a relatively high likelihood of recurrence and suggested that the microenvironment of the invaded blood vessels inside primary tumors might be capable of predicting the likelihood of metastasis in patients with pathological stage I adenocarcinoma. A more mechanistically oriented experimental approach is required to clarify the key regulators of the intravascular microenvironment, possibly leading to useful therapeutic options in the future.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- α‐SMA
α‐smooth muscle actin
- CEA
carcinoembryonic antigen
- CI
confidence interval
- EMT
epithelial–mesenchymal transition
- HR
hazard ratio
- NSCLC
non‐small‐cell lung cancer
- VI
vascular invasion
- VVG
Victoria blue van Gieson
Acknowledgments
This work was supported by the National Cancer Center Research and Development Fund (23‐A‐12 and 23‐K‐18), the Foundation for the Promotion of Cancer Research, 3rd‐Term Comprehensive 10‐Year Strategy for Cancer Control, Program for the Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation, and JSPS KAKENHI (24659185).
(Cancer Sci, doi: 10.1111/cas.12219, 2013)
References
- 1. Kessler R, Gasser B, Massard G et al Blood vessel invasion is a major prognostic factor in resected non‐small cell lung cancer. Ann Thorac Surg 1996; 62: 1489–93. [DOI] [PubMed] [Google Scholar]
- 2. Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res 1974; 34: 997–1004. [PubMed] [Google Scholar]
- 3. Rigau V, Molina TJ, Chaffaud C et al Blood vessel invasion in resected non small cell lung carcinomas is predictive of metastatic occurrence. Lung Cancer 2002; 38: 169–76. [DOI] [PubMed] [Google Scholar]
- 4. Lee AK, DeLellis RA, Silvermann ML, Heatley GJ, Wolfe HJ. Prognostic significance of peritumoral lymphatic and blood vessel invasion in node‐negative carcinoma of the breast. J Clin Oncol 1990; 8: 1457–65. [DOI] [PubMed] [Google Scholar]
- 5. Ouchi K, Sugawara T, Ono H et al Histologic features and clinical significance of venous invasion in colorectal carcinoma with hepatic metastasis. Cancer 1996; 78: 2313–7. [DOI] [PubMed] [Google Scholar]
- 6. Kroeger N, Rampersaud EN, Patard JJ et al Prognostic value of microvascular invasion in predicting the cancer specific survival and risk of metastatic disease in renal cell carcinoma: a multicenter investigation. J Urol 2012; 187: 418–23. [DOI] [PubMed] [Google Scholar]
- 7. Sobin LH, Wittekind C, eds. International Union Against Cancer (UICC) TNM Classification of Malignant Tumours, 6th edn New York, NY: Wiley‐Liss, 2002; 99–103. [Google Scholar]
- 8. Greene FL, Page DL, Fleming ID, eds. American Joint Committee on Cancer (AJCC) Cancer Staging Handbook, 6th edn New York, NY: Springer, 2002; 191–203. [Google Scholar]
- 9. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature 2008; 454: 436–44. [DOI] [PubMed] [Google Scholar]
- 10. Matsumura Y, Ishii G, Aokage K et al Morphophenotypic characteristics of intralymphatic cancer and stromal cells susceptible to lymphogenic metastasis. Cancer Sci 2012; 103: 1342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009; 59: 225–49. [DOI] [PubMed] [Google Scholar]
- 12. Yoshida J, Nagai K, Asamura H et al Visceral pleura invasion impact on non‐small cell lung cancer patient survival: its implications for the forthcoming TNM staging based on a large‐scale nation‐wide database. J Thorac Oncol 2009; 4: 959–63. [DOI] [PubMed] [Google Scholar]
- 13. Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male: female differences diminishing and adenocarcinoma rates rising. Int J Cancer 2005; 117: 294–9. [DOI] [PubMed] [Google Scholar]
- 14. Asamura H, Goya T, Koshiishi Y et al A Japanese Lung Cancer Registry study: prognosis of 13,010 resected lung cancers. J Thorac Oncol 2008; 3: 46–52. [DOI] [PubMed] [Google Scholar]
- 15. Yoshizawa A, Motoi N, Riely GJ et al Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma:prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011; 24: 653–64. [DOI] [PubMed] [Google Scholar]
- 16. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis‐correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 17. Thiery JP. Epithelial‐mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442–54. [DOI] [PubMed] [Google Scholar]
- 18. Ohtaki Y, Ishii G, Nagai K et al Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol 2010; 5: 1507–15. [DOI] [PubMed] [Google Scholar]
- 19. Kojc N, Zidar N, Vodopivec B, Gale N. Expression of CD34, alpha‐smooth muscle actin, and transforming growth factor beta 1 in squamous intraepithelial lesions and squamous cell carcinoma of the hypopharynx. Hum Pathol 2005; 36: 16–21. [DOI] [PubMed] [Google Scholar]
- 20. Tenderenda M, Rutkowski P, Jesoniek‐Kupnicka D, Kubiak R. Expression of CD34 in gastric cancer and its correlation with histology, stage, proliferation activity, p53 expression and apoptotic index. Pathol Oncol Res 2001; 7: 129–34. [DOI] [PubMed] [Google Scholar]
- 21. Korkolopoulou P, Konstantinidou AE, Kavantzas N et al Morphometric microvascular characteristics predict prognosis in superficial and invasive bladder cancer. Virchows Arch 2001; 438: 603–11. [DOI] [PubMed] [Google Scholar]
- 22. Duda DG, Duyverman AM, Kohno M et al Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA 2010; 107: 21677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E‐cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 2008; 68: 3645–54. [DOI] [PubMed] [Google Scholar]
- 24. Ono K, Sugio K, Uramoto H et al Discrimination of multiple primary lung cancers from intrapulmonary metastasis based on the expression of four cancer‐related proteins. Cancer 2009; 115: 3489–500. [DOI] [PubMed] [Google Scholar]
- 25. Imai T, Horiuchi A, Shiozawa T et al Elevated expression of E‐cadherin and alpha‐, beta‐, and gamma‐catenins in metastatic lesions compared with primary epithelial ovarian carcinomas. Hum Pathol 2004; 35: 1469–76. [DOI] [PubMed] [Google Scholar]
- 26. Ishii G, Ito TK, Aoyagi K et al Presence of human circulating progenitor cells for cancer stromal fibroblasts in the blood of lung cancer patients. Stem Cells 2007; 25: 1469–77. [DOI] [PubMed] [Google Scholar]


