Abstract
Epirubicin is widely used to treat various human tumors. However, it is difficult to achieve a sufficient antitumor effect because of dosage limitation to prevent cardiotoxicity. We hypothesized that epirubicin‐incorporating micelle would reduce cardiotoxicity and improve the antitumor effect. NC‐6300 comprises epirubicin covalently bound to PEG polyaspartate block copolymer through an acid–labile hydrazone bond. The conjugate forms a micellar structure of 40–80 nm in diameter in an aqueous milieu. NC‐6300 (10, 15 mg/kg) and epirubicin (10 mg/kg) were given i.v. three times to mice bearing s.c. or liver xenograft of human hepatocellular carcinoma Hep3B cells. Cardiotoxicity was evaluated by echocardiography in C57BL/6 mice that were given NC‐6300 (10 mg/kg) or epirubicin (10 mg/kg) in nine doses over 12 weeks. NC‐6300 showed a significantly potent antitumor effect against Hep3B s.c. tumors compared with epirubicin. Moreover, NC‐6300 also produced a significantly longer survival rate than epirubicin against the liver orthotopic tumor of Hep3B. With respect to cardiotoxicity, epirubicin‐treated mice showed significant deteriorations in fractional shortening and ejection fraction. In contrast, cardiac functions of NC‐6300 treated mice were no less well maintained than in control mice. This study warrants a clinical evaluation of NC‐6300 in patients with hepatocellular carcinoma or other cancers.
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third largest cause of cancer mortality worldwide.1, 2 The range of available oncological treatment for HCC is sometimes limited due to poor liver function caused by concomitant chronic liver disease, especially liver cirrhosis, which is mainly the result of hepatitis virus infection. Surgical resection is widely considered the mainstay for curative treatment and yields a certain survival rate. However, <20% of patients with HCC can undergo surgical resection.3, 4 With the exception of patients at an early stage and with adequate liver function, recurrence rates after surgical resection are unfortunately high. High recurrence rates are also seen in patients treated by other local treatment options, such as ablation, percutaneous ethanol injection, and trans‐arterial chemoembolization.5 For advanced HCC, the only available option is sorafenib, a tyrosine kinase inhibitor, which was recently approved; however, the survival rate associated with its use is far from satisfactory.6, 7, 8
Anthracyclines such as epirubicin (EPI) and doxorubicin (DXR) are widely used and highly effective anticancer agents for the treatment of various human tumors including HCC, breast cancer, and gastric cancer.9, 10, 11, 12, 13 However, anthracyclines induce several adverse effects, among which acute or chronic cardiotoxicity sometimes causes patients irreversible damage.14, 15, 16, 17 Cardiotoxicity of EPI is approximately 66% of that of DXR, but it is still the most serious problem in oncological treatment; this side‐effect can sometimes require cessation of EPI treatment, resulting in an insufficient antitumor effect.18, 19, 20 To address these issues, various anthracyclines have been developed to reduce cardiotoxicity without loss of the antitumor effect. However, to date, no such anthracyclines are available in a clinical context.
Against this background, NC‐6300 was synthesized. NC‐6300 comprises EPI covalently bound to PEG polyaspartate block copolymer through an acid–labile hydrazone bond. The conjugate spontaneously forms a micellar structure with a diameter of 40–80 nm in aqueous media, as reported previously.21 In vitro findings indicated that it showed pH‐dependent EPI release, namely, the release of EPI from NC‐6300 accelerated under increasingly acidic conditions. NC‐6300 is stable in the bloodstream for a long time; therefore, it accumulates selectively in tumor tissues through the enhanced permeability and retention effect.21, 22 Following the extravasation of NC‐6300, NC‐6300 or its disintegrated EPI‐bound unimers reach and enter cancer cells. Then, under the acidic conditions within lysosomes, EPI may be released, leading to the subsequent death of the cancer cells.
In this study, we evaluated the antitumor effect of NC‐6300 using mouse models with human HCC Hep3B cells. Moreover, we examined the cardiotoxicity of NC‐6300, particularly in terms of cardiac function, by using echocardiography over a long period.
Materials and Methods
Drugs
NC‐6300 was supplied and synthesized by NanoCarrier (Kashiwa, Japan) (Fig. 1). The synthesis was described elsewhere.21 NC‐6300 was stored in a frozen state at −80°C and melted on ice immediately before inoculation into mice. Then, the NC‐6300 was prepared with distilled water at 1.0 mg/mL (epirubicin‐equivalent dose). NC‐6300 is stable at low temperatures under neutral conditions, but gradually begins to release the encapsulated EPI in an acidic environment.21 The EPI was purchased from Pfizer Japan (Tokyo, Japan).
Figure 1.
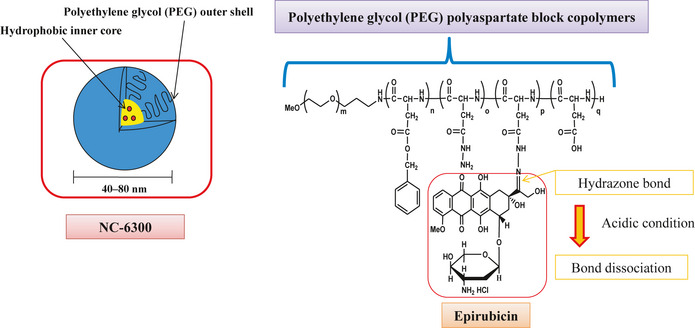
NC‐6300 comprises epirubicin covalently bound to the polyaspartate chain of PEG polyaspartate block copolymer by an acid–labile hydrazone bond. The conjugate spontaneously forms a micellar structure in an aqueous milieu. The diameter of NC‐6300 is 40–80 nm, and the micellar formulation is stable under physiological conditions.
Cell culture
The human HCC cell line Hep3B was obtained from the European Collection of Cell Culture (ECACC, Salisbury, UK). Hep3B cells were cultured in DMEM supplemented with 10% FBS (Cell Culture Technologies, Gaggenau‐Hoerden, Germany), penicillin, streptomycin, and amphotericin B (100 units/mL, 100 μg/mL, and 25 μg/mL, respectively; Sigma, St. Louis, MO, USA) in humidified 5% CO2 at 37°C.
Establishment of Hep3B cell line stably expressing firefly luciferase
For the in vivo bioluminescence imaging of liver orthotopic tumors, the Hep3B cell line stably expressing firefly luciferase (Hep3B/Luc) was established. Briefly, the coding sequence for firefly luciferase was subcloned into the pcDNA3.1(+) vector (Invitrogen, Carlsbad, CA, USA) to generate plasmids of pcDNA3.1/luciferase. Hep3B cells (2 × 105) were seeded onto 3‐cm dishes 24 h before transfection. The cells were transfected with 2.5 μg plasmid DNA using Lipofectamine LTX Reagent and PLUS Reagent (Invitrogen) in accordance with the manufacturer's instructions, then incubated for 48 h at 37°C. The cells were then passaged in medium containing G418 (1 mg/mL; Invitrogen) to select for the neomycin‐resistance gene integrated in the pcDNA3.1(+) plasmids. The accuracy of a quantitative bioluminescence image as an indicator of Hep3B/Luc cell number was analyzed using the IVIS Kinetic Imaging system (Caliper Life Sciences, Hopkinton, MA, USA) in vitro, as described below. This analysis showed a clear correlation between a quantitative bioluminescence image and cell number. The sensitivity of Hep3B/Luc cells to each anticancer drug was similar to that of parental Hep3B cells (data not shown).
Orthotopic tumor model
Six‐ to eight‐week‐old female BALB/c nude mice (CLEA Japan, Tokyo, Japan) each weighing approximately 20 g at the time of surgery were used for in vivo studies. The animals were maintained under specific pathogen‐free conditions in cages, provided with standard food, and given free access to sterilized water. All animal procedures were carried out in compliance with the Guidelines for the Care and Use of Experimental Animals established by the Committee for Animal Experimentation of the National Cancer Center, Japan; these guidelines meet the ethical standards required by law and also comply with the guidelines for the use of experimental animals in Japan.
The HCC model was created by direct intrahepatic inoculation of Hep3B/Luc cells. The mice were anesthetized by i.p. injection of 0.15 mL/g body weight of 2,2,2‐tribromoethanol in 2‐methyl‐2‐butanol. The stock solution of the anesthetic is 1.0 g 2,2,2‐tribromoethanol (Wako, Osaka, Japan) dissolved in 1.0 mL 2‐methyl‐2‐butanol (Sigma). Just before use, 1 mL stock solution was diluted with 40 mL H2O. A short median laparotomy was carried out with disinfection of the abdominal skin. Then, 5 × 106 Hep3B/Luc cells suspended in 20 μL Matrigel (BD Biosciences, San Jose, CA, USA) were directly injected into the left lower lobe of the liver using a 29‐gauge needle. To prevent either bleeding or dissemination of tumor cells, puncture sites were ablated with a bipolar cautery.
In vivo tumor growth inhibition assay
Experiment 1
Six‐week‐old BALB/c nude mice (CLEA Japan) were s.c. inoculated with 1 × 107 Hep3B/Luc cells in the flank region. When the tumor volume reached 150 mm3, the mice were randomly divided into test groups consisting of six mice per group (day 0). Drug was given i.v. on days 0, 7, and 14 through the lateral tail vein. NC‐6300 was given at 10 and 15 mg/kg and the reference drug, EPI, was given at its maximum tolerated dose of 10 mg/kg. The length (a) and width (b) of s.c. tumors and body weight were measured twice a week, and tumor volume (TV) was calculated as follows: TV = (a × b 2)/2. Relative body weight (RBW) on day n was calculated according to the following formula: RBW = BWn/BW0, where BWn is the body weight on day n and BW0 is the body weight on day 0.
Experiment 2
Two weeks after direct hepatic inoculation of Hep3B/Luc cells, mice were randomly divided into three tests groups consisting of 10 mice per group (day 0). Randomization was carried out on the basis of bioluminescence images, and we confirmed that the mean values of count per minute in the images were not significantly different between groups. The mice were given i.v. injections into the lateral tail vein (200 μL) at 10 mg/kg or 15 mg/kg NC‐6300, or 10 mg/kg EPI, on days 0, 7, and 14. Control mice were injected with 200 μL PBS following the same schedule. To evaluate the progression of orthotopic liver tumor, in vivo bioluminescence imaging was carried out with the IVIS Kinetic Imaging system (Caliper Life Sciences) every week from the day of treatment initiation, and the body weight of each mouse was also measured. Mortality and morbidity were checked daily and the mice were maintained until each one showed signs of morbidity (massive ascites or hepatic tumor that could be observed through skin, jaundice, and 20% weight loss), at which point they were killed.
Pharmacokinetics of EPI and NC‐6300 in mice
Four weeks after direct tumor inoculation of Hep3B/Luc in the liver, either EPI (10 mg/kg) or NC‐6300 (10 mg/kg) was injected i.v. Before sampling, the mice were anesthetized with ether. Plasma, tumor, liver, heart, kidney, spleen, and lung were obtained and weighed at 1, 6, 12, 24, and 72 h. Tissue samples were rinsed with saline and stored at −80°C until use.
Tissue samples were suspended in 0.1 M sodium phosphate buffer (pH 7.4) at a concentration of 25% w/w and homogenized on ice using Precellys 24 (Bertin Technologies, Montigny‐le‐Bretonneux, France). Using aliquots of the homogenates and plasma (50 μL), concentrations of the EPI released from NC‐6300 in vivo and the total EPI (free EPI released from NC‐6300 in vivo and the polymer‐bound EPI) were determined.
To determine the concentration of released EPI, the homogenates and plasma samples (50 μL) were treated with acetonitrile (125 μL) to precipitate proteins. Then, 1% Triton X‐100 (25 μL) was added and the sample was vortexed and centrifuged for 5 min at 5000 g at 4°C. Subsequently, 75 μL daunorubicin HCL (2 μg/mL)/20 mM sodium phosphate buffer (pH 7.4) was added to the supernatant (75 μL) as an internal control. The prepared mixture was analyzed by HPLC. To determine the total EPI concentration, the samples (50 μL) were treated with acetonitrile (130 μL) and acidified with 1 N HCL (20 μL) for 1 h at room temperature to allow the complete release of EPI from NC‐6300. Next, 1% Triton X‐100 (25 μL) was added and the sample was vortexed and centrifuged for 5 min at 5000 g at 4°C. Then, 75 μL daunorubicin HCL (2 μg/mL)/25 mM ammonium formate buffer (pH 3.0) was added to the supernatant (75 μL) as an internal control. The prepared mixture was analyzed by HPLC.
Reversed‐phase HPLC was carried out at 40°C on a Tosoh TSK‐gel ODS‐80Tm (4.6 φ × 150 mm; Tosoh, Tokyo, Japan) with a Tosoh ODS‐80Tm guard cartridge. The EPI was eluted with 25 mM ammonium formate buffer (pH 3.0):acetonitrile (70:30, v/v) using a Waters Alliance System (Waters Corporation, Milford, MA, USA) at a flow rate of 1.0 mL/min. Detection was carried out using a Waters fluorescence detector with excitation and emission wavelengths of 488 and 560 nm, respectively.
Echocardiography for mice
To investigate the acute and chronic cardiotoxicity induced by EPI, we designed the following experimental scheme. Five‐week‐old female C57BL/6 mice were given NC‐6300 at a dose of 10 mg/kg or EPI at 10 mg/kg (n = 6) on days 0, 7, and 14, and the mice were left for an interval of 7 days. We repeated this schedule three times; namely, mice were given each drug nine times in total. We evaluated the cardiac function using echocardiography on days 0, 7, 14, 21, 49, and 77.
Echocardiography was carried out using a high‐resolution Micro Ultrasound system (Vevo 770; VisualSonics, Toronto, Canada), equipped with a 40‐MHz ultrasound probe (RMV704; VisualSonics). Mice were kept under light sedation with 1–2% isoflurane until the heart rate stabilized at between 400 and 500 beats per minute. Left ventricular dimensions and wall thicknesses were determined from parasternal short‐axis M‐mode echocardiography images, and fractional shortening (FS) and ejection fraction (EF) were automatically calculated with Vevo 770 software.
Statistical analysis
Data are expressed as the mean ± SD. To evaluate antitumor effects, changes in body weight, and changes in cardiac function, repeated‐measures anova was carried out using StatView 5.0 software (Statview, Hulinks, Tokyo, Japan). Survival was assessed by the Kaplan–Meier method using spss version 19 software (SPSS Inc., Chicago, IL, USA). For all tests, P‐values <0.05 were considered to be statistically significant. All statistical tests were two‐sided.
Results
Antitumor activity of NC‐6300 and epirubicin in mice bearing Hep3B s.c. tumor
NC‐6300 given at 10 or 15 mg/kg three times with a 7‐day interval appeared to possess significantly superior antitumor activity compared with EPI given at 10 mg/kg with the same schedule. The group treated with EPI at 10 mg/kg showed a significant decrease in body weight as compared with the groups treated with NC‐6300 at 10 and 15 mg/kg (Fig. 2).
Figure 2.
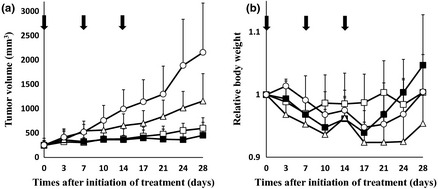
Antitumor effects of NC‐6300 and epirubicin (EPI) in mice bearing s.c. Hep3B xenografts. Hep3B cells (1 × 107 cells) were inoculated s.c. on the back. NC‐6300 or conventional EPI was injected i.v. on days 0, 7, and 14. Day 0 indicates the day when tumor volume reached 150 mm3 (n = 6). The dose of NC‐6300 is expressed as dose equivalent of EPI. ○, Control; □, NC‐6300 at 10 mg/kg; ■, NC‐6300 at 15 mg/kg; ∆, EPI at 10 mg/kg. (a) Antitumor activity of NC‐6300 or EPI was evaluated by measuring the tumor volume. The anova test between NC‐6300 (10 mg/kg) and EPI (10 mg/kg), P = 0.0017; NC‐6300 (15 mg/kg) and EPI (10 mg/kg), P < 0.001. (b) Changes in relative body weight. Data were derived from the same mice as those used in the treatment experiment. The anova test between NC‐6300 (10 mg/kg) and EPI (10 mg/kg), P = 0.0053; NC‐6300 (15 mg/kg) and EPI (10 mg/kg), P < 0.001. Arrows, drug injections; bars, SD; points, mean.
Antitumor activity of NC‐6300 and epirubicin in mice bearing Hep3B/Luc liver orthotopic tumors
Antitumor activity was observed in mice bearing Hep3B/Luc xenografts implanted orthotopically following treatment with NC‐6300 at 10 or 15 mg/kg or EPI at 10 mg/kg (Fig. 3). There was a significant difference between the control group and the groups treated with EPI and NC‐6300 (Fig. 3b). Comparison of the relative photon count revealed significant differences between NC‐6300 at 15 mg/kg and EPI at 10 mg/kg (Fig. 3b). Kaplan–Meier analysis showed that there was a significant improvement in the survival rate in the group given NC‐6300 at 10 mg/kg compared with that in the group given EPI at 10 mg/kg (P = 0.002), as well as for the group given NC‐6300 at 15 mg/kg compared with the group given EPI at 10 mg/kg (P = 0.004) (Fig. 3c).
Figure 3.
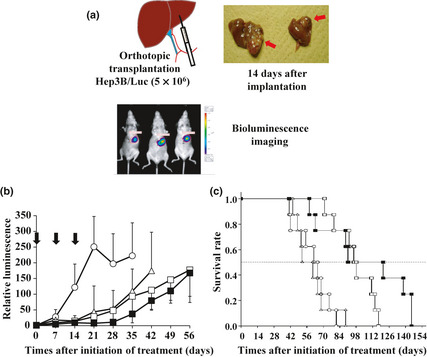
(a) Orthotopic liver tumor xenografts and evaluation of tumor growth using bioluminescence imaging. Hep3B/Luc cells (5 × 106) suspended in 20 μL Matrigel were directly injected into the left lower lobe of the liver in mice using a 29‐gauge needle. To visualize and evaluate the transplanted hepatic tumor of Hep3B/Luc cells, in vivo bioluminescence imaging was carried out. (b) Antitumor effect of NC‐6300 and epirubicin (EPI) in mice bearing Hep3B/Luc xenografts. Each drug was given on days 0, 7, and 14 after tumor implantation (n = 8). The antitumor activity was evaluated by determining the relative photon count. The anova test between NC‐6300 (15 mg/kg) and EPI (10 mg/kg), P = 0.025. Arrows, drug injections; bars, SD; points, mean. (c) Kaplan–Meier curves. Log–rank test, NC‐6300 (10 mg/kg) group versus EPI (10 mg/kg) group, P = 0.002; NC‐6300 (15 mg/kg) group versus EPI (10 mg/kg) group, P = 0.004. ○, Control; □, NC‐6300 at 10 mg/kg; ■, NC‐6300 at 15 mg/kg; ∆, EPI at 10 mg/kg.
Tissue distribution of NC‐6300 and epirubicin in mice bearing orthotopic Hep3B/Luc tumor
Tissue distribution experiments were carried out to evaluate the toxicity and efficacy data obtained for both NC‐6300 and EPI in terms of the plasma and tissue concentrations of each formulation. The concentration–time profiles in plasma and various tissues were obtained (Fig. 4) and the results were similar to previous data.21 After the injection of EPI, its concentration declined rapidly. On the other hand, NC‐6300 showed significantly slower clearance. The clearance rate of NC‐6300 in orthotopic Hep3B tumors was significantly slower than that of other normal organs. In a similar manner to other drugs categorized in the drug delivery system, NC‐6300 showed higher accumulation in organs of the reticuloendothelial system. In heart, a significantly higher concentration of EPI was obtained for several hours after treatment with conventional EPI compared with that of NC‐6300. To compare the tissue accumulation of EPI released from NC‐6300 with that of EPI after treatment with conventional EPI, the area under the time–concentration curve (AUC) values in each tissue were determined, as summarized in Table 1. NC‐6300 produced increases in EPI concentration, particularly in the plasma, liver, spleen, and tumor, whereas NC‐6300 decreased the free EPI concentration in kidney, lung, and heart.
Figure 4.
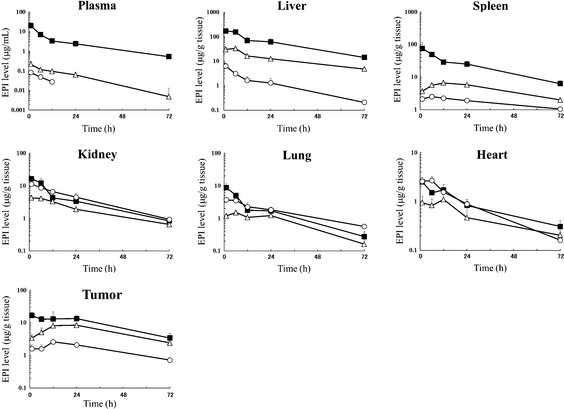
Plasma and tissue concentration–time profiles of total (■) and released (∆) epirubicin (EPI) after i.v. injection of NC‐6300 (10 mg/kg) into mice bearing Hep3B/Luc orthotopic liver tumors. Plasma and tissue concentration–time profiles of EPI after conventional EPI (10 mg/kg) (○) injection. Data points are the means of three mice.
Table 1.
Area under the time–concentration curve (AUC) values in plasma and tissues after injection of NC‐6300 at 10 mg/kg or epirubicin (EPI) at 10 mg/kg in mice bearing orthotopic Hep3B/Luc liver xenografts
| AUC (μg/g tissue or mL × h) | |||||
|---|---|---|---|---|---|
| NC‐6300 | Conventional EPI | Released/Conventional EPI AUC ratio | |||
| Released EPI | Total EPI | % Released | |||
| Plasma | 4.1 | 213.0 | 1.9 | 0.6 | 6.95 |
| Liver | 920.5 | 4246.8 | 21.7 | 94.9 | 9.70 |
| Spleen | 323.3 | 1657.3 | 19.5 | 121.9 | 2.65 |
| Kidney | 136.2 | 273.5 | 49.8 | 295.2 | 0.46 |
| Lung | 61.4 | 127.2 | 48.3 | 118.7 | 0.52 |
| Heart | 36.2 | 63.7 | 56.9 | 66.9 | 0.54 |
| Tumor | 422.5 | 724.2 | 58.3 | 116.8 | 3.62 |
Echocardiogram following treatment of NC‐6300 and epirubicin
Cardiotoxicity was evaluated by echocardiography in C57BL/6 mice during and following a total of nine treatments with NC‐6300 (10 mg/kg) and conventional EPI (10 mg/kg) over 12 weeks. The EF of mice treated with conventional EPI (10 mg/kg) was significantly reduced compared with those of the control (P = 0.0019) and NC‐6300 (10 mg/kg) treatment groups (P = 0.0081). The FS was also significantly lower in the conventional EPI treatment group (Fig. 5).
Figure 5.
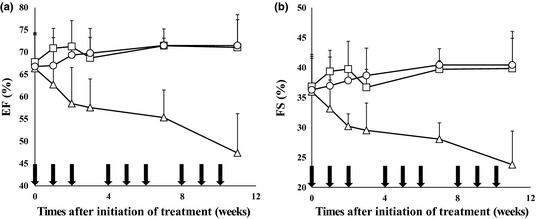
Echocardiography for evaluating cardiotoxicity. C57BL/6 mice were given NC‐6300 (10 mg/kg) or conventional epirubicin (EPI; 10 mg/kg) on days 0, 7, and 14 every 4 weeks, for a total of nine injections of each drug over 12 weeks (n = 6). ○, Control; □, NC‐6300 at 10 mg/kg; ∆, EPI at 10 mg/kg. Left ventricular dimensions and wall thicknesses were determined from the echocardiography images, and ejection fraction (EF) and fractional shortening (FS) were automatically calculated. (a) Changes in EF; anova test between NC‐6300 (10 mg/kg) and EPI (10 mg/kg), P = 0.0081. (b) Changes in FS; anova test between NC‐6300 (10 mg/kg) and EPI (10 mg/kg), P = 0.0114. Arrows, drug injections; bars, SD; points, mean.
Discussion
NC‐6300 was constructed to enhance antitumor activity and to reduce the adverse effects of EPI.21 Previous data clearly showed that NC‐6300 possessed superior antitumor activity to conventional EPI in mice bearing MDA‐MB‐231 human breast s.c. tumor.21 In the present study, NC‐6300 (10 or 15 mg/kg) also exerted significantly superior antitumor activity to EPI (10 mg/kg) both in s.c. HCC Hep3B xenografts and in the orthotopic liver tumor model. From the present pharmacokinetic analysis, we hypothesize that NC‐6300 selectively accumulates in tumor tissue owing to the enhanced permeability and retention effect and directly reaches the cancer cells in order to attack them. Alternatively, the formulation spontaneously disintegrates while it is retained within the tumor tissue. Disintegrated EPI‐bound unimers immediately reach and enter cancer cells. Then, under the acidic conditions in lysosomes, EPI is released to kill the cancer cells.
In terms of available preclinical data, the most prominent show that NC‐6300 was found to cause marked reduction in the cardiotoxicity of conventional EPI. This was evaluated by precise echocardiography during and after a total of nine treatments with NC‐6300 or EPI. The present pharmacokinetic study showed that the AUC value of released EPI in the heart was 36.2 μg h/g when NC‐6300 (10 mg/kg) was given. The AUC value of EPI in the heart was 66.9 μg h/g in the case of conventional EPI treatment. The ratio of the AUC of EPI released from NC‐6300 to that of native EPI was 0.54. In addition, the heart C max of EPI was remarkably higher in the case of conventional EPI compared with NC‐6300 treatment. In the echocardiographic examination, mice treated with conventional EPI showed significantly deteriorated EF and FS. In contrast, no mice treated with NC‐6300 experienced cardiotoxicity in terms of EF and FS. Combining the results of the pharmacokinetic study and echocardiography, NC‐6300 was found to markedly reduce cardiotoxicity. Among the various kinds of anticancer agents used in a clinical context, anthracyclines are one of the most important and commonly used drug groups in oncology.9, 10, 11, 12, 13 Like other anticancer agents, drugs categorized into the anthracyclines have several adverse effects, including bone marrow toxicity, gastrointestinal toxicity, general fatigue, and hair loss. The most problematic adverse effect of the anthracyclines is undoubtedly cardiotoxicity14, 15, 16, 17 because, different from other adverse effects, it cannot be predicted when this cardiotoxicity will occur and how serious it will be. Furthermore, cardiotoxicity is occasionally unmanageable by any medical treatment. Although EPI has been developed in order to reduce the cardiotoxicity of DXR, EPI is still associated with serious cardiotoxicity.18 However, the present data on NC‐6300 suggest its potential to resolve this difficult clinical problem.
In the present preclinical study, NC‐6300 appeared to show a strong antitumor effect in HCC xenografts compared with EPI. Furthermore, NC‐6300 was found to reduce the cardiotoxicity of EPI markedly. Data from the present study warrant a clinical evaluation of NC‐6300.
Disclosure Statement
NanoCarrier Co. Ltd holds the patent for NC‐6300 (Japanese patent number 4781435). M. Harada and H. Saito are the inventors of the product. NanoCarrier Co. Ltd received licensing fees from Kowa Co. Ltd (Nagoya, Japan) according to the license agreement.
Acknowledgments
This work was supported by the Funding Program for World‐Leading Innovative R&D on Science and Technology (FIRST Program) (Y.M.), the National Cancer Center Research and Development Fund (Y.M.), and the Ministry of Health, Labor and Welfare, Health and Labour Science Research Grants, Third Term Comprehensive Control Research for Cancer (Y.M.). We thank Mrs K. Shiina for her secretarial assistance.
(Cancer Sci 2013; 104: 920–925)
References
- 1. El‐Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007; 132: 2557–76. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Shin HR, Bray F et al Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–917. [DOI] [PubMed] [Google Scholar]
- 3. Hung H. Treatment modalities for hepatocellular carcinoma. Curr Cancer Drug Targets 2005; 5: 131–8. [DOI] [PubMed] [Google Scholar]
- 4. Johnson PJ. Hepatocellular carcinoma: is current therapy really altering outcome? Gut 2002; 51: 459–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takayama T, Makuuchi M, Hirohashi S et al Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology 1998; 28: 1241–6. [DOI] [PubMed] [Google Scholar]
- 6. Llovet JM, Ricci S, Mazzaferro V et al Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–90. [DOI] [PubMed] [Google Scholar]
- 7. Nowak AK, Chow PK, Findlay M. Systemic therapy for advanced hepatocellular carcinoma: a review. Eur J Cancer 2004; 40: 1474–84. [DOI] [PubMed] [Google Scholar]
- 8. Cheng AL, Kang YK, Chen Z et al Efficacy and safety of sorafenib in patients in the Asia‐Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double‐blind, placebo‐controlled trial. Lancet Oncol. 2009; 10: 25–34. [DOI] [PubMed] [Google Scholar]
- 9. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 2003; 37: 429–42. [DOI] [PubMed] [Google Scholar]
- 10. Biganzoli L. Doxorubicin and paclitaxel versus doxorubicin and cyclophosphamide as first‐line chemotherapy in metastatic breast cancer: The European Organization for Research and Treatment of Cancer 10961 Multicenter Phase III Trial. J Clin Oncol 2002; 20: 3114–21. [DOI] [PubMed] [Google Scholar]
- 11. Martin M, Rodriguez‐Lescure A, Ruiz A et al Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by Paclitaxel for early breast cancer. J Natl Cancer Inst 2008; 100: 805–14. [DOI] [PubMed] [Google Scholar]
- 12. Roche H, Fumoleau P, Spielmann M et al Sequential adjuvant epirubicin‐based and docetaxel chemotherapy for node‐positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol 2006; 24: 5664–71. [DOI] [PubMed] [Google Scholar]
- 13. Cunningham D, Starling N, Rao S et al Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008; 358: 36–46. [DOI] [PubMed] [Google Scholar]
- 14. Bristow MR, Billingham ME, Mason JW et al Clinical spectrum of anthracycline antibiotic cardiotoxicity. Cancer Treat Rep 1978; 62: 873–9. [PubMed] [Google Scholar]
- 15. Lefrak EA, Pitha J, Rosenheim S et al A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973; 32: 302–14. [DOI] [PubMed] [Google Scholar]
- 16. Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart 2008; 94: 525–33. [DOI] [PubMed] [Google Scholar]
- 17. Singal PK, Iliskovic N. Doxorubicin‐induced cardiomyopathy. N Engl J Med 1998; 339: 900–5. [DOI] [PubMed] [Google Scholar]
- 18. de Azambuja E, Paesmans M, Beauduin M et al Long‐term benefit of high‐dose epirubicin in adjuvant chemotherapy for node‐positive breast cancer: 15‐year efficacy results of the Belgian multicentre study. J Clin Oncol 2009; 27: 720–5. [DOI] [PubMed] [Google Scholar]
- 19. Salvatorelli E, Menna P, Lusini M et al Doxorubicinolone formation and efflux: a salvage pathway against epirubicin accumulation in human heart. J Pharmacol Exp Ther 2009; 329: 175–84. [DOI] [PubMed] [Google Scholar]
- 20. Innocenti F, Iyer L, Ramirez J et al Epirubicin glucuronidation is catalyzed by human UDP‐glucuronosyltransferase 2B7. Drug Metab Dispos 2001; 29: 686–92. [PubMed] [Google Scholar]
- 21. Harada M, Bobe I, Saito H et al Improved anti‐tumor activity of stabilized anthracycline polymeric micelle formulation, NC‐6300. Cancer Sci 2011; 102: 192–9. [DOI] [PubMed] [Google Scholar]
- 22. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986; 46: 6387–92. [PubMed] [Google Scholar]


