Abstract
Triple negative breast cancer (TNBC) is defined by estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 negativity. Patients with TNBC frequently undergo an aggressive clinical course due to the unavailability of specific targeted therapies. Androgen receptor (AR) was reported to be expressed in up to 60% of TNBC cases but there have been controversies as to the roles of androgen signaling through AR in TNBC. Therefore, in this study, we analyzed the status of AR in combination with androgen synthesizing enzymes (5α‐reductase type 1 (5αR1) and 17β‐hydroxysteroid dehydrogenase type 5 (17βHSD5)] in order to further understand androgenic actions in TNBC. Androgen receptor, 5αR1, and 17βHSD5 were immunolocalized in a cohort of 203 TNBC patients from Thailand and Japan. We then correlated the findings with clinicopathological characteristics (age, stage, tumor diameter, lymph node invasion, metastatic spread, Ki‐67 labeling index, disease‐free survival, and overall survival) of the patients. Univariate analysis revealed that AR+/enzyme+ cases were associated with a significantly lower Ki‐67 labeling index than AR−/enzyme− samples. Multivariate analysis indicated the presence of significant positive correlations between AR and enzyme status in tumor cells, and between tumor diameter, lymph node invasion, and distant metastasis. Significant negative correlations were also detected between Ki‐67 labeling index and AR status (P = 0.04) or 5αR1 (P < 0.001). Cox proportional hazards analysis showed that Ki‐67 labeling index and stage were the only factors predicting disease‐free and overall survival of the patients, although univariate Kaplan–Meier analysis revealed AR/5αR1 negativity suggested a more adverse clinical course up to 80 months after surgery. These results suggest that the presence of androgen synthesizing pathways in addition to AR expression in tumor cells could confer a better clinical outcome through suppression of cell proliferation.
Breast cancer is the most common malignancy in women1 and, although recent advances in clinical management have significantly improved the survival rates of the great majority of breast cancer patients,2 one subtype, so‐called triple negative breast cancer (TNBC) continues to be associated with an adverse prognosis.3 Triple negative breast cancer is characterized by the absence of estrogen receptor‐α (ERα), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression in the tumor cells and constitutes approximately 6–60% of all breast cancer cases, depending on the cohort evaluated.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 This subtype is considered to be far more diverse compared to other subtypes of breast malignancy.24, 25, 26 Triple negative breast cancer is generally associated with relatively adverse clinical outcome27, 28, 29, 30 primarily due to the lack of specific therapies, higher rates of tumor cell proliferation, and more aggressive behavior.31
Numerous published studies have attempted to identify biomarkers that could further subclassify TNBC into disease subtypes. For instance, growth factors such as epidermal growth factor receptor32 and insulin‐like growth factor‐133 have been studied and higher expression was reported to be associated with adverse clinical outcomes in TNBC patients. Other biomarkers reported so far include Numb protein,34 chromosomal instability,35 EZHR,36 and miR34b.37 Among these markers, one of the most extensively investigated but also one of the most controversial is the androgen receptor (AR).
The AR in triple negative breast tumor cells has been reported to be expressed at a relatively lower rate than in other types of breast cancer or even in breast cancer as a whole (50–100% in non‐subtype specific38, 39, 40, 41, 42, 43; 0–53% in TNBC4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 21, 22, 44, 45). However, the mere presence of AR in even a subset of TNBC patients suggests the manipulation of androgenic pathways in tumor cells could serve as a therapeutic option, at least for some TNBC patients. In addition, the availability of AR targeted agents, developed primarily for the treatment of prostate cancer, potentially makes the manipulation of androgenic pathways in TNBC patients more appealing as such treatment could be incorporated into clinical practice with less difficulty compared to other modes of target‐specific therapies. The use of such therapies, however, is dependent on a clear understanding of the role of androgenic pathways in the biological behavior of TNBC.
The biological roles of androgens in TNBC are in dispute. Immunohistochemical studies looking at the presence of AR in tumor cells have reported conflicting results in terms of the correlation between AR and clinical outcome; some studies indicated a survival advantage,11, 46, 47 others showed no significant effects of AR expression in tumor cells on survival of the patients.20, 43, 48 However it is also true that, in contrast to these clinical studies, results of in vitro investigations consistently showed that AR may replace ER and PR as a driver of tumor proliferation and growth in TNBC cell lines.48, 49, 50, 51, 52, 53, 54, 55, 56, 57 The suggestion that AR expression could be an adverse marker is partially supported by one clinical study using gene expression profiles rather than immunoreactivity to define AR+ TNBC using luminal androgen receptor (LAR) gene expression profiles.56 Based on these findings, clinical trials of AR antagonist have been initiated,58 but further investigations are considered necessary to establish the precise roles of AR in TNBC patients.
We previously reported that AR expression in tandem with the presence of androgen synthesizing enzymes could eventually determine the clinical outcome for breast cancer patients as a whole, regardless of their intrinsic subtypes.59 Therefore, we examined AR and androgen metabolizing enzymes in whole tissue sections of a cohort of 203 triple negative surgical breast cancer specimens obtained from both Japanese and Thai cohorts in order to evaluate the influence or effects of AR status in tumor cells on the Ki‐67 labeling index of tumor cells, as well as on overall survival (OS) and disease‐free survival (DFS) of the patients, in order to further extrapolate our findings on the receptor and enzymes in this study.
Materials and Methods
Patient cohorts
Following approval from the relevant institutional review boards or ethical committees (Japan, Tohoku University School of Medicine ID: 2012‐1‐185; Thailand, Mahidol University, Faculty of Medicine Ramathibodi Hospital ID: 01‐54‐50), archival materials of TNBC patients were retrieved from the surgical pathology files. The status of triple negativity in tumor cells was confirmed by reviewing the ER/PR/HER2 stained slides based on American Society of Clinical Oncology/College of American Pathologists guidelines. From these cohorts, a total of 203 TNBC specimens with whole tissue availability (Japan, 86 cases; Thailand, 117 cases) were examined. Clinical data including patient age, stage, tumor diameter, lymph node involvement, and metastatic spread was available in both cohorts after review of the charts of individual patients.
Immunohistochemistry
Archival tissue blocks were serially sectioned at a thickness of 4 μM and placed on pre‐cleaned glue‐coated glass slides for immunohistochemistry (IHC). Immunostaining of serial tissue sections for AR, 5α‐reductase type 1 (5αR1) and 17β‐hydroxysteroid dehydrogenase type 5 (17βHSD5) was carried out as described previously.59, 60 In brief, antibodies against all three targets (AR, AR441 1:50 [Dako, Carpinteria, CA, USA]; 5αR1, 1:1000, provided by Dr D.W. Russell [University of Texas Southwestern Medical Center, Dallas, TX, USA]; 17βHSD5, NP6.G6.A6 1:200 [Sigma, St. Louis, MO, USA]) were used in conjunction with a streptavidin–biotin visualization method (Histofine kit; Nichirei, Tokyo, Japan). A control tissue (AR, prostate; 5αR1, liver; 17βHSD5, adrenal) was included in all runs of immunostaining in order to confirm the specificity of immunostaining. Ki‐67 immunostaining was carried out as described previously.59
Evaluation of immunoreactivity
Immunoreactivity was evaluated as follows. In an evaluation of AR in tumor cells, immunoreactivity was assessed by the H score.61 In brief, the H score was obtained by assessing immunointensity (on a scale of 0–3) and prevalence in 100 cells over five different areas throughout the tumor (scale, 0–300). Cytoplasmic (5αR1, 17βHSD5) immunoreactivity was evaluated using a semiquantitative scale (0–2) which divided tumor cells into categories: no immunoreactivity; 0.1–50% immunoreactivity; and 50.1–100% immunoreactivity.60 All slides were counted three times in order to assess intra‐observer variability and variation was found to be less than 10%. In addition, a subset of tissue slides was assessed by at least two of the authors (KM, YN, KT) in order to assess inter‐observer variability and inter‐observer differences. Variation for the H score was less than 12%, and for the enzyme score less than 5%. For Ki‐67 more than 500 tumor cells were counted in each case at the sites of hot spots. Hot spots were identified after evaluating the stained slides at low magnification. The immunoreactivity was quantified using a labeling index (LI). All the results were assessed by two authors (TY, HS) in order to assess reproducibility of the data, and found to be in agreement. When dichotomous variables were needed we used a cut‐off point of 10% LI (converted from the H score) for AR, a score of 2 (>50% positivity) in enzyme staining.
Statistical analysis
Statistical analyses were carried out using jmp software (JMP Pro 9.0.2; SAS Institute, Tokyo, Japan). To assess the overlap between enzymes and receptor expression, as well as significant differences between categorical variables by country, a χ2‐test was used. Differences in linear variables between two different cohorts were tested using Student's t‐test. Analysis of the effect of AR and enzyme expression on the Ki‐67 LI was tested by stratifying the groups by AR/5αR1 status and using anova followed by the Tukey–Kramer HSD post‐hoc test. Correlation analysis was carried out using the Pearson correlation in order to compare the correlations among AR, enzyme expression, and clinical and pathological markers. Interactions between receptor and enzyme status and survival were tested using a multivariate Cox proportional hazards model, and statistical significance of individual factors examined was tested using Kaplan–Meier survival analysis.
Results
Androgen receptor, 5αR1, and 17βHSD5 in TNBC tumor cells
Androgen receptor immunoreactivity was detected in the nuclei of tumor cells and 17βHSD5 and 5αR1 immunoreactivity was detected in the cytoplasm of tumor cells. Immunostaining in serial tissue sections indicated that in the great majority of the cases examined, AR, 5αR1, and 17βHSD5 immunoreactivity, where present, was detected in comparable areas (Fig. 1).
Figure 1.
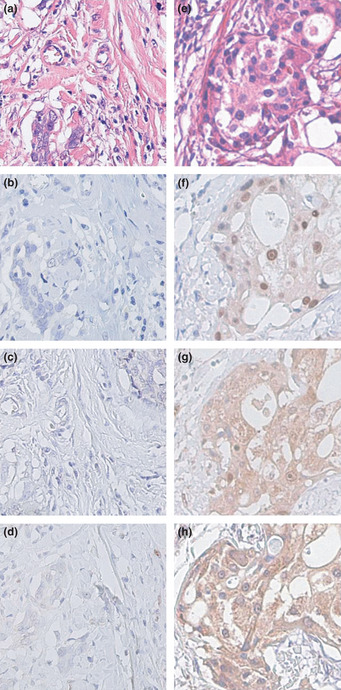
Representative photographs of androgen receptor (AR), 5α‐reductase type 1 (5αR1), and 17β‐hydroxysteroid dehydrogenase type 5 (17βHSD5) immunohistochemistry in AR−5αR1−17βHSD5− (a–d) and AR+5αR1+17βHSD5+ (e–h) triple negative breast carcinomas. Hematoxylin–eosin staining (a,e). Androgen receptor was immunolocalized in the nuclei of carcinoma cells at variable immunoreactivity (b,f). Both 5αR1 (c,g) and 17βHSD5 (d,h) were immunolocalized in the cytoplasm of carcinoma cells. Stromal cells were negative in areas either adjacent or distal to carcinoma. Original magnification, x200.
Differences in clinicopathological parameters between cohorts
In order to asses any possible differences between the two separate cohorts, χ2 assessment of the distribution of values between the Thai and the Japanese cohorts was undertaken. The number of patients and the percentage of populations (brackets) are given in Table 1. Immunoreactivity of 17βHSD5, lymph metastasis, and distant metastasis did not vary between Japanese and Thai cases but other factors such as age, stage, Ki‐67, and tumor diameter were significantly different.
Table 1.
Summary of distribution of clinicopathological features in Thai, Japanese, and combined cohorts of patients with triple negative breast cancer (n = 203)
| Thai, n (%) | Japanese, n (%) | Combined | P‐value | |
|---|---|---|---|---|
| 5αR1 | ||||
| Negative | 26 (22.2) | 31 (36.4) | 57 (28.2) | 0.03 |
| <50% positivity | 28 (23.9) | 23 (27.0) | 51 (25.2) | |
| ≥50% positivity | 63 (53.8) | 31 (36.4) | 94 (46.5) | |
| 17βHSD5 | ||||
| Negative | 34 (29.1) | 27 (31.1) | 61 (30.3) | 0.61 |
| <50% positivity | 53 (45.2) | 42 (48.8) | 95 (46.7) | |
| ≥50% positivity | 30 (25.6) | 17 (19.7) | 47 (23.1) | |
| AR | ||||
| Positive (>10% LI) | 20 (17.1) | 31 (36.1) | 51 (25.1) | 0.002 |
| Negative (≥10% LI) | 97 (82.9) | 55 (63.9) | 152 (74.9) | |
| TNM stage | ||||
| I | 32 (27.8) | 30 (37.0) | 62 (31.6) | 0.01 |
| IIA | 46 (40.0) | 27 (33.3) | 73 (37.2) | |
| IIB | 11 (9.6) | 8 (9.9) | 19 (9.7) | |
| IIIA | 14 (12.2) | 6 (7.4) | 20 (10.2) | |
| IIIB | 9 (7.8) | 2 (2.4) | 11 (5.6) | |
| IIIC | 1 (0.9) | 8 (9.9) | 9 (4.6) | |
| IV | 2 (1.7) | 0 (0) | 2 (1.0) | |
| Tumor size | ||||
| <20 mm | 38 (33.9) | 46 (56.7) | 84 (43.5) | 0.005 |
| 20.1–50 mm | 63 (56.2) | 31 (38.3) | 94 (48.7) | |
| >50.1 mm | 11 (9.8) | 4 (4.9) | 15 (7.7) | |
| Lymph invasion | ||||
| No | 74 (65.5) | 50 (62.5) | 124 (64.3) | 0.62 |
| Yes | 39 (34.5) | 30 (37.5) | 69 (35.8) | |
| Presence of distant metastasis | ||||
| Yes | 2 (1.7) | 0 (0) | 2 (1.0) | 0.23 |
| No | 113 (98.3) | 81 (100) | 194 (99.0) | |
| Age | ||||
| <50 years | 59 (50.4) | 29 (33.3) | 88 (43.1) | 0.04 |
| ≥50 years | 58 (49.6) | 58 (66.7) | 116 (56.9) | |
| Ki‐67 | ||||
| <25% | 75 (63.6) | 30 (37.0) | 105 (52.8) | 0.003 |
| ≥25% | 43 (36.4) | 51 (63.0) | 94 (47.2) | |
Bold indicates significant value. 5αR1, 5α‐reductase type 1; 17βHSD5, 17β‐hydroxysteroid dehydrogenase type 5; AR, androgen receptor; LI, labeling index.
Prevalence of AR, 5αR1, and 17βHSD5 in TNBC patients
In 203 TNBC cases examined in this study, positive AR immunoreactivity in the tumor cells (defined as >10% LI) was detected in 51 cases (25%). The immunoreactivity of 5αR1 and 17βHSD5 in breast cancer cases was greater than that of AR, with 71.7% of cancers positive for 5αR1 and 69.8% for 17βHSD5. There were significant differences between the Thai and Japanese cohorts in terms of AR positivity, AR H score, and 5αR1 score (P < 0.03) but not in 17βHSD5 score (P = 0.61). These results are summarized in Table 1.
Correlation between AR, enzymes, and clinicopathological factors in TNBC
Using the χ2‐test, the status of AR and 17βHSD5 in tumor cells was significantly correlated (P = 0.001) (either double negative or double positive), and also that of AR and 5αR1 (P = 0.04) in the whole cohort. A significant positive correlation was also detected between these two enzymes in the whole cohort (P = 0.001, 53% of samples showing the same enzymatic score, 70% when classified as negative or positive). In the whole cohort of patients, 40% were receptor and enzymes double negative (AR−/5αR2−/17βHSD5−) and 8% were receptor/enzymes double positive (AR+/5αR2+/17βHSD5+) using a cut‐off value of 2 (>50% immunoreactivity) for the enzymes and 10% LI for AR.
Pearson's correlation was used to assess the strength (Pearson's Rho; Table 2, first line for each parameter), and the significance (P‐value; Table 2, second line for each parameter, values in parentheses) of correlations between various histological and clinical parameters. Table 2 shows strong and significant (indicated in bold italics) or near significant (bold) correlations found between androgenic pathways as well as between androgenic pathways and indicators of tumor proliferation (AR and 5αR2, P = 0.002; AR and 17βHSD5, P = 0.001; AR and age, P < 0.001; 5αR1 and 17βHSD5, P < 0.0001), and also between the three clinicopathological factors used to define TNM breast cancer stage (tumor diameter, lymph node invasion, and metastatic spread). Significant negative correlations were also detected between Ki‐67 LI and AR (P = 0.048) or 5αR1 (P = 0.004) status in tumor cells. The AR status tended to be inversely correlated with tumor diameter but this correlation did not reach statistical significance (P = 0.055). When stratified by country, the correlation coefficients obtained showed similar trends but not all significant associations remained.
Table 2.
Pearson's correlations between clinicopathological features and androgen receptor (AR), 5α‐reductase type 1 (5αR1), and 17β‐hydroxysteroid dehydrogenase type 5 (17βHSD5) expression in combined cohort of patients with triple negative breast cancer from Japan and Thailand (n = 203)
| 5αR1 score | 17βHSD5 score | Age | Ki‐67 | Tumor diameter | Lymph node metastasis | Presence of distant metastasis | ||
|---|---|---|---|---|---|---|---|---|
| AR H score | 0.2151 (0.0020) | 0.2438 (0.0010) | 0.2948 (<0.0001) | –0.1414 (0.0480) | −0.1390 (0.0550) | 0.0920 (0.2040) | −0.0190 (0.7890) | |
| 5αR1 score | 0.4062 (<0.0010) | 0.0511 (0.4470) | –0.2037 (0.0040) | 0.0405 (0.5780) | 0.0343 (0.6380) | −0.0845 (0.2420) | ||
| 17βHSD5 score | 0.0740 (0.3010) | −0.0546 (0.4460) | 0.0210 (0.7730) | −0.0292 (0.6870) | −0.0606 (0.4000) | |||
| Age | −0.0665 (0.3530) | −0.1274 (0.0780) | 0.0319 (0.6600) | −0.1104 (0.1230) | ||||
| Ki‐67 | −0.0205 (0.7780) | −0.0272 (0.7080) | 0.0005 (0.9950) | |||||
| Tumor diameter | 0.2808 (<0.0001) | 0.1492 (0.0400) | ||||||
| Lymph node invasion | 0.1395 (0.0540) |
Correlation strength was calculated using Pearson's Rho; significance (P‐values) shown in parentheses. Bold, near significant correlation; bold italics, significant correlation.
In order to further assess the effects of AR/enzyme action on Ki‐67 LI in tumor cells in TNBC cases, we subclassified the cases according to the AR/5αR1 status and compared the Ki‐67 LI among these different groups of TNBC patients. We did not include 17βHSD5 in this stratification because of the relatively small cohort, and the close correlation between 17βHSD5 and 5αR1. In this analysis AR+/5αR1+ cases had the lowest Ki‐67 LI and AR−/5αR1− the highest Ki‐67 LI. (Fig. 2). The tendency among the four different groups still remained even if the Thai and Japanese cohorts were analyzed separately, but only the Japanese cohort showed significance (P = 0.002) in separate analysis.
Figure 2.
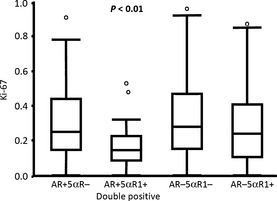
Correlation between androgen receptor (AR) H score and Ki‐67 labeling index (LI) and effects on Ki‐67 LI when triple negative breast cancer cases were subdivided according to receptor and enzyme status. The AR+ 5α‐reductase type 1 (5αR1)+ group was significantly different from the AR−5αR1− group (P = 0.004, Tukey–Kramer HSD), based on the cut‐off points >50% immunopositivity in 5αR1 and >10% labeling index for AR. If these results were stratified by country of origin, only the Japanese cohorts showed statistically significant differences. Thai cohorts showed a similar trend but the correlation did not reach statistical significance. The circles above the columns represent outlying points.
Analysis of the effects of AR and enzyme expression on DFS and OS
Interactions between AR, enzyme expression, and clinicopathological factors were modelled using a multivariate Cox proportional hazards model (Table 3). For OS, a total of 176 patients had complete data with 31 events; for DFS, a total of 174 patients were available with complete data with 35 events. For linear factors the risk ratio represents the change in risk per unit of the regressor, for ordinal factors the risk ratio is standardized to the lowest (i.e., in the least developed stage) grouping. In the stratification by country, the risk ratios were standardized to the Japanese cohort. Both models were significant predictors of outcome (OS, P < 0.0001; DFS, P = 0.0005). This analysis showed that the only robust and significant factors were tumor TNM stage at the time of diagnosis (which accounts for tumor diameter, lymph node involvement, and presence of distant metastasis), and Ki‐67 LI (P < 0.001 and P = 0.017 respectively).
Table 3.
Interactions between androgenic pathways and clinical factors in determining survival outcomes in triple negative breast cancer patients from Thailand and Japan (n = 203)
| Overall survival | Disease‐free survival | |||||||
|---|---|---|---|---|---|---|---|---|
| P‐value | Risk ratio | CI lower | CI upper | P‐value | Risk ratio | CI lower | CI upper | |
| Ki‐67 | 0.0175 | 6.80 | 1.42 | 40.75 | 0.0404 | 4.798 | 1.07 | 22.45 |
| TNM stage | ||||||||
| IIa | <0.0001 | 0.81 | 0.24 | 2.86 | <0.0001 | 1.18 | 0.42 | 3.59 |
| IIb | 5.17 | 1.37 | 20.39 | 3.72 | 0.99 | 13.42 | ||
| IIIa | 5.86 | 1.30 | 25.32 | 5.19 | 1.42 | 18.33 | ||
| IIIb | 3.61 | 0.17 | 26.59 | 3.95 | 0.55 | 19.15 | ||
| IIIc | 6.95 | 1.98 | 25.48 | 6.99 | 2.08 | 23.64 | ||
| IV | 401.97 | 34.79 | 4646.53 | 98.81 | 11.40 | 658.97 | ||
| Country | 0.8429 | 0.88 | 0.23 | 3.03 | 0.3792 | 0.64 | 0.23 | 1.72 |
| AR H score | 0.4268 | 1.00 | 0.99 | 1.01 | 0.7099 | 1.00 | 0.99 | 1.01 |
| 5αR1 score | ||||||||
| 1 | 0.5886 | 1.54 | 0.48 | 4.89 | 0.6304 | 1.47 | 0.52 | 4.17 |
| 2 | 0.93 | 0.31 | 2.88 | 0.97 | 0.35 | 2.66 | ||
| 17βHSD5 score | ||||||||
| 1 | 0.8235 | 0.75 | 0.29 | 1.99 | 0.6275 | 0.67 | 0.29 | 1.58 |
| 2 | 0.97 | 0.29 | 3.04 | 0.93 | 0.29 | 2.67 | ||
| Age | 0.4145 | 2.89 | 0.98 | 1.05 | 0.7953 | 1.00 | 0.97 | 1.04 |
Bold indicates significant value. 5αR1, 5α‐reductase type 1; 17βHSD5, 17β‐hydroxysteroid dehydrogenase type 5; AR, androgen receptor; CI, confidence interval.
Results of univariate Kaplan–Meier analysis showed no significant effects on OS or DFS in evaluating AR+5αR1+ groups compared to others, but a significantly worse (P < 0.05) survival outcome was detected in AR‐5αR1− patients in an 80‐month follow‐up period, which corresponds to the longest length of follow‐up for patients in the Thai cohort (Fig. 3). The Ki‐67 LI also predicted survival in the TNBC group in this analysis (Fig. 3).
Figure 3.
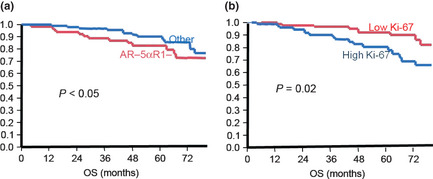
Survival curves according to Ki‐67 and androgen receptor (AR)−/5α‐reductase type 1 (5αR1)− status of breast cancer patients. Kaplan–Meier survival curves analyzed according to 5αaR1‐/AR‐ (a) and Ki‐67 labeling index (LI) (b). Double negative cases were tentatively defined as having less than 10% LI for AR and less than 50% of carcinoma areas immunohistochemically positive for 5αR1. Low Ki‐67 cases were tentatively defined as less than 25% Ki‐67 LI, with high Ki‐67 cases greater than 25% LI (median value). Survival curves and analysis were truncated at 80 months because this was the longest time to follow up in the Thai cohort. If not truncated the survival curve of androgen action negative groups the patients crossed at approximately 90 months, but the Ki‐67 survival curves did not cross until 150 months (the longest time of clinical follow‐up in this study).
Discussion
This study was undertaken to address controversies regarding the possible roles of androgenic pathways in the biological behavior of TNBC patients. In addition, we also hypothesized that assessing enzyme status in combination with receptor status could illuminate why contradictory results existed regarding the roles of androgen signalling in TNBC because of the biological importance of the intratumoral production of active steroids in situ (intracrinology).62 In particular, we have previously shown that only the combination of the enzyme and AR expression (i.e., an intact androgen synthesis and signalling pathway) in tumor cells could predict a better clinical outcome for AR+ breast cancer patients, whereas AR status in tumor cells alone failed to indicate any significant effect of androgen signalling on prognosis of breast cancer patients.59
In our present study, all associations were tested by being stratified according to each cohort examined, as well as combined because of the differences in a number of clinicopathological factors between these two cohorts. This study was not designed to answer any possible cause of differences between the two cohorts, however, there are many factors we can speculate may be the cause, including but not limited to difference in ethnicity, and differences in methodological approaches between different hospitals. Despite these differences, the same trend was evident in terms of the possible correlation between various clinicopathological variables and those factors as related to androgenic signalling in tumor cells. Therefore, the underlying differences between these two cohorts are reasonably postulated not to hamper the validity of the conclusions in our study either when separated or combined.
Androgen receptor nuclear immunoreactivity was detected in approximately 25% of all TNBC cases examined, which fell into the ranges previously reported for TNBC.45 In addition, AR immunoreactivity was associated with enzyme status, which is consistent with our previous findings in the whole series of breast cancer not stratified by individual subtypes.59 The status of both AR and enzymes was independently associated with lower rates of Ki‐67 LI of tumor cells and AR tended to be associated with a smaller tumor diameter, although the tendency did not reach statistical significance. These findings were also consistent with results of previously published studies regarding the correlation between AR and reduced Ki‐67 LI in TNBC,44, 56 ER−,23 and general breast cancer groups,20 as well as the correlation between growth suppression in response to androgen therapy in vivo.63, 64 In addition, results of our present study indicated the presence of androgen synthesis enzymes in the tumors, which conferred an additional contribution to decreased cell proliferation. It is also interesting to note that the lowest Ki‐67 LI was indeed associated with AR+/5αR1+ cases followed by AR−/5aR1+, AR+/5aR1−, and AR−/5aR1− (Table 2). The statistically significant correlation between Ki‐67 LI and survival was previously reported in TNBC cohorts,65 which also suggests that AR and AR/enzyme expression may confer a survival advantage through the suppression of cell proliferation in TNBC.
In this study, it is also true that we could not show a significant effect of AR or enzyme expression on the overall clinical outcome of the patients, although the AR−5αR1− groups conferred an aggressive clinical course upon the patients examined in this study. One explanation for this finding could be that AR and androgen metabolism may not be the only factors responsible for cell proliferation, as in luminal A type breast cancer, but merely two of many governing cell proliferation. Hence, they may not necessarily be sufficient to significantly affect survival in our cohort, with its limited numbers of patients, especially in the AR+/enzyme+ group. The lack of correlation between AR and survival is not contradictory to published reports as, despite many studies showing a survival advantage associated with AR expression,11, 46, 47 others have been unable to find a significant effect of AR expression on survival outcomes for patients.20, 43, 48
Results of the correlation analysis revealed that, aside from Ki‐67, the only other clinical factor showing a statistically significant correlation with AR was patient age. This correlation has been detected in previous studies examining AR expression in breast cancer non‐ stratified by subtype,7 ER− breast cancer,46 and in TNBC,56 although these results are not necessarily consistent.19 A positive association between an increased level of circulating androgens in relation to estrogens and AR expression in the breast has been also reported in female to male transsexuals66 and in prepubescent and postmenopausal primate breast tissue,67 which suggest that correlations between age and AR expression may be explained by changes in the availability of circulating sex steroids. Further studies are needed to investigate what effect, if any, this increase in AR receptor expression with age may have on the underlying biology of TNBC. The lack of correlation between AR or enzyme expression and distant metastasis status or lymph node metastasis suggest that androgen signalling in TNBC cells might not play important roles in the process of tumor cell invasion and/or metastasis either to the lymphatic system or distant from the original tumor.
One of the major inconsistencies currently present in TNBC patients is the discrepancy between the majority of IHC results in TNBC cases and the results reported in AR+ TNBC cell lines. Although many clinical cohorts11, 46, 47 and transient transfection63 studies have shown clinically beneficial effects of AR expression in tumor cells, the great majority of AR+ TNBC cell lines, with the exception of MFM223 cells,64 show growth stimulation or proliferation responses to androgen treatment,57, 68 mediated through activation of ER target genes54 via androgen receptor actions.48, 52, 53, 54, 55 Results of these in vivo studies suggest that AR expression in triple negative disease would be detrimental for the patients. The results are also in agreement with gene expression studies selecting for androgen‐overexpressing ER− tumors, which indicated adverse clinical outcomes in OS69 and disease recurrence.56 Clinically these inconsistencies have posed serious problems in terms of deciding whether androgen inhibition or stimulation would prove beneficial in TNBC patients. Some potential explanations for the contradictions could be as follows.
First, there are often‐raised differences between results in cell lines and human tissue specimens, as in other malignancies.70, 71, 72 The three most widely used AR+ TNBC cell lines have significant mutations associated with intracellular signalling that are not necessarily recapitulated in the majority of TNBC specimens.56, 70, 73, 74 In addition, the most widely characterized TNBC cell line, MBD‐MB‐453, carries recently discovered mutations in the AR that alter its promiscuity to other ligands.75 At this point the frequency of the mutation in the TNBC cancer population is totally unknown.
A second possible explanation is that the effects of AR expression in TNBC may not be homogeneous, or that gene expression profiling and IHC data of individual cases may not be selecting the same populations of patients. Gene expression profiling was initially used to show that breast cancer can be subtyped into categories that have meaningful clinical outcomes based upon their gene expression profiles.76, 77 Subsequent studies using gene expression profiles found groupings within breast cancer that are defined by the expression of AR in the absence of ERa. These groupings have been termed molecular apocrine and LAR.54, 56 The molecular apocrine subtype is defined by the lack of a complete luminal or basal gene signature (as defined by Perou)78 in combination with increment in AR signalling, whereas the LAR subtype is defined by enrichment of hormonally regulated pathways. It should be noted that the molecular apocrine classification included both HER2/ERBB2 enriched cancers in addition to triple negative cancers, and the triple negativity in the complete LAR set was defined by gene expression levels rather than a direct assessment of IHC reactivity of ER/PR and HER2. In both analyses, although the gene expression profiles selected for subgroups contained high levels of AR,56, 69 not all AR‐expressing tumors were included in the LAR and molecular apocrine gene expression profiles (see Farmer et al., fig. 3A69 and Lehmann et al., fig. S1156). This suggests that gene expression profiling is not just selecting for AR‐expressing tumors but a subset of AR‐expressing tumors that have an underlying biological signature. Interestingly, the gene profiles of the AR‐expressing and AR growth‐stimulated triple negative cell lines mentioned above all corresponded to the LAR subtype,56 suggesting the currently available AR‐expressing triple negative cell lines available might not be representative of the full spectrum of AR‐expressing TNBC. Therefore, it is important to note that AR immunoreactivity in TNBC specimens does not necessarily indicate that these cases correspond to the androgen‐enriched (LAR, molecular apocrine) subtypes of TNBC. Therefore, the possibility for divergent effects of AR action in TNBC could account for the current apparent contradictions in published reports between gene expression profile studies and clinical pathology studies. Further research into potential sub‐subtyping of AR‐expressing TNBC, including assessment of additional steroid receptors known to activated by androgen derivatives such as ERβ, may help to clarify the underlying biology.
In conclusion, results of our present study suggest that AR and androgen signalling pathways within the tumor may be beneficial to the clinical outcome of TNBC breast cancer patients through the inhibition of cellular proliferation. If borne out by further investigations, this could provide a subset of TNBC patients access to more targeted therapies than those currently available.
Disclosure Statement
The authors have no conflicts of interest.
Acknowledgments
Keely McNamara is supported by a Japan Society for the Promotion of Science – Australian Academy of Science postdoctoral fellowship. We would also like to acknowledge the support and assistance of the members of the Department of Anatomical Pathology, Tohoku University School of Medicine.
(Cancer Sci 2013; 104: 639–646)
References
- 1. Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol 2012; 36: 237–48. [DOI] [PubMed] [Google Scholar]
- 2. Maxmen A. The hard facts. Nature 2012; 485: S50–1. [DOI] [PubMed] [Google Scholar]
- 3. Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J 2009; 15: 593–602. [DOI] [PubMed] [Google Scholar]
- 4. Meijnen P, Peterse JL, Antonini N, Rutgers EJ, van de Vijver MJ. Immunohistochemical categorisation of ductal carcinoma in situ of the breast. Br J Cancer 2008; 98: 137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanley K, Wang J, Bourne P et al Lack of expression of androgen receptor may play a critical role in transformation from in situ to invasive basal subtype of high‐grade ductal carcinoma of the breast. Hum Pathol 2008; 39: 386–92. [DOI] [PubMed] [Google Scholar]
- 6. Wei B, Wang J, Bourne P et al Bone metastasis is strongly associated with estrogen receptor‐positive/progesterone receptor‐negative breast carcinomas. Hum Pathol 2008; 39: 1809–15. [DOI] [PubMed] [Google Scholar]
- 7. Ogawa Y, Hai E, Matsumoto K et al Androgen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkers. Int J Clin Oncol 2008; 13: 431–5. [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez‐Angulo AM, Stemke‐Hale K, Palla SL et al Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res 2009; 15: 2472–8. [DOI] [PubMed] [Google Scholar]
- 9. Park S, Koo J, Park HS et al Expression of androgen receptors in primary breast cancer. Ann Oncol 2010; 21: 488–92. [DOI] [PubMed] [Google Scholar]
- 10. Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor‐positive tumors and in estrogen receptor‐negative tumors with apocrine differentiation. Mod Pathol 2010; 23: 205–12. [DOI] [PubMed] [Google Scholar]
- 11. Luo X, Shi YX, Li ZM, Jiang WQ. Expression and clinical significance of androgen receptor in triple negative breast cancer. Chin J Cancer 2010; 29: 585–90. [DOI] [PubMed] [Google Scholar]
- 12. Masuda H, Masuda N, Kodama Y et al Predictive factors for the effectiveness of neoadjuvant chemotherapy and prognosis in triple‐negative breast cancer patients. Cancer Chemother Pharmacol 2011; 67: 911–7. [DOI] [PubMed] [Google Scholar]
- 13. Aleskandarany MA, Rakha EA, Ahmed MA, Powe DG, Ellis IO, Green AR. Clinicopathologic and molecular significance of phospho‐Akt expression in early invasive breast cancer. Breast Cancer Res Treat 2011; 127: 407–16. [DOI] [PubMed] [Google Scholar]
- 14. Micello D, Marando A, Sahnane N, Riva C, Capella C, Sessa F. Androgen receptor is frequently expressed in HER2‐positive. ER/PR‐negative breast cancers. Virchows Arch 2010; 457: 467–76. [DOI] [PubMed] [Google Scholar]
- 15. Koo JS, Jung W, Jeong J. The predictive role of E‐cadherin and androgen receptor on in vitro chemosensitivity in triple‐negative breast Cancer. Jpn J Clin Oncol 2009; 39: 560–8. [DOI] [PubMed] [Google Scholar]
- 16. Pristauz G, Petru E, Stacher E et al Androgen receptor expression in breast cancer patients tested for BRCA1 and BRCA2 mutations. Histopathology 2010; 57: 877–84. [DOI] [PubMed] [Google Scholar]
- 17. Chen J, Zhang X, Tian R et al Expression of androgen receptor in breast carcinoma and its relationship with estrogen receptor, progesterone receptor and HER2 status. Zhonghua Bing Li Xue Za Zhi 2010; 39: 743–6. [PubMed] [Google Scholar]
- 18. Chae BJ, Lee A, Bae JS, Song BJ, Jung SS. Expression of nuclear receptor DAX‐1 and androgen receptor in human breast cancer. J Surg Oncol 2011; 103: 768–72. [DOI] [PubMed] [Google Scholar]
- 19. He J, Peng R, Yuan Z et al Prognostic value of androgen receptor expression in operable triple‐negative breast cancer: a retrospective analysis based on a tissue microarray. Med Oncol 2011; 29: 406–10. [DOI] [PubMed] [Google Scholar]
- 20. Park S, Koo JS, Kim MS et al Androgen receptor expression is significantly associated with better outcomes in estrogen receptor‐positive breast cancers. Ann Oncol 2011; 22: 1755–62. [DOI] [PubMed] [Google Scholar]
- 21. Tang D, Xu S, Zhang Q, Zhao W. The expression and clinical significance of the androgen receptor and E‐cadherin in triple‐negative breast cancer. Med Oncol 2011; 29 (2): 526–33. [DOI] [PubMed] [Google Scholar]
- 22. Loibl S, Muller BM, von Minckwitz G et al Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat 2011; 130: 477–87. [DOI] [PubMed] [Google Scholar]
- 23. Qi JP, Yang YL, Zhu H et al Expression of the androgen receptor and its correlation with molecular subtypes in 980 chinese breast cancer patients. Breast Cancer (Auckl) 2012; 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Irshad S, Ellis P, Tutt A. Molecular heterogeneity of triple‐negative breast cancer and its clinical implications. Curr Opin Oncol 2011; 23: 566–77. [DOI] [PubMed] [Google Scholar]
- 25. Perou CM. Molecular stratification of triple‐negative breast cancers. Oncologist 2011; 16(Suppl. 1): 61–70. [DOI] [PubMed] [Google Scholar]
- 26. Weigman VJ, Chao HH, Shabalin AA et al Basal‐like Breast cancer DNA copy number losses identify genes involved in genomic instability, response to therapy, and patient survival. Breast Cancer Res Treat 2012; 133: 865–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bauer K, Parise C, Caggiano V. Use of ER/PR/HER2 subtypes in conjunction with the 2007 St Gallen Consensus Statement for early breast cancer. BMC Cancer 2010; 10: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwentner L, Wolters R, Koretz K et al Triple‐negative breast cancer: the impact of guideline‐adherent adjuvant treatment on survival‐a retrospective multi‐centre cohort study. Breast Cancer Res Treat 2011; 132: 1073–80. [DOI] [PubMed] [Google Scholar]
- 29. Razzak AR, Lin NU, Winer EP. Heterogeneity of breast cancer and implications of adjuvant chemotherapy. Breast Cancer 2008; 15: 31–4. [DOI] [PubMed] [Google Scholar]
- 30. Carey LA. Directed therapy of subtypes of triple‐negative breast cancer. Oncologist 2011; 16(Suppl. 1): 71–8. [DOI] [PubMed] [Google Scholar]
- 31. Bosch A, Eroles P, Zaragoza R, Vina JR, Lluch A. Triple‐negative breast cancer: molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev 2010; 36: 206–15. [DOI] [PubMed] [Google Scholar]
- 32. Peiro G, Adrover E, Sanchez‐Tejada L et al Increased insulin‐like growth factor‐1 receptor mRNA expression predicts poor survival in immunophenotypes of early breast carcinoma. Mod Pathol 2011; 24: 201–8. [DOI] [PubMed] [Google Scholar]
- 33. Nogi H, Kobayashi T, Suzuki M et al EGFR as paradoxical predictor of chemosensitivity and outcome among triple‐negative breast cancer. Oncol Rep 2009; 21: 413–7. [PubMed] [Google Scholar]
- 34. Rennstam K, McMichael N, Berglund P et al Numb protein expression correlates with a basal‐like phenotype and cancer stem cell markers in primary breast cancer. Breast Cancer Res Treat 2010; 122: 315–24. [DOI] [PubMed] [Google Scholar]
- 35. Smid M, Hoes M, Sieuwerts AM et al Patterns and incidence of chromosomal instability and their prognostic relevance in breast cancer subtypes. Breast Cancer Res Treat 2011; 128: 23–30. [DOI] [PubMed] [Google Scholar]
- 36. Hussein YR, Sood AK, Bandyopadhyay S et al Clinical and biological relevance of enhancer of zeste homolog 2 in triple‐negative breast cancer. Hum Pathol 2011; 43: 1638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Svoboda M, Sana J, Redova M et al MiR‐34b is associated with clinical outcome in triple‐negative breast cancer patients. Diagn Pathol 2012; 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moinfar F, Okcu M, Tsybrovskyy O et al Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer 2003; 98: 703–11. [DOI] [PubMed] [Google Scholar]
- 39. Hall RE, Aspinall JO, Horsfall DJ et al Expression of the androgen receptor and an androgen‐responsive protein, apolipoprotein D, in human breast cancer. Br J Cancer 1996; 74: 1175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Isola JJ. Immunohistochemical demonstration of androgen receptor in breast cancer and its relationship to other prognostic factors. J Pathol 1993; 170: 31–5. [DOI] [PubMed] [Google Scholar]
- 41. Kuenen‐Boumeester V, Van der Kwast TH, van Putten WL, Claassen C, van Ooijen B, Henzen‐Logmans SC. Immunohistochemical determination of androgen receptors in relation to oestrogen and progesterone receptors in female breast cancer. Int J Cancer 1992; 52: 581–4. [DOI] [PubMed] [Google Scholar]
- 42. Lea OA, Kvinnsland S, Thorsen T. Improved measurement of androgen receptors in human breast cancer. Cancer Res 1989; 49: 7162–7. [PubMed] [Google Scholar]
- 43. Hu R, Dawood S, Holmes MD et al Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res 2011; 17: 1867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsutsumi Y. Apocrine Carcinoma as Triple‐negative Breast Cancer: novel Definition of Apocrine‐type Carcinoma as Estrogen/Progesterone Receptor‐negative and Androgen Receptor‐positive Invasive Ductal Carcinoma. Jpn J Clin Oncol 2012; 42: 375–86. [DOI] [PubMed] [Google Scholar]
- 45. McNamara K, Yoda T, Takagi K, Miki Y, Suzuki T, Sasano H. Androgen receptor in triple negative breast cancer. J Steroid Biochem Mol Biol 2013; 133: 66–76. [DOI] [PubMed] [Google Scholar]
- 46. Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen receptor expression in estrogen receptor‐negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol 2003; 120: 725–31. [DOI] [PubMed] [Google Scholar]
- 47. Rakha EA, El‐Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple‐negative breast cancer. Cancer 2007; 109: 25–32. [DOI] [PubMed] [Google Scholar]
- 48. Peters AA, Buchanan G, Ricciardelli C et al Androgen receptor inhibits estrogen receptor‐alpha activity and is prognostic in breast cancer. Cancer Res 2009; 69: 6131–40. [DOI] [PubMed] [Google Scholar]
- 49. Ormandy CJ, Clarke CL, Kelly PA, Sutherland RL. Androgen regulation of prolactin‐receptor gene expression in MCF‐7 and MDA‐MB‐453 human breast cancer cells. Int J Cancer 1992; 50: 777–82. [DOI] [PubMed] [Google Scholar]
- 50. Hall RE, Tilley WD, McPhaul MJ, Sutherland RL. Regulation of androgen receptor gene expression by steroids and retinoic acid in human breast‐cancer cells. Int J Cancer 1992; 52: 778–84. [DOI] [PubMed] [Google Scholar]
- 51. Birrell SN, Bentel JM, Hickey TE et al Androgens induce divergent proliferative responses in human breast cancer cell lines. J Steroid Biochem Mol Biol 1995; 52: 459–67. [DOI] [PubMed] [Google Scholar]
- 52. Bentel JM, Birrell SN, Pickering MA, Holds DJ, Horsfall DJ, Tilley WD. Androgen receptor agonist activity of the synthetic progestin, medroxyprogesterone acetate, in human breast cancer cells. Mol Cell Endocrinol 1999; 154: 11–20. [DOI] [PubMed] [Google Scholar]
- 53. Mitchell S, Abel P, Madaan S et al Androgen‐dependent regulation of human MUC1 mucin expression. Neoplasia 2002; 4: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Doane AS, Danso M, Lal P et al An estrogen receptor‐negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006; 25: 3994–4008. [DOI] [PubMed] [Google Scholar]
- 55. Robinson JL, MacArthur S, Ross‐Innes CS et al Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. EMBO J 2011; 30: 3019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lehmann BD, Bauer JA, Chen X et al Identification of human triple‐negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011; 121: 2750–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ni M, Chen Y, Lim E et al Targeting androgen receptor in estrogen receptor‐negative breast cancer. Cancer Cell 2011; 20: 119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gucalp A, Traina TA. Triple‐negative breast cancer: role of the androgen receptor. Cancer J 2010; 16: 62–5. [DOI] [PubMed] [Google Scholar]
- 59. Suzuki T, Miki Y, Moriya T et al 5α‐reductase type 1 and aromatase in breast carcinoma as regulators of in situ androgen production. Int J Cancer 2007; 120: 285–91. [DOI] [PubMed] [Google Scholar]
- 60. Chanplakorn N, Chanplakorn P, Suzuki T et al Increased 5alpha‐reductase type 2 expression in human breast carcinoma following aromatase inhibitor therapy: the correlation with decreased tumor cell proliferation. Horm Cancer 2011; 2: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McCarty KS Jr, Szabo E, Flowers JL et al Use of a monoclonal anti‐estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res 1986; 46: 4244s–8s. [PubMed] [Google Scholar]
- 62. Sasano H, Suzuki T, Miki Y, Moriya T. Intracrinology of estrogens and androgens in breast carcinoma. J Steroid Biochem Mol Biol 2008; 108: 181–5. [DOI] [PubMed] [Google Scholar]
- 63. Garay JP, Karakas B, Abukhdeir AM et al The growth response to androgen receptor signaling in ERalpha‐negative human breast cells is dependent on p21 and mediated by MAPK activation. Breast Cancer Res 2012; 14: R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hackenberg R, Hawighorst T, Filmer A et al Regulation of androgen receptor mRNA and protein level by steroid hormones in human mammary cancer cells. J Steroid Biochem Mol Biol 1992; 43: 599–607. [DOI] [PubMed] [Google Scholar]
- 65. Miyashita M, Ishida T, Ishida K et al Histopathological subclassification of triple negative breast cancer using prognostic scoring system: five variables as candidates. Virchows Arch 2011; 458: 65–72. [DOI] [PubMed] [Google Scholar]
- 66. Grynberg M, Fanchin R, Dubost G et al Histology of genital tract and breast tissue after long‐term testosterone administration in a female‐to‐male transsexual population. Reprod Biomed Online 2010; 20: 553–8. [DOI] [PubMed] [Google Scholar]
- 67. Stute P, Sielker S, Wood CE et al Life stage differences in mammary gland gene expression profile in non‐human primates. Breast Cancer Res Treat 2012; 133: 617–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hackenberg R, Luttchens S, Hofmann J, Kunzmann R, Holzel F, Schulz KD. Androgen sensitivity of the new human breast cancer cell line MFM‐223. Cancer Res 1991; 51: 5722–7. [PubMed] [Google Scholar]
- 69. Farmer P, Bonnefoi H, Becette V et al Identification of molecular apocrine breast tumours by microarray analysis. Oncogene 2005; 24: 4660–71. [DOI] [PubMed] [Google Scholar]
- 70. Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res 2011; 13: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mehta JP, O'Driscoll L, Barron N, Clynes M, Doolan P. A microarray approach to translational medicine in breast cancer: how representative are cell line models of clinical conditions? Anticancer Res 2007; 27: 1295–300. [PubMed] [Google Scholar]
- 72. Ross DT, Perou CM. A comparison of gene expression signatures from breast tumors and breast tissue derived cell lines. Dis Markers 2001; 17: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vranic S, Gatalica Z, Wang ZY. Update on the molecular profile of the MDA‐MB‐453 cell line as a model for apocrine breast carcinoma studies. Oncol Lett 2011; 2: 1131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Capes‐Davis A, Theodosopoulos G, Atkin I et al Check your cultures! A list of cross‐contaminated or misidentified cell lines. Int J Cancer 2010; 127: 1–8. [DOI] [PubMed] [Google Scholar]
- 75. Moore NL, Buchanan G, Harris J et al An androgen receptor mutation in the MDA‐MB‐453 cell line model of molecular apocrine breast cancer compromises receptor activity. Endocr Relat Cancer 2012; 19 (4): 599–613. [DOI] [PubMed] [Google Scholar]
- 76. Sorlie T, Perou CM, Tibshirani R et al Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98: 10869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sorlie T, Tibshirani R, Parker J et al Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 2003; 100: 8418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Perou CM, Sorlie T, Eisen MB et al Molecular portraits of human breast tumours. Nature 2000; 406: 747–52. [DOI] [PubMed] [Google Scholar]


