Abstract
The association between schizophrenia and cancer risk is contentious in the clinical and epidemiological literature. Studies from different populations, tumor sites, or health care systems have provided inconsistent findings. In the present study, we examined a less well‐investigated hypothesis that age plays a crucial role in cancer risk in schizophrenia. We conducted a nationwide cohort study using Taiwan's National Health Insurance Research Database (NHIRD) between 1995 and 2007. Overall, gender‐, and age‐stratified standardized incidence ratios (SIR) were used to investigate the pattern of cancer risk by age. Of the 102 202 schizophrenic patients, 1738 developed cancer after a diagnosis of schizophrenia (SIR = 0.92; 95% confidence interval [CI] 0.90–0.96). However, the age‐stratified SIR declined with age (e.g. SIR [95% CI] = 1.97 [1.85–2.33], 0.68 [0.65–0.78], and 0.36 [0.34–0.45] for those aged 20–29, 60–69, and ≥70 years, respectively) in both genders and for major cancers. Cancer risks in schizophrenic patients were lower for cancers that are more likely to develop at an older age in the general population (e.g. stomach cancer [SIR = 0.62; 95% CI 0.57–0.80], pancreatic cancer [SIR = 0.49; 95% CI 0.39–0.84], and prostate cancer [SIR = 0.35; 95% CI 0.29–0.58]). In contrast, cancer risks were higher for cancers that have a younger age of onset, such as cancers of the nasopharynx (SIR = 1.18; 95% CI 1.08–1.49), breast (SIR = 1.50; 95% CI 1.44–1.66) and uterine corpus (SIR = 2.15; 95% CI 1.98–2.74). The unique age structures and early aging potential of schizophrenia populations may contribute to the observed inverse relationship between age and cancer risk. Higher cancer comorbidity in young schizophrenic patients deserves more attention.
Schizophrenia, a devastating brain disorder with high morbidity and mortality, affects approximately 1% of the population worldwide.1 In addition to suicide and accidents, cardiovascular, respiratory, and metabolic diseases are frequently comorbid with schizophrenia.2, 3 However, whether schizophrenic patients have a higher risk of cancers remains contentious. Although some studies have found that cancer risk in patients with schizophrenia was higher relative to the general population,4 others have found either lower cancer risk5, 6, 7, 8 or no difference.9 In general, findings vary greatly with regard to the direction and size of the difference according to tumor sites,10 study sites, racial and/or ethnic groups, and populations.11
Several hypotheses have been proposed to explain the differential cancer risk in the schizophrenic population. Patients with schizophrenia may appear to have lower risk because they are less likely to be screened for cancer, due to their more limited access to health care.3, 12 It is also possible that prolonged hospitalization, where smoking is prohibited and diets are controlled, can protect patients from developing cancer.5 Others have suggested that genetic factors may play a prote‐ctive role.13, 14 In contrast, some patient characteristics (e.g. unhealthy lifestyle, poor self‐care, drug abuse, smoking and heavy drinking) can elevate the risk of cancer.15, 16 For example, Lichtermann et al.4 reported that patients with schizophrenia have a higher risk of lung cancer in psychiatric care settings where smoking was allowed.
The present study examines the effects of age on cancer risk among patients with schizophrenia, which is an important yet less well‐studied issue. It is well established that cancer is a disease of aging. However, age may affect the pattern of cancer risk in the schizophrenic population in several ways. For example, the schizophrenic population has a relatively low proportion of older people because of its high mortality at an early age.17 The average age at the time of death for schizophrenic patients (57.3–65.5 years old18) is lower than that of the general population, with the life expectancy of schizophrenic patients shorter by 16.3–18.7 years.19 Therefore, it is possible that cancer risks may be lower among older schizophrenic patients due to competitive mortality; that is, schizophrenic patients may die earlier because of other diseases and/or conditions, including accidents or suicide. Thus, few schizophrenic patients live to an older age when cancers begin to develop. It is also possible that younger schizophrenic patients may experience earlier aging. Kirkpatrick et al.20 have posited that physiological changes associated with aging occur at an earlier age in people with schizophrenia than in the general population. This early aging hypothesis is supported by the fact that schizophrenic patients with early adulthood‐onset schizophrenia exhibit different clinical manifestations and pathophysiology compared with those who develop schizophrenia after middle age.1, 21, 22 These factors may contribute to different age‐related cancer risks between the schizophrenic and general populations. Specifically, the unique age structure and the early aging hypothesis of schizophrenia may lead to a pattern of decreasing cancer risks with age. In the present study, we sought to document the relationships between age and cancer risk in patients with schizophrenia.
Material and Methods
Data source
The National Health Insurance Research Database (NHIRD) is a comprehensive dataset on more than 99% of the 23.74 million people in Taiwan enrolled in the National Health Insurance (NHI) program.23 The NHIRD offers a rich set of patient and clinical information, including demographics, diagnostic codes, dates and types of procedures, prescription drugs, and expenditures.24, 25 The data used in the present study came from a subset of the NHIRD, namely the Registry for Catastrophic Illness Patient Database (RCIPD). Patients who qualify as having a “catastrophic illness” (including schizophrenia and cancer) are waived their copayments for services. The diagnosis and enrollment of schizophrenic patients into the RCIPD are quite accurate because of the incentive for patients to obtain benefits and care, as well as the rigorous regulatory review and verification of the clinical information.
Study subjects
To be enrolled in the RCIPD, patients with schizophrenia must have been admitted to an acute psychiatric ward for at least 1 month or have been followed regularly by board‐certified psychiatrists in an outpatient setting for at least 6 months. The subjects of the present study were patients who had been diagnosed and registered with schizophrenia (ICD‐9 CM code 295) between March 1, 1995, and December 31, 2007, excluding schizophreniform disorders (ICD‐9 CM code 295.8), which do not meet the qualifying criteria for the RCIPD.26 Patients younger than 20 years of age and those with previous cancers were excluded from the study. These patients with schizophrenia thus identified were then tracked until the occurrence of the first cancer, death, or the end of the study, whichever occurred first. Diagnoses of cancers in the present study are also quite reliable because specialists must supply ICD codes with pathological and imaging results for RCIPD verification. Cancers were identified by ICD‐9 CM codes 140–208. Metastatic malignancies were excluded from the study because the stability of these diseases is relatively low.
The present study was approved by the Institutional Review Board of China Medical University Hospital.
Statistical analysis
Analysis of age structure
We provide two sets of statistics to investigate the relationship between age and cancer risks in patients with schizophrenia. First, we illustrated the difference in underlying age distributions between the schizophrenic and general populations during the study period (1995–2007). We graphed the proportion of total person‐years contributed by each age group in the schizophrenic and general populations separately. The proportion of total person‐years by age was calculated as the total person‐years observed in each age strata divided by the total person‐years contributed by all patients in the respective population.
Analysis of cancer risk
We calculated overall and age‐specific standardized incidence ratios (SIR) to investigate overall cancer risk and the pattern of cancer risk by age among the patients with schizophrenia. The SIR were calculated by dividing the actual observed number of cancer cases that emerged among the patients with schizophrenia by the expected number of cancer cases. The expected number of cancer cases was derived by multiplying the age‐, gender‐, and follow‐up year‐specific group population of schizophrenic patients by the cancer incidence of the corresponding group in the general population. The population of each age and sex strata and the corresponding stratum‐specific incidence rates of cancers for the entire population were based on the Taiwan population census and National Cancer Registry cancer registry data, respectively. The 95% confidence intervals (CI) of SIR and trend analysis were calculated assuming a Poisson distribution.27 The SIR trends for cancers were calculated across age categories. P trend < 0.05 was considered significant. Cancers for which fewer than 10 cases were observed in the schizophrenia group were excluded from SIR analysis. These cancer sites included the eye, nasal cavities, middle ear and accessory sinuses, salivary gland, larynx, small intestine, duodenum, gallbladder, extrahepatic bile ducts, retroperitoneum, peritoneum, pleura, thymus, heart, mediastinum, malignant melanoma, other nervous system, bone, Hodgkin's disease, connective and other soft tissue, other endocrine glands and related structures, and male breast, penis, and testis. Stratified SIR were not calculated if the gender and/or age stratum had fewer than 10 observed cases.
All analyses were performed using sas for Windows, version 9.1 (SAS Institute, Cary, NC, USA).
Results
Age distribution in the schizophrenia versus general population
In all, 102 202 schizophrenic patients were identified on the RCIPD, of whom 55 755 (54.55%) were men and 46 447 (45.45%) were women. The mean duration of follow‐up was 7.58 years, with a total of 774 691 person‐years. The 12‐year prevalence of schizophrenia in the present study was 4.6 per 1000. Figure 1 shows the age distribution in the schizophrenic and general populations. As shown in Figure 1, there was a higher percentage of younger subjects in the schizophrenic compared with general population, with a quicker drop‐off of the number of older people. The proportion of total person‐years contributed by the 50–59, 60–69, and ≥70 years age groups in the general population was 28.6%, 15.94%, and 5.38%, respectively, compared with 14.58%, 5.82%, and 1.52%, respectively, in the schizophrenic population.
Figure 1.
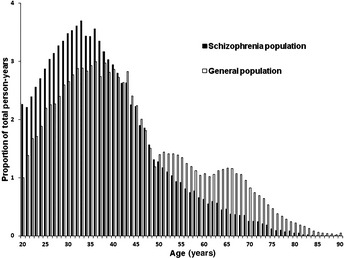
Age structure in the schizophrenic and general populations in Taiwan. The age structure in the schizophrenic and general populations is presented as the follow‐up time at risk for cancer in both populations during the present study (1995–2007) according to age.
Age of onset of cancer in the general Taiwanese population
Consistent with the rest of the world, cancer is a disease of old age in Taiwan. Table S1 presents cancer incidence according to age at onset and cancer sites in the general Taiwanese population. Prostate, pancreatic, liver, stomach, lung, and colorectal cancers have an older (≥60 years) age of onset, whereas cancers of the nasopharynx, breast, and uterine corpus have a much younger (40–59 years) age of onset.
Overall, site‐specific, and gender‐specific SIR in schizophrenia
Of the patients with schizophrenia, 1738 were diagnosed with cancer during the study period. The mean (±SD) age of schizophrenic patients at the time of RCIPD registration was 39.03 ± 12.81 years, whereas the mean (±SD) age at the time of cancer diagnosis was 52.94 ± 12.53 years. Compared with the general population, schizophrenic patients had a significantly lower relative risk of cancer overall (SIR = 0.92; 95% CI 0.90–0.96; Table 1). In stratified analysis according to tumor site, SIR varied greatly. For example, SIR were >1 for cancers of the nasopharynx, brain, breast, uterine cervix (invasive), uterine corpus, ovary, and other uterine adnexa, but were <1 for cancers of the lip, oral cavity and pharynx, stomach, colorectum, liver, pancreas, lung, thyroid, other skin, and prostate.
Table 1.
Overall, site‐specific, and gender‐specific standardized incidence ratios in schizophrenia
| Cancer site | All patients | Male patients | Female patients | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Observed no. cancer cases | Expected no. cancer cases† | SIR (95% CI) | Observed no. cancer cases | Expected no. cancer cases† | SIR (95% CI) | Observed no. cancer cases | Expected no. cancer cases† | SIR (95% CI) | |
| All cancer sites | 1738 | 1898 | 0.92 (0.90–0.96) | 691 | 1027 | 0.67 (0.66–0.72) | 1047 | 871 | 1.20 (1.18–1.28) |
| 480‡ | 506‡ | 0.95 (0.92–1.04) | |||||||
| Lip, oral cavity, and pharynx | 114 | 142 | 0.80 (0.75–0.96) | 102 | 130 | 0.78 (0.73–0.95) | 12 | 12 | 0.98 (0.79–1.71) |
| Nasopharynx | 67 | 57 | 1.18 (1.08–1.49) | 45 | 44 | 1.03 (0.93–1.38) | 22 | 13 | 1.66 (1.42–2.52) |
| Esophagus | 29 | 38 | 0.76 (0.66–1.08) | 28 | 35 | 0.79 (0.69–1.14) | 1 | 3 | – |
| Stomach | 60 | 97 | 0.62 (0.57–0.80) | 29 | 61 | 0.48 (0.42–0.68) | 31 | 36 | 0.86 (0.76–1.23) |
| Colorectum | 182 | 216 | 0.84 (0.80–0.97) | 79 | 121 | 0.65 (0.60–0.81) | 103 | 95 | 1.09 (1.01–1.32) |
| Liver | 190 | 269 | 0.71 (0.67–0.81) | 134 | 202 | 0.66 (0.63–0.79) | 56 | 67 | 0.84 (0.76–1.09) |
| Pancreas | 13 | 27 | 0.49 (0.39–0.84) | 4 | 15 | – | 9 | 11 | – |
| Lung | 144 | 177 | 0.81 (0.77–0.96) | 79 | 113 | 0.70 (0.65–0.87) | 65 | 64 | 1.02 (0.94–1.30) |
| Kidney | 31 | 41 | 0.76 (0.67–1.08) | 18 | 22 | 0.82 (0.69–1.29) | 13 | 19 | 0.70 (0.57–1.20) |
| Bladder | 39 | 43 | 0.90 (0.81–1.23) | 22 | 31 | 0.71 (0.60–1.07) | 17 | 12 | 1.41 (1.18–2.26) |
| Thyroid | 33 | 56 | 0.59 (0.52–0.83) | 8 | 14 | – | 25 | 42 | 0.59 (0.51–0.87) |
| Brain (malignant) | 32 | 18 | 1.82 (1.60–2.56) | 12 | 10 | 1.17 (0.94–2.04) | 20 | 7 | 2.72 (2.31–4.21) |
| Non‐Hodgkin's disease | 41 | 45 | 0.91 (0.82–1.24) | 17 | 25 | 0.68 (0.57–1.09) | 24 | 20 | 1.20 (1.04–1.79) |
| Leukemia | 34 | 34 | 1.00 (0.89–1.40) | 20 | 21 | 0.95 (0.81–1.47) | 14 | 13 | 1.10 (0.90–1.84) |
| Other skin | 22 | 54 | 0.41 (0.35–0.62) | 15 | 29 | 0.51 (0.42–0.84) | 7 | 24 | – |
| Breast | 341 | 228 | 1.50 (1.44–1.66) | ||||||
| Uterine cervix (invasive) | 124 | 79 | 1.58 (1.48–1.88) | ||||||
| Uterine corpus | 65 | 30 | 2.15 (1.98–2.74) | ||||||
| Ovary and other uterine adnexa | 40 | 28 | 1.42 (1.27–1.93) | ||||||
| Prostate | 15 | 43 | 0.35 (0.29–0.58) | ||||||
†Expected cancer cases were calculated on the basis of the age‐ and gender‐specific cancer incidence of the general population in Taiwan. ‡Female‐specific cancers, including breast, cervix, uterine corpus, ovary, and other uterine adnexa, were excluded. Cancers with fewer than 10 observed cases in schizophrenia were excluded from standardized incidence ratio (SIR) analysis. These cancer sites included the eye, nasal cavities, middle ear and accessory sinuses, salivary gland, larynx, small intestine, duodenum, gallbladder, extrahepatic bile ducts, retroperitoneum, peritoneum, pleura, thymus, heart, mediastinum, malignant melanoma, other nervous system, bone, Hodgkin's disease, connective and other soft tissue, other endocrine glands and related structures, male breast, penis, and testis. Similarly, SIR are not provided if the observed number of cases according to gender was <10. CI, confidence interval.
Stratifying cancer risk according to gender revealed that female patients with schizophrenia had a higher overall cancer risk compared with the general population (SIR = 1.20; 95% CI 1.18–1.28; Table 1), whereas male schizophrenic patients had a lower overall cancer risk (SIR = 0.67; 95% CI 0.66–0.72; Table 1). After excluding women‐specific cancers, the cancer risk of female patients schizophrenia was found to be similar to that of the general population (SIR = 0.95; 95% CI 0.92–1.04). Thus, the higher relative risk of cancer among female patients with schizophrenia overall appears to be driven primarily by a greater risk of cancer of the breast, uterine cervix (invasive), uterine corpus, and ovary.
Higher cancer risks in younger schizophrenic patients
To understand the pattern of cancer risk by age, we plotted SIR according to age group (Fig. 2). The relative risk of cancer in schizophrenia is highest among those aged 20–29 years and declines steadily with increasing age. For example, the SIR (95% CI) for younger schizophrenic patients aged 20–29 and 30–39 was 1.97 (1.85–2.33) and 1.42 (1.38–1.57), respectively. However, the SIR (95% CI) for those aged 60–69 and ≥70 years was 0.68 (0.65–0.78) and 0.36 (0.34–0.45), respectively. Moreover, higher cancer risks in younger patients with schizophrenia were observed for both genders, even following the exclusion of female‐specific cancers. Statistical analysis revealed that the SIR for cancer in both genders were highest for those in the younger age groups and declined significantly with age (P trend < 0.0001).
Figure 2.
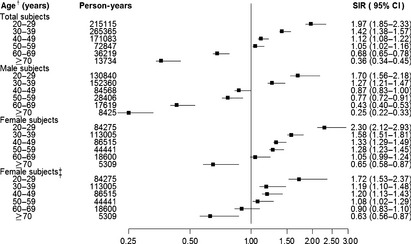
Overall and gender‐specific standardized incidence ratios (SIR) according to age group in the schizophrenic population. †Age refers to the age at which the person was registered as having schizophrenia on the Registry for Catastrophic Illness Patient Database. ‡Excluding specific cancers sites in women (i.e. breast, uterine cervix, uterine corpus, ovary, and other uterine adnexa). CI, confidence interval.
We further investigated whether the age‐related patterns for the major cancers in Taiwan were observed across all cancers. Table 2 provides age‐specific SIR according to cancer. Essentially, the same age‐related pattern for overall cancer risk shown in Figure 2 was observed for most of major cancers examined (Table 2). Specifically, patients with schizophrenia with the youngest age at onset (i.e. 20–39 years) were found to have the highest SIR, with SIR declining thereafter with age. This pattern was found for all common cancers in Taiwan, including cancers of the lip, oral cavity and pharynx, stomach, colorectum, liver, lung, breast, and uterine corpus. Therefore, the pattern of declining risk with increasing age is not cancer specific and is likely to be driven by common factors that exist in the schizophrenic population.
Table 2.
Age‐specific standardized incidence ratios in schizophrenia according to cancer type
| Cancer type | Age (years)† | Observed no. cancer cases | Expected no. cancer cases‡ | SIR (95%CI) | P trend |
|---|---|---|---|---|---|
| Lip, oral cavity, and pharynx | 20–29 | 12 | 3 | 3.61 (2.90–6.30) | <0.0001 |
| 30–39 | 45 | 30 | 1.5 (1.35–2.01) | ||
| 40–49 | 32 | 53 | 0.6 (0.53–0.85) | ||
| 50–59 | 15 | 29 | 0.52 (0.43–0.86) | ||
| 60–69 | 9 | 17 | – | ||
| ≥70 | 1 | 5 | – | ||
| Stomach | 20–29 | 2 | 1 | – | 0.0196 |
| 30–39 | 13 | 10 | 1.36 (1.10–2.33) | ||
| 40–49 | 14 | 18 | 0.8 (0.65–1.33) | ||
| 50–59 | 10 | 16 | 0.63 (0.49–1.16) | ||
| 60–69 | 10 | 20 | 0.51 (0.40–0.93) | ||
| ≥70 | 11 | 20 | 0.55 (0.44–0.98) | ||
| Colorectum | 20–29 | 8 | 6 | – | <0.0001 |
| 30–39 | 31 | 21 | 1.46 (1.28–2.07) | ||
| 40–49 | 43 | 37 | 1.17 (1.05–1.58) | ||
| 50–59 | 46 | 41 | 1.11 (1.00–1.48) | ||
| 60–69 | 41 | 49 | 0.84 (0.75–1.13) | ||
| ≥70 | 13 | 36 | 0.36 (0.29–0.61) | ||
| Liver | 20–29 | 14 | 5 | 2.89 (2.36–4.85) | <0.0001 |
| 30–39 | 42 | 36 | 1.18 (1.06–1.59) | ||
| 40–49 | 55 | 55 | 1 (0.91–1.31) | ||
| 50–59 | 43 | 56 | 0.77 (0.69–1.04) | ||
| 60–69 | 31 | 64 | 0.49 (0.43–0.69) | ||
| ≥70 | 5 | 29 | – | ||
| Lung | 20–29 | 5 | 1 | – | <0.0001 |
| 30–39 | 19 | 11 | 1.78 (1.50–2.78) | ||
| 40–49 | 31 | 26 | 1.2 (1.05–1.70) | ||
| 50–59 | 38 | 30 | 1.25 (1.12–1.72) | ||
| 60–69 | 37 | 44 | 0.83 (0.74–1.15) | ||
| ≥70 | 14 | 39 | 0.36 (0.20–0.60) | ||
| Breast | 20–29 | 18 | 4 | 4.53 (3.80–7.16) | 0.0501 |
| 30–39 | 85 | 45 | 1.88 (1.75–2.33) | ||
| 40–49 | 118 | 92 | 1.27 (1.19–1.52) | ||
| 50–59 | 84 | 54 | 1.55 (1.44–1.92) | ||
| 60–69 | 34 | 20 | 1.7 (1.50–2.37) | ||
| ≥70 | 2 | 4 | – | ||
| Uterine cervix (invasive) | 20–29 | 5 | 1 | – | 0.3843 |
| 30–39 | 25 | 15 | 1.68 (1.45–2.48) | ||
| 40–49 | 46 | 28 | 1.67 (1.50–2.23) | ||
| 50–59 | 33 | 18 | 1.88 (1.66–2.63) | ||
| 60–69 | 10 | 10 | 1.01 (0.79–1.86) | ||
| ≥70 | 5 | 4 | – | ||
| Uterine corpus | 20–29 | 3 | 1 | – | 0.0026 |
| 30–39 | 19 | 4 | 4.39 (3.70–6.85) | ||
| 40–49 | 24 | 10 | 2.38 (2.06–3.55) | ||
| 50–59 | 15 | 10 | 1.54 (1.27–2.53) | ||
| 60–69 | 4 | 3 | – | ||
| ≥70 | 0 | – | – |
†The age at which schizophrenia was registered on the National Health Insurance Research Database. ‡Expected cancer cases were calculated on the basis of the age‐ and gender‐specific cancer incidence of the general population in Taiwan. Stratified standardized incidence ratios (SIR) were not calculated if the age group contained fewer than 10 observed cases. Cancers are omitted from the table if there were fewer than three analyzable strata (with ≥10 cases per stratum) for that cancer. CI, confidence interval.
Discussion
There are two important findings of the present 12‐year nationwide cohort study into cancer risk according to age in patients with schizophrenia in Taiwan. First, among schizophrenic patients, cancer risk appears to decrease with age, which is in contrast with the pattern in the general population, in which cancer risk increases with age. In particular, the relative risk of cancer was highest among those schizophrenic patients aged 20–29 years, and declined thereafter. This pattern was observed in both genders and for all common types of cancers. Second, compared with the general population, younger schizophrenic patients (20–39 years) have higher risk relative of cancer than their corresponding age group in the general population. In contrast, older patients (≥50 years) had lower relative cancer risks. We think that a significant shift in the age distribution towards younger age and potential early aging occurring among patients with early adulthood onset schizophrenia may contribute to these patterns.
As Shiels et al.28 discussed in the comparison of patients with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) and the general population, fundamental differences in the age composition of populations could lead to biases in estimated cancer risks. Similar to HIV/AIDS, schizophrenia is associated with a relatively high mortality for younger‐aged individuals, such that only 14.58% of the population survives to 50 years.17, 18 The sharp decrease in the number of older patients with schizophrenia is likely to contribute to the pattern of declining cancer risk with increasing age because of competing mortality: patients may have died as a result of other reasons before being diagnosed with cancer. The very small older schizophrenic population may also lead to unstable estimates of SIR. However, because we found the same pattern for all types of cancer and for both genders, it is most likely due to a common underlying factor (e.g. the age structure of the population) rather than cancer‐ or gender‐specific factor(s). Although other studies have documented declining cancer risks with age in schizophrenia and have proposed several hypotheses to account for the observations,29, 30 the present study is the first to examine the role of age structure. Because of the different age composition of the populations, the age structure hypothesis can also explain why some studies using different schizophrenia study populations at different times, locations, and countries found higher cancer risk for schizophrenic patients, whereas others have reported lower risks.
Another important finding consistent with the shift in the age structure of the schizophrenic population is that the SIR are driven by the age of onset of the cancer. Because the SIR weights cancer incidence in the general population with the age distribution of the study population, the overall SIR will be underestimated if the study population has a very small proportion of people in whom cancer incidence is high and vice versa. The age structure hypothesis is consistent with our finding that for the schizophrenia population the SIR is <1 for cancers with an old (>60 years) age of onset in the general population (e.g. stomach cancer [SIR = 0.62], pancreatic cancer [SIR = 0.49], and prostate cancer [SIR = 0.35]). For the same reason, the SIR was >1 for cancers with a younger (<50 years) age of onset in the general population (e.g. cancers of the nasopharynx [SIR = 1.18], breast [SIR = 1.50], and uterine corpus [SIR = 2.15]).
For almost all cancers examined, female schizophrenic patients appeared to have higher cancer risks than male schizophrenic patients, which is in line with many previous studies that have performed gender analyses.5, 31 Female schizophrenic patients have an increased risk of cancers of the breast, ovary, uterine corpus, and uterine cervix compared with the general female population in Taiwan, consistent with other studies that have reported that breast cancer is the leading type of cancer in women with schizophrenia in other countries.5, 7, 31 It was interesting to find that women with schizophrenia had higher risks of both cancer of the uterine corpus (usually endometrial cancer32) and invasive cervical cancer (Table 1), even though the pathogenesis of the two cancers differs. Endometrial cancer, along with breast and ovarian cancers, is related to hormonal factors.32 It has been suggested that the prolactin‐releasing side effect of some antipsychotics may be related to galactorrhea, menstrual irregularities, sexual dysfunction, infertility, decreased bone mineral density,33 and a higher risk of endometrial and breast cancer.34, 35 Other risk factors, such as obesity, tobacco use, and a sedentary lifestyle, may also explain, in part, the higher overall risk of breast, endometrial, and ovarian cancers in women with schizophrenia.35 In contrast, cervical cancer is related to infection with the human papillomavirus (HPV),37 and its invasive progression can be prevented by early detection of cervical dysplasia.32 Cervical cancer screening programs (papanicolaou test) have significantly reduced the incidence of invasive cervical cancer (a reduction of 48.0% from 1995 to 2006) in the general population in Taiwan; this reduction is greater than that seen with screening programs for other cancers.38 However, women with schizophrenia are less likely to have a papanicolaou test and therefore benefit from the program.39 The present findings of an increased risk of invasive cervical cancer in women with schizophrenia compared with the general population may be due to the screening gap (Tables 1, 2). More studies are needed to examine the trend regarding the risk of cervical cancer in relation to the papanicolaou test in women with schizophrenia.
In the present study, the finding of different cancer risks according to gender is likely also driven by underlying differences in age structure between the two groups. Women live longer, thus resulting in a higher proportion of older female patients with schizophrenia, which will likely lead to a higher overall SIR relative to that for male patients. In addition, female‐specific cancers tend to occur at younger ages. For example, it has been reported that breast cancer is a cancer of younger age for Asian women.40 This, too, will lead to higher overall SIR for women. Indeed, when we excluded women‐specific cancers (i.e. cancer of the breast, uterine cervix, uterine corpus, ovary, and other uterine adnexa), the SIR for female schizophrenic patients became <1, further supporting our age structure hypothesis.
The second major finding of the present study is that, compared with the general population, schizophrenic patients seem to have higher risks of cancer in younger age groups and lower risks of cancer in older age groups based on SIR. This trend is very consistent among overall cancer risk, by cancer type, or by gender for the major cancers we investigated. Although the age distribution in schizophrenia suggests a decreasing trend of cancer risk among patients with older age, it does not necessarily indicate elevated cancer risks relative to the general population among younger people. Therefore, the hypothesis that schizophrenia is a disease of accelerated aging20 appears to better describe the higher risks of cancers among younger schizophrenic patients observed in the present study. It was demonstrated that individuals with schizophrenia are more likely to have some sort of abnormalities,41 are less responsive to medications, exhibit more impaired functioning, and have a poorer quality of life, particularly in the case of those with early adulthood onset schizophrenia.42 Because neuropsychiatric and physical degeneration may occur at a younger age, cancer screening should probably begin at a younger age for schizophrenic patients to avoid excessive mortality.18, 43
It may be that schizophrenic patients are more likely to be positive for risk factors for cancer development, such as cigarette smoking,16 alcohol and drug use, poor dietary habits,44 obesity,45 and less physical activity and exercise.46 However, these risk behaviors need time to exert a cumulative carcinogenic effect and tend to affect older patients; therefore, the factors cannot explain the increased cancer risk in younger schizophrenic patients in the present study. Genetic factors may also contribute to cancer risk. It has been hypothesized that the tumor suppressor gene is a candidate susceptibility gene in schizophrenia13 based on the decreased cancer risks in patients with schizophrenia5 and their parents and siblings.4 However, this hypothesis was regarded as premature47 and is not supported by other research29 or the findings of the present study, which shows an increased risk for cancer in younger patients with schizophrenia.
The present study has the highest number of person‐year observations of existing studies, which allowed us to conduct analyses according to gender and cancer type, the results of which were consistent with those published in the literature.5, 7, 31 However, there are several limitations to the present study. First, the NHIRD database does not contain any personal information, such as information regarding lifestyle and family history. However, our finding of an age effect is unlikely to be affected by these factors. Second, the present study only analyzed patients with schizophrenia who had purchased NHI. However, it should be kept in mind that the insurance rate reached 99% and the 12‐year prevalence of treated schizophrenia was 4.6 per 1000, which is close to that in other countries.48 These 12‐year statistics support the accuracy of both the diagnosis and enrollment of schizophrenic patients in the present study. Third, some patients may not have been diagnosed immediately during the early stages of schizophrenia, thus resulting in a lag in the schizophrenia database. This may results in lower SIR for schizophrenic patients because younger schizophrenic patients would have been categorized into an older age group. However, this would not change the trend for younger schizophrenic patients to have a higher cancer risk. Fourth, following most previous research, the present study only included those patients who were first diagnosed with schizophrenia, then cancer. The selection bias may ignore patients who were diagnosed with cancer before being diagnosed with schizophrenia, resulting in competitive mortality due to cancer; thus, the overall SIR for cancer may have been underestimated. Fifth, the overall sample size of the present study is relatively large, but we have limited power to analyze SIR by cancer type and/or by gender for some cancers with low prevalence. Although SIR are supposed to produce less bias for rare diseases and we did not perform analyses for cancers with fewer than 10 cases, some of the results may be unstable when the number of observed cases is small. Sixth, the subjects of the present study were Taiwanese, mainly Han Chinese. Although the clinical manifestations of schizophrenia are similar across races, cancer risks in younger schizophrenic patients among races deserves further investigation.
In conclusion, the present study examined the hypothesis that the particular age structure in schizophrenia may affect the estimated cancer risks for schizophrenic patients and found several pieces of evidence to support this hypothesis. The findings that cancer risk decreases with age, is higher for cancers with an earlier age at onset, and is higher among women can all be consistently explained by the significant shift in the distribution of the schizophrenic population towards younger age. In addition, comparing the relative risks of cancer in schizophrenia relative to the general population in Taiwan, younger schizophrenic patients presented higher cancer risks for nearly all the major cancers we examined. Early aging in schizophrenic patients with younger‐onset schizophrenia may be a potential explanation for this observation. For schizophrenic patients, both neuropsychiatric and physical degeneration may occur at a younger age than in the general population. More attention to higher physical comorbidity, including cancer, in young schizophrenic patients is warranted.
Disclosure Statement
The authors have no conflicts of interest to declare.
Supporting information
Table S1. Cancer incidence by onset age and cancer sites in the general Taiwan population.
Acknowledgments
The present study is based on a subset of data from the NHIRD provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institute (Taiwan). This study was supported, in part, by the Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH102‐TD‐B‐111‐004, PH‐100‐PP‐54, PH‐101‐PP‐23).
(Cancer Sci, doi: 10.1111/cas.12094, 2013)
References
- 1. Amereican Psychiatry Association . Diagnostic and Stastistical Manual of Mental Disorders, 4th edn Washington, DC: American Psychiatric Press, 1994. [Google Scholar]
- 2. Carney C, Jones L, Woolson R. Medical comorbidity in women and men with schizophrenia: a population‐based controlled study. J Gen Intern Med 2006; 21: 1133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leucht S, Burkard T, Henderson J, Maj M, Sartorius N. Physical illness and schizophrenia: a review of the literature. Acta Psychiatr Scand 2007; 116: 317–33. [DOI] [PubMed] [Google Scholar]
- 4. Lichtermann D, Ekelund J, Pukkala E, Tanskanen A, Lonnqvist J. Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry 2001; 58: 573–8. [DOI] [PubMed] [Google Scholar]
- 5. Mortensen PB. The incidence of cancer in schizophrenic patients. J Epidemiol Community Health 1989; 43: 43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barak Y, Achiron A, Mandel M, Mirecki I, Aizenberg D. Reduced cancer incidence among patients with schizophrenia. Cancer 2005; 104: 2817–21. [DOI] [PubMed] [Google Scholar]
- 7. Grinshpoon A, Barchana M, Ponizovsky A et al Cancer in schizophrenia: is the risk higher or lower? Schizophr Res 2005; 73: 333–41. [DOI] [PubMed] [Google Scholar]
- 8. Chou FH, Tsai K, Su C, Lee C. The incidence and relative risk factors for developing cancer among patients with schizophrenia: a nine‐year follow‐up study. Schizophr Res 2011; 129: 97–103. [DOI] [PubMed] [Google Scholar]
- 9. Goldacre MJ, Kurina LM, Wotton CJ, Yeates D, Seagroat V. Schizophrenia and cancer: an epidemiological study. Br J Psychiatry 2005; 187: 334–8. [DOI] [PubMed] [Google Scholar]
- 10. Hippisley‐Cox J, Vinogradova Y, Coupland C, Parker C. Risk of malignancy in patients with schizophrenia or bipolar disorder: nested case‐control study. Arch Gen Psychiatry 2007; 64: 1368–76. [DOI] [PubMed] [Google Scholar]
- 11. Gulbinat W, Dupont A, Jablensky A. Cancer incidence of schizophrenic patients: results of record linkage studies in 3 countries. Br J Psychiatry 1992; 161(Suppl): 75–83. [PubMed] [Google Scholar]
- 12. Jeste DV, Gladsjo JA, Lindamer LA, Lacro JP. Medical comorbidity in schizophrenia. Schizophr Bull 1996; 22: 413–30. [DOI] [PubMed] [Google Scholar]
- 13. Catts V, Catts S. Apoptosis and schizophrenia: is the tumour suppressor gene, p53, a candidate susceptibility gene? Schizophr Res 2000; 41: 405–15. [DOI] [PubMed] [Google Scholar]
- 14. Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003; 4: 915–25. [DOI] [PubMed] [Google Scholar]
- 15. Margolese HC, Malchy L, Negrete JC, Tempier R, Gill K. Drug and alcohol use among patients with schizophrenia and related psychoses: levels and consequences. Schizophr Res 2004; 67: 157–66. [DOI] [PubMed] [Google Scholar]
- 16. de Leon J, Diaz FJ. A meta‐analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res 2005; 76: 135–57. [DOI] [PubMed] [Google Scholar]
- 17. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007; 64: 1123–31. [DOI] [PubMed] [Google Scholar]
- 18. Brown S, Kim M, Mitchell C, Inskip H. Twenty‐five year mortality of a community cohort with schizophrenia. Br J Psychiatry 2010; 196: 116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Urban Ö, Correia N, Brandt L, Ekbom A, Sparén P. Mortality and causes of death in schizophrenia in Stockholm County, Sweden. Schizophr Res 2000; 45: 21–8. [DOI] [PubMed] [Google Scholar]
- 20. Kirkpatrick B, Messias E, Harvey PD, Fernandez‐Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophr Bull 2008; 34: 1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris MJ, Jeste DV. Late‐onset schizophrenia: an overview. Schizophr Bull 1988; 14: 39–55. [DOI] [PubMed] [Google Scholar]
- 22. Howard R, Rabins PV, Seeman MV, Jeste DV. Late‐onset schizophrenia and very‐late‐onset schizophrenia‐like psychosis: an international consensus. Am J Psychiatry 2000; 157: 172–8. [DOI] [PubMed] [Google Scholar]
- 23. Cheng T. Taiwan's new national health insurance program: genesis and experience so far. Health Aff 2003; 22: 61–76. [DOI] [PubMed] [Google Scholar]
- 24. Wu CY, Wu MS, Kuo KN, Wang CB, Chen YJ, Lin JT. Effective reduction of gastric cancer risk with regular use of nonsteroidal anti‐inflammatory drugs in Helicobacter pylori‐infected patients. J Clin Oncol 2010; 28: 2952–7. [DOI] [PubMed] [Google Scholar]
- 25. Wu CY, Kuo KN, Wu MS, Chen YJ, Wang CB, Lin JT. Early Helicobacter pylori eradication decreases risk of gastric cancer in patients with peptic ulcer disease. Gastroenterology 2009; 137: 1641–8. [DOI] [PubMed] [Google Scholar]
- 26. ICD9Data.com . Schizophrenic disorders. 2012. [Cited 5 Dec 2012]. Available from URL: http://www.icd9data.com/2012/Volume1/290-319/295-299/295/default.htm
- 27. Department of Health NYS . Guidance for Health Outcome Data Review and Analysis Relating to Nysdec Environmental Justice and Permitting: Appendix B: Calculating Confidence Intervals, 2011. [Cited 5 Nov 2012.] Available from URL: http://www.health.ny.gov/environmental/investigations/environmental_justice/appendix_b.htm
- 28. Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med 2010; 153: 452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dalton SO, Laursen TM, Mellemkjaer L, Johansen C, Mortensen PB. Risk for cancer in parents of patients with schizophrenia. Am J Psychiatry 2004; 161: 903–8. [DOI] [PubMed] [Google Scholar]
- 30. Lin GM, Chen YJ, Kuo DJ et al Cancer incidence in patients with schizophrenia or bipolar disorder: a nationwide population‐based study in Taiwan, 1997–2009. Schizophr Bull 2011; doi: 10.1093/schbul/sbr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dalton SO, Mellemkjaer L, Thomassen L, Mortensen PB, Johansen C. Risk for cancer in a cohort of patients hospitalized for schizophrenia in Denmark, 1969–1993. Schizophr Res 2005; 75: 315–24. [DOI] [PubMed] [Google Scholar]
- 32. Barakat RR, Markman M, Randall ME. Principles and Practice of Gynecologic Oncology. Philadelphia, PA: Lippincott Williams & Wilkins, 2009. [Google Scholar]
- 33. Lin CH, Huang KH, Chang YC et al Clozapine protects bone mineral density in female patients with schizophrenia. Int J Neuropsychopharmacol 2011; 15: 897–906. [DOI] [PubMed] [Google Scholar]
- 34. Yamazawa K, Matsui H, Seki K, Sekiya S. A case‐control study of endometrial cancer after antipsychotics exposure in premenopausal women. Oncology 2003; 64: 116–23. [DOI] [PubMed] [Google Scholar]
- 35. Wang PS, Walker AM, Tsuang MT et al Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatry 2002; 59: 1147–54. [DOI] [PubMed] [Google Scholar]
- 36. Akdeniz F. Female‐specific health problems in mental patients. Curr Opin Psychiatry 2010; 23: 378–82. [DOI] [PubMed] [Google Scholar]
- 37. Sasagawa T, Dong YZ, Saijoh K, Satake SI, Tateno M, Inoue M. Human papillomavirus infection and risk determinants for squamous intraepithelial lesion and cervical cancer in Japan. Cancer Sci 1997; 88: 376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen Y, You S, Chen C et al Effectiveness of national cervical cancer screening programme in Taiwan: 12‐year experiences. Br J Cancer 2009; 101: 174–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin HC, Yang LY, Chiu WT. Cervix cancer screening of women with schizophrenia in Taiwan. Psychiatr Serv 2010; 61: 327–8. [DOI] [PubMed] [Google Scholar]
- 40. Lee SY, Jeong SH, Kim YN et al Cost‐effective mammography screening in Korea: high incidence of breast cancer in young women. Cancer Sci 2009; 1020: 1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fernandez‐Egea E, García‐Rizo C, Miller B et al Testosterone in newly diagnosed, antipsychotic‐naive men with nonaffective psychosis: a test of the accelerated aging hypothesis. Psychosom Med 2011; 73: 643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vahia I, Palmer B, Depp C et al Is late‐onset schizophrenia a subtype of schizophrenia? Acta Psychiatr Scand 2010; 122: 414–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tran E, Rouillon F, Loze J‐Y et al Cancer mortality in patients with schizophrenia. Cancer 2009; 115: 3555–62. [DOI] [PubMed] [Google Scholar]
- 44. McCreadie R, Macdonald E, Blacklock C et al Dietary intake of schizophrenic patients in Nithsdale, Scotland: case‐control study. BMJ 1998; 317: 784–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Allison DB, Fontaine KR, Heo M et al The distribution of body mass index among individuals with and without schizophrenia schizophrenia. J Clin Psychiatry 1999; 60: 215–20. [DOI] [PubMed] [Google Scholar]
- 46. Daumit GL, Goldberg RW, Anthony C et al Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis 2005; 193: 641–6. [DOI] [PubMed] [Google Scholar]
- 47. Jablensky A, Lawrence D. Schizophrenia and cancer: is there a need to invoke a protective gene? Arch Gen Psychiatry 2001; 58: 579–80. [DOI] [PubMed] [Google Scholar]
- 48. Sadock B, Sadock V. Kaplan & Sadock's Synopsis of Psychiatry, 9th edn New York: Williams & Wilkins, 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cancer incidence by onset age and cancer sites in the general Taiwan population.


