Abstract
Purple corn color is a widely used food colorant that was reported to have attenuating effects on hypertension, diabetes, and to have anti‐cancer effects on colon and breast cancer. Our study is the first on its possible chemoprevention effects against prostate cancer. For this purpose an androgen‐dependent prostate cancer cell line, LNCaP, was used to examine effects in vitro. Purple corn color inhibited the proliferation of LNCaP cells by decreasing the expression of Cyclin D1 and inhibiting the G1 stage of the cell cycle. Thirty‐six male transgenic rats for adenocarcinoma of prostate were fed basic diet or diet with purple corn color for 8 weeks. Purple corn color decreased the incidence of adenocarcinoma in the lateral prostate and slowed down the progression of prostate cancer. A lower Ki67 positive rate, a decrease of the expression of Cyclin D1, and downregulation of the activation of Erk1/2 and p38 MAPK were observed in the group consuming purple corn color in the diet. Since purple corn color is a mixture, determining its active component should help in the understanding and usage of purple corn color for prostate cancer chemoprevention. Therefore, the three major anthocyanins in purple corn color, cyanidin‐3‐glucoside, pelargonidin‐3‐glucoside and peonidin‐3‐glucoside, were tested with LNCaP cells. The results suggested that cyanidin‐3‐glucoside and pelargonidin‐3‐glucoside are the active compounds.
Cancer is a major public health problem in developed and some developing countries. In 2012, prostate cancer (PCa) was estimated to be the No.1 cause of new cancer cases in males and the second highest cause of cancer related deaths in the USA,1 and PCa is rapidly increasing in Asia. Although considerable effort has been expended on searching for early screening markers and curing PCa, the main treatment remains androgen ablation therapy, which was developed in the early 1940s.2, 3 However, even though more than 80% of PCa respond to this therapy, almost all of these cases relapse in less than a decade and become refractory to treatment.3 Brachytherapy, radiotherapy, and prostatectomy of PCa prior to metastasis can affect a cure, but these procedures can dramatically alter the quality‐of‐life of the patient.4, 5 Therefore, prevention of PCa is especially important.
The field of chemoprevention, using natural or laboratory‐made substances to prevent cancer, has become increasingly studied in recent years. Researchers have investigated numerous chemicals that may have chemopreventive effects on PCa in vitro and in vivo. Three large‐scale randomized, controlled clinical trials have been conducted: the SELECT trial found that neither selenium nor Vitamin E reduced the risk of PCa in healthy men at average risk;6 the PCPT trial found that finasteride, a 5α‐reductase inhibitor, reduced the risk of PCa of Gleason 6 or less, whereas there was an increased risk of high grade disease with Gleason 7 or more;7 the REDUCE trial also encountered similar difficulties with dutasteride, another 5 α‐reductase inhibitor.8 Therefore, the search for an appropriate chemopreventor for PCa needs to be continued.
Purple corn has a long history as a food product. Nowadays its color is widely used as a food colorant in Japan. Previous studies have provided evidence that purple corn color (PCC) has anti‐cancer effects on colon and breast cancer.9, 10 It also has attenuating effects on some life style diseases, for example, hypertension, hyperglycemia, and diabetes.11, 12 The present study was conducted as an initial investigation on PCC's effects on PCa. As a result we found that PCC inhibited the proliferation of the androgen‐dependent cell line LNCaP in vitro and inhibited prostate carcinogenesis in vivo in the Transgenic Rat for Adenocarcinoma of Prostate (TRAP) model. The TRAP rat model, in which expression of the Simian virus 40 T antigen is under control of the probasin gene promoter, was established in our laboratory. These animals develop high‐grade prostatic intraepithelial neoplasia (HG‐PIN) and well‐differentiated adenocarcinomas with high incidence in all prostate lobes at 15 weeks of age, all lesions being completely androgen‐dependent.13, 14 The model provides an ideal tool to gain insights into possible mechanisms of PCa prevention in relatively short‐term studies.15, 16, 17, 18, 19
Purple corn color is a mixture, which contains several anthocynins. To determine its active component, the three major anthocyanins in PCC, cyanidin‐3‐glucoside (C3G), pelargonidin‐3‐glucoside (Pg3G) and peonidin‐3‐glucoside (P3G), were tested using LNCaP cells. By comparing the effects of these anthocyanins to the effect of the mixture, we found that C3G and Pg3G are the active compounds.
To our knowledge, the present study provides the first evidence that PCC inhibits prostate carcinogenesis in a rat model closely mimicking the human disease. The clues obtained as to the molecular basis of action are of critical importance as first steps towards human clinical trials.
Materials and Methods
Chemicals, reagents, plasmids and cell line
Purple corn color was provided by San‐Ei Gen F.F.I. (Osaka, Japan). Lot No. 100413, anthocyanin concentration 12.5% was used for the in vitro study. Lot No. 110418, anthocyanin concentration 20.9% was used for the in vivo study. C3G (Cyanidin‐3‐O‐glucoside chloride) and P3G (Peonidin‐3‐O‐glucoside chloride) were purchased from Tokiwa Phytochemical (Chiba, Japan). Pg3G (Pelargonidin‐3‐O‐glucoside chloride) was purchased from Extrasynthese (Genay Cedex, France). The chemical structures of C3G, P3G and Pg3G are shown in Supplementary Figure S1. The LNCaP human PCa cell line (androgen‐dependent) was from the American Type Culture Collection (Manassas, VA, USA). The pGL3‐PSA luciferase expression vector (pGL3/PSA‐Luc) was donated by Dr Chawnshang Chang, University of Rochester Medical Center.
Animals
Male heterozygous TRAP rats with a Sprague–Dawley genetic background were used in the present study. They housed three animals per cage on wood‐chip bedding in an air‐conditioned animal room at 23 ± 2°C and 50 ± 10% humidity. Food and tap water were available ad libitum. The Institutional Animal Care and Use Committees of the Nagoya City University (Nagoya, Japan) specifically approved this study.
Experimental protocol
A total of 36 heterozygous male TRAP rats at 6 weeks of age were randomly divided into three groups. Rats in the control group (n = 13) received powdered basal diet (Oriental MF, Oriental Yeast, Tokyo, Japan). The rats in the other two groups received 0.1% (n = 12) or 1% PCC (n = 11) in the diet for 8 weeks. At the end of week 8, the rats were killed under deep anesthesia. Each prostate was removed and halves of the ventral and lateral lobes were immediately frozen in liquid nitrogen and stored at −80°C until processed; the remaining prostates was fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned. Testosterone and estrogen levels in the serum were analyzed by radioimmunoassay by SRL (Tokyo, Japan). All experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of Nagoya City University Graduate School of Medical Sciences.
Assessment of prostate neoplastic lesion development
Neoplastic lesions in the prostate glands of TRAP rats were evaluated as previously described.19 Briefly, neoplastic lesions were classified into three types: low‐grade prostatic intraepithelial neoplasia (LG‐PIN), HG‐PIN and adenocarcinoma. The relative numbers of acini with the histological characteristics of each type, that is, LG‐PIN, HG‐PIN and adenocarcinoma, were quantified with reference to the total acini in each prostatic lobe.
Immunoblot analysis
The immunoblotting analysis was performed as described previously.19 Briefly, LNCaP cells or frozen ventral prostate tissues were homogenized in radioimmunoprecipitation assay buffer (150 mM NaCl, 50 mM Tris–HCl [pH 8.0], 1% NP‐40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM phenylmethylsulphonyl fluoride, 1 mM sodium orthovanadate, and protease inhibitor cocktail [Complete, Roche, Mannheim, Germany]) and subjected to immunoblot analysis using standard techniques. The antibodies used were Cyclin D1 and androgen receptor (Santa Cruz Biotechnology, Santa Cruz, CA, USA), cleaved caspase 3, cleaved caspase 7, Erk1/2, phospho‐Erk1/2, p38 MAPK and phospho‐p38 MAPK (Cell Signaling Technology, Boston, MA, USA), prostate‐specific antigen (DAKO, Tokyo, Japan) and β‐actin (Sigma‐Aldrich, St. Louis, MO, USA). The density of the bands was semi‐quantified using ImageJ (version 1.42q, National Institute of Health, Bethesda, MD, USA).
Immunohistochemistry
Deparaffinized sections were incubated with antibodies for Ki‐67 (Novocastra Laboratories, Newcastle, UK) and SV40 T antigen (Santa Cruz Biotechnologies). Apoptotic cells in the prostate were detected using an In Situ Apoptosis Detection kit (TUNEL method) according to the manufacturer's instructions (Takara Bio, Ohtsu, Japan). Labeling indices were determined as the positive percentage for Ki‐67 or TUNEL by randomly picking eight fields of view in the whole ventral/lateral prostate and counting over 1000 prostate epithelial cells under a microscope at high magnification.
Cell proliferation assay
Cell proliferation of LNCaP cells was assessed by manual counting under trypan blue staining. Briefly, LNCaP cells were seeded in 96‐well plates at 10 000 cells/well in 200 μL of RPMI media; PCC, C3G, Pg3G or P3G were added 24 h after seeding and the cells were incubated for 72 h; and the cells were removed from the plate with trypsin‐EDTA and counted.
RNA extraction, cDNA preparation, and quantitative real‐time PCR
Total RNAs of LNCaP cells or frozen prostate tissue was isolated using an RNeasy Mini kit (Qiagen, Valencia, CA, USA) and reverse‐transcribed with the Thermoscript first‐strand synthesis system (Invitrogen Corporation, Carlsbad, CA, USA). Real‐time RT‐PCR was performed using Syber Premix Ex Taq II (Takara) in a LightCycler (Roche Diagnostics GmbH). The primers used were: human GAPDH, 60°C, 5′‐AACGGATTTGGTCGTATTGG‐3′ and 5′‐CATACTTCTCATGGTT‐CACA‐3′; human Cyclin D1, 60°C, 5′‐CCGAGAAGCTGTGCATCTAC‐3′ and 5′‐CAGGTTCAGGCCTTGCACTG‐3′; rat GAPDH, 59°C, 5′‐GAATGGGAAGCTGGT‐CATCA‐3′ and 5′‐TGGATGCAGGGATGATGTTC‐3′; rat probasin, 60°C, 5′‐ACTTCCGTCGCATTGAGTGT‐3′ and 5′‐GTAAACGTCTTGGGATCTCC‐3′; rat GK11, 59°C, 5′‐GCAGCACCAAACCCCTGGAT‐3′ and 5′‐TGAGATCTGTCACCTTCTCA‐3′.
Cell cycle analysis
LNCaP cells were seeded in 6‐well plates at 150 000 cells/well. Purple corn color, C3G or Pg3G were added 24 h after seeding, incubated for 72 h, collected and analyzed with propidium iodide (Guava cell cycle reagent; Guava Technologies, Hayward, CA, USA) according to the Guava Cell Cycle Assay protocol. The cell cycle phase distribution was determined on a Guava PCA Instrument using CytoSoft Software.
Reporter gene assays
LNCaP cells were transfected with the pGL3/PSA‐Luc using Nucleofector II. 24 h later, 5 nM DHT and/or PCC were added. After 48 h incubation, the cells were lysed with the buffer supplied in the kit. Luciferase assays were conducted using the dual‐luciferase reporter assay system (Promega, Madison, WI, USA), and the phRL‐TK vector (Promega) as an internal control, according to the manufacturer's protocol. Data shown are means and SD of four independent data points.
Statistical analysis
All data presented are mean ± SD values. Statistical comparisons were performed with one‐way anova followed by Dunnett's test. Correlations were assessed by Spearman correlation coefficient analysis. A P‐value of < 0.05 was considered to be significant. All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA).
Results
PCC inhibition of LNCaP proliferation and slowing of the cell cycle
When the androgen‐dependent cell line LNCaP was incubated with increasing levels of PCC for 72 h, the proliferation of the cells was inhibited in a dose‐dependent manner (Fig. 1A). Cell cycle analysis showed that PCC increased the proportion of cells in G0/G1 slightly but significantly (Fig. 1B). Western blotting showed a 15–30% decrease in the protein level of Cyclin D1 in the PCC treated cells (Fig. 1C), and reverse‐transcription PCR also showed that the mRNA level of Cyclin D1 was significantly decreased by PCC (Fig. 1D). Purple corn color did not affect androgen receptor (AR) expression, but PSA expression was dramatically decreased (Fig. 1C). Since the PSA gene is a target of AR, we used a luciferase assay to examine the influence of PCC on the activity of the PSA promoter. Purple corn color inhibited functional AR transcriptional activity in a dose‐dependent manner (Fig. S2).
Figure 1.
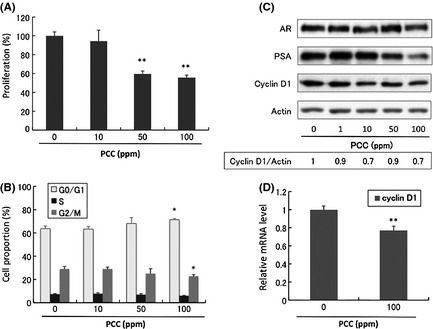
Effects of purple corn color (PCC) on LNCaP cells. (A) Inhibitory effects on LNCaP cell proliferation (72 h) (n = 3). (B) Effects on the cell cycle of LNCaP cells (n = 3). (C) Protein changes assessed by Western blotting analysis of LNCaP cells after incubation with PCC for 72 h. The density of the bands of Cyclin D1 and actin were semi‐quantified by ImageJ. (D) mRNA levels of Cyclin D1 analyzed by reverse‐transcription polymerase chain reaction (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001.
No toxic effects of PCC were observed in the TRAP rat model
Body weights, relative organ weights (ventral prostate, liver and kidney), and food consumption were not affected by administration of PCC in the diet to TRAP rats (Table S1). The average PCC intakes were 25 mg/rat per day and 267 mg/rat per day for the 0.1% and 1% PCC groups (Table S1). Purple corn color did not have any effect on the serum levels of testosterone or estradiol (Table S1).
PCC inhibition of prostate carcinogenesis in the TRAP rat model
With the expression of SV40T antigen under the control of AR, TRAP rats developed prostate cancer with high incidence. In the end of the experiment, all the acini in both ventral and lateral prostate of TRAP rats had developed precancerous or cancerous lesions. Lesions were divided into three stages: LG‐PIN, HG‐PIN, and adenocarcinoma (Fig. S3). The total percentage of LG‐PIN, HG‐PIN and adenocarcinoma is 100%. In this study, adenocarcinomas were observed in ventral prostate of all the rats, that is, the incidence of adenocarcinoma was 100% in all three groups. However, the rats consuming PCC had a lower percentage of adenocarcinoma and a higher percentage of LG‐PIN (Table 1), which suggests that PCC retarded the progression of PCa and more acini lesions remained in the relatively benign LG‐PIN stage. The increased LG‐PIN percentage and decreased adenocarcinoma percentage showed a strong correlation to the dose of PCC. In the lateral prostate, we also observed this retardation of PCa progression. Of critical importance in this study is that PCC also significantly decreased the incidence of adenocarcinoma in the lateral prostate (Table 1). These results suggest that PCC inhibited tumorigenesis in the prostates of TRAP rats.
Table 1.
Quantitative evaluation of neoplastic lesions in prostates of TRAP rats treated with PCC
| No. of animal | Incidence of adenocarcinoma | Proportion of acini in different stages of carcinogenesis (%) | |||
|---|---|---|---|---|---|
| LG‐PIN | HG‐PIN | Adenocarcinoma | |||
| Ventral prostate | |||||
| Control | 13 | 13 (100%) | 4.4 ± 2.8 | 89.2 ± 3.5 | 6.4 ± 4.0 |
| 0.1% PCC | 12 | 12 (100%) | 5.9 ± 3.4** | 90.6 ± 3.8 | 3.5 ± 1.7*** |
| 1% PCC | 11 | 11 (100%) | 9.1 ± 3.9** | 88.2 ± 3.4 | 2.7 ± 2.0*** |
| Lateral prostate | |||||
| Control | 13 | 12 (92%) | 24.7 ± 12.4 | 72.8 ± 11.7 | 2.5 ± 2.8 |
| 0.1% PCC | 12 | 7 (58%) | 17.8 ± 5.6 | 81.2 ± 5.6 | 1.0 ± 1.0** |
| 1% PCC | 11 | 3 (27%)* | 21.1 ± 6.8 | 78.0 ± 6.2 | 0.9 ± 1.6** |
AC, adenocarcinoma; HG, high grade; LG‐PIN, low grade prostatic intraepithelial neoplasia. *P < 0.01 versus control (Dunnett's test). **P < 0.01 and ***P < 0.001 versus control, respectively (Spearman's rank correlation coefficient analysis).
PCC inhibition of the cell growth pathways in the TRAP rat model
Carcinogenesis in the TRAP rat is induced by SV40T antigen expressed under the control of the probasin promoter, which is regulated by the AR. Since PCC downregulated AR activity in vitro, we examined whether SV40T expression was downregulated by PCC. Immunohistochemical analysis showed that there was no overt inhibition of SV40T expression by PCC (Fig. 2A,B). We used RT‐PCR to examine mRNA levels in the VP of the androgen responsive genes probasin and GK11, an ortholog of human PSA. These results also indicated that AR activity was not inhibited by PCC (Fig. 2C).
Figure 2.
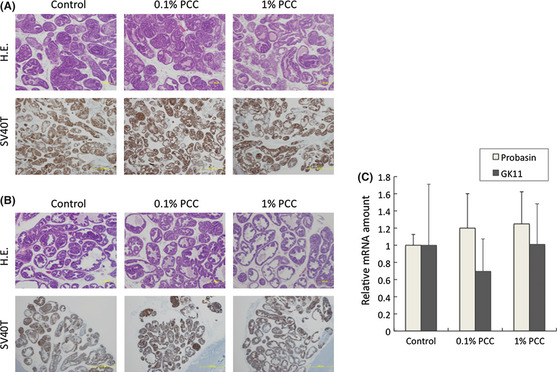
Purple corn color (PCC)‐mediated inhibition of carcinogenesis is not due to down regulation of androgen receptor (AR) activity. (A) H&E staining (4 × magnification) and SV40T antigen expression (4 × magnification) in the ventral prostate. (B) H&E staining (4 × magnification) and SV40T antigen expression (4 × magnification) in the lateral prostate. (C) mRNA of the ventral prostate was used for reverse‐transcription polymerase chain reaction. mRNA levels of probasin and GK11 (the rat ortholog of human PSA), which are AR target genes, were checked. Scale bars, 500 μm.
Figure 3A shows that the Ki67 index was decreased by PCC in both VP and LP. On the other hand, the TUNEL staining index was not affected by PCC (Fig. 3B), in agreement with our in vitro studies in which no apoptosis was observed (data not shown).
Figure 3.
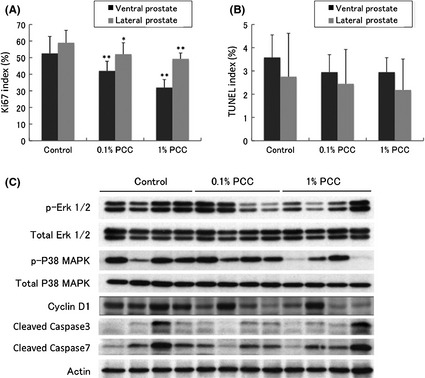
Effects of purple corn color (PCC) on Ki67 and terminal deoxynucleotidyl transferase‐mediated dUTP nick end labeling (TUNEL) indices in the prostate. (A) Purple corn color decreased the Ki67 index significantly in both the ventral and lateral prostate (samples from all the rats were used for analysis). (B) Purple corn color did not affect the TUNEL index in either the ventral or lateral prostate (samples from all the rats were used for analysis). (C) Western blot of proteins related to cell growth and apoptosis using samples from the ventral prostate.
Immunoblotting demonstrated factors involved in cell growth pathways, Erk1/2 and p38 MAPK phosphorylation and Cyclin D1, to be downregulated by PCC. In agreement with the results of TUNEL staining, PCC had no affect on the levels of cleaved caspases 3 or 7 (Fig. 3C).
Search for active compounds in PCC
The compounds that give PCC its purple color are anthocyanins. C3G, Pg3G and P3G are the three major components of PCC. When their effects on LNCaP cells were tested, C3G and Pg3G dose‐dependently inhibited the proliferation of LNCaP cells, while P3G had no effect (Fig. 4A). The differences of the chemical structures of these three chemicals (Fig. S1) suggest that the hydroxyl radical may play an important role in the inhibitory activity on PCa. Both C3G and Pg3G upregulated AR expression. However, PSA expression, an indicator of AR activity, remained the same (Fig. 4B). This effect on AR activity is in contrast to the effect of PCC in vitro but similar to that in vivo. Both C3G and Pg3G decreased the expression of Cyclin D1 (Fig. 4C), while increasing the proportion of cells in G0/G1 (Fig. 4D,E), again in line with the PCC effect.
Figure 4.
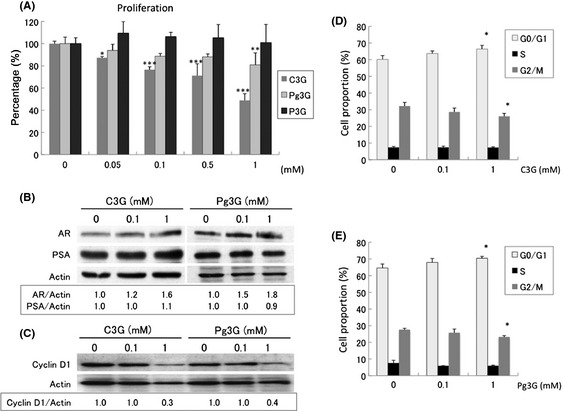
Anthocyanins in the purple corn color (PCC) mixture, cyanidin‐3‐glucoside (C3G), pelargonidin‐3‐glucoside (Pg3G) and peonidin‐3‐glucoside (P3G), were tested using LNCaP cells. (A) Effects of C3G, Pg3G and P3G on LNCaP cell proliferation. (B,C) Findings of Western blotting. The density of the bands of androgen receptor (AR), PSA and actin were semi‐quantified by ImageJ. (D) Effects of C3G on the cell cycle of LNCaP cells. (E) Effects of Pg3G on the cell cycle of LNCaP cells. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Purple corn color is reported to have anti‐cancer effect on colon and breast cancer,9, 10 and it also has attenuating effects on some life style diseases, for example, hypertension, hyperglycemia, and diabetes.11, 12 Like breast cancer, PCa is hormone‐related. Prostate cancer is also closely associated with a high‐fat diet.20, 21 Finally, in the TRAP model, hypertension is positively associated with PCa development.22 Therefore, we investigated the possibility that PCC could have inhibitory effects on PCa. The results of our experiments, both in vitro and in vivo, supported this hypothesis. Purple corn color not only showed antiproliferative effects on an androgen‐dependent PCa cell line, it also inhibited prostate carcinogenesis in vivo. Importantly, dietary PCC did not have any observable toxic effects: there were no significant changes in the final body weights or relative liver or kidney weights in rats fed PCC in their diets. This suggests that PCC could be used as a long‐term dietary supplement for chemoprevention of PCa. The success of PCC in the TRAP rat model demonstrates that PCC may inhibit prostate carcinogenesis not only in a simple in vitro tissue culture system but also in the complex system of living experimental animals.
To date, an enormous effort has been made to find means to prevent PCa, but an ideal chemopreventor active by itself has yet to be found. The trend has therefore been to put chemicals that target different pathways together to form a “cocktail” that would be more effective. Our laboratory has been focused on looking for PCa chemopreventors. We previously reported that resveratrol could inhibit PCa genesis by targeting the AR pathway,19 while γ‐tocopherol exerts inhibitory effects though activation of caspase‐signaling.23 In the present study, we found that PCC targets cell growth. A reasonable expectation is that combining PCC, resveratrol and γ‐tocopherol could have a greater effect than using any of the compounds singly. This is a direction for future studies.
To better understand the chemopreventive effects of PCC on PCa, identifying the active components is essential. Cell proliferation assays here indicated that both C3G and Pg3G dose‐dependently inhibited the growth of LNCaP. Similarly to PCC, both C3G and Pg3G decreased the expression of Cyclin D1 and increased the percentage of LNCaP cells in G0/G1. The increased percentage of cells in G0/G1 was about 1.1 times compared to the control. Importantly, the cell proliferation was inhibited by approximately 50%. This suggests that while the effect of C3G and Pg3G on the cell proliferation is rather small, the cumulative effect over time can be substantial. Intriguingly, although the PCC mixture downregulated AR activity, C3G and Pg3G did not show the same effect. Notably, PCC inhibited carcinogenesis in the TRAP model without downregulating AR activity. Therefore, it is likely that PCC does not inhibit PCa through downregulation of AR. The downregulation observed with the PCC mixture in vitro might be a byproduct of other compounds. Taken together, these results suggest that C3G and Pg3G are the probable active compounds contained in PCC.
Carcinogenesis is a complex and long‐term progress. Therefore, finding a chemopreventor and using it to inhibit the progression of PCa will be of general benefit. Our study has proved: (i) the safety of relatively long‐term PCC consumption; (ii) PCC can inhibit PCa both in vitro and in vivo, and modulation of cell growth pathways may possibly be involved in suppressive effects of PCC. Other studies have reported that C3G and Pg3G, the active compounds in PCC, have antioxidant and free‐radical‐scavenging effects, which may protect cells from oxidative damage and reduce the risk of diabetes, cardiovascular diseases and cancer.24, 25, 26, 27 With further study, we can expect to determine whether the mechanism of PCC inhibition of PCa also involves these pathways. Modulation of multiple pathways can increase the chance of PCC effectively preventing PCa in humans. Taking the available information into account, PCC, a widely used food colorant, appears to be a promising chemopreventor for PCa.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. The chemical structures of C3G, Pg3G and P3G.
Fig. S2. PCC down regulated the activity of the PSA promoter.
Fig. S3. The different stages of carcinogenesis in the prostate of TRAP rat.
Table S1. Final body and organ weights, serum hormone levels, and average PCC intake.
Acknowledgments
This work was supported by a Grant‐in‐Aid for the 3rd Term Comprehensive 10‐year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan and a grant from the Society for Promotion of Pathology of Nagoya, Japan. The authors thank Koji Kato and Junko Takekawa for their technical assistance with immunohistochemistry.
(Cancer Sci, doi: 10.1111/cas.12078, 2013)
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10–29. [DOI] [PubMed] [Google Scholar]
- 2. Huggins C, Hodges CV. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1941; 1: 293–7. [DOI] [PubMed] [Google Scholar]
- 3. Rubin MA. Targeted therapy of cancer: new roles for pathologists‐prostate cancer. Mod Pathol 2008; 21(Suppl. 2): S44–55. [DOI] [PubMed] [Google Scholar]
- 4. Wei JT, Dunn RL, Sandler HM et al Comprehensive comparison of health‐related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol 2002; 20: 557–66. [DOI] [PubMed] [Google Scholar]
- 5. Sanda MG, Dunn RL, Michalski J et al Quality of life and satisfaction with outcome among prostate‐cancer survivors. N Engl J Med 2008; 358: 1250–61. [DOI] [PubMed] [Google Scholar]
- 6. Lippman SM, Klein EA, Goodman PJ et al Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin E cancer prevention trial (SELECT). JAMA 2009; 301: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thompson IM, Tangen CM, Goodman PJ, Lucia MS, Klein EA. Chemoprevention of prostate cancer. J Urol 2009; 182(2): 499–507; discussion 508. [DOI] [PubMed] [Google Scholar]
- 8. Andriole GL, Bostwick DG, Brawley OW et al Effect of dutasteride on the risk of prostate cancer. N Engl J Med 2010; 362: 1192–202. [DOI] [PubMed] [Google Scholar]
- 9. Hagiwara A, Miyashita K, Nakanishi T et al Pronounced inhibition by a natural anthocyanin, purple corn color, of 2‐amino‐1‐methyl‐6‐phenylimidazo[4,5‐b]pyridine (PhIP)‐associated colorectal carcinogenesis in male F344 rats pretreated with 1,2‐dimethylhydrazine. Cancer Lett 2001; 171: 17–25. [DOI] [PubMed] [Google Scholar]
- 10. Fukamachi K, Imada T, Ohshima Y, Xu J, Tsuda H. Purple corn color suppresses Ras protein level and inhibits 7,12‐dimethylbenz[a]anthracene‐induced mammary carcinogenesis in the rat. Cancer Sci 2008; 99: 1841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shindo M, Kasai T, Abe A, Kondo Y. Effects of dietary administration of plant‐derived anthocyanin‐rich colors to spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo) 2007; 53: 90–3. [DOI] [PubMed] [Google Scholar]
- 12. Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3‐O‐beta‐D‐glucoside‐rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr 2003; 133: 2125–30. [DOI] [PubMed] [Google Scholar]
- 13. Asamoto M, Hokaiwado N, Cho YM et al Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res 2001; 61: 4693–700. [PubMed] [Google Scholar]
- 14. Cho YM, Takahashi S, Asamoto M et al Age‐dependent histopathological findings in the prostate of probasin/SV40 T antigen transgenic rats: lack of influence of carcinogen or testosterone treatment. Cancer Sci 2003; 94: 153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng Y, Yokohira M, Saoo K et al Inhibition of prostate carcinogenesis in probasin/SV40 T antigen transgenic rats by raloxifene, an antiestrogen with anti‐androgen action, but not nimesulide, a selective cyclooxygenase‐2 inhibitor. Carcinogenesis 2005; 26: 1109–16. [DOI] [PubMed] [Google Scholar]
- 16. Kandori H, Suzuki S, Asamoto M et al Influence of atrazine administration and reduction of calorie intake on prostate carcinogenesis in probasin/SV40 T antigen transgenic rats. Cancer Sci 2005; 96: 221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Said MM, Hokaiwado N, Tang M et al Inhibition of prostate carcinogenesis in probasin/SV40 T antigen transgenic rats by leuprorelin, a luteinizing hormone‐releasing hormone agonist. Cancer Sci 2006; 97: 459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang M, Ogawa K, Asamoto M et al Protective effects of citrus nobiletin and auraptene in transgenic rats developing adenocarcinoma of the prostate (TRAP) and human prostate carcinoma cells. Cancer Sci 2007; 98: 471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seeni A, Takahashi S, Takeshita K et al Suppression of prostate cancer growth by resveratrol in the transgenic rat for adenocarcinoma of prostate (TRAP) model. Asian Pac J Cancer Prev 2008; 9: 7–14. [PubMed] [Google Scholar]
- 20. De Nunzio C, Aronson W, Freedland SJ, Giovannucci E, Parsons JK. The correlation between metabolic syndrome and prostatic diseases. Eur Urol 2012; 61: 560–70. [DOI] [PubMed] [Google Scholar]
- 21. Hoda MR, Mohammed N, Theil G, Fischer K, Fornara P. Obesity and prostate cancer: role of adipocytokines and clinical implications. Urologe A 2012; 51: 1253–60. [DOI] [PubMed] [Google Scholar]
- 22. Takeshita K, Takahashi S, Tang M, Seeni A, Asamoto M, Shirai T. Hypertension is positively associated with prostate cancer development in the TRAP transgenic rat model. Pathol Int 2011; 61: 202–9. [DOI] [PubMed] [Google Scholar]
- 23. Takahashi S, Takeshita K, Seeni A et al Suppression of prostate cancer in a transgenic rat model via gamma‐tocopherol activation of caspase signaling. Prostate 2009; 69: 644–51. [DOI] [PubMed] [Google Scholar]
- 24. Sun CD, Zhang B, Zhang JK et al Cyanidin‐3‐glucoside‐rich extract from Chinese bayberry fruit protects pancreatic beta cells and ameliorates hyperglycemia in streptozotocin‐induced diabetic mice. J Med Food 2012; 15: 288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Delazar A, Khodaie L, Afshar J, Nahar L, Sarker SD. Isolation and free‐radical‐scavenging properties of cyanidin 3‐O‐glycosides from the fruits of Ribes biebersteinii Berl . Acta Pharm 2010; 60: 1–11. [DOI] [PubMed] [Google Scholar]
- 26. Xu M, Bower KA, Wang S et al Cyanidin‐3‐glucoside inhibits ethanol‐induced invasion of breast cancer cells overexpressing ErbB2. Mol Cancer 2010; 9: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goulas V, Manganaris GA. The effect of postharvest ripening on strawberry bioactive composition and antioxidant potential. J Sci Food Agric 2011; 91: 1907–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The chemical structures of C3G, Pg3G and P3G.
Fig. S2. PCC down regulated the activity of the PSA promoter.
Fig. S3. The different stages of carcinogenesis in the prostate of TRAP rat.
Table S1. Final body and organ weights, serum hormone levels, and average PCC intake.


