Abstract
Receptor tyrosine kinases MET and RON (MST1R) form non‐covalent complexes on the cell surface, a critical step in tumor progression. A recent study suggested a prognostic role for MET expression in diffuse large B‐cell lymphoma (DLBCL). The aim of this study was to examine the impact of MET and RON expression in uniformly treated DLBCL patients. The expression of MET and RON was retrospectively examined by immunohistochemistry in 120 DLBCL patients treated with rituximab combined with a CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone). The median follow‐up time was 42.5 months (range, 1–89 months). Thirty‐two (26%) and 30 patients (25%) expressed MET or RON, respectively. Seventy‐five patients (62.5%) were negative for both MET and RON (MET − RON −). MET negativity was associated with worse overall survival (P = 0.029). In multivariate analysis, negativity for both MET and RON (MET − RON −) was strongly associated with inferior overall survival (P = 0.008). Interestingly, the MET − RON − phenotype retained its prognostic impact after subgroup analysis according to the international prognostic index or by the cell of origin by immunohistochemical algorithm by Choi et al. This study suggests that the MET − RON − phenotype is an independent prognostic factor in DLBCL patients receiving R‐CHOP, and may identify a subgroup of DLBCL patients who require more intensive therapy.
Diffuse large B‐cell lymphoma (DLBCL), the most common subtype of lymphoid neoplasm, is an aggressive tumor with heterogeneous clinical behavior.1, 2 When rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) became the standard treatment for DLCBL, prognosis significantly improved.3, 4
Despite important advances in treatment, 40% of patients with DLBCL will relapse within a short time after initial remission and will eventually die as a result of the disease. The standard stratification system for survival in patients with DLBCL is the international prognostic index (IPI), but it does not represent the biologic spectrum of DLBCL. Several biologic factors have been proposed as predictors of clinical outcome in DLBCL patients, including Bcl‐2, Myc, LMO2, mutated p53, vascular endothelial growth factor receptor‐2, hypoxia‐inducible factor‐1α, Ki‐67, and CD5.5 However, their prognostic value in patients with DLBCL has not been conclusively determined.
The receptor tyrosine kinase MET is implicated in tumor cell proliferation, survival, invasion, and metastasis through a paracrine system involving its ligand, hepatocyte growth factor (HGF).6 However, other studies indicated that the HGF/MET pathway has an antiproliferative effect in malignant tumors.7, 8, 9 Previous studies show that MET is overexpressed in gastric, colon, ovary, kidney, and thyroid carcinomas.10 MET and HGF expression levels have prognostic significance in many malignant tumors.11, 12, 13
A receptor tyrosine kinase with homology to MET, RON (MST1R), is involved in tumor progression and metastasis.14, 15 RON is overexpressed in human epithelial malignancies,16, 17 and the expression of RON is associated with poor clinical outcome in breast18 and gastroesophageal cancer.19
Both MET and RON have been detected in human DLBCL tissue.20, 21 Recently, MET expression was reported to be a prognostic factor in DLBCL, but results are conflicting.20, 22 Although a relationship between RON and MET is observed in several malignancies,23, 24 no study has examined their prognostic significance in DLBCL. Furthermore, no attempts have been made to validate the prognostic significance of MET and RON in a large cohort of uniformly treated patients. Thus, the aim of the present study was to determine the clinical significance of MET and RON protein expression in patients with DLBCL receiving R‐CHOP therapy.
Materials and Methods
Patients
The present report comprised a retrospective study of 120 patients with DLBCL diagnosed at Asan Medical Center (University of Ulsan College of Medicine, Seoul, Korea) between 2004 and 2011. All the patients met the following criteria: pathologically confirmed DLBCL; treatment with an R‐CHOP regimen; no previous treatment with biologic therapy or chemotherapy; no previous history of malignancy; and absence of HIV infection. Paraffin‐embedded tumor tissues and follow‐up data were available for all included patients.
Clinical information, including age, gender, stage (using the Ann Arbor system), presence or absence of B symptoms, IPI, performance status, extranodal site involvement, serum lactate dehydrogenase levels, and survival data were obtained from patients' medical records. The median follow‐up time for surviving patients was 42.5 months (range, 1–89 months). Responses were assessed using Cheson's criteria.25 Routine follow‐up imaging analyses were carried out every 3 months for the first 2 years, every 6 months for the next 3 years, and then annually (or whenever clinically indicated) thereafter. The present research was approved by the Internal Review Board of the Asan Medical Center.
Histopathological analysis and immunohistochemistry
Histological data from all patients was reviewed by three pathologists (JH, YWK, and HSH). Histological subtype was determined according to World Health Organization criteria.
A representative tumor paraffin block was collected from each case, and three tumor cores (1 mm in diameter) were obtained with a trephine apparatus (Seoungkohn, Seoul, Korea). Trephinated tissue cores were consecutively placed in tissue array molds (Seoungkohn), which were filled with liquid paraffin and cooled.
An immunohistochemistry protocol for formalin‐fixed, paraffin‐embedded tissue sections was carried out using an automatic staining device (Benchmark XT; Ventana Medical Systems, Tucson, AZ, USA). Briefly, 5‐μm‐thick sections were transferred onto poly‐l‐lysine‐coated adhesive slides and dried at 62°C for 30 min. After standard heat epitope retrieval for 30 min in EDTA (pH 8.0) in the autostainer, the samples were incubated with antibodies against cleaved CD10 (1:50 dilution; Novocastra, Newcastle, UK), Bcl‐6 (1:100 dilution; Cell Marque, Rocklin, CA, USA), FOXP1 (1:500 dilution; Abcam, Cambridge, UK), GCET1 (1:25 dilution; Abcam), MUM1 (1:50 dilution; Dako, Glostrup, Denmark), Ki‐67 (1:100; Zymed Laboratories, San Francisco, CA, USA), MET (1:50 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), or RON (1:25 dilution; Santa Cruz Biotechnology). The sections were subsequently incubated with biotinylated anti‐mouse immunoglobulin, peroxidase‐labeled streptavidin (LSAB kit; Dako), and 3,3′‐diaminobenzidine. Slides were counterstained with Harris hematoxylin.
Various cut‐off points for MET and RON expression were examined (from the 10th to the 35th percentile, in 5% increments; i.e., 10%, 15%, 20%, 25%, 30%, and 35%) (Table S1). The most significant difference in overall survival (OS) was observed at a cut‐off of 30% for MET and RON, using the log–rank test. All interpretations of MET and RON staining patterns were carried out by a colleague blinded to the patients' clinical outcomes. Immunoperoxidase results for CD10, Bcl‐6, FOXP1, GCET1, and MUM1 were used to classify the cases into germinal center B‐cell‐like (GCB) or non‐GCB DLBCL according to the algorithm by Choi et al.,26 as previously described.
Statistical analysis
Overall survival was defined as the time between the date of diagnosis and the date of death from any cause. For still‐living patients, the OS was considered the time between the diagnosis and the latest follow‐up date. The OS was analyzed with Kaplan–Meier curves, which were compared by log–rank testing.
Multivariate prognostic analyses were carried out on OS data with the Cox proportional hazards regression model. Categorical variables were compared using the χ2‐test. Continuous variables were compared using the Mann–Whitney U‐test. All statistical analyses were carried out using the spss statistical software program (version 18.0; SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Patient characteristics
The clinical characteristics of the 120 patients included in the present study are summarized in Table 1. Patients ranged in age from 25 to 83 years (median, 59 years). 32 patients experienced death during the course of the study. The estimated 5‐year OS was 67.2%.
Table 1.
Demographic and clinical characteristics of patients with diffuse large B‐cell lymphoma (DLBCL) treated with R‐CHOP (n = 120)
| Characteristic at diagnosis | No. of patients (%) |
|---|---|
| Age, median (range, years) | 59.5 (25–83) |
| Male gender | 75 (62.5%) |
| Performance status ≥2 | 10 (8.3%) |
| Histologic subtype | |
| DLBCL, NOS | 113 (94.2%) |
| T‐cell/histiocyte‐rich DLBCL | 6 (5.0%) |
| EBV‐positive DLBCL of the elderly | 1 (0.8%) |
| Ann Arbor stage | |
| I | 17 (14.2%) |
| II | 44 (36.7%) |
| III | 16 (13.3%) |
| IV | 43 (35.8%) |
| LDH ≥250 U/L | 55 (45.8%) |
| B symptoms present | 19 (15.8%) |
| Extranodal site involvement ≥2 | 35 (29.2%) |
| International prognostic index ≥3 (high risk) | 39 (32.5%) |
| GCB type | 50 (41.7%) |
| Primary treatment | |
| R‐CHOP + radiotherapy | 17 (14.2%) |
| R‐CHOP | 103 (85.8%) |
EBV, Epstein–Barr virus; GCB, germinal center B‐cell‐like; LDH, lactate dehydrogenase; NOS, not otherwise specified; R‐CHOP, rituximab plus cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisolone cyclophosphamide.
MET and RON protein expression in DLBCL tissue
Thirty‐two (26%) and 30 patients (25%) showed cytoplasmic or membranous positivity for MET or RON, respectively (Fig. 1a,b). Coexpression of MET and RON in tumor cells was observed in 15 cases (12.5%). Fifteen cases (12.5%) showed MET expression only, 15 cases (12.5%) showed RON expression only, and 75 (62.5%) cases were negative for both MET and RON. Neither MET nor RON expression was associated with any of the clinicopathological factors evaluated (Table S2). There was no correlation between MET and Ki‐67 expression (P = 0.658) or between RON and Ki‐67 expression (P = 0.912).
Figure 1.
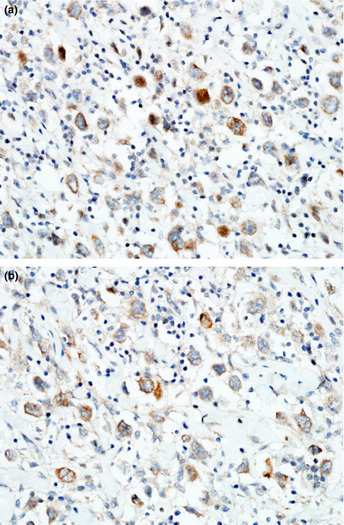
Expression of MET and RON in diffuse large B‐cell lymphoma (DLBCL) tissue. (a) DLBCL cells showing strong MET immunostaining on the cell membrane (original magnification, ×400). (b) DLBCL RON expression on the cell membrane (original magnification, ×400).
Prognostic significance of MET and RON protein expression
Negativity of MET protein expression was associated with lower 3‐year OS (69.9% vs 89%, P = 0.029; Fig. 2a). Negativity of RON protein expression was associated with a trend toward a lower 3‐year OS rate, but this did not reach statistical significance (71.6% vs 88.2%, P = 0.191; Fig. 2b)
Figure 2.
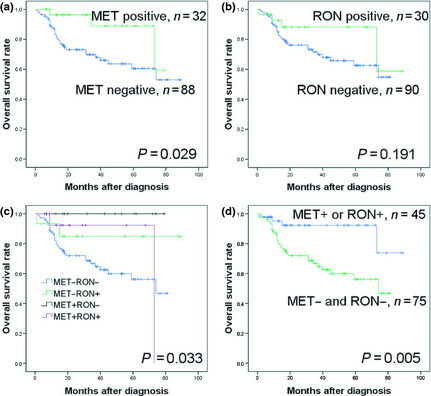
Comparison of overall survival (OS) rates according to MET and RON expression in patients with diffuse large B‐cell lymphoma. (a) OS according to MET expression. (b) OS according to RON expression. (c) OS according to the MET/RON expression pattern. (d) OS in the group with MET − and RON − and group with MET + or RON +.
To evaluate the relative importance of MET and RON, the dichotomized MET and RON data was combined, and patients were stratified into four groups (MET+RON+, MET−RON+, MET+RON−, and MET−RON−). Patients with tumors negative for both proteins (the MET−RON− phenotype) had significantly worse OS (P = 0.033; Fig. 2c) than patients with the other expression patterns. Because the survival curves of the MET+RON+ and the single‐positive groups converged, expression patterns were categorized into two groups, MET−RON− and MET+ or RON+. The MET−RON− phenotype was associated with worse OS than either the MET+ or the RON+ phenotype (3‐year OS, 66.7% vs 92.3%, P = 0.005; Fig. 2d). The MET−RON− phenotype included more patients receiving chemoradiotherapy (20% vs 4.4%, P = 0.028) compared with either the MET+ or RON+ phenotype (Table S3).
To further assess the prognostic value of MET and RON expression, subgroup analyses were carried out according to IPI and the cell of origin using Choi's algorithm. In the low IPI group (<3), cases with a MET−RON− phenotype showed a worse OS rate than cases with either a MET+ or a RON+ phenotype (P = 0.086; Fig. 3a). In the high IPI group (≥3), cases with a MET−RON− phenotype also had an inferior OS rate (P = 0.04; Fig. 3b). In the GCB group, a MET−RON− phenotype showed a worse OS rate than cases with either a MET+ or RON+ phenotype, (P = 0.069; Fig. 3c). In the non‐GCB group, cases with MET−RON− phenotype was also associated with an inferior OS rate (P = 0.032; Fig. 3d) compared to either MET+ or RON+ cases.
Figure 3.
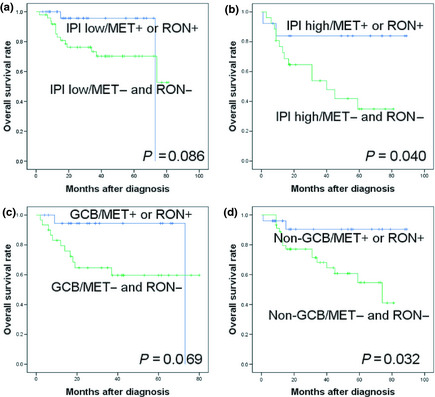
Comparison of overall survival rates according to expression of MET and RON (MET − and RON − vs either MET + or RON +) in patients with diffuse large B‐cell lymphoma with a low international prognostic index (IPI) (<3) (a) or a high IPI (≥3) (b), or in patients with germinal center B‐cell (GCB)‐like type (c) or non‐GCB‐like type of disease (d).
In univariate analysis, IPI shows a marginal significance for OS (Table 2). In multivariate analysis, a MET−RON− phenotype was an independent prognostic marker for OS, along with a high‐risk IPI and a non‐GCB type (Table 3).
Table 2.
Univariate analyses for overall survival in patients with diffuse large B‐cell lymphoma treated with R‐CHOP (n = 120)
| Prognostic factor | HR | 95% CI | P‐valuea | |
|---|---|---|---|---|
| Age, years | ≤60 vs >60 | 1.718 | 0.85–3.45 | 0.129 |
| Gender | Female vs male | 1.621 | 0.76–3.44 | 0.209 |
| Performance status | <2 vs ≥2 | 5.876 | 2.57–13.42 | <0.001 |
| Ann Arbor stage | ≤2 vs >2 | 1.986 | 0.09–4.20 | 0.073 |
| LDH (U/L) | Normal vs abnormal | 1.385 | 0.68–2.78 | 0.362 |
| B symptom | (−) vs (+) | 1.914 | 0.85–4.27 | 0.113 |
| Extranodal site involvement | <2 vs ≥2 | 1.568 | 0.77–3.17 | 0.212 |
| IPI | <3 vs ≥3 | 1.980 | 0.98–3.98 | 0.055 |
| GCB type | GCB vs non‐GCB | 0.936 | 0.46–1.09 | 0.854 |
| Radiation therapy | (−) vs (+) | 1.185 | 0.48–2.88 | 0.709 |
| MET expression | (−) vs (+) | 0.289 | 0.08–0.94 | 0.041 |
| RON expression | (−) vs (+) | 0.505 | 0.17–1.44 | 0.201 |
| MET/RON | MET+ or RON+ vs MET− and RON− | 3.975 | 1.39–11.30 | 0.010 |
Cox univariate analysis. CI, confidence interval; GCB, germinal center B‐cell‐like; HR, hazard ratio; IPI, International Prognostic Index; LDH, lactate dehydrogenase; R‐CHOP, rituximab plus cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisolone cyclophosphamide.
Table 3.
Multivariate analysis for overall survival in patients with diffuse large B‐cell lymphoma treated with R‐CHOP (n = 120)
| Prognostic factor | HR | 95% CI | P‐valuea | |
|---|---|---|---|---|
| IPI | <3 vs ≥3 | 2.080 | 1.038–4.168 | 0.039 |
| GCB type | GCB vs non‐GCB | 0.893 | 0.440–1.812 | 0.754 |
| MET/RON | MET+ or RON+ vs MET− and RON− | 4.127 | 1.444–11.790 | 0.008 |
Cox multivariate analysis. CI, confidence interval; GCB, germinal center B‐cell‐like; HR, hazard ratio; IPI, International Prognostic Index; R‐CHOP, rituximab plus cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisolone cyclophosphamide.
Discussion
In this analysis of 120 DLBCL samples from patients receiving R‐CHOP therapy, survival analysis reveals that MET expression is a favorable prognostic factor for OS. Interestingly, negativity for both MET and RON (the MET−RON− phenotype) was strongly associated with poor OS in a multivariate analysis. Subgroup analysis showed that the MET−RON− phenotype predicts high‐risk DLBCL patients, independently of the IPI or the cell of origin. Therefore, cases showing a MET−RON− phenotype warrant closer and more meticulous follow‐up examination. Limitations of this study include the retrospective nature of the study design, the short follow‐up period, and the relatively small sample size.
One previous study also reported the favorable impact of MET expression in the prognosis of patients with DLBCL.20 However, the patients' chemotherapy regimens were not specified in that study. Here, we confirm the favorable prognostic impact of MET expression. We also carried out a survival analysis in patients who received CHOP. In CHOP‐treated patients, RON expression was associated with better OS (Fig. 4a); however, MET expression was not correlated with OS (Fig. 4b). CHOP‐treated patients negative for both MET and RON (the MET–RON– phenotype) had a worse OS by univariate analysis (Fig. 4c); however, it did not retain prognostic significance in the multivariate analysis (Table S4).
Figure 4.
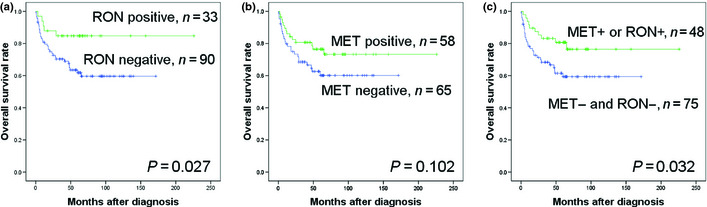
Comparison of overall survival (OS) rates according to MET and RON expression in patients who received CHOP therapy. (a) OS according to RON expression. (b) OS according to MET expression. (c) OS in the MET − RON − group and the MET + or RON + group.
In stark contrast to our results in DLBCL, MET and HGF expression have been associated with unfavorable survival outcomes in solid malignancies,11, 12, 13, 27, 28, 29 although a few studies reported a favorable prognostic impact for MET.20, 30 Expression of RON is also an unfavorable predictor of outcome in various malignancies.19, 31 The favorable prognostic impact of the expression of MET and RON shown in our study and the study by Uddin et al.20 raises the question of the mechanism of action of these molecules in lymphomagenesis. Uddin et al. ascribed the superior survival of MET‐positive DLBCL to increased sensitivity to chemotherapy because of higher proliferation rates of MET+ cases in their series, as shown by the Ki‐67 labeling index. In the present study, however, the Ki‐67 proliferation index was not associated with MET or RON. The reason for this discrepancy is unknown. As the cases in the study by Uddin et al. were not stratified by treatment, differences in the patient population may be one explanation. Furthermore, although it is well known that deregulation of the MET/HGF pathway plays an important role in unchecked overproliferation, previous studies have reported an antiproliferative effect of HGF on melanoma and hepatocellular carcinoma cell lines, suggesting an antitumor effect for this growth factor.7 A marked inhibition of cell growth after treatment with HGF was also noted in other epithelial malignancies.8, 9 Therefore, HGF/MET signaling can have opposing roles in cell growth and apoptosis in malignant tumors. Furthermore, in a recent study, pediatric B‐cell acute lymphoblastic leukemia cells prestimulated with HGF had a higher apoptotic rate than non‐HGF prestimulated samples.32 Although a previous study reported that siRNA targeted against MET triggered caspase‐dependent apoptosis in DLBCL cell lines,20 MET directly binds to the pro‐apoptotic protein FAS, proposing that this association might have an effect on the apoptotic activity of FAS in some epithelial cancer cells.33, 34 Further studies are needed to delineate the mechanism through which MET influences prognosis in DLBCL.
Amplification, mutation, and overexpression of MET or RON have been described in various malignancies, and such dysregulation was associated with tumor transformation and progression.19, 35, 36, 37 Furthermore, amplification of 3p, which is the chromosome region containing RON, is a common event in various solid tumors, occurring in 15–42.5% of the samples examined.38 MET/HGF signaling is mainly mediated by the RAS–MAPK and PI3K–Akt pathways and affects gene expression and cell cycle progression.39, 40 Uddin et al.20 also reported that MET expression was associated with increased activation of p‐Akt in DLBCL patients. RON is also a strong inducer of both the MAPK and PI3K signaling pathways.41
Intriguingly, patients who were negative for both MET and RON showed inferior clinical outcomes compared to those with expression of at least one of the two proteins. MET and RON exist as a preformed dimer in the cell membrane before ligand stimulation. A bidirectional transphosphorylation between MET and RON occurs after exposure of cells to either HGF or macrophage‐stimulating protein.42 Although RON appears to be less efficient than MET as a kinase, the formation of MET/RON complexes leads to more efficient RON transphosphorylation by MET, resulting in a more sustained signal than is induced by RON/RON homodimers. This cooperative activation of both MET and RON may induce a synergistic response to their ligands. Patients with tumors coexpressing MET and RON reportedly have an inferior clinical outcome than those with single receptor‐positive tumors.24, 43, 44 Considering the opposing roles of HGF/MET, the MET and RON pathways may induce different cellular responses depending on cell type, cell environment, and protein interactions. The synergy between MET and RON pathways may contribute to the inhibition of tumor progression in different environments.
The HGF/MET signaling pathway has conflicting roles in cell growth and apoptosis in malignant tumors. In a previous study, the MET inhibitor PHA665752 was highly selective for tumor cells with high MET expression and had no effect on normal cells, thereby avoiding potential systemic side‐effects. Uddin et al.20 also showed that fatty acid synthase, the enzyme responsible for the de novo synthesis of fatty acids, was closely associated with the expression of MET kinase in DLBCL cell lines, and siRNA targeted against fatty acid synthase induced caspase‐dependent apoptosis and suppressed the expression of MET kinase.45 However, previous studies indicate that stimulation of the HGF/MET pathway induced apoptosis in epithelial and hematologic malignancies.8, 9, 32 To clarify the conflicting results from previous reports, clinical trials should be carried out.
A previous study showed a correlation between MET expression and the cell of origin in DLBCL patients.20 In the present study, no correlation was observed between MET expression and the cell of origin. This discrepancy may be due to the use of different criteria for GCB classification among the different studies, different reading criteria for immunohistochemical staining, and/or different therapeutic and follow‐up approaches. No previously reported studies have examined the correlation between RON expression and GCB status in DLBCL patients. Our study found no correlation between RON expression and GCB status. These results suggest that MET and RON expression are associated with survival independent of the cell of origin.
In summary, our results suggest the prognostic significance of MET and RON negativity in DLBCL patients, independent of IPI or GCB type. The lack of both MET and RON expression can be used to identify a subgroup of DLBCL patients who are at a high risk of recurrence or progression and who may benefit from aggressive chemotherapy. Further studies, including prospective clinical trials, are needed to investigate the effects of MET and RON expression on clinical outcome and to confirm the present findings.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Table S1. MET and RON vs overall survival (OS).
Table S2. Correlation of MET and RON protein expression with clinicopathologic variables.
Table S3. Correlation of MET/RON pattern with clinicopathologic variables.
Table S4. Multivariate analysis for MET and RON protein expression and overall survival (OS) in patients who received CHOP therapy.
Acknowledgments
This study was supported by a grant (2011‐090) from the Asan Institute for Life Sciences, Seoul, Korea.
(Cancer Sci, 2013; 103: 000–000)
References
- 1. Moskowitz C. Diffuse large B cell lymphoma: how can we cure more patients in 2012? Best Pract Res Clin Haematol 2012; 25: 41–7. [DOI] [PubMed] [Google Scholar]
- 2. Pileri SA, Agostinelli C, Sabattini E et al Lymphoma classification: the quiet after the storm. Semin Diagn Pathol 2011; 28: 113–23. [DOI] [PubMed] [Google Scholar]
- 3. Sehn LH. A decade of R‐CHOP. Blood 2010; 116: 2000–1. [DOI] [PubMed] [Google Scholar]
- 4. Fu K, Weisenburger DD, Choi WW et al Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B‐cell‐like and non‐germinal center B‐cell‐like subtypes of diffuse large B‐cell lymphoma. J Clin Oncol 2008; 26: 4587–94. [DOI] [PubMed] [Google Scholar]
- 5. Sehn LH. Paramount prognostic factors that guide therapeutic strategies in diffuse large B‐cell lymphoma. Hematology Am Soc Hematol Educ Program 2012; 2012: 402–9. [DOI] [PubMed] [Google Scholar]
- 6. Benvenuti S, Comoglio PM. The MET receptor tyrosine kinase in invasion and metastasis. J Cell Physiol 2007; 213: 316–25. [DOI] [PubMed] [Google Scholar]
- 7. Tajima H, Matsumoto K, Nakamura T. Hepatocyte growth factor has potent anti‐proliferative activity in various tumor cell lines. FEBS Lett 1991; 291: 229–32. [DOI] [PubMed] [Google Scholar]
- 8. Shiota G, Rhoads DB, Wang TC, Nakamura T, Schmidt EV. Hepatocyte growth factor inhibits growth of hepatocellular carcinoma cells. Proc Natl Acad Sci U S A 1992; 89: 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ronen D, Altstock RT, Firon M et al Met‐HGF/SF mediates growth arrest and differentiation in T47D breast cancer cells. Cell Growth Differ 1999; 10: 131–40. [PubMed] [Google Scholar]
- 10. Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 2008; 7: 504–16. [DOI] [PubMed] [Google Scholar]
- 11. Masuya D, Huang C, Liu D et al The tumour‐stromal interaction between intratumoral c‐Met and stromal hepatocyte growth factor associated with tumour growth and prognosis in non‐small‐cell lung cancer patients. Br J Cancer 2004; 90: 1555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aune G, Lian AM, Tingulstad S et al Increased circulating hepatocyte growth factor (HGF): a marker of epithelial ovarian cancer and an indicator of poor prognosis. Gynecol Oncol 2011; 121: 402–6. [DOI] [PubMed] [Google Scholar]
- 13. Tanimoto S, Fukumori T, El‐Moula G et al Prognostic significance of serum hepatocyte growth factor in clear cell renal cell carcinoma: comparison with serum vascular endothelial growth factor. J Med Invest 2008; 55: 106–11. [DOI] [PubMed] [Google Scholar]
- 14. Chen YQ, Zhou YQ, Angeloni D, Kurtz AL, Qiang XZ, Wang MH. Overexpression and activation of the RON receptor tyrosine kinase in a panel of human colorectal carcinoma cell lines. Exp Cell Res 2000; 261: 229–38. [DOI] [PubMed] [Google Scholar]
- 15. Wang MH, Wang D, Chen YQ. Oncogenic and invasive potentials of human macrophage‐stimulating protein receptor, the RON receptor tyrosine kinase. Carcinogenesis 2003; 24: 1291–300. [DOI] [PubMed] [Google Scholar]
- 16. Bardella C, Costa B, Maggiora P et al Truncated RON tyrosine kinase drives tumor cell progression and abrogates cell‐cell adhesion through E‐cadherin transcriptional repression. Cancer Res 2004; 64: 5154–61. [DOI] [PubMed] [Google Scholar]
- 17. Wang MH, Kurtz AL, Chen Y. Identification of a novel splicing product of the RON receptor tyrosine kinase in human colorectal carcinoma cells. Carcinogenesis 2000; 21: 1507–12. [PubMed] [Google Scholar]
- 18. Thangasamy A, Rogge J, Ammanamanchi S. Regulation of RON tyrosine kinase‐mediated invasion of breast cancer cells. J Biol Chem 2008; 283: 5335–43. [DOI] [PubMed] [Google Scholar]
- 19. Catenacci DV, Cervantes G, Yala S et al RON (MST1R) is a novel prognostic marker and therapeutic target for gastroesophageal adenocarcinoma. Cancer Biol Ther 2011; 12: 9–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uddin S, Hussain AR, Ahmed M et al Inhibition of c‐MET is a potential therapeutic strategy for treatment of diffuse large B‐cell lymphoma. Lab Invest 2010; 90: 1346–56. [DOI] [PubMed] [Google Scholar]
- 21. Renne C, Willenbrock K, Martin‐Subero JI et al High expression of several tyrosine kinases and activation of the PI3K/AKT pathway in mediastinal large B cell lymphoma reveals further similarities to Hodgkin lymphoma. Leukemia 2007; 21: 780–7. [DOI] [PubMed] [Google Scholar]
- 22. Kawano R, Ohshima K, Karube K et al Prognostic significance of hepatocyte growth factor and c‐MET expression in patients with diffuse large B‐cell lymphoma. Br J Haematol 2004; 127: 305–7. [DOI] [PubMed] [Google Scholar]
- 23. Chen Q, Seol DW, Carr B, Zarnegar R. Co‐expression and regulation of Met and Ron proto‐oncogenes in human hepatocellular carcinoma tissues and cell lines. Hepatology 1997; 26: 59–66. [DOI] [PubMed] [Google Scholar]
- 24. Cheng HL, Liu HS, Lin YJ et al Co‐expression of RON and MET is a prognostic indicator for patients with transitional‐cell carcinoma of the bladder. Br J Cancer 2005; 92: 1906–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheson BD, Pfistner B, Juweid ME et al Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–86. [DOI] [PubMed] [Google Scholar]
- 26. Choi WW, Weisenburger DD, Greiner TC et al A new immunostain algorithm classifies diffuse large B‐cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res 2009; 15: 5494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakajima M, Sawada H, Yamada Y et al The prognostic significance of amplification and overexpression of c‐met and c‐erb B‐2 in human gastric carcinomas. Cancer 1999; 85: 1894–902. [DOI] [PubMed] [Google Scholar]
- 28. Kammula US, Kuntz EJ, Francone TD et al Molecular co‐expression of the c‐Met oncogene and hepatocyte growth factor in primary colon cancer predicts tumor stage and clinical outcome. Cancer Lett 2007; 248: 219–28. [DOI] [PubMed] [Google Scholar]
- 29. Ramirez R, Hsu D, Patel A et al Over‐expression of hepatocyte growth factor/scatter factor (HGF/SF) and the HGF/SF receptor (cMET) are associated with a high risk of metastasis and recurrence for children and young adults with papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2000; 53: 635–44. [DOI] [PubMed] [Google Scholar]
- 30. Nakopoulou L, Gakiopoulou H, Keramopoulos A et al c‐met tyrosine kinase receptor expression is associated with abnormal beta‐catenin expression and favourable prognostic factors in invasive breast carcinoma. Histopathology 2000; 36: 313–25. [DOI] [PubMed] [Google Scholar]
- 31. Hui MK, Lai KK, Chan KW et al Prognostic significance of phosphorylated RON in esophageal squamous cell carcinoma. Med Oncol 2012; 29: 1699–706. [DOI] [PubMed] [Google Scholar]
- 32. Accordi B, Pillozzi S, Dell'Orto MC et al Hepatocyte growth factor receptor c‐MET is associated with FAS and when activated enhances drug‐induced apoptosis in pediatric B acute lymphoblastic leukemia with TEL‐AML1 translocation. J Biol Chem 2007; 282: 29384–93. [DOI] [PubMed] [Google Scholar]
- 33. Shen K, Novak RF. Fas‐signaling and effects on receptor tyrosine kinase signal transduction in human breast epithelial cells. Biochem Biophys Res Commun 1997; 230: 89–93. [DOI] [PubMed] [Google Scholar]
- 34. Wang X, DeFrances MC, Dai Y et al A mechanism of cell survival: sequestration of Fas by the HGF receptor Met. Mol Cell 2002; 9: 411–21. [DOI] [PubMed] [Google Scholar]
- 35. Di Renzo MF, Olivero M, Giacomini A et al Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res 1995; 1: 147–54. [PubMed] [Google Scholar]
- 36. Di Renzo MF, Olivero M, Martone T et al Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene 2000; 19: 1547–55. [DOI] [PubMed] [Google Scholar]
- 37. Peace BE, Hughes MJ, Degen SJ, Waltz SE. Point mutations and overexpression of Ron induce transformation, tumor formation, and metastasis. Oncogene 2001; 20: 6142–51. [DOI] [PubMed] [Google Scholar]
- 38. Senchenko VN, Liu J, Loginov W et al Discovery of frequent homozygous deletions in chromosome 3p21.3 LUCA and AP20 regions in renal, lung and breast carcinomas. Oncogene 2004; 23: 5719–28. [DOI] [PubMed] [Google Scholar]
- 39. Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003; 4: 915–25. [DOI] [PubMed] [Google Scholar]
- 40. Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends Cell Biol 2009; 19: 542–51. [DOI] [PubMed] [Google Scholar]
- 41. Danilkovitch A, Donley S, Skeel A, Leonard EJ. Two independent signaling pathways mediate the antiapoptotic action of macrophage‐stimulating protein on epithelial cells. Mol Cell Biol 2000; 20: 2218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Follenzi A, Bakovic S, Gual P, Stella MC, Longati P, Comoglio PM. Cross‐talk between the proto‐oncogenes Met and Ron. Oncogene 2000; 19: 3041–9. [DOI] [PubMed] [Google Scholar]
- 43. Lee WY, Chen HH, Chow NH, Su WC, Lin PW, Guo HR. Prognostic significance of co‐expression of RON and MET receptors in node‐negative breast cancer patients. Clin Cancer Res 2005; 11: 2222–8. [DOI] [PubMed] [Google Scholar]
- 44. Lee CT, Chow NH, Su PF, Lin SC, Lin PC, Lee JC. The prognostic significance of RON and MET receptor coexpression in patients with colorectal cancer. Dis Colon Rectum 2008; 51: 1268–74. [DOI] [PubMed] [Google Scholar]
- 45. Uddin S, Hussain AR, Ahmed M et al Inhibition of fatty acid synthase suppresses c‐Met receptor kinase and induces apoptosis in diffuse large B‐cell lymphoma. Mol Cancer Ther 2010; 9: 1244–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. MET and RON vs overall survival (OS).
Table S2. Correlation of MET and RON protein expression with clinicopathologic variables.
Table S3. Correlation of MET/RON pattern with clinicopathologic variables.
Table S4. Multivariate analysis for MET and RON protein expression and overall survival (OS) in patients who received CHOP therapy.


