Abstract
The goal of the present study was to compare the efficacy of the combination of cetuximab and irinotecan to the combination of oxaliplatin and fluoropyrimidines as second‐line chemotherapy in patients with irinotecan‐refractory and oxaliplatin‐naïve metastatic colorectal cancer (mCRC) harboring wild‐type KRAS. The study included 120 patients with mCRC who had progressed after irinotecan‐containing first‐line chemotherapy and were never treated with oxaliplatin; 40 patients with wild‐type KRAS were accrued prospectively in the experimental arm (arm A), and 80 patients accrued retrospectively were divided into control arms B (n = 46) and C (n = 34) according to KRAS genotype. Second‐line treatments consisted of cetuximab plus irinotecan for arm A, and oxaliplatin plus either 5‐fluorouracil (FOLFOX) or capecitabine (CapeOX) for the control arms. The median progression‐free survival (PFS) was 8.3, 5.8 and 3.9 months, for arms A, B and C, respectively, with statistical significance favoring arm A (P = 0.007). Differences in overall survival did not reach statistical significance (18.3 vs 12.6 vs 12.9, P = 0.138), although there was a trend toward longer overall survival in arm A. In terms of benefit from oxaliplatin‐containing regimens either as second‐line or third‐line therapy, the median PFS was 5.0 months in arms B and C as second‐line therapy, and 4.0 months in arm A as third‐line therapy, with no statistical significance (P = 0.385). Second‐line cetuximab plus irinotecan is a valid treatment strategy for mCRC patients with irinotecan‐refractory and oxaliplatin‐naïve tumors harboring wild‐type KRAS. Oxaliplatin‐containing chemotherapy resulted in equivalent PFS both as a second‐line and a third‐line therapy, enabling delay of the administration of FOLFOX and CapeOX until subsequent treatment cycles.
At present, a limited number of active drugs are available for the treatment of metastatic colorectal cancer (mCRC), and the upfront doublet combination of fluoropyrimidines plus either oxaliplatin or irinotecan is regarded as the reference strategy for patients for whom intensive therapy is appropriate.1, 2, 3 Before the era of targeted agents, treatment strategies in terms of the combination or sequence of cytotoxic agents were rather simple; survival outcomes did not differ according to either the administration sequence of oxaliplatin or irinotecan, and whether sequential or combination chemotherapy was applied in the treatment continuum was inconsequential.4, 5, 6, 7 Treatment strategies have become more complicated in the era of targeted agents.
Bevacizumab plus chemotherapy as initial chemotherapy improved efficacy without significant adverse events and was proven effective as second‐line continuation beyond progression;8, 9, 10, 11, 12 however, limited overall survival (OS) benefit was observed when bevacizumab was combined with optimal doublet chemotherapy.13
As a first‐line treatment, cetuximab has shown OS benefit when combined with irinotecan plus infusional 5‐fluorouracil and leucovorin (FOLFIRI), and improved OS even in mCRC patients who failed all standard treatments.14, 15, 16 However, only patients whose tumors harbor wild‐type KRAS benefit from cetuximab, and no survival benefit was observed upon combination with oxaliplatin‐containing chemotherapy in large, randomized trials conducted recently.17, 18
Second‐line chemotherapy with oxaliplatin plus fluoropyrimidines is generally accepted as a strategy following first progression with irinotecan‐based chemotherapy in mCRC patients; however, the survival benefit from second‐line oxaliplatin‐based chemotherapy has not been satisfactory. Indeed, the use of cetuximab is appropriate with irinotecan‐based chemotherapy, and has an advantage in terms of overcoming irinotecan‐refractoriness; cetuximab plus irinotecan in patients who progressed after irinotecan‐containing chemotherapy has been proven more effective than cetuximab alone.19, 20 We recently reported promising efficacy in a prospective phase II study of second‐line cetuximab plus irinotecan in mCRC patients whose tumors were irinotecan‐refractory, oxaliplatin‐naïve and harboring wild‐type KRAS.21 Based on these results, the present study was designed to: (i) compare two different second‐line treatment strategies, cetuximab plus irinotecan versus oxaliplatin plus fluoropyrimidines, in patients with irinotecan‐refractory mCRC and oxaliplatin‐naïve mCRC patients; and (ii) to investigate whether the oxaliplatin plus fluoropyrimidines regimen could be delayed to third‐line therapy in the treatment continuum of these patients.
Patients and Methods
Patient population
Patients were divided into three arms. Arm A, the experimental arm, consisted of 40 patients who participated in a previous prospective multicenter study (NCT00637091),21 which was conducted between March 2008 and October 2009 and determined the feasibility of cetuximab plus irinotecan as second‐line chemotherapy after failure of irinotecan plus fluoropyrimidines in oxaliplatin‐naïve, wild‐type KRAS tumors with or without epidermal growth factor receptor (EGFR) expression (measured by immunohistochemistry). For the control arms, 199 patients who received oxaliplatin plus fluoropyrimidines as second‐line chemotherapy were screened during the same time period as the study described above, and 80 patients with the following matched variables were found eligible: (i) histologically confirmed adenocarcinoma; (ii) ≥1 measurable lesion(s) according to Response Evaluation Criteria In Solid Tumors (RECIST, version 1.0); (iii) progressive disease during or within 3 months after termination of irinotecan plus fluoropyrimidines with or without bevacizumab; (iv) oxaliplatin‐naïve; (v) age ≥20 years with life expectancy of ≥3 months; and (vi) adequate hematologic, hepatic and renal function. Patients were excluded from this analysis if they had undergone metastasectomy with curative intent; received upfront cetuximab or first‐line treatments other than irinotecan‐containing regimens; received second‐line treatments other than oxaliplatin‐containing regimens; or progressed beyond 3 months after termination of irinotecan‐containing first‐line chemotherapy. The 80 patients in the control arms were classified into two arms according to KRAS genotype: arm B consisted of patients with wild‐type KRAS tumors and arm C of patients with mutant KRAS tumors (Fig. 1). The survival outcomes and subsequent treatments for patients in arm A from our previous publication were updated, and the medical records of patients in arms B and C were retrospectively reviewed for this analysis. The study protocol was approved by the institutional review boards.
Figure 1.
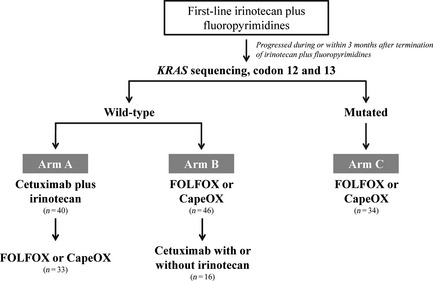
Patient population.
KRAS mutation analysis
For detecting KRAS mutations, tumor samples were obtained from paraffin‐embedded blocks of primary or metastatic sites. DNA was extracted from one to three sections of each tumor sample using a DEXPAT kit (TaKaRa, Kyoto, Japan). PCR amplification and direct DNA sequencing of KRAS exon 1 were performed as in Di Fiore et al.22 All PCR samples were subjected to automated sequencing using an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Study design and treatment
The purpose of this retrospective study was to compare two different second‐line treatment strategies in patients who progressed after a first‐line irinotecan‐containing regimen and were oxaliplatin‐naïve. Patients with wild‐type KRAS tumors were divided into different second‐line strategies: patients in arm A received cetuximab plus irinotecan, even after failure of irinotecan‐containing chemotherapy, with planned administration of oxaliplatin plus fluoropyrimidines as a third‐line therapy; patients in arm B received oxaliplatin plus fluoropyrimidines after first‐line failure of irinotecan‐containing chemotherapy, which is considered the reference strategy, with planned administration of third‐line cetuximab if possible; and patients in arm C, with mutant KRAS, received oxaliplatin plus fluoropyrimidines as second‐line chemotherapy. The primary endpoint was progression‐free survival (PFS) and the secondary endpoints were response rate, OS, and suitability of oxaliplatin plus fluoropyrimidines as a third‐line therapy, especially for patients in arm A.
For patients in arm A, second‐line treatment consisted of biweekly administration of cetuximab plus irinotecan (500 mg/m2 cetuximab and 180 mg/m2 irinotecan on day 1, as previously described).21 For patients in arms B and C, second‐line treatment consisted of oxaliplatin plus either 5‐fluorouracil or capecitabine, FOLFOX or CapeOX; FOLFOX consisted of 200 mg/m2 leucovorin on day 1, 400 mg/m2 5‐FU bolus infusion on day 1 and 2400 mg/m2 5‐FU continuous infusion for 46 h, with 85 mg/m2 oxaliplatin on day 1, repeated every 2 weeks. CapeOX consisted of 1000 mg/m2 capecitabine twice daily on days 1–14 and 130 mg/m2 oxaliplatin on day 1 and again every 3 weeks.
Assessments
Treatment responses were evaluated every 6 weeks (three cycles of 2‐week regimens or two cycles of 3‐week regimens) or earlier if disease progression was suspected. Objective tumor responses were assessed according to RECIST (version 1.0). OS was defined as the duration from the date of study treatment to death. PFS was defined as the time between the date of initiation of the study treatment and disease progression.
Statistical analysis
Patients in arm A, the experimental arm, were accrued prospectively and the results from our previous publication were updated.21 For patients in arms B and C, the control arms, medical records were reviewed retrospectively. Descriptive statistics are presented as proportions and median values. OS and PFS, along with 95% confidence interval (CI) for median time to event, were assessed using the Kaplan–Meier method. The hazard ratio (HR) or the odds ratio (OR) of clinical events and corresponding 95% CI were estimated using the Cox proportional hazards regression model or a linear regression model, respectively. All statistical analyses were performed using the spss software version 20.0 (IBM Statistics, Armonk, NY, USA).
Results
Patient characteristics
Between March 2008 and October 2009, 40 patients, among those who had completed a previous prospective multicenter phase II study of cetuximab plus irinotecan as a second‐line treatment and whose tumors were oxaliplatin‐naïve and harboring wild‐type KRAS, were enrolled in arm A.21 For the controls arms, 80 patients who were treated with second‐line oxaliplatin plus fluoropyrimidines during the same time period were accrued retrospectively and their medical records were reviewed: 46 patients with wild‐type KRAS tumors were included in arm B and 34 patients with mutant KRAS tumors were included in arm C (Fig. 1). Second‐line chemotherapy with oxaliplatin plus fluoropyrimidines included FOLFOX for 55 patients (with bevacizumab in eight patients) and CapeOX for 25 patients, for arms B and C, respectively. Patient characteristics are listed in Table 1. Most demographic variables, tumor characteristics and treatment outcomes from first‐line irinotecan‐based chemotherapy were similar between the arms.
Table 1.
Patient characteristics
| Arm A | Arm B | Arm C | P‐value | ||||
|---|---|---|---|---|---|---|---|
| n = 40 | % (100) | n = 46 | % (100) | n = 34 | % (100) | ||
| Gender | |||||||
| Male | 30 | 75.0 | 29 | 63.0 | 19 | 55.9 | 0.215 |
| Female | 10 | 25.0 | 17 | 37.0 | 15 | 44.1 | |
| Age, years (median) (range) | 55.5 (23–75) | 55.5 (26–73) | 54.0 (20–75) | 0.845 | |||
| ECOG PS | |||||||
| 0 | 7 | 17.5 | 11 | 23.9 | 11 | 32.4 | 0.239 |
| 1 | 33 | 82.5 | 33 | 71.7 | 23 | 67.6 | |
| 2 | 0 | 0 | 2 | 4.3 | 0 | 0 | |
| Primary site | |||||||
| Rectum | 21 | 52.5 | 23 | 50.0 | 14 | 41.2 | 0.599 |
| Colon | 19 | 47.5 | 23 | 50.0 | 20 | 58.8 | |
| Presentation of initial disease | |||||||
| Initial metastatic | 28 | 70.0 | 32 | 69.6 | 17 | 50.0 | 0.126 |
| Relapsed | 12 | 30.0 | 14 | 30.4 | 17 | 50.0 | |
| Tumour differentiation | |||||||
| Well differentiated | 5 | 12.5 | 4 | 8.7 | 5 | 14.7 | 0.319 |
| Moderately differentiated | 24 | 60.0 | 35 | 76.1 | 25 | 73.5 | |
| Poorly differentiated | 7 | 17.5 | 5 | 10.9 | 2 | 5.9 | |
| Mucinous/SRS | 4 | 10.0 | 0 | 0.0 | 1 | 2.9 | |
| Undetermined | 0 | 0.0 | 2 | 4.3 | 1 | 2.9 | |
| Site of metastasis | |||||||
| Liver | 27 | 67.5 | 31 | 67.4 | 27 | 79.4 | 0.430 |
| Lung | 18 | 45.0 | 15 | 32.6 | 18 | 52.9 | 0.177 |
| Lymph node | 25 | 62.5 | 32 | 69.6 | 27 | 79.4 | 0.285 |
| Peritoneum | 8 | 20.0 | 13 | 28.3 | 11 | 32.4 | 0.465 |
| Bone | 2 | 5.0 | 4 | 8.7 | 1 | 2.9 | 0.534 |
| Number of metastatic organs | |||||||
| 1 organ | 10 | 25.0 | 9 | 19.6 | 2 | 5.9 | 0.070 |
| 2 organs | 19 | 47.5 | 17 | 37.0 | 15 | 44.1 | |
| ≥3 organs | 11 | 27.5 | 20 | 43.5 | 17 | 50.0 | |
| First‐line chemotherapy | |||||||
| Duration of treatment (median) (range) | 6.7 (1.4–17.9) | 6.2 (1.4–18.9) | 6.0 (2.1–11.7) | 0.483 | |||
| Bevacizumab‐containing | 3 | 7.5 | 0 | 0.0 | 3 | 8.8 | 0.136 |
| ORR (95% CI) | 52.5% (37.0–68.0) | 52.2% (37.8–66.6) | 52.9% (36.1–69.7) | 1.000 | |||
| PFS‐1, months (95% CI) | 6.8 (6.4–7.2) | 7.5 (4.9–10.1) | 6.1 (5.1–7.2) | 0.253 | |||
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; ORR, overall response rate; PFS‐1, progression‐free survival of first‐line treatments; SRS, signet ring cell.
Efficacy of second‐line treatments
The efficacy of second‐line treatments is shown in Table 2 and Figure 2. After 15.3 months of median follow up (interquartile range: 9.7–24.5), the median PFS was 8.3 months (95% CI: 6.3–10.2) for arm A, 5.8 months (95% CI: 4.9–6.7) for arm B and 3.9 months (95% CI: 2.1–5.8) for arm C, respectively, and there was statistical significance favoring arm A (P = 0.007, Fig. 2A). The PFS benefit favoring arm A was maintained when compared to arms B and C together: the median PFS was 8.3 months for arm A and 5.0 months (95% CI: 3.8–6.1) for arms B and C (HR 1.67 [1.10–2.55], P = 0.015, Fig. 2B). The median OS was 18.3 months (95% CI: 15.2–21.5) for arm A, 12.6 months (95% CI: 10.1–15.0) for arm B and 12.9 months (95% CI: 10.6–15.2) for arm C, respectively, and there was no statistical significance (P = 0.138, Fig. 2C,D). The overall response rate (ORR) was 45.0% (95% CI: 29.6–60.4) with three complete responses and 15 partial responses (PRs) for arm A, 28.8% (95% CI: 18.8–38.7) with 23 PRs for arms B and C, respectively, and there was no statistical significance (OR 2.03 [0.92–4.46], P = 0.077).
Table 2.
Efficacy of second‐line treatments
| Arm A (n = 40) | Arm B (n = 46) | Arm C (n = 34) | ||||
|---|---|---|---|---|---|---|
| Treatment response | ||||||
| CR | 3 | 7.5% | 0 | 0.0% | 0 | 0.0% |
| PR | 15 | 37.5% | 14 | 30.4% | 9 | 26.5% |
| SD | 15 | 37.5% | 26 | 56.5% | 13 | 38.2% |
| PD | 7 | 17.5% | 6 | 13.0% | 11 | 32.4% |
| NA | 0 | 0.0% | 0 | 0.0% | 1 | 2.9% |
| ORR (95% CI) | ||||||
| A vs B vs C | 45.0% (29.6–60.4) | 30.4% (17.1–43.7) | 26.5% (11.7–41.3) | |||
| P = 0.195 | ||||||
| A vs B + C | 45.0% (29.6–60.4) | 28.8% (18.9–38.7) | ||||
| OR 2.03 (0.92–4.46), P = 0.077 | ||||||
| PFS, months (median) (95% CI) | ||||||
| A vs B vs C | 8.3 (6.3–10.2) | 5.8 (4.9–6.7) | 3.9 (2.1–5.8) | |||
| HR (95% CI) | 1 | 1.41 (0.88–2.26) | 2.19 (1.33–3.59) | |||
| P = 0.007 | ||||||
| A vs B + C | 8.3 (6.3–10.2) | 5.0 (3.8–6.1) | ||||
| HR (95% CI) | 1 | 1.67 (1.10–2.55) | ||||
| P = 0.015 | ||||||
| OS, months (median) (95% CI) | ||||||
| A vs B vs C | 18.3 (15.2–21.5) | 12.6 (10.1–15.0) | 12.9 (10.6–15.2) | |||
| HR (95% CI) | 1 | 1.27 (0.79–2.04) | 1.65 (1.00–2.72) | |||
| P = 0.138 | ||||||
| A vs B + C | 18.3 (15.2–21.5) | 12.7 (10.5–15.0) | ||||
| HR (95% CI) | 1 | 1.15 (0.93–2.16) | ||||
| P = 0.101 | ||||||
CI, confidence interval; CR, complete response; HR, hazard ratio; OR, odds ratio; ORR, overall response rate; OS, overall survival; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Figure 2.
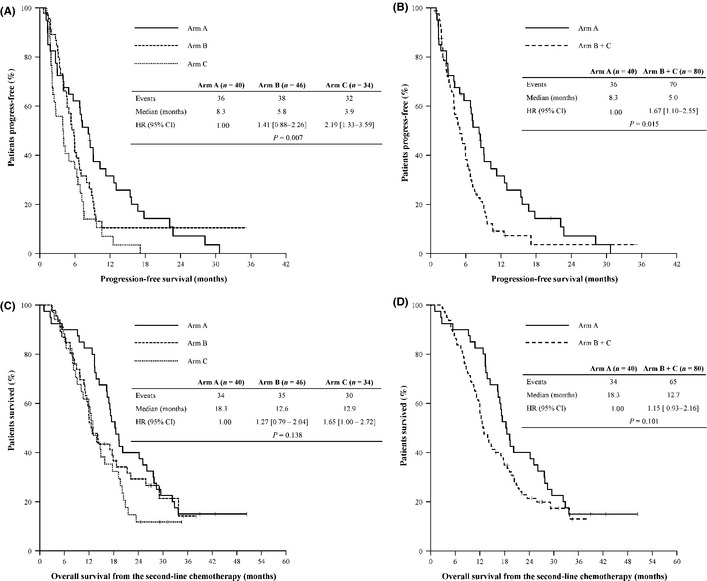
Efficacy of second‐line treatments. Comparison of progression‐free survival between the three arms (A) and between arm A and B + C (B), and comparison of overall survival between the three arms (C) and between arm A and B + C (D). CI, confidence interval; HR, hazard ratio.
Adverse events
The significant adverse events are listed in Table 3. Grade 3 or 4 hematologic adverse events were not statistically different between groups; however, a trend toward a higher incidence of leukopenia (2.5% vs 12.5%, P = 0.097), neutropenia (12.5% vs 26.3%, P = 0.103) and thrombocytopenia (0% vs 5.0%, P = 0.299) was observed in patients who received FOLFOX or CapeOX. Non‐hematologic adverse events were similar between arms except acneiform rash, which occurred only in patients treated with cetuximab plus irinotecan (10.0% vs 0%, P = 0.011).
Table 3.
Comparison of significant adverse events from second‐line treatments
| Grade 3 or 4 | Arm A | Arm B + C | P‐valuea | ||
|---|---|---|---|---|---|
| (n = 40) | % (100) | (n = 80) | % (100) | ||
| Hematologic AE | |||||
| Anemia | 0 | 0.0 | 3 | 3.8 | 0.550 |
| Leukopenia | 1 | 2.5 | 10 | 12.5 | 0.097 |
| Neutropenia | 5 | 12.5 | 21 | 26.3 | 0.103 |
| Febrile neutropenia | 2 | 5.0 | 2 | 2.5 | 0.599 |
| Thrombocytopenia | 0 | 0.0 | 4 | 5.0 | 0.299 |
| Non‐hematologic AE | |||||
| Asthenia | 2 | 5.0 | 0 | 0.0 | 0.109 |
| Nausea | 2 | 5.0 | 2 | 2.5 | 0.599 |
| Vomiting | 1 | 2.5 | 0 | 0.0 | 0.333 |
| Diarrhoea | 2 | 5.0 | 1 | 1.3 | 0.257 |
| Allergic reaction | 1 | 2.5 | 0 | 0.0 | 0.333 |
| Acneiform rash | 4 | 10.0 | 0 | 0.0 | 0.011 |
| Nail toxicity or HFS | 1 | 2.5 | 1 | 1.3 | 1.000 |
| Sensory neuropathy | 0 | 0.0 | 3 | 3.8 | 0.550 |
P‐values by Fisher's exact test. AE, adverse events; HFS, hand–foot syndrome.
Efficacy of third‐line treatments
Of the patients, 49 out of 120 received third‐line chemotherapy; 33 patients (82.5%) in arm A received oxaliplatin plus fluoropyrimidines, and 16 patients (34.8%) in arm B received either cetuximab plus irinotecan (12 patients) or cetuximab monotherapy (four patients) as third‐line therapy (Table 4). Patients in arm C did not receive any third‐line treatment. The ORR was 9.1% (95% CI: 0.0–18.9) in arm A and 18.8% in arm B. The median PFS (PFS‐3) was 4.0 months (95% CI: 3.2–4.8) for arm A and 2.5 months (95% CI: 0.0–5.5) for arm B, and the median OS from the third‐line treatment was 11.7 months (95% CI: 8.0–15.4) for arm A and 6.0 months (95% CI: 4.8–7.2) for arm B (P = 0.398). The benefit from oxaliplatin plus fluoropyrimidines in terms of PFS was preserved regardless of the timing of administration: the median PFS was 5.0 months (95% CI: 3.8–6.1) in arms B and C as a second‐line therapy, and 4.0 months (95% CI: 3.2–4.8) in arm A as a third‐line therapy, with no statistical significance (HR 0.83 [0.54–1.28], P = 0.385, Table 5, Fig. 3). However, the benefit from oxaliplatin‐based chemotherapy in terms of ORR was higher when oxaliplatin was administered as a second‐line therapy (28.8%) than as a third‐line therapy (9.1%) with an OR of 0.20 (95% CI: 0.06–0.72, P = 0.009).
Table 4.
Efficacy of third‐line treatments
| Arm A (n = 40) | Arm B (n = 46) | Arm C (n = 34) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Third‐line chemotherapy | |||||||||
| None | 7 | 17.5% | 30 | 65.2% | 34 | 100% | |||
| FOLFOX | 17 | 42.5% | — | — | |||||
| CapeOX | 16 | 40.0% | — | — | |||||
| Cetuximab plus irinotecan | — | 12 | 26.1% | — | |||||
| Cetuximab monotherapy | — | 4 | 8.7% | — | |||||
| Treatment response | (n = 33) | (n = 16) | |||||||
| CR | 0 | 0.0% | 0 | 0.0% | — | ||||
| PR | 3 | 9.1% | 3 | 18.8% | — | ||||
| SD | 17 | 51.5% | 7 | 43.8% | — | ||||
| PD | 10 | 30.3% | 6 | 37.5% | — | ||||
| NA | 3 | 9.1% | 0 | 0.0% | — | ||||
| ORR (95% CI) | 9.1% (0.0–18.9) | 18.8% (0.0–37.9) | — | ||||||
| PFS‐3, median (95% CI) | 4.0 months (3.2–4.8) | 2.5 months (0.0–5.5) | — | ||||||
| OS‐3, median (95% CI) | 11.7 months (8.0–15.4) | 6.0 months (4.8–7.2) | — | ||||||
CapeOX, oxaliplatin/capecitabine; CI, confidence interval; CR, complete response; FOLFOX, oxaliplatin/5‐fluorouracil/leucovorin; NA, response evaluation was not available; ORR, overall response rate; OS‐3, overall survival from third‐line treatments; PD, progressive disease; PFS‐3, progression‐free survival from third‐line treatments; PR, partial response; SD, stable disease; –, not applicable.
Table 5.
Comparison of the efficacy of oxaliplatin plus fluoropyrimidines as second‐line and third‐line treatment
| Third‐line in arm A (n = 33) | Second‐line in arms B and C (n = 80) | |||
|---|---|---|---|---|
| Treatment response | ||||
| CR | 0 | 0.0% | 0 | 0.0% |
| PR | 3 | 9.1% | 23 | 28.8% |
| SD | 17 | 51.5% | 39 | 48.8% |
| PD | 10 | 30.3% | 17 | 21.3% |
| NA | 3 | 9.1% | 1 | 1.3% |
| ORR (95% CI) | 9.1% (0.0–18.9) | 28.8% (18.9–38.7) | ||
| OR 2.01 (0.06–0.72), P = 0.009 | ||||
| PFS‐OX, months (median) (95% CI) | 4.0 months (3.2–4.8) | 5.0 months (3.8–6.1) | ||
| HR 0.83 (0.54–1.28), P = 0.385 | ||||
CI, confidence interval; CR, complete response; NA, response evaluation was not available; ORR, overall response rate; PD, progressive disease; PFS‐OX, progression‐free survival from oxaliplatin‐containing chemotherapy; PR, partial response; SD, stable disease.
Figure 3.
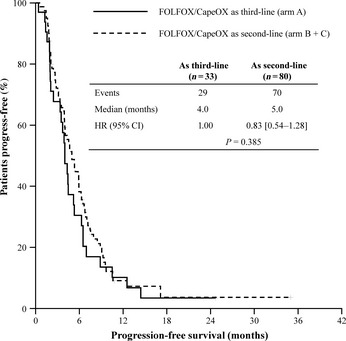
Progression‐free survival benefit from oxaliplatin plus fluoropyrimidines and comparison between administrations as second‐line and third‐line treatment progression‐free survival.
Univariate and multivariate analyses for overall survival
Univariate and multivariate analyses using the Cox regression method are listed in Table 6. In the univariate analysis, treatment response in the first‐line, PFS and treatment responses both in the second‐line and third‐line treatments were significant factors for better OS. In the multivariate analysis, however, PFS improvements in the second‐line or third‐line treatments were independent factors for better OS.
Table 6.
Cox regression analysis for overall survival from study treatments (included patients with wild‐type KRAS only, arms A and B)
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Age | ||||
| <60 | 1 | 0.140 | — | — |
| ≥60 | 0.67 (0.39–1.14) | — | — | |
| Gender | ||||
| Female | 1 | 0.642 | — | — |
| Male | 1.13 (0.67–1.92) | — | — | |
| ECOG performance status | ||||
| 0 | 1 | 0.216 | — | — |
| ≥1 | 0.69 (0.38–1.24) | — | — | |
| Number of metastatic organs | ||||
| 1 | 1 | 0.433 | — | — |
| ≥2 | 1.27 (0.70–2.23) | — | — | |
| First‐line PFS (months) | ||||
| ≤6 | 1 | 0.156 | — | — |
| >6 | 0.71 (0.44–1.14) | — | — | |
| First‐line responses | ||||
| CR/PR | 1 | 0.049 | 1 | 0.895 |
| SD/PD | 1.61 (1.00–2.60) | 0.96 (0.50–1.84) | ||
| Second‐line PFS (months) | ||||
| ≤4 | 1 | <0.001 | 1 | <0.001 |
| >4 | 0.19 (0.11–0.33) | 0.16 (0.07–0.36) | ||
| Second‐line responses | ||||
| CR/PR | 1 | 0.002 | 1 | 0.659 |
| SD/PD | 2.23 (1.33–3.74) | 1.19 (0.55–2.55) | ||
| Third‐line PFS (months) | ||||
| ≤3 | 1 | 0.007 | 1 | 0.006 |
| >3 | 0.43 (0.23–0.79) | 0.40 (0.21–0.77) | ||
| Third‐line responses | ||||
| CR/PR | 1 | 0.027 | 1 | 0.480 |
| SD/PD | 3.81 (1.16–12.5) | 1.68 (0.40–7.09) | ||
| Exposed to cetuximab during treatment continuum | ||||
| Yes | 1 | 0.744 | — | — |
| No | 1.10 (0.61–1.99) | — | — | |
CI, confidence interval; CR, complete response; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; PD, progressive disease; PFS, progression‐free survival; PR, partial response; SD, stable disease; –, not included in the multivariate analysis.
Discussion
Second‐line cetuximab plus irinotecan showed longer PFS (8.3 vs 5.8 months, HR 1.41 [0.88–2.26], P = 0.007) than FOLFOX or CapeOX in patients with oxaliplatin‐naïve, wild‐type KRAS mCRC who progressed after first‐line irinotecan plus fluoropyrimidines. There was a trend toward higher ORR (45.0% vs 28.8%) and longer OS (18.3 vs 12.7 months) upon administration of second‐line cetuximab plus irinotecan, although without statistical significance. The PFS of FOLFOX and CapeOX were similar whether these regimens were administered as second‐line or third‐line treatments.
The addition of targeted agents to the doublet combination chemotherapy of fluoropyrimidines with either irinotecan or oxaliplatin is now regarded as standard of care in mCRC. In the first‐line setting, the targeted agents can easily be selected: bevacizumab improves efficacy regardless of the cytotoxic agent with which it is combined,9, 10, 11, 13 and cetuximab improves overall efficacy, including OS, when combined with first‐line FOLFIRI.14, 15 Panitumumab plus FOLFOX also improves PFS in these patients.23 However, the selection of targeted agents is more difficult in second‐line therapy.
Cetuximab with or without irinotecan‐based chemotherapy is frequently considered in the second‐line setting or later in the treatment continuum,19, 24 and recent studies demonstrate that the addition of aflibercept25 or panitumumab26 to second‐line FOLFIRI is reasonable in mCRC that has progressed after first‐line oxaliplatin plus fluoropyrimidines. On the basis of the present results, patients who have progressed after first‐line FOLFOX or CapeOX with or without bevacizumab still have reasonable choices of targeted agents as a second‐line treatment.
However, the selection of targeted agents, especially for patients who have progressed after FOLFIRI with or without targeted agents and for patients for whom second‐line FOLFOX or CapeOX was planned, is now difficult because cetuximab is no longer recommended in combination with oxaliplatin‐based chemotherapy17, 18 and because aflibercept and panitumumab have been proven effective as a second‐line therapy only when combined with FOLFIRI.25, 26
The present study involved patients who became refractory to first‐line irinotecan plus fluoropyrimidines. Second‐line treatment strategies were compared according to KRAS genotype: patients with mutant KRAS followed crossover cytotoxic doublet regimens of FOLFOX or CapeOX, and those with wild‐type KRAS followed second‐line cetuximab plus irinotecan or FOLFOX or CapeOX regimens. Second‐line cetuximab plus irinotecan improved efficacy compared to crossover FOLFOX or CapeOX, even in patients who progressed after irinotecan‐based regimens, and the efficacy of third‐line FOLFOX or CapeOX was comparable to that of second‐line FOLFOX or CapeOX. Furthermore, the efficacy of second‐line cetuximab plus irinotecan in the present study (ORR 45.0%, PFS 8.3 months) was very promising compared to that in previous studies involving second‐line aflibercept (RR 19.8%, PFS 6.9 months)25 or panitumumab (RR 35%, PFS 5.9 months).26
In the present study, the median OS from the second‐line treatments was 18.3 months for arm A, 12.6 months for arm B and 12.9 months for arm C, respectively, and there was a trend favoring arm A without statistical significance (P = 0.138, Fig. 2C,D). The different proportions between arms of patients who could be treated with third‐line treatments might act as one of the confounding factors for the longer OS favoring arm A; of note, more patients in arm A (82.5%) entered into the third‐line treatments with compared to those in arm B (34.8%). A recent trial of first‐line chemotherapy in Korean patients showed that the proportion of patients who could receive subsequent treatments was relatively limited; approximately 60% of patients could enter into second‐line treatments, and only around 18% of patients could enter into third‐line treatments.27 The ML18147 study, which studied bevacizumab continuation as second‐line chemotherapy, also demonstrated that only 39.8% of patients could receive third‐line treatment with either cetuximab or panitumumab, regardless of KRAS status.12 Therefore, the proportion of patients (34.8%) who could receive third‐line treatment with cetuximab‐based chemotherapy in arm B is comparable with results reported in previous publications. In fact, the OS from the third‐line treatment was 11.7 months for arm A and 6.0 months for arm B, and these differences might contribute to the trend favoring arm A in terms of OS.
Anti‐EGFR agents offer survival benefits in patients with wild‐type KRAS later in the treatment continuum,16, 28 and some authors point out that the absolute benefit of OS is not different between first‐line and subsequent administrations of cetuximab.29 Recently, another targeted agent, regorafenib, was approved by US FDA on September 27, 2012 for mCRC patients who have failed all standard treatments.30 Because regorafenib improved PFS and OS in these heavily‐treated patients, earlier administration of cetuximab may be a reasonable strategy in the treatment continuum.
The present study has limitations, including a small patient population and the retrospective design of the control arms, in which patients were treated with FOLFOX or CapeOX, although the experimental arm, in which patients were treated with cetuximab plus irinotecan, was accrued prospectively. In addition, only a few patients could be treated with bevacizumab either as a first‐line therapy in the experimental arm or as a second‐line therapy in the control arm, representing another limitation of the study in terms of comparison of survival outcome after optimized treatment continuum. Due to cost, only a small number of patients were treated with bevacizumab; bevacizumab (and other targeted agents) is yet to be covered by National Insurance; thus, most mCRC patients cannot afford to include targeted agents in their treatment. Of note, the number of patients in arm B who were treated with anti‐EGFR agents as their third‐line treatment was rather small; only 34.8% (16/46) of patients received cetuximab‐based chemotherapy and this might be the main reason for similar OS between arms B and C.
In conclusion, cetuximab plus irinotecan as second‐line chemotherapy could be an alternative treatment option for irinotecan‐refractory and oxaliplatin‐naïve mCRC with wild‐type KRAS. Delayed administration of crossover oxaliplatin‐based chemotherapy as a third‐line treatment did not impair efficacy. KRAS status should be considered when selecting treatment with targeted agents or when deciding on a sequence of cytotoxic agents.
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare (A102059), and by a grant from the Covering Research Center Program, Ministry of Education, Science and Technology (2011K000871), Korea.
Disclosure Statement
The authors have no conflict of interest to declare.
(Cancer Sci 2013; 104: 473–480)
References
- 1. Hoff PM, Ansari R, Batist G et al Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first‐line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 2001; 19: 2282–92. [DOI] [PubMed] [Google Scholar]
- 2. de Gramont A, Figer A, Seymour M et al Leucovorin and fluorouracil with or without oxaliplatin as first‐line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938–47. [DOI] [PubMed] [Google Scholar]
- 3. Douillard JY, Cunningham D, Roth AD et al Irinotecan combined with fluorouracil compared with fluorouracil alone as first‐line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000; 355: 1041–7. [DOI] [PubMed] [Google Scholar]
- 4. Koopman M, Antonini NF, Douma J et al Randomised study of sequential versus combination chemotherapy with capecitabine, irinotecan and oxaliplatin in advanced colorectal cancer, an interim safety analysis. A Dutch Colorectal Cancer Group (DCCG) Phase III Study. Ann Oncol 2006; 17: 1523–8. [DOI] [PubMed] [Google Scholar]
- 5. Seymour MT, Maughan TS, Ledermann JA et al Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet 2007; 370: 143–52. [DOI] [PubMed] [Google Scholar]
- 6. Seymour MT, Thompson LC, Wasan HS et al Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open‐label, randomised factorial trial. Lancet 2011; 377: 1749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tournigand C, Andre T, Achille E et al FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004; 22: 229–37. [DOI] [PubMed] [Google Scholar]
- 8. Grothey A, Sugrue MM, Purdie DM et al Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 2008; 26: 5326–34. [DOI] [PubMed] [Google Scholar]
- 9. Hurwitz H, Fehrenbacher L, Novotny W et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–42. [DOI] [PubMed] [Google Scholar]
- 10. Tebbutt NC, Wilson K, Gebski VJ et al Capecitabine, bevacizumab, and mitomycin in first‐line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol 2010; 28: 3191–8. [DOI] [PubMed] [Google Scholar]
- 11. Van Cutsem E, Rivera F, Berry S et al Safety and efficacy of first‐line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 2009; 20: 1842–7. [DOI] [PubMed] [Google Scholar]
- 12. Bennouna J, Sastre J, Arnold D et al Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013; 14: 29–37. [DOI] [PubMed] [Google Scholar]
- 13. Saltz LB, Clarke S, Diaz‐Rubio E et al Bevacizumab in combination with oxaliplatin‐based chemotherapy as first‐line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008; 26: 2013–9. [DOI] [PubMed] [Google Scholar]
- 14. Van Cutsem E, Kohne CH, Hitre E et al Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009; 360: 1408–17. [DOI] [PubMed] [Google Scholar]
- 15. Van Cutsem E, Kohne CH, Lang I et al Cetuximab plus irinotecan, fluorouracil, and leucovorin as first‐line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011; 29: 2011–9. [DOI] [PubMed] [Google Scholar]
- 16. Karapetis CS, Khambata‐Ford S, Jonker DJ et al K‐ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359: 1757–65. [DOI] [PubMed] [Google Scholar]
- 17. Maughan TS, Adams RA, Smith CG et al Addition of cetuximab to oxaliplatin‐based first‐line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011; 377: 2103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tveit KM, Guren T, Glimelius B et al Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first‐line treatment of metastatic colorectal cancer: the NORDIC‐VII study. J Clin Oncol 2012; 30: 1755–62. [DOI] [PubMed] [Google Scholar]
- 19. Cunningham D, Humblet Y, Siena S et al Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan‐refractory metastatic colorectal cancer. N Engl J Med 2004; 351: 337–45. [DOI] [PubMed] [Google Scholar]
- 20. Wilke H, Glynne‐Jones R, Thaler J et al Cetuximab plus irinotecan in heavily pretreated metastatic colorectal cancer progressing on irinotecan: MABEL study. J Clin Oncol 2008; 26: 5335–43. [DOI] [PubMed] [Google Scholar]
- 21. Kang MJ, Hong YS, Kim KP et al Biweekly cetuximab plus irinotecan as second‐line chemotherapy for patients with irinotecan‐refractory and KRAS wild‐type metastatic colorectal cancer according to epidermal growth factor receptor expression status. Invest New Drugs 2012; 30: 1607–13. [DOI] [PubMed] [Google Scholar]
- 22. Di Fiore F, Blanchard F, Charbonnier F et al Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer 2007; 96: 1166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Douillard JY, Siena S, Cassidy J et al Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first‐line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010; 28: 4697–705. [DOI] [PubMed] [Google Scholar]
- 24. Sobrero AF, Maurel J, Fehrenbacher L et al EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26: 2311–9. [DOI] [PubMed] [Google Scholar]
- 25. Van Cutsem E, Tabernero J, Lakomy R et al Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin‐based regimen. J Clin Oncol 2012; 30: 3499–506. [DOI] [PubMed] [Google Scholar]
- 26. Peeters M, Price TJ, Cervantes A et al Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second‐line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010; 28: 4706–13. [DOI] [PubMed] [Google Scholar]
- 27. Hong YS, Park YS, Lim HY et al S‐1 plus oxaliplatin versus capecitabine plus oxaliplatin for first‐line treatment of patients with metastatic colorectal cancer: a randomised, non‐inferiority phase 3 trial. Lancet Oncol 2012; 13: 1125–32. [DOI] [PubMed] [Google Scholar]
- 28. Van Cutsem E, Peeters M, Siena S et al Open‐label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy‐refractory metastatic colorectal cancer. J Clin Oncol 2007; 25: 1658–64. [DOI] [PubMed] [Google Scholar]
- 29. Rother MJ. Survival benefit from up‐front fluorouracil, leucovorin, and irinotecan/cetuximab in metastatic colorectal cancer: is it just a now‐or‐never result? J Clin Oncol 2011; 29: 4207. [DOI] [PubMed] [Google Scholar]
- 30. Grothey A, Van Cutsem E, Sobrero A et al Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo‐controlled, phase 3 trial. Lancet 2013; 381: 303–12. [DOI] [PubMed] [Google Scholar]


