Abstract
The current criteria for evaluating antiangiogenic efficacy is insufficient as tumor shrinkage occurs after blood perfusion decreases. Tumor blood volume (BV) in computed tomography perfusion imaging and circulating endothelial cells (CEC) might predict the status of angiogenesis. The present study aimed to validate their representation as feasible predictors in non‐small‐cell lung carcinoma (NSCLC). A total of 74 patients was categorized randomly into two arms undergoing regimens of vinorelbine and cisplatin (Navelbine and platinum [NP]) with rh‐endostatin or single NP. The response rate, perfusion imaging indexes and activated CEC (aCEC) during treatment were recorded. Progression‐free survival (PFS) was determined through follow up. Correlations among the above indicators, response and PFS were analyzed: aCEC increased significantly in cases of progressive disease after single NP chemotherapy (P = 0.024). Tumor BV decreased significantly in cases with a clinical benefit in the combined arm (P = 0.026), whereas inverse correlations existed between ∆aCEC (post‐therapeutic value minus the pre‐therapeutic value) and PFS (P = 0.005) and between ∆BV and PFS (P = 0.044); a positive correlation existed between ∆aCEC and ∆BV. Therefore, both aCEC and tumor BV can serve as predictors, and detection of both indicators can help evaluate the chemo‐antiangiogenic efficacy in NSCLC more accurately.
As lung cancer is a malignancy with the highest morbidity and mortality,1 in most cases patients receive chemotherapy on diagnosis because most tumors. However, achieving better efficacy by increasing the dosage is not realistic because of the intolerable toxicity and recurrent resistance to chemotherapy.
Since Folkman2, 3 first proposed the concept of tumoral angiogenesis, it has been proven as the foundation of tumor progression, recurrence and metastasis. Therefore, blockage of tumor angiogenesis prohibits the development and progression of tumor. Antiangiogenic reagents, which mainly include inhibitors of vascular endothelial growth factor (VEGF) and its receptor (VEGFR) or matrix metalloproteinase (MMP) and endostatin, have been introduced clinically, including bevacizumab and rh‐endostatin.4, 5 The former is a monoclonal antibody against VEGF and the latter is the unique human recombinant endostatin approved for clinical administration after Chinese scientists remodeled endostatin to improve its stability.6 They have been proven to prolong the progression‐free survival (PFS) and survival of patients with non‐small‐cell lung cancer (NSCLC).6, 7, 9 However, the antiangiogenic therapeutic efficacy remains doubtful because of its defects, including a short therapeutic period less than three cycles without maintenance in clinical trials in China.8, 9 Moreover, clinical studies have shown that only some patients benefit from antiangiogenesis, suggesting it is difficult to define the optimal patient population for treatment and predict the efficacy beforehand. This is accounted for by the fact that antiangiogenic agents mainly effect the micrangium rather than tumor volume per se, and such an effect could cause an early diversified response of neoplastic cells including depression or even activation without a visible change in tumor volume.10 Nevertheless, current efficacy assessment criteria set by the World Health Organization (WHO) and Response Evaluation Criteria in Solid Tumors (RECIST) are both based on shrinkage or expansion of the tumor,11 so that the status of angiogenesis in a tumor cannot be gauged as early as possible to determine whether to continue or stop antiangiogenic treatment. Specific methods are urgently needed to screen for the optimal patient population for antiangiogenic treatment and to predict its efficacy in order to enable doctors to avoid insufficient or excess treatment.
Therefore, we have assessed levels of tumoral angiogenic factors (TAF) such as VEGF, basic fibroblast growth factor (bFGF) and platelet derived growth factor during treatment. However, no significant correlation was observed between changes of TAF levels and efficacy.12 This is probably due to the easy degradation of such factors in the blood as well as antagonism by many endogenous inhibitors of angiogenesis, so that changes of only a few TAF cannot possibly reflect the global status of angiogenesis. Therefore, attention was shifted to the downstream target cells of TAF and antiangiogenic therapy, the endothelial cells of micrangium, which can indicate the angiogenic status and antiangiogenic effect more directly.13, 14 Circulating endothelial cells (CEC), which mainly consist of endothelial progenitor cells (EPC) mobilized by TAF from the bone marrow and endothelial cells shed from the walls of blood vessels, are rarely found in the blood of a healthy body, but increase dramatically during tumor progression where they play an essential role in tumor angiogenesis.15 Circulating endothelial cells also decrease significantly after effective chemotherapy or tumor resection.16 Apparently, changes in CEC levels could reflect development and suppression of angiogenesis more directly. Chu et al.17 has reported that a decrease in CEC after paclitaxel and carboplatin regimen with rh‐endostatin treatment was closely associated with longer PFS and survival even though CEC were observed within only 6 weeks. Similarly, doubts on CEC remain since CEC could fluctuate vigorously because of various factors, especially during early treatment, until the final moment when tumor growth is suppressed by longer therapy.
A combination of computed tomography (CT) and computer software enables a functional and morphological diagnosis, with a typical sample being multiple slice computed tomography (MSCT) perfusion.18, 19 The CT perfusion examination detects blood perfusion in vivo with a repeated contrast scan in the region of interest to obtain a time density curve in pixels from the slices. Blood flow (BF), blood volume (BV), mean transit time (MTT) and permeability of the capillary vessel surface (PS) can then be calculated based on the curves using appropriate mathematical models, thereby assessing quantitatively the blood perfusion status of the organ to give diagnosis and evaluation of efficacy. It has been reported that metastatic renal carcinoma cases with higher basal data can benefit more significantly from antiangiogenic therapy with a conspicuous decline in BV and BF.20 The declines of BV in the therapeutic arm were compared with the control arm, and longer overall survival (OS) was achieved in cases with a >50% BV decline than with a <50% BV decline (20 vs 13 months).
Although these two kinds of indicators offer expectation of continuous assessment of blood supply status in tumors and prediction of antiangiogenic efficacy, only a few studies have been conducted regarding the pattern of their changes during antiangiogenic therapy, the correlation between them and the correlation with efficacy. Simultaneously, to redeem the deficiency in previous studies that did not have enough therapy, longer antiangiogenic administration should be performed after tumor response. Therefore, we investigated more sensitive and reliable approaches to forecast antiangiogenic efficacy through measurement of pre‐ and post‐therapeutic CEC and blood perfusion data in a trial of multiple‐cycle chemotherapy with rh‐endostatin in order to find a therapeutic effect in longer therapy and to discover more reliable data on changes in CEC and blood perfusion indexes.
Material and Methods
Patients
A prospective randomized and two‐arm controlled study was conducted at the Cancer Institute and Hospital of Tianjin Medical University from August 2007 to March 2011. The designated number of subjects was 150, 75 for each arm. The primary end‐point of the trial was PFS. Secondary end‐points included response rate (RR), toxicity and quality of life. To search for potential predictors of efficacy, we planned to observe a dynamic change in CEC and perfusion image data of BF, BV, MTT and PS in tumors during the trial as an exploratory end‐point. Stratification factors were defined as CEC, BF, BV, MTT and PS because other factors had been analyzed in previous studies.8, 9 Each patient was required to meet the following criteria: (i) age range of 18–75 years; (ii) stage IIIb, IV NSCLC with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; (iii) naïve to endostatin and chemotherapy or with previous chemotherapy of only a single drug other than vinorelbine and cisplatin if completed ≥4 weeks before enrollment and recovery from previous chemotherapeutic toxicity; (iv) at least one lesion with measurable diameters of ≥10 mm as identified by span CT; (v) no pre‐existing cardiovascular conditions, such as symptomatic congestive heart failure, unstable angina pectoris or cardiac arrhythmia; (vi) no history of gross hemoptysis; (vii) no concomitant diseases such as ischemic heart disease, systemic vasculitis, pulmonary hypertension or serious complications such as infectious disease or diabetes; (viii) no known uncontrolled central nervous system metastases; (ix) no contraindications for chemotherapy, that is, WBC ≥4.0 × 109/L, PLT ≥80 × 109/L, Hb ≥90 g/L; Cr ≤2.0 × UNL; BIL ≤2.0 × UNL, ALT/AST ≤5.0 × UNL; and (x) awareness and signed informed consent. The Committee of Tianjin Medical University Cancer Institute and Hospital approved the study (approval number E2007012.1).
Therapy schedule
Patients were randomized into combined or single chemotherapy arms. In these two groups, regimens were based on the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines on Oncology (2007) as Navelbine and platinum (NP) (25 mg/m2 vinorelbine, days 1, 8; 25 mg/m2 cisplatin, days 2–4) with or without Rh‐endostatin (National Medicine Permit No.: S20080307, 7.5 mg/m2, i.v., days 1–14; Simcere Pharmaceutical Group, Jiangsu, China). Treatment was administered in both arms every 3–4 weeks until patients met the criteria for progressive disease (RECIST criteria), experienced unacceptable toxicity or achieved six therapeutic cycles.
Evaluation of efficacy using CT scans
Enhanced CT scans were performed prior to the first chemotherapy administration and every two cycles during the therapeutic regimen, or at any time during therapy when necessary, and after completion of the therapy. Efficacy was evaluated using a CT scan at least every two cycles according to the RECIST criteria as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Confirmation for responders was done 4 weeks later. The PFS, which is defined as the moment from the randomization of patients to the end of treatment for any reason, was also recorded. The RR was calculated as (CR + PR)/total × 100%. The clinical benefit rate (CBR) was calculated as (CR + PR + SD)/total × 100%.
Sample assay for activated circulating endothelial cells (aCEC)
Blood samples were obtained 2 days before the beginning of each therapeutic cycle and the last blood sample was collected on the 8th day after completion of the last cycle. All blood samples were anticoagulated with EDTA, stored at 4°C and processed within 36 h after collection.
Flow cytometry was used to determine the aCEC (CD45−CD146+CD105+).20, 21, 23 All antibodies were purchased from Beckman Coulter (Los Angeles, CA, USA), except for CD105, which was purchased from Chemicon (Billerica, MA, USA). Whole anticoagulated peripheral blood (100 μL) was added in the isotype control tube and incubated for 30 min in the dark with 10 μL each of the following basic combinations of fluorescein isothiocyanate (FITC), phycoerythrin (PE) and phycoerythrin cyanine 5 (PE‐Cy5) IgG1 isotype control mouse antibodies. The same procedure was performed in the test tube incubated for 30 min in the dark with 10 μL each of CD45‐PE‐Cy5, CD146‐PE and CD105‐FITC antibodies, respectively. After incubation, the red blood cells were lysed with lysis solution A (Beckman Coulter) for 30 s and gently vortexed. Lysis solution B was then added for 10 s and gently vortexed. The cells were washed thrice in phosphate‐buffered saline using centrifugation. Using the forward scatter/side scatter (FS/SS) gating strategy, acquisition was performed using flow cytometry (EPICS‐XL; Beckman Coulter) equipped with a 488 nm argon ion laser. A minimum of 100 000 events were collected for each sample.
All cells were gated by gate A in FS vs SS 2‐D dots plot. In next 2‐D dot plot, X axis means CD146‐PE, Y axis means CD45‐PECy5. The population in gating region G was CD146bright CD45negative cells; analyzing cells from gate G and cells in the gating region H were CD105bright and CD146bright, in this dot plot, X axis means CD146‐PE, Y axis means CD105‐ FITC. These cells are identified as aCEC.
Data from each sample were analyzed using Software System II (Beckman Coulter). The aCEC count difference (∆aCEC) is defined as the difference in aCEC, that is, the post‐therapeutic count minus the pre‐therapeutic count.
Perfusion CT imaging
Perfusion CT examinations were performed using enhanced CT scans. All perfusion images were obtained using 128 multi‐slice spiral CT (SOMATOM, Siemens, Erlangon, Germany). The perfusion scan covers a width of 207 mm and allows quantitative 3‐D evaluation of dynamic CT data of whole lungs and tumors or lymphadenopathies within the thorax. Iohexol (100 mL, 300 mg/mL) was injected using a high‐pressure syringe at a rate of 4 mL/s and perfusion scans were started 5 s after injection.
The imaging data were transmitted to a Syngo MultiModality Workplace (Siemens) and processed with the Lung Tumor Perfusion software included in the Syngo Volume Perfusion CT Body package. The aorta in the same layer was used as reference. By providing imaging data of BF, BV, MTT and PS from one set of dynamic CT scans, the Lung Tumor Perfusion software allows the assessment of perfusion disturbances and perfusion changes during therapy.
Statistical analysis
All analyses were performed using the spss 17.0 statistical software (SPSS Inc., Chicago, IL, USA). The results were expressed as means ± standard deviation or median and interquartile range (M, P25–P75) for continuous variables, as well as counts and proportions for categorical variables. The RR to treatment were compared between groups using a Chi‐squared test. The aCEC counts between groups were compared using a t‐test of two independent groups of samples, while aCEC counts between pre‐ and post‐therapeutic, which are normalized using log transformation, were analysed using the paired t‐test. Spearman's correlation analysis was performed to determine correlations among aCEC count, BV, BF, MTT, PS and PFS. Survival analysis was done using the Kaplan–Meier method. A log‐rank test was used to assess the survival difference between the groups. Differences were considered statistically significant at P < 0.05 of a two‐tailed test. In addition, the multivariate Cox's proportional hazards regression analysis was also used to estimate the impact of ∆aCEC and ∆BV on survival.
Results
Response to therapy
Because the trial was ended by the supporting pharmaceuticals company due to results that were considered to be sufficient to confirm the efficacy of rh‐endostatin from a phase IV trial of chemotherapy with rh‐endostatin, only a final total of 74 valid patients were enrolled in the study, with 38 cases in the combined arm and 36 in the single NP arm. Characteristics of the study population are summarized in Table 1. The median age was 57.3 years (range, 25–75 years). No statistical difference was found between the characteristics of the groups (all P > 0.05).
Table 1.
General characteristics of the patients
| Characteristics | Combined arm (%) | Single NP arm (%) | P |
|---|---|---|---|
| No. cases | 38 | 36 | — |
| Sex | |||
| Male | 20 (52.6) | 20 (55.6) | 0.820 |
| Female | 18 (47.4) | 16 (44.4) | |
| Age (years) | |||
| Median | 56.37 | 58.86 | 0.326 |
| Range | 35–74 | 25–75 | |
| Histology | |||
| Adenocarcinoma | 23 (60.5) | 22 (61.1) | 0.946 |
| Squamous cell carcinoma | 11 (28.9) | 11 (30.6) | |
| Other | 4 (10.5) | 3 (8.3) | |
| TNM stage | |||
| IIIB | 16 (42.1) | 11 (30.6) | 0.342 |
| IV | 22 (57.9) | 25 (69.4) | |
| Prior therapy | |||
| No | 24 (63.2) | 24 (66.7) | 0.811 |
| Yes | 14 (36.8) | 12 (33.3) | |
| ECOG | |||
| 1 | 35 (92.1) | 32 (88.9) | 0.707 |
| 2 | 3 (7.9) | 4 (11.1) | |
All prior therapies were performed using non‐standard single drug chemotherapy excluding vinorelbine and cisplatin. ECOG, Eastern Cooperative Oncology Group; NP, Navelbine and platinum.
—, unavailable.
Therapy was performed to two cycles in 37.8% of patients, four cycles in 56.8% of patients and five cycles or more in 5.4% of total patients, respectively. Mean therapeutic cycles of single NP and combined therapy were 2.98 and 3.97, respectively. The RR was 11.1% in the single NP arm and 13.2% in the combined arm, while the CBR was 58.3% and 73.7% in the single NP and combined arm respectively. No significant differences between the two arms were found in RR and CBR (P = 0.787 and 0.220). However, the mean therapeutic cycles of single NP and combined therapy for cases with a clinical benefit (CBR cases) after two therapeutic cycles were 3.40 and 4.66, respectively.
Progression‐free survival
The cut‐off date for data collection was November 2011. The median follow‐up period for PFS was 330 days, with follow up from 28 to 700 days. The PFS was 196 and 136 days in the combined arm and single NP arm, respectively. No significant difference in PFS in both the naïve or recurrent cases between the two arms was found, except a longer PFS was found in the combined arm compared with the single NP arm (P = 0.059). For cases with a clinical benefit (no PD) after two therapeutic cycles, the PFS was 239.57 and 165.20 days in the combined arm and single NP arm, respectively (P = 0.034). For cases with PD after two therapeutic cycles, the PFS was 52.00 and 48.00 days in the combined arm and single NP arm, respectively (P = 0.660).
aCEC
Blood samples for aCEC measurement were obtained from 63 of 74 patients until termination of the fourth cycle of NP and fifth cycle of combined therapy at a minimum, respectively, with the remaining 11 patients declining to provide blood samples, and from 30 healthy volunteers with informed consent. Males accounted for 54.1% of patients and 50.0% of healthy volunteers, respectively. The median age of patients and healthy volunteers was 58.5 and 54.5 years, respectively. No significant difference was found between the two groups in age, gender, race, smoking status and other population characteristics. The results showed a significant difference in baseline aCEC between the patients and healthy volunteers (144.38/105 vs 59.87/105, P = 0.006) as Bidard et al.24 reported, but not between patients in the two arms (159.95/105 vs 122.23/105, P = 0.516). A significant increase in aCEC was observed in the PD cases in the single NP group (P = 0.018), but not in the other subgroups (Table 2). The changes in aCEC counts could be modeled as a categorical variable (categorized to ≤0, 0–10, 11–100 and >100, as aCEC1, aCEC2, aCEC3 and aCEC4, respectively). The correlation analysis showed an inverse correlation between ∆aCEC and PFS in the combined arm (r = −0.461, P = 0.005), but not in the single NP group (r = −0.205, P = 0.372; Table 3). For all 63 cases with aCEC data, we initially considered five covariates for model 1, which included ∆aCEC, TNM stage, histology, patient age and number of therapeutic cycles. ∆aCEC were incorporated into the final model as shown in Table 4. Cox regression analysis proved that ∆aCEC was the significant indicator of PFS for the combined therapeutic group.
Table 2.
Change in aCEC and BV between pre‐ and post‐therapy among groups
| lg aCEC (/105) | P | BV (mL/100 g) | P | |||||
|---|---|---|---|---|---|---|---|---|
| n | Pre‐therapy | Post‐therapy | n | Pre‐therapy | Post‐therapy | |||
| Total | 63 | 1.75 ± 0.69 | 5.07 ± 1.55 | 0.005 | 60 | 5.26 ± 2.00 | 4.96 ± 1.97 | 0.250 |
| Combined arm | 37 | 1.81 ± 0.66 | 4.81 ± 1.54 | 0.115 | 29 | 5.39 ± 2.11 | 4.93 ± 1.73 | 0.107 |
| Non‐PD | 28 | 1.84 ± 0.63 | 4.54 ± 1.23 | 0.611 | 23 | 5.46 ± 2.18 | 4.76 ± 1.68 | 0.034 |
| PD | 9 | 1.74 ± 0.78 | 5.60 ± 2.10 | 0.142 | 6 | 5.10 ± 1.96 | 5.57 ± 1.92 | 0.374 |
| Single NP arm | 26 | 1.65 ± 0.73 | 5.44 ± 1.51 | 0.005 | 31 | 5.14 ± 1.92 | 4.99 ± 2.21 | 0.729 |
| Non‐PD | 15 | 1.68 ± 0.72 | 4.88 ± 1.51 | 0.089 | 17 | 5.20 ± 2.30 | 4.59 ± 2.39 | 0.382 |
| PD | 11 | 1.61 ± 0.77 | 6.22 ± 1.17 | 0.018 | 14 | 5.07 ± 1.40 | 5.48 ± 1.93 | 0.389 |
aCEC, activated circulating endothelial cells; BV, blood volume; NP, Navelbine and platinum; PD, progressive disease.
Table 3.
Correlation among aCEC, BV and PFS
| Combined arm | Single NP arm | |||||
|---|---|---|---|---|---|---|
| n | r | P | n | r | P | |
| aCEC | 36 | −0.461 | 0.005 | 21 | −0.205 | 0.372 |
| BV | 28 | −0.384 | 0.044 | 24 | −0.282 | 0.182 |
aCEC, activated circulating endothelial cells; BV, blood volume; NP, Navelbine and platinum; PFS, progression‐free survival.
Table 4.
Hazard ratios for aCEC from the Cox PH model for treatment arms
| Combined arm | Single NP arm | |||||||
|---|---|---|---|---|---|---|---|---|
| β | P | HR | 95% CI | β | P | HR | 95% CI | |
| aCEC1 | — | 0.048 | — | — | — | 0.466 | — | — |
| aCEC2 | 1.447 | 0.085 | 4.252 | 0.820–22.053 | −0.132 | 0.849 | 0.876 | 0.225–3.413 |
| aCEC3 | 1.114 | 0.184 | 3.047 | 0.589–15.751 | 0.500 | 0.504 | 1.649 | 0.380–7.147 |
| aCEC4 | 3.050 | 0.006 | 21.119 | 2.416–184.601 | 0.590 | 0.474 | 1.805 | 0.358–9.085 |
| No. therapeutic cycles | −0.551 | 0.070 | 0.576 | 0.317–1.046 | −0.265 | 0.166 | 0.768 | 0.528–1.116 |
aCEC, activated circulating endothelial cells; CI, confidence interval; COX PH model, the COX proportional hazards model; HR, hazard ratio; NP, Navelbine and platinum; —, unavailable.
Computed tomography perfusion imaging
Perfusion data were acquired from 60 cases because of failure in obtaining perfusion imaging in the other 14 patients. The results exhibited a significant decline in non‐PD cases in the combined arm (P = 0.034) and non‐significant decline in the single NP arm (P = 0.382) (Table 2). No significant changes in other perfusion data were observed in any subgroups (data not shown). Correlation analysis showed an inverse correlation between BV and PFS only in the combined arm (r = −0.384, P = 0.044; Table 3), but not in the single NP group (r = −0.282, P = 0.182). We also tried to evaluate the possible predictive effect of BV on PFS adjusted for the treatment duration (Table 5). Model 2 used the PFS data on the 60 subjects whose BV data had been collected. While the independent variables are different, the outcome variables for both models are the same PFS. Because there was no complete coincidence in subjects who provided BV and aCEC, the BV and number of therapeutic cycles were entered into another model of Cox regression (model 2). Cox regression analysis proves that ∆BV is a significant indicator of PFS in the combined therapy group (HR = 1.805, P = 0.040). Moreover, no significant difference between the PFS of the PR and SD cases was observed with decreased BV (P = 0.736).
Table 5.
Hazard ratios for BV from the Cox PH model for treatment arms
| Combined arm | Single NP arm | |||||||
|---|---|---|---|---|---|---|---|---|
| β | P | HR | 95% CI | β | P | HR | 95% CI | |
| BV | 0.591 | 0.040 | 1.805 | 1.029–3.168 | 0.094 | 0.241 | 1.099 | 0.939–1.286 |
| No. therapeutic cycles | −0.428 | 0.163 | 0.652 | 0.358–1.188 | −0.222 | 0.241 | 0.801 | 0.552–1.161 |
BV, blood volume; CI, confidence interval; HR, hazard ratio; NP, Navelbine and platinum.
The enhanced CT scan and perfusion imaging of a case treated with combined NP and rh‐endostatin is shown in Figure 1. The red/yellow region accounted for the majority of the internal tumor area before treatment, which represented abundant blood volume inside the tumor. After two cycles of treatment, we observed expansion of the blue region with shrinkage of the red/yellow region, suggesting a reduction in tumor blood perfusion, but we did not observe shrinkage of the tumor volume. After two more subsequent cycles of treatment, the blue region increased and the red/yellow region decreased substantially, accompanied by obvious shrinkage of the tumor volume as a partial response.
Figure 1.
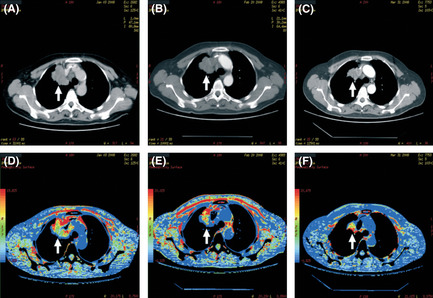
Imaging of the contrast computed tomography (CT) scan and perfusion of a case treated with combined Navelbine and platinum (NP) and Re‐endostatin. (A) Contrast CT image before treatment. (B) Contrast CT image after two cycles of treatment without tumor shrinkage (arrow). (C) Contrast CT image after four cycles of treatment with tumor shrinkage (arrow). (D) Blood volume inside the tumor before treatment (arrow). (E) Decreased blood volume inside the tumor (arrow) after two cycles of treatment. (F) Continuously decreased blood volume inside the tumor after four cycles of treatment (arrow).
Correlation between perfusion indexes and aCEC
The same tendency of change was found in aCEC and BV with positive correlation between ∆aCEC and ∆BV in the combined arm (r = 0.430, P = 0.022) but not in the single NP arm (r = 0.295, P = 0.162).
Data of safety and quality of life are not shown in this article.
Discussion
Although platinum‐based chemotherapy has been recognized as the standard treatment for advanced NSCLC, the PFS only reaches 4–6 months.25 Antiangiogenic agents are believed to delay the recovery of residual tumor from chemotherapeutic impairment by suppressing blood supply to the tumor.4 Endostatin was capable of inhibiting endothelial cell proliferation and migration leading to inhibition of tumor growth and metastasis. Rh‐endostatin, a novel recombinant humanized endostatin, improved stability of the protein.5 In clinical trials in China, the combination of rh‐endostatin and chemotherapy was shown to have significantly improved OS and PFS of advanced NSCLC.8, 9 However, doubts remained in those trials due to few therapeutic cycles (less than three cycles, without maintenance). Therefore, we designed a trial of longer therapy in order to confirm or modify the previous conclusion. Despite the unexpected end of the trial, in the present study the obtained CBR in the single chemotherapy arm and combined arms achieved 58.3% and 73.7% with PFS of 136 and 196 days, respectively, with insignificant differences (P = 0.220 and 0.059, respectively). The results other than that from a phase III trial of NP with or without rh‐endostatin (PFS was 6.3 and 3.6 months in NP plus rh‐endostatin and single NP, respectively, with P‐value 0.00) might be ascribed to the limited number of subjects or more therapeutic cycles in our trial. However, the PFS for cases with a clinical benefit achieved 239.57 days (8 months) and 165.20 days (5.5 months) in the combined arm and single NP arm (P = 0.034), respectively, whereas the PFS for cases with PD was 52.00 and 48.00 days in the combined arm and single NP arm, respectively. This implies that only some “optimal individuals” could benefit from continuous antiangiogenesis when induced treatment was effective. It is necessary to distinguish the optimal group from the total patient population by way of predicting their response to therapy as early as possible to avoid insufficient or excessive antiangiogenic therapy. Because antiangiogenic drugs mainly suppress growth of tumor blood vessels rather than tumor tissue per se, tumor volume shrinkage always occurs after repression of tumor blood supply.26 Since both WHO and the RECIST criteria estimate efficacy for solid tumors based on volume change that is usual later events during treatment, much earlier predicting approaches are urgently needed to provide a more timely assessment of the treatment efficacy.
In the present study, we investigated early predictors of response and PFS, consideration of age, sex, histology, stage, prior therapy, ECOG, number of therapeutic cycles, aCEC and BV were incorporated into the multivariate Cox's proportional hazards model. However, significance was only found on the latter three ones. Therefore, they were reincorporated into the multivariate Cox's proportional hazards models and significance was only found on aCEC and BV. In addition, although it was not included in model 1 (P = 0.07), the number of therapeutic cycles is of significant importance and further study is needed to determine whether an increased number of therapeutic cycles can prolong PFS.
aCEC for predicting the efficacy of antiangiogenic therapy
Although antiangiogenic efficacy was proven in the E4599 trials, the endostatin and the thalidomide trial, it remains to be determined whether efficacy can be assessed based on changes of TAF, such as serum VEGF and bFGF levels.25, 27, 29 This might be due to the unstable balance resulting from confrontation of the TAF and antiangiogenic factors, suggesting more downstream markers other than TAF should be searched.
The CEC, as discovered by Bouvier in the 1970s, are circulating endothelial cells that directly reflect injury to the vascular endothelium in vivo through its alteration in the blood levels.30 The CEC include mainly EPC that originate from the bone marrow, mature endothelial cells shed from blood vessel walls and cancer‐derived cells with endothelial function.31 These cells need to be activated by TAF so that they can home to tumor sites, adhere and form new tumoral vasculature.32 Many markers have been used to identify CEC because of the diversity of their origin and complexity of their differentiation. The currently recognized markers are CD45−CD146+Flk1+.33, 34 In addition, Beaudry introduced CD117 to identify EPC and Mancuso19, 21, 23, 34 chose CD105 to distinguish activated functional CEC (aCEC), which are considered more relevant to angiogenesis than the total CEC. A previous study showed that EPC level is usually undetectable because of its scarcity in the circulation,35 but the correlation was observed between changes in CD105+ aCEC and efficacy.36 Given that activated mature endothelial cells (CD45−CD146+CD105+) are viable and continue to exhibit proliferative capacity despite their terminal differentiation,31 CD45−CD146+CD105+ was used to identify aCEC in the present study in accordance with the literature.19, 20, 21
Even though both CEC and EPC provide the potentiality to reveal the angiogenetic status and predict antiangiogenic therapeutic efficacy, controversy remains due to the variability of CEC and EPC levels in different studies. The aCEC decrease after a complete response to chemotherapy or mastectomy was proven by Mancuso.16, 21 However, elevation of both CEC and EPC several days after antiangiogenic therapy and/or chemotherapy was reported, especially after taxane drug treatment in some studies. This is because of mobilization of EPC from bone marrow by platelet‐released stromal cell‐derived factor induced by taxane.37 Nevertheless, a decrease in both CEC and EPC was reported with longer PFS or OS in the majority of reports after effective antiangiogenic therapy and/or chemotherapy, such as bevacizumab with non‐taxane and metronomic chemotherapy.38, 39 Furthermore, after taxane treatment, a higher elevation in apoptotic/dead CEC compared with EPC was reported.30 These results imply a complex equilibrium between mobilization of EPC and apoptosis of CEC induced by different regimens and drugs.
Another probable explanation for a short increase in CEC after treatment might be “normalization” of tumoral vasculature. Normalization of tumor vasculature is a common effect of antiangiogenic agents, including a series of antiangiogenic events in which suppression of MMP by antiangiogenic agents induces decreased degradation and low permeability of the micrangium basal membrane, reduction of fluid leakage and interstitial fluid pressure.40 Thus, the dilated and tortuous vessels in the tumor are constricted and stretched, allowing chemotherapeutic drugs in the blood to enter tumors more easily.41 Because endothelial cells in tumoral vasculature are more vulnerable to damage by antiangiogenic agents and are easy to shed, both endothelial retraction/apoptosis and tumor vasculature shrinkage might simultaneously induce endothelial cells to shed from the blood vessel walls, allowing for easier penetration by chemotherapeutic agents.34
In the present study, to eliminate possible disturbance from transient elevation of aCECs caused by chemotherapeutic or antiangiogenic drugs,38, 42 the blood samples were obtained after completion of the previous therapeutic cycle and prior to the beginning of next therapeutic cycle until completion of therapy (other than three blood samples drawn in another trial17) in order to obtain the ‘dynamic tendency’ and confirmed terminal data of aCECs during therapy. The results showed aCEC increased on tumor progression, especially in the single NP arm, but did not increase or slightly increased in SD and PR, which showed a tumor response. Apart from a possible deviation caused by the limited number of cases, this is probably due to the fact that effective antiangiogenic therapy also increases aCEC through “vasculature normalization” separately from decreasing aCEC by suppression of EPC mobilization and induction of aCEC apoptosis,33 which renders aCEC to be kept stable or slightly increased in the SD and PR patients. However, the “window” of vasculature normalization is only 1–2 weeks after antiangiogenic therapy, after which it turns into vascular insufficiency induced by suppression of tumor angiogenesis.42 These two effects of antiangiogenesis could alternate during therapy leading to CEC fluctuation and a gradual decline along with tumor shrinkage and decreasing circulating TAF after effective prolonged therapy, resulting in the inverse correlation between ∆aCEC and either PFS or BV in the present study, which also suggests a need for multiple inspections of CEC during therapy. These results are consistent with a previous report by Fürstenberger et al. showing that effective treatment induced robust apoptosis of CEC and that chemotherapy based on platinum and non‐taxane drugs did not mobilize EPC from bone marrow.14, 35 Furthermore, ∆aCEC was confirmed as a significant indicator of PFS in the combined antiangiogenesis regimen using Cox regression in model 1.
Value of CT perfusion imaging in predicting efficacy
Bellomi et al.41 proposed the concept of CT perfusion imaging whereby rapid dynamic scans are conducted at a single location after a bolus of contrast medium injection. The imaging data are calculated using an enhanced level of each pixel in each layer and are displayed in grayscale to form the quantitative or semi‐quantitative perfusion images of the organ.
Computed tomography perfusion imaging has been used for early diagnosis of brain infarction and cardiovascular and renal diseases, using BF, BV, MTT and PS as the main indexes.19, 44 The perfusion indexes have been proven to be higher in cancerous lung tissues than normal peripheral tissue and increasing BV indicates angiogenesis.43 Better efficacy has been reported in cases with higher baseline BV. Changes in the perfusion indexes, such as BV, indicate changes in tumor vasculature, suggesting that they could be used to monitor antiangiogenic therapy.
In the results of present study, decreased BV could indicate clinical benefits, particularly in the combined arm. Chemotherapy mainly causes shrinkage of tumor in addition to vasculature impairment. In contrast, effective antiangiogenic therapy leads to vasculature normalization by constricting dilated and tortuous tumor vessels to reduce the vasculature area. Considering the decreased blood volume from vascular normalization and the subsequent inadequacy resulting in vascular bed reduction, a significant decrease in BV was observed only in the combined arm but not in the single NP arm. In contrast, poor antiangiogenic efficacy could at least partially be ascribed to enhanced tumor angiogenesis induced by TAF and migration of tumor cells along the blood vessels (perivascular invasion) into normal tissues to evade antiangiogenesis.45 Clearly, predicting tumor invasion and progression based only on BV change in the regions of interest inside tumors is difficult in the above case. This might explain the non‐significant increase in BV during tumor progression. Unlike previous reports, no significant connection between changes in other perfusion indexes, such as BF, MMT and PS, was found in the current study. This might be due to a limited sample pool or the fact that BV might be a more optimal indicator of antiangiogenic efficacy than the other parameters, which might warrant further investigation.
In the long‐term observation, an inverse correlation was observed between ∆BV and PFS (r = −0.461, P = 0.005), suggesting that a continuous decrease of BV might indicate long‐term efficacy, as does aCEC. The results show that ∆BV is a significant indicator of PFS in the combined therapy group (P = 0.019). In addition, no significant difference was found between PFS in cases of SD and PR, as defined by the RECIST criteria, implying that predicting antiangiogenic efficacy using ∆BV is more reliable than tumor volume and a longer stable disease duration could be achieved in some no‐shrink tumors with significantly decreased BV.
Although some reports showed better responses to chemotherapy could be observed in cases with higher baseline levels of CEC and BV, no significant difference in baseline CEC and BV was found between cases of PD and non‐PD in the present study.46, 47 Further investigation is needed to clarify whether baseline CEC and BV can serve as ideal predictors.
Correlation between BV and aCEC
The correlation between ∆aCEC and ∆BV was only found in the combined arm (r = 0.430, P = 0.022); the positive but not very close correlation implies that their similar trend could reflect the progression and regression of tumoral angiogenesis and provide a way for accurate prediction of antiangiogenic efficacy through combined detection. It suggested that blood volume in tumor is “determined” by the growth of micrangium formed by aCEC. Considering that rh‐endostatin could suppress many targets in endothelial cells compared with bevacizumab, which only inhibits VEGF, changes in the VEGF level alone might be insufficient to reflect rh‐endostatin's efficacy, as demonstrated in a previous study,12 therefore multiple markers should be detected. Besides, suppression of micrangium around the tumor could have different effects on neoplastic cells, including depression or enhancement of growth.10 Therefore, not only baseline but dynamic detection of markers must be done during therapy and follow up. Nevertheless, further prospective study with a larger patient pool is needed for validation.
To eliminate disturbance from confounding factors due to insufficient subjects in the study and to determine real influential factors for PFS, we made a multivariate Cox's proportional hazards regression analysis to disclose all possible factors by inputting variables such as age, sex, history, TNM stage, prior therapy and ECOG performance into a model besides CEC and perfusion indexes, and only CEC and BV were defined as significant indicators of PFS in the combined therapy group.
Limits of present research
Some reports proposed that the exceptionally high CEC counts detected with the commonly used flow cytometry test (FCT)‐based assay are attributed to the fact that the majority of cells designated as CEC are actually large platelets.48 To avoid such artifact, combined detection through both magnetic isolation and FCT assays has been initiated in our center to determine total CEC, aCEC and apoptotic CEC.
In conclusion, both aCEC and BV can predict antiangiogenic efficacy and their combined detection is helpful in predicting antiangiogenic efficacy more sensitively and reliably than plain or enhanced CT scans alone. Optimization of the detection method to elucidate changes in the subgroups of CEC during antiangiogenic therapy, as well as the connection between CEC and efficacy in further prospective trials, are necessary.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This study was supported by a grant from Tianjin Science and Technology Project (Crucial Special Anticancer Program “Creation of New Ways to Assess Targeted Therapeutic Efficacy of Antiangiogenesis”) and a grant from CSCO (Y‐X2011‐001). The authors gratefully acknowledge Simcere Pharmaceutical Group (Jiangsu, China) for their sincere support by cordially providing the Rh‐endostatin.
(Cancer Sci 2013; 104: 445–452)
References
- 1. Jemal A, Siegel R, Ward E et al Cancer statistics 2008. CA Cancer J Clin 2008; 58: 71–96. [DOI] [PubMed] [Google Scholar]
- 2. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–6. [DOI] [PubMed] [Google Scholar]
- 3. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990; 82: 4–6. [DOI] [PubMed] [Google Scholar]
- 4. Wu JM, Staton CA. Anti‐angiogenic drug discovery: lessons from the past and thoughts for the future. Expert Opin Drug Discov 2012; 7: 723–43. [DOI] [PubMed] [Google Scholar]
- 5. Sim BK, Fogler WE, Zhou XH et al Zinc ligand‐disrupted recombinant human endostatin: potent inhibition of tumor growth, safety and pharmacokinetic profile. Angiogenesis 1999; 3: 41–51. [DOI] [PubMed] [Google Scholar]
- 6. Zhao X, Mei K, Cai X et al A randomized phase II study of recombinant human endostatin plus gemcitabine/cisplatin compared with gemcitabine/cisplatin alone as first‐line therapy in advanced non‐small‐cell lung cancer. Invest New Drugs 2012; 30: 1144–9. [DOI] [PubMed] [Google Scholar]
- 7. Sandler A, Gray R, Perry MC et al Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med 2006; 355: 2542–50. [DOI] [PubMed] [Google Scholar]
- 8. Wang JW, Sun Y, Liu YY et al Results of randomized, multicenter, double‐blind phase III trial of rh‐endostatin (YH‐16) in treatment of advanced non‐small cell lung cancer patients. Chin J Lung Cancer (Chin) 2005; 8: 283–90. [DOI] [PubMed] [Google Scholar]
- 9. Han B, Xiu Q, Wang H et al A multicenter, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy of paclitaxel‐carboplatin alone or with endostar for advanced non‐small cell lung cancer. J Thorac Oncol 2011; 6: 1104–6. [DOI] [PubMed] [Google Scholar]
- 10. Casanovas O. Cancer: limitations of therapies exposed. Nature 2012; 484: 44–6. [DOI] [PubMed] [Google Scholar]
- 11. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 12. Herbst RS, Hess KR, Tran HT et al Phase I study of recombinant human endostatin in patients with advanced solid tumors. J Clin Oncol 2002; 20: 3792–803. [DOI] [PubMed] [Google Scholar]
- 13. Cavallo T, Sade R, Folkman J, Cotran RS. Tumor angiogenesis. Rapid induction of endothelial mitoses demonstrated by autoradiography. J Cell Biol 1972; 54: 408–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keegan A, Hill C, Kumar S, Phillips P, Schor A, Weiss J. Purified tumour angiogenesis factor enhances proliferation of capillary, but not aortic, endothelial cells in vitro . J Cell Sci 1982; 55: 261–76. [DOI] [PubMed] [Google Scholar]
- 15. Duong HT, Erzurum SC, Asosingh K. Pro‐angiogenic hematopoietic progenitor cells and endothelial colony‐forming cells in pathological angiogenesis of bronchial and pulmonary circulation. Angiogenesis 2011; 14: 411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fürstenberger G, von Moos R, Lucas R et al Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer 2006; 94: 524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chu TQ, Ding H, Garfield DH et al Candetermination of circulating endothelial cells and serum caspase‐cleaved CK18 predict for response and survival in patients with advanced non–small‐cell lung cancer receiving endostatin and paclitaxel–carboplatin chemotherapy? A retrospective study J Thorac Oncol 2012; 7: 1781–9. [DOI] [PubMed] [Google Scholar]
- 18. Li Y, Yang ZG, Chen TW, Yu JQ, Sun JY, Chen HJ. First‐pass perfusion imaging of solitary pulmonary nodules with 64‐detector row CT: comparison of perfusion parameters of malignant and benign lesions. Br J Radiol 2010; 83: 785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sitartchouk I, Roberts HC, Pereira AM, Bayanati H, Waddell T, Roberts TP. Computed tomography perfusion using first pass methods for lung nodule characterization. Invest Radiol 2008; 43: 349–58. [DOI] [PubMed] [Google Scholar]
- 20. Fournier LS, Oudard S, Thiam R et al Metastatic renal carcinoma: evaluation of antiangiogenic therapy with dynamic contrast‐enhanced CT. Radiology 2010; 256: 511–8. [DOI] [PubMed] [Google Scholar]
- 21. Mancuso P, Burlini A, Pruneri G, Goldhirsch A, Martinelli G, Bertolini F. Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood 2001; 97: 3658–61. [DOI] [PubMed] [Google Scholar]
- 22. Ali AM, Ueno T, Tanaka S, Ishiguro H, Abdellah AZ, Toi M. Determining circulating endothelial cells using Cell Search system during preoperative systemic chemotherapy in breast cancer patients. Eur J Cancer 2011; 47: 2265–72. [DOI] [PubMed] [Google Scholar]
- 23. Rowand JL, Martin G, Doyle GV et al Endothelial cells in peripheral blood of healthy subjects and patients with metastatic carcinomas. Cytometry A 2007; 71: 105–13. [DOI] [PubMed] [Google Scholar]
- 24. Bidard FC, Mathiot C, Degeorges A et al Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Ann Oncol 2010; 21: 1765–71. [DOI] [PubMed] [Google Scholar]
- 25. Mok TS, Wu YL, Thongprasert S et al Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 26. Tudorica A, Thomas CR Jr, Huang W. Invited commentary. Radiographics 2010; 30: 716–9. [DOI] [PubMed] [Google Scholar]
- 27. Young RJ, Tin AW, Brown NJ, Jitlal M, Lee SM, Woll PJ. Analysis of circulating angiogenic biomarkers from patients in two phase III trials in lung cancer of chemotherapy alone or chemotherapy and thalidomide. Br J Cancer 2012; 106: 1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Niers TM, Richel DJ, Meijers JC, Schlingemann RO. Vascular endothelial growth factor in the circulation in cancer patients may not be a relevant biomarker. PLoS ONE 2011; 6: e19873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bernaards C, Hegde P, Chen D et al Circulating vascular endothelial growth factor (VEGF) as a biomarker for bevacizumab based therapy in metastatic colorectal, non‐small cell lung, and renal cell cancers: analysis of phase III studies. J Clin Oncol 2010; 28(Suppl. 15): abstract 10519. [Google Scholar]
- 30. Hunting CB, Noort WA, Zwaginga JJ. Circulating endothelial (progenitor) cells reflect the state of the endothelium: vascular injury, repair and neovascularization. Vox Sang 2005; 88: 1–9. [DOI] [PubMed] [Google Scholar]
- 31. Ricci‐Vitiani L, Pallini R, Biffoni M et al Tumour vascularization via endothelial differentiation of glioblastoma stem‐like cells. Nature 2010; 468: 824–8. [DOI] [PubMed] [Google Scholar]
- 32. Asahara T, Takahashi T, Masuda H et al VEGF contributes to postnatal neovascularization by mobilizing bone marrow‐derived endothelial progenitor cells. EMBO J 1999; 18: 3964–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li H, Raia V, Bertolini F, Price DK, Figg WD. Circulating endothelial cells as a therapeutic marker for thalidomide in combined therapy with chemotherapy drugs in a human prostate cancer model. BJU Int 2008; 101: 884–8. [DOI] [PubMed] [Google Scholar]
- 34. Beaudry P, Force J, Naumov GN et al Differential effects of vascular endothelial growth factor receptor‐2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res 2005; 11: 3514–22. [DOI] [PubMed] [Google Scholar]
- 35. Norden‐Zfoni A, Desai J, Manola J et al Blood‐based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib‐resistant gastrointestinal stromal tumor. Clin Cancer Res 2007; 13: 2643–50. [DOI] [PubMed] [Google Scholar]
- 36. Wang J, Huang C, Wei XY et al Changes of activated circulating endothelial cells and surviving in patients with non‐small cell lung cancer after antiangiogenesis therapy. Chin Med J (Engl) 2008; 121: 2334–40. [PubMed] [Google Scholar]
- 37. Shaked Y, Henke E, Roodhart JM et al Rapid chemotherapy‐induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 2008; 14: 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roodhart JM, Langenberg MH, Vermaat JS et al Late release of circulating endothelial cells and endothelial progenitor cells after chemotherapy predicts response and survival in cancer patients. Neoplasia 2010; 12: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bertolini F, Paul S, Mancuso P et al Maximum tolerable dose and low‐dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 2003; 63: 4342–6. [PubMed] [Google Scholar]
- 40. Lu N, Ling Y, Gao Y et al Endostar suppresses invasion through downregulating the expression of matrix metalloproteinase‐2/9 in MDA‐MB‐435 human breast cancer cells. Exp Biol Med (Maywood) 2008; 233: 1013–20. [DOI] [PubMed] [Google Scholar]
- 41. Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 2004; 64: 3731–6. [DOI] [PubMed] [Google Scholar]
- 42. Starlinger P, Brugger P, Reiter C et al Discrimination between circulating endothelial cells and blood cell populations with overlapping phenotype reveals distinct regulation and predictive potential in cancer therapy. Neoplasia 2011; 13: 980–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bellomi M, Viotti S, Preda L, D'Andrea G, Bonello L, Petralia G. Perfusion CT in solid body‐tumours part II. Clinical applications and future development. Radiol Med 2010; 115: 858–74. [DOI] [PubMed] [Google Scholar]
- 44. Eastwood JD, Lev MH, Provenzale JM. Perfusion CT with iodinated contrast material. AJR Am J Roentgenol 2003; 180: 3–12. [DOI] [PubMed] [Google Scholar]
- 45. Bergers G, Hanahan D. Modes of resistance to anti‐angiogenic therap. Nat Rev Cancer 2008; 8: 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kawaishi M, Fujiwara Y, Fukui T et al Circulating endothelial cells in non‐small cell lung cancer patients treated with carboplatin and paclitaxel. J Thorac Oncol 2009; 4: 208–13. [DOI] [PubMed] [Google Scholar]
- 47. Bellomi M, Petralia G, Sonzogni A, Zampino MG, Rocca A. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology 2007; 244: 486–93. [DOI] [PubMed] [Google Scholar]
- 48. Strijbos MH, Kraan J, den Bakker MA, Lambrecht BN, Sleijfer S, Gratama JW. Cells meeting our immunophenotypic criteria of endothelial cells are large platelets. Cytometry B Clin Cytom 2007; 72: 86–93. [DOI] [PubMed] [Google Scholar]


