Abstract
Aberrant β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression in malignant tissues has been reported to be involved in the development of various cancers and their progression, through altering N‐glycan branching. We aimed to investigate the clinical and prognostic values of MGAT5 and improve the risk stratification in patients with gastric cancer. MGAT5 expression was retrospectively analyzed by immunohistochemistry in three independent sets comprising 313 patients from China with gastric adenocarcinoma. Results were assessed for association with clinical features and overall survival using Kaplan–Meier analysis. Prognostic values of MGAT5 expression and clinical outcomes were evaluated by Cox regression analysis. A molecular prognostic stratification scheme incorporating MGAT5 expression was determined in patients with late‐stage gastric cancer by using receiver operating characteristic analysis. The results show that low intratumoral MGAT5 density, which was associated with poor differentiation, N classification, TNM stage, and Kiel stage, was an independent prognosticator for poor overall survival. The combination of intratumoral MGAT5 expression and TNM or Kiel staging systems had a better predictive power for overall survival. Applying the prognostic value of intratumoral MGAT5 density to TNM stage III+IV and Kiel stage IIIB+IV groups showed a better risk stratification for overall survival in patients with late‐stage gastric cancer. In conclusion, integrating intratumoral MGAT5 density that was recognized as an independent prognostic marker into current clinical staging systems significantly improved prognostic stratification of patients with late‐stage gastric cancer. This refined risk stratification scheme might aid in appropriate therapeutic options and ultimately improve the outcomes of patients with advanced‐stage disease.
Gastric cancer is the fourth most common neoplasm worldwide and the second most frequent cause of cancer‐related mortality, partially due to the loss of curative therapeutic opportunities at the time of initial diagnosis, with advanced stage disease with lymph node metastases already present in the vast majority of patients.1, 2 The TNM classification of the International Union Against Cancer and Kiel's proposal of stage grouping systems (Fig. S1), based on the postoperative clinicopathological status of patients with gastric cancer, establish predictive nomograms for accurately predicting patient survival, guiding therapy decisions (Fig. 1a), and stratifying patients in clinical trials.3, 4, 5 However, there are noted heterogeneous clinical outcomes among patients with the same stage and similar treatment regimens in these two staging systems, especially non‐surgical patients with end‐stage gastric cancer who are the focus of clinical trials.6 Therefore, molecular approaches to stratifying patients with gastric cancer, through incorporation of molecular information, including post‐translational modification, into conventional predictive nomograms will improve current prognostic stratification and identify non‐surgical patients who are more likely to benefit from adjuvant therapies.
Figure 1.
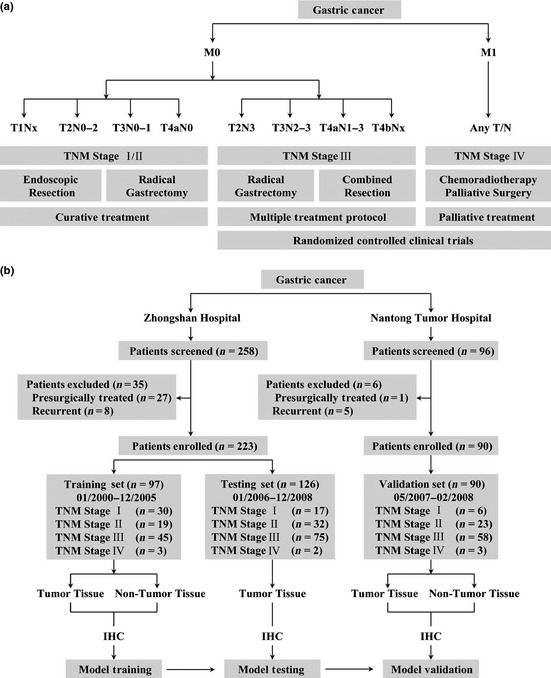
Treatment guidelines for gastric cancer and study design. (a) Treatment guidelines for gastric cancer. Patients with early or intermediate gastric cancer (TNM stage I/II) are candidates for curative treatment (endoscopic resection, radical gastrectomy). Patients within TNM stage III might benefit from radical gastrectomy, extended surgery, and neoadjuvant or adjuvant chemoradiotherapy. Patients with end‐stage disease (TNM stage IV) will receive palliative treatment. Patients with TNM stage III/IV disease are candidates for randomized controlled clinical trials. Modified from Japanese gastric cancer treatment guidelines 2010 (version 3). (b) Study design. In the training set, tumor and non‐tumor tissues were analyzed separately and each was assessed with the clinicopathologic characteristics and overall survival of patients with gastric cancer. The model based on non‐tumor tissue in the training set was validated with the use of an independent validation set. IHC, immunohistochemistry.
As the most extensive and complex form of protein post‐translational modifications, glycosylation produces an abundant, diverse, and highly regulated repertoire of cellular glycans as an “ensemble” of structures that mediate function. Unlike protein–protein interactions, which can be generally viewed as “digital” in regulating function, glycan–protein interactions impinge on biological functions in a more “analogue” fashion that can in turn “fine‐tune” a biological response. In the tumor microenvironment, changes in glycosylation allow neoplastic cells to usurp many of the events that occur in development (for example, receptor activation, cell adhesion, and cell motility), which allows tumor cells to invade and spread throughout the organism.7 Glycosylation alterations, including both underexpression and overexpression of naturally‐occurring glycans, as well as neo‐expression of glycans normally restricted to embryonic tissues, most often arise from changes in the expression levels of glycosyltransferases in the Golgi compartment of cancerous cells.8 One of the most common changes is an increase in the size and branching of N‐linked glycans, which is often attributed to increased activity of β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) during colon and breast carcinoma progression.9, 10, 11 In the Golgi compartment, this enzyme transfers a GlcNAc residue onto growing N‐linked glycans so that subsequent glycosylation results in “multi‐antennary” chains. The presence of such complex β1, 6‐branched N‐glycans on tumor cell E‐cadherin reduces tumor cell–cell adhesion. Therefore, increased expression of this enzyme promotes cell detachment and invasion.7, 8 Moreover, the MGAT5‐deficient background suppresses the oncogenic potency of polyomavirus middle T oncogene transgene in mice.12 In opposition to the abovementioned oncogenic function of MGAT5, low MGAT5 expression was associated with poor prognosis in bladder and non‐small‐cell lung cancers.13, 14 However, a comprehensive analysis of MGAT5 expression in relation to survival of patients with gastric cancer remains largely unknown and needs to be further established.
In this study, low intratumoral MGAT5 expression in gastric cancer was examined by immunohistochemical analysis, and its correlation with poor prognosis of patients with gastric cancer was evaluated. Moreover, integration of intratumoral MGAT5 expression into TNM staging or Kiel's staging system could refine prognostic stratification of patients with late‐stage gastric cancer.
Materials and Methods
Clinical specimens
We prospectively recruited consecutive patients with gastric cancer, collected the clinicopathologic data and the specimens, and retrospectively analyzed the samples for markers correlating with survival and their role in refining gastric cancer prognostic stratification. Three independent sets comprising 354 patients who had undergone total or partial gastrectomy for gastric adenocarcinoma from two institutional clinical centers were enrolled in the study (Fig. 1b). Patients with presurgically treated gastric cancer (n = 28) and recurrent gastric cancer (n = 13) were excluded. Specimens of a training set (n = 97) and testing set (n = 126) were obtained from Zhongshan Hospital (Shanghai, China) between January 2000 and December 2008. Specimens of a validation set (n = 90) were obtained from Nantong Tumor Hospital (Jiangsu, China) between May 2007 and February 2008 with the same criteria of the training and testing sets. Non‐tumor gastric tissues of the training set and validation set were obtained at least 5 cm from the tumor. All specimens were pathologically reassessed independently by two gastroenterology pathologists blinded to the clinical data. Institutional review board approval from each hospital and written informed consent from all patients were obtained for this study.
Patient characteristics
Detailed clinical characteristics of three independent sets, including 313 gastric cancer specimens obtained for this study, are summarized in Table 1. There were more patients with late‐stage gastric cancer (TNM III and IV, 67.77% vs 61.11% vs 49.48%, respectively; Kiel IIIB and IV, 71.11% vs 64.29% vs 53.61%, respectively) and shorter survival time (median, 35 months vs >60 months vs >60 months, respectively) in the validation set compared with the training and testing sets. Such heterogeneity may help to ensure that the predictor has real‐world applicability across heterogeneous populations of patients from different districts.
Table 1.
Clinical characteristics of patients with gastric cancer in three independent sets
| Factor | Training set | Testing set | Validation set | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| All patients | 97 | 100 | 126 | 100 | 90 | 100 |
| Age, years | ||||||
| Mean | 60.35 | 65.04 | 65.47 | |||
| SD | 11.37 | 11.22 | 10.69 | |||
| Gender | ||||||
| Female | 35 | 36.08 | 37 | 29.37 | 23 | 25.56 |
| Male | 62 | 63.92 | 89 | 70.63 | 67 | 74.44 |
| Localization | ||||||
| Proximal | 10 | 10.31 | 28 | 22.22 | 25 | 27.78 |
| Middle | 45 | 46.39 | 48 | 38.10 | 28 | 31.11 |
| Distal | 42 | 43.30 | 50 | 39.68 | 37 | 41.11 |
| Differentiation | ||||||
| Well | 5 | 5.16 | 17 | 13.49 | 1 | 1.11 |
| Moderate | 36 | 37.11 | 34 | 26.99 | 36 | 40.00 |
| Poor | 56 | 57.73 | 75 | 59.52 | 53 | 58.89 |
| Lauren classification | ||||||
| Intestinal type | 71 | 73.20 | 87 | 69.05 | 61 | 67.78 |
| Diffuse type | 26 | 26.80 | 39 | 30.95 | 29 | 32.22 |
| T classification | ||||||
| T1 | 26 | 26.80 | 10 | 7.94 | 4 | 4.44 |
| T2 | 10 | 10.31 | 12 | 9.52 | 8 | 8.89 |
| T3 | 4 | 4.13 | 14 | 11.11 | 9 | 10.00 |
| T4 | 57 | 58.76 | 90 | 71.43 | 69 | 76.67 |
| N classification | ||||||
| N0 | 38 | 39.18 | 42 | 33.33 | 20 | 22.22 |
| N1 | 19 | 19.59 | 14 | 11.11 | 16 | 17.78 |
| N2 | 14 | 14.43 | 22 | 17.46 | 26 | 28.89 |
| N3 | 26 | 26.80 | 48 | 38.10 | 28 | 31.11 |
| Distant metastasis | ||||||
| No | 94 | 96.91 | 124 | 98.41 | 87 | 96.67 |
| Yes | 3 | 3.09 | 2 | 1.59 | 3 | 3.33 |
| TNM stage | ||||||
| I | 30 | 30.93 | 17 | 13.49 | 6 | 6.67 |
| II | 19 | 19.59 | 32 | 25.40 | 23 | 25.56 |
| III | 45 | 46.39 | 75 | 59.52 | 58 | 64.44 |
| IV | 3 | 3.09 | 2 | 1.59 | 3 | 3.33 |
| Tumor size, cma | ||||||
| <3.5 | 56 | 57.73 | 59 | 46.83 | 20 | 22.22 |
| ≥3.5 | 41 | 42.27 | 67 | 53.17 | 70 | 77.78 |
| Kiel stage | ||||||
| I | 21 | 21.65 | 7 | 5.55 | 2 | 2.22 |
| II | 17 | 17.52 | 33 | 26.19 | 17 | 18.89 |
| IIIA | 7 | 7.22 | 5 | 3.97 | 7 | 7.78 |
| IIIB | 27 | 27.84 | 39 | 30.96 | 37 | 41.11 |
| IV | 25 | 25.77 | 42 | 33.33 | 27 | 30.00 |
| Survival, months | ||||||
| Median | >60 | >60 | 35 | |||
| Range | 5–139 | 1–72 | 1–51 | |||
Kiel stage, Kiel proposal of stage grouping; SD, standard deviation.
Split at median.
Tissue microarray and immunohistochemistry
Tissue microarrays were constructed as previously described.15 Primary anti‐MGAT5 antibody (Abcam, Cambridge, MA, USA) was used for immunohistochemistry (IHC) staining. Before IHC staining, antibody specificity was ascertained by Western blot analysis in two human gastric cancer cell lines (AGS and HGC27) after transfection with control pcDNA3.1 vector and pcDNA3.1‐MGAT5 plasmid (a generous gift from Dr. Jianguo Gu, Tohoku Pharmaceutical University, Miyagi, Japan), respectively (Fig. S2). The intensity of IHC staining of MGAT5 was scored independently by two gastroenterology pathologists using the semiquantitative immunoreactivity scoring (IRS) system as previously described.16 Briefly, category A documented the intensity of IHC staining as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). Category B documented the percentage of immunoreactive cells as 1 (0–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). Multiplication of category A and B resulted in an IRS ranging from 0 to 12 for each specimen. The area under the receiver operating characteristic curves (AUC) at different cut‐off values of overall survival time was calculated to determine the optimal cut‐off value of the MGAT5 IRS in tumors. In the training set, the optimal value of the cut‐off point was eight, due to its best predictive value (AUC, 0.722; 95% confidence interval [CI], 0.622–0.808; P < 0.001; Fig. S3). Under these conditions, specimens with IRS 0–8 and IRS 9–12 were classified as low and high expression of MGAT5 both in tumors and non‐tumors, respectively. After establishing the IHC assessment criteria in the training set, expression of MGAT5 in the testing and validation sets was scored in exactly the same way. Negative controls were included in all assays and treated identically but with the primary antibody omitted.
Statistical analysis
Statistical analysis was carried out using MedCalc Software (version 11.4.2.0; MedCalc, Mariakerke, Belgium). Numerical data were analyzed using Student's t‐test, whereas categorical data were studied using the χ 2‐test or Fisher's exact test. Cumulative survival time was calculated by the Kaplan–Meier method and analyzed by the log–rank test. Numbers at risk were calculated for the beginning of each time period. The Cox proportional hazards regression model was used to carry out univariate and multivariate analyses. Receiver operating characteristic (ROC) analysis was used to compare the sensitivity and specificity for the prediction of overall survival by the parameters. All P‐values were two sided, and differences were considered significant at values of P < 0.05. Results are reported according to the Reporting Recommendations for Tumor Marker Prognostic Studies guidelines.17
Results
Immunohistochemical findings
As shown in Figure 2, MGAT5 staining was mainly on the cytoplasm of gastric epithelia and cancer cells. Most of the stroma cells were negatively stained, although sporadic positive staining on these cells was also observed (Fig. 2). Compared with higher peritumoral MGAT5 density in gastric epithelial cells (Fig. 2a,b), intratumoral MGAT5 expression in gastric cancer cells decreased gradually, accompanied with disease progression from TNM stage I (Fig. 2c), TNM stage II (Fig. 2d), TNM stage III (Fig. 2e), to TNM stage IV (Fig. 2f). Collectively, these observations suggested that decreased MGAT5 expression in tumor cells might be associated with the progression of gastric cancer.
Figure 2.
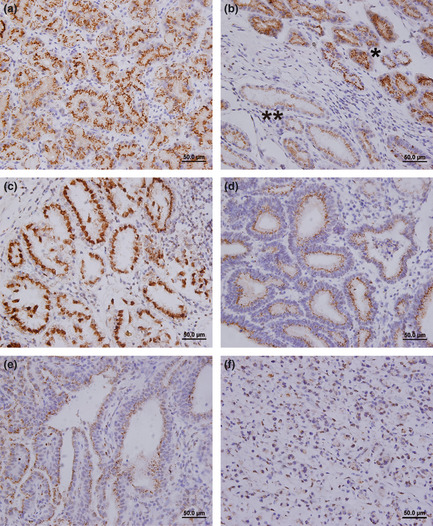
Representative photomicrographs of β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression in sections of gastric cancer and non‐tumor tissues. (a) Non‐tumor tissue shows strong expression of MGAT5. (b) Gastric cancer and adjacent peritumoral tissues. *Gastric peritumoral epithelial cells with strong expression of MGAT5. **Gastric cancer tissue with moderate MGAT5 expression. (c) TNM stage I, strong MGAT5 expression. (d) TNM stage II, moderate MGAT5 expression. (e) TNM stage III, moderate to weak MGAT5 expression. (f) TNM stage IV, weak MGAT5 expression. Scale bar = 50.0 μm.
Associations between MGAT5 expression and clinicopathologic features
Specimen tissues with an MGAT5 IRS value less than or equal to 8 were considered to have low MGAT5 expression. According to this criterion, approximately 54.64% (53 of 97), 51.59% (65 of 126), and 61.11% (55 of 90) tumors were scored with low MGAT5 expression in the training, testing, and validation sets, respectively. As shown in Table 2, intratumoral MGAT5 expression was associated with tumor cell differentiation (P = 0.030, P < 0.001, and P < 0.001, respectively), N classification (P = 0.009, P = 0.004, and P = 0.042, respectively), TNM stage (P = 0.006, P = 0.014, and P = 0.004, respectively), and Kiel stage (P = 0.026, P = 0.003, and P = 0.013, respectively) in three independent sets. In addition, in the training set, intratumoral MGAT5 expression was also associated with T classification (P = 0.013). In the testing and validation sets, intratumoral MGAT5 expression was associated with Lauren classification (P = 0.002 and P = 0.001, respectively). However, non‐tumor MGAT5 expression was not associated with any clinicopathologic factors of gastric cancer patients in the training or validation sets (Table S1).
Table 2.
Relation between intratumoral β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression and clinical characteristics in three independent sets of patients with gastric cancer
| Factor | Training set | Testing set | Validation set | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MGAT5 expression | MGAT5 expression | MGAT5 expression | |||||||
| High | Low | P | High | Low | P | High | Low | P | |
| All patients | 44 | 53 | 61 | 65 | 35 | 55 | |||
| Age, years† | 0.321 | 0.802 | 0.682 | ||||||
| ≤63 | 23 | 33 | 24 | 27 | 15 | 26 | |||
| >63 | 21 | 20 | 37 | 38 | 20 | 29 | |||
| Gender | 0.426 | 0.973 | 0.640 | ||||||
| Female | 14 | 21 | 18 | 19 | 8 | 15 | |||
| Male | 30 | 32 | 43 | 46 | 27 | 40 | |||
| Localization | 0.799 | 0.076 | 0.611 | ||||||
| Proximal | 4 | 6 | 18 | 10 | 9 | 16 | |||
| Middle | 22 | 23 | 18 | 30 | 9 | 18 | |||
| Distal | 18 | 24 | 25 | 25 | 17 | 21 | |||
| Differentiation | 0.030 | <0.001 | <0.001 | ||||||
| Well | 3 | 2 | 13 | 4 | 1 | 0 | |||
| Moderate | 22 | 14 | 23 | 11 | 22 | 14 | |||
| Poor | 19 | 37 | 25 | 50 | 12 | 41 | |||
| Lauren classification | 0.198 | 0.002 | 0.001 | ||||||
| Intestinal type | 35 | 36 | 50 | 37 | 31 | 30 | |||
| Diffuse type | 9 | 17 | 11 | 28 | 4 | 25 | |||
| T classification | 0.013 | 0.153 | 0.195 | ||||||
| T1 | 18 | 8 | 7 | 3 | 3 | 1 | |||
| T2 | 6 | 4 | 8 | 4 | 3 | 5 | |||
| T3 | 1 | 3 | 8 | 6 | 5 | 3 | |||
| T4 | 19 | 38 | 38 | 52 | 24 | 46 | |||
| N classification | 0.009 | 0.004 | 0.042 | ||||||
| N0 | 25 | 13 | 28 | 14 | 13 | 7 | |||
| N1 | 6 | 13 | 7 | 7 | 6 | 10 | |||
| N2 | 6 | 8 | 12 | 10 | 9 | 17 | |||
| N3 | 7 | 19 | 14 | 34 | 7 | 21 | |||
| Distant metastasis | 0.311 | 0.504 | 0.422 | ||||||
| No | 44 | 50 | 61 | 63 | 35 | 52 | |||
| Yes | 0 | 3 | 0 | 2 | 0 | 3 | |||
| TNM stage | 0.006 | 0.014 | 0.004 | ||||||
| I | 21 | 9 | 12 | 5 | 5 | 1 | |||
| II | 8 | 11 | 20 | 12 | 13 | 10 | |||
| III | 15 | 30 | 29 | 46 | 17 | 41 | |||
| IV | 0 | 3 | 0 | 2 | 0 | 3 | |||
| Tumor size, cm† | 0.137 | 0.576 | 0.248 | ||||||
| <3.5 | 29 | 27 | 27 | 32 | 10 | 10 | |||
| ≥3.5 | 15 | 26 | 34 | 33 | 25 | 45 | |||
| Kiel stage | 0.026 | 0.003 | 0.013 | ||||||
| I | 16 | 5 | 6 | 1 | 2 | 0 | |||
| II | 9 | 8 | 21 | 12 | 11 | 6 | |||
| IIIA | 2 | 5 | 2 | 3 | 4 | 3 | |||
| IIIB | 11 | 16 | 21 | 18 | 11 | 26 | |||
| IV | 6 | 19 | 11 | 31 | 7 | 20 | |||
Kiel stage, Kiel proposal of stage grouping. †Split at median.
Prognostic factors
Kaplan–Meier survival analysis indicated that the overall survival of gastric cancer patients with low intratumoral MGAT5 expression was significantly poorer than those patients with high MGAT5 intratumoral expression in the three independent sets (all P < 0.001; Fig. 3a–c). Univariate analysis of prognostic significance of intratumoral MGAT5 expression was carried out for overall survival in three independent sets of gastric cancer patients. As shown in Table S2, low intratumoral MGAT5 expression is a significant negative predictor for overall survival in the training set (hazard ratio [HR], 7.45; 95% CI, 2.60–21.33; P < 0.001), testing set (HR, 4.37; 95% CI, 2.23–8.55; P < 0.001), and validation set (HR, 4.65; 95% CI, 2.39–9.04; P < 0.001). To evaluate the robustness of the prognostic value of intratumoral MGAT5 expression, Cox multivariate regression analysis was carried out to derive risk estimates related to overall survival, with the same covariates of the training set showing significance in univariate analysis to the control for confounders. As shown in Table S3, intratumoral MGAT5 expression (P = 0.003, P < 0.001, P < 0.001, respectively) and TNM stage (P < 0.001, P < 0.001, P = 0.019, respectively) were both recognized as independent prognostic factors for overall survival in three independent sets. Moreover, we did the same analysis with inclusion of Kiel stage as a covariate and found that intratumoral MGAT5 expression was also an independent predictor of overall survival in the three independent sets (P = 0.003, P < 0.001, P < 0.001, respectively; Table S3), whereas Kiel stage was only identified as an independent prognostic factor in the training and testing sets (P < 0.001 and P = 0.001, respectively; Table S3). However, Kaplan–Meier survival analysis showed that non‐tumor MGAT5 expression was not associated with overall survival of gastric cancer patients in the training and validation sets (Table S4, Fig. S4).
Figure 3.
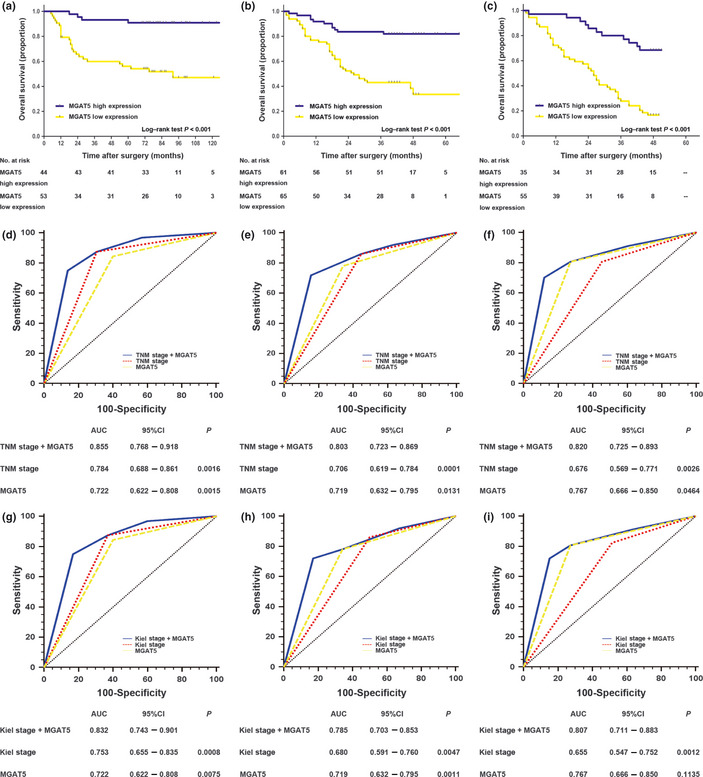
Analysis of overall survival according to the expression of intratumoral β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) in patients with gastric cancer. (A–C) Kaplan–Meier analyses of overall survival according to intratumoral MGAT5 expression in patients with gastric cancer in the training set (n = 97) (a), testing set (n = 126) (b), and validation set (n = 90) (c). (d–i) Receiver operating characteristic (ROC) analyses of the sensitivity and specificity for the prediction of overall survival by the combined MGAT5 expression and TNM stage or Kiel stage models, the TNM stage model alone, the Kiel stage model alone, and the MGAT5 expression model alone in the training set (n = 97) (d,g), testing set (n = 126) (e,h), and validation set (n = 90) (f,i). P‐values show the area under the ROC curves of the combined MGAT5 expression and TNM stage or Kiel stage models versus the area under the ROC curves of the TNM stage model, the Kiel stage model, or the MGAT5 expression model. CI, confidence interval.
Extension of current prognostic models with intratumoral MGAT5 expression
To improve the prognostic value of current predictive nomograms for overall survival, ROC analysis was carried out by integrating intratumoral MGAT5 expression into the TNM and Kiel staging systems. The combination of intratumoral MGAT5 expression and TNM stage (AUC [95% CI], 0.855 [0.768–0.918], 0.803 [0.723–0.869], and 0.820 [0.725–0.893], respectively) showed a better prognostic value than did TNM stage alone (AUC [95% CI], 0.784 [0.688–0.861], 0.706 [0.619–0.784], and 0.676 [0.569–0.771], respectively) or intratumoral MGAT5 expression alone (AUC [95% CI], 0.722 [0.622–0.808], 0.719 [0.632–0.795], and 0.767 [0.666–0.850], respectively) in three independent sets (Fig. 3d–f). Moreover, the combination of intratumoral MGAT5 expression and Kiel stage (AUC [95% CI], 0.832 [0.743–0.901], 0.785 [0.703–0.853], and 0.807 [0.711–0.883], respectively) also showed a better prognostic value than did Kiel stage alone (AUC [95% CI], 0.753 [0.655–0.835], 0.680 [0.591–0.760], and 0.655 [0.547–0.752], respectively) or intratumoral MGAT5 expression alone (AUC [95% CI], 0.722 [0.622–0.808], 0.719 [0.632–0.795], and 0.767 [0.666–0.850], respectively) alone in three independent sets (Fig. 3g–i).
Improvement of late‐stage stratification with intratumoral MGAT5 expression
To investigate whether intratumoral MGAT5 expression could stratify patients within each TNM stage stratum, we evaluated the prognostic value of intratumoral MGAT5 expression in each stratum. As shown in Table 3, only the patients within TNM stage III could be significantly stratified by intratumoral MGAT5 expression (P = 0.010, P < 0.001, and P < 0.001, respectively) in three independent sets. The analysis of TNM stage IV was not applicable, because there were only eight patients within it, and all showed low intratumoral MGAT5 expression. We further combined TNM stages I and II as early‐stage disease, and TNM stages III and IV as late‐stage disease. We then found that among patients with late‐stage gastric cancer, the overall survival differed significantly according to intratumoral MGAT5 expression (P = 0.003, P < 0.001, and P < 0.001, respectively; Table 3; Fig. S5A–C) in three independent sets, but early‐stage disease did not. In order to validate this result, we did the same analysis under the Kiel staging system and drew the same conclusion, that only the overall survival of patients with Kiel stage IIIB and IV disease could be significantly stratified by intratumoral MGAT5 expression (P = 0.003, P < 0.001, and P < 0.001, respectively; Table 3; Fig. S5G–I). In addition, ROC analysis showed that intratumoral MGAT5 expression had an excellent ability to discriminate the outcomes of patients with late‐stage gastric cancer (Fig. S5D–F, S5J–L).
Table 3.
Log–rank test on overall survival for TNM stage and Kiel stage split by intratumoral β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression in three independent sets
| Factor | Overall survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Training set | Testing set | Validation set | |||||||
| Patients | Patients | Patients | |||||||
| n | % | P | n | % | P | n | % | P | |
| All patients | 97 | 100 | 126 | 100 | 90 | 100 | |||
| TNM stage I | 30 | 30.93 | 17 | 13.49 | 6 | 6.67 | |||
| MGAT5 expression | 0.127 | 0.468 | 0.247 | ||||||
| High | 21 | 21.65 | 12 | 9.53 | 5 | 5.56 | |||
| Low | 9 | 9.28 | 5 | 3.96 | 1 | 1.11 | |||
| TNM stage II | 19 | 19.59 | 32 | 25.40 | 23 | 25.56 | |||
| MGAT5 expression | 0.217 | 0.963 | 0.213 | ||||||
| High | 8 | 8.25 | 20 | 15.87 | 13 | 14.45 | |||
| Low | 11 | 11.34 | 12 | 9.53 | 10 | 11.11 | |||
| TNM stage III | 45 | 46.39 | 75 | 59.52 | 58 | 64.44 | |||
| MGAT5 expression | 0.010 | <0.001 | <0.001 | ||||||
| High | 15 | 15.46 | 29 | 23.02 | 17 | 18.89 | |||
| Low | 30 | 30.93 | 46 | 36.50 | 41 | 45.55 | |||
| TNM stage IV | 3 | 3.09 | 2 | 1.59 | 3 | 3.33 | |||
| MGAT5 expression | NA | NA | NA | ||||||
| High | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |||
| Low | 3 | 3.09 | 2 | 1.59 | 3 | 3.33 | |||
| TNM stage I+II | 49 | 50.52 | 49 | 38.89 | 29 | 32.22 | |||
| MGAT5 expression | 0.149 | 0.646 | 0.117 | ||||||
| High | 29 | 29.90 | 32 | 25.40 | 18 | 20.00 | |||
| Low | 20 | 20.62 | 17 | 13.49 | 11 | 12.22 | |||
| TNM stage III+IV | 48 | 49.48 | 77 | 61.11 | 61 | 67.77 | |||
| MGAT5 expression | 0.003 | <0.001 | <0.001 | ||||||
| High | 15 | 15.46 | 29 | 23.02 | 17 | 18.89 | |||
| Low | 33 | 34.02 | 48 | 38.09 | 44 | 48.88 | |||
| Kiel stage I+II+IIIA | 45 | 46.39 | 45 | 35.71 | 26 | 28.89 | |||
| MGAT5 expression | 0.139 | 0.705 | 0.152 | ||||||
| High | 27 | 27.83 | 29 | 23.02 | 17 | 18.89 | |||
| Low | 18 | 18.56 | 16 | 12.69 | 9 | 10.00 | |||
| Kiel stage IIIB+IV | 52 | 53.61 | 81 | 64.29 | 64 | 71.11 | |||
| MGAT5 expression | 0.003 | <0.001 | <0.001 | ||||||
| High | 17 | 17.52 | 32 | 25.40 | 18 | 20.00 | |||
| Low | 35 | 36.09 | 49 | 38.89 | 46 | 51.11 | |||
Kiel stage, Kiel proposal of stage grouping; NA, not applicable.
Refinement of subgroup stratification with intratumoral MGAT5 expression
To further characterize whether intratumoral MGAT5 expression could refine patient stratification within different gastric cancer subgroups, we stratified the participants of three independent sets by human epidermal growth factor receptor 2 (HER2) classification of positive or negative,18 histological subtype of differentiated or undifferentiated, and Lauren classification of intestinal type or diffuse type. The intratumoral MGAT5 expression could strongly predict patient survival within each gastric cancer subtype in all three independent sets (HER2 positive: P = 0.018, P = 0.025, and P = 0.027, respectively; HER2 negative: P < 0.001, P < 0.001, and P < 0.001, respectively; differentiated: P < 0.001, P = 0.012, and P = 0.007, respectively; undifferentiated: P = 0.029, P < 0.001, and P < 0.001, respectively; intestinal type: P < 0.001, P < 0.001, and P < 0.001, respectively; diffuse type: P = 0.041, P = 0.019, and P = 0.049, respectively; Table S5, Figs S6–S8).
Discussion
To our knowledge, this is the first study to identify low intratumoral MGAT5 expression as an independent poor prognostic factor for overall survival following gastrectomy for gastric cancer. In order to robustly validate our results, we used three independent datasets from two clinical centers. Moreover, in this study, incorporation of intratumoral MGAT5 density into current clinicopathologic predictive models improved prognostic value for overall survival. In addition, intratumoral MGAT5 expression had good discriminatory power as a supplementary risk factor in patients with late‐stage gastric cancer. Therefore, patients with low intratumoral MGAT5 expression should have aggressive therapies and a closer follow‐up. Intratumoral MGAT5 expression might also serve as a new stratification factor for randomized controlled clinical trials. However, the results of integration of intratumoral MGAT5 expression into current prognostic models and the potential clinical practice changing should be validated in an independent and larger dataset.
Profound alterations in cellular glycosylation contribute to malignant transformation and cancer progression in various tumors.19 During early stages of malignant transformation of gastric epithelia, the loss of epithelial cell polarity results in the expression of O‐glycosylated carcinoma mucins, which can block cell–cell interactions by steric hindrance through glycans.20, 21 Thus, the early stages of malignant transformation of gastric epithelial cells might be only the individual cell behavior. During late stages of cancer progression such as tumor invasion and metastasis, aberrant N‐glycan glycosylation may affect the cross‐talk between the tumor cells and play vital roles in tumor progression. Among the N‐glycosyltransferases involved in biosynthesis of oligosaccharides, MGAT5 is one of the most intensively characterized enzymes, which had been shown to be relevant with cancer progression in different tumor entities. 9, 10, 11 Although Tian and colleagues showed previously that MGAT5 expression might be correlated with a poor prognosis in gastric cancer patients due to metastases, and the survival rate was only significantly different between MGAT5‐positive and ‐negative patients with TNM stage I and II gastric cancer,22 our present study indicates that low intratumoral MGAT5 expression was correlated with poor prognosis in patients with late‐stage gastric cancer, but not in patients with early‐stage disease. These contrary findings might reflect the different genetic background of patients with gastric cancer, and the profound molecular roles of MGAT5 in gastric cancer progression remain far from fully understood and merit further investigation.
The expression level of MGAT5 in the original tissue might determine its clinical implications in tumor malignancy.13 Enhanced expression of MGAT5 was detected in carcinomas of mammary and colon, which showed no MGAT5 expression in the respective normal epithelia.9, 10, 11 However, MGAT5 expression was detected in a majority of normal tissues, including lung, bladder, and stomach.23 In the cancers originated from such tissues, MGAT5 expression might be a good predictor. But the mechanisms underlying the aberrant expression of MGAT5 in gastric cancer remain poorly determined and await further characterization.
The TNM classification of gastric cancer is an essential guide to treatment selection and enables the evaluation of therapeutic options.24 Recently, Warneke and colleagues proposed a revised staging system, named the Kiel proposal of stage grouping of gastric cancer, in order to provide an exact distinction of lymph node‐positive and lymph node‐negative patients (Fig. S1).5 However, there is a prominent heterogeneity among patients within the same stage.6 New molecular markers are urgently needed to lead a more accurate classification of patients with gastric cancer. Patients with late‐stage gastric cancer classified TNM stages III and IV have emerged as the standard patient populations to be included in gastric cancer randomized controlled clinical trials.18, 25, 26 If clinicians could have a better handle on the molecular profile of patients, they would provide them with an optimal initial treatment. In the present study, patients with late‐stage gastric cancer could be significantly stratified by intratumoral MGAT5 expression. This could potentially lead to a more accurate classification under the TNM staging system and suggest that patients with high intratumoral MGAT5 expression might have a more favorable prognosis if surgery is done first, whereas others might benefit from aggressive adjuvant therapies before and after surgery, although the role of adjuvant therapy is controversial.27 Moreover, patients of noted heterogeneity within different gastric cancer subgroups included in randomized controlled clinical trials, such as HER2 positive/negative, differentiated/undifferentiated, and intestinal type/diffuse type, could also be significantly stratified by intratumoral MGAT5 expression. Collectively, the above analyses indicate that patients with gastric cancer should be prestratified before clinicians determine their initial treatment strategies.
In summary, our results show that decreased intratumoral MGAT5 expression independently predicts poor postoperative overall survival of patients with gastric cancer. Integration of intratumoral MGAT5 density into current clinicopathologic TNM or Kiel staging systems might add prognostic information for patient survival, refine stratification of late‐stage gastric cancer, and ultimately improve gastric cancer outcome.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Table S1. Relation between non‐tumor β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression and clinical characteristics in the training set and validation set.
Table S2. Univariate Cox regression analyses for overall survival in three independent sets.
Table S3. Multivariate Cox regression analyses for overall survival in three independent sets.
Table S4. Log–rank test on overall survival for non‐tumor β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression in the training set and validation set.
Table S5. Log–rank test on overall survival in three independent sets of gastric cancer subtypes.
Fig. S1. Kiel proposal of stage grouping and TNM classification of gastric cancer.
Fig. S2. β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) antibody specificity in human gastric cancer cells.
Fig. S3. Optimal cut‐off value of β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) immunoreactivity scoring was obtained by receiver operating characteristic analysis.
Fig. S4. Comparison of overall survival in gastric cancer patients according to non‐tumor β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression.
Fig. S5. Analysis of overall survival according to the expression of intratumoral β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) in patients with late‐stage gastric cancer.
Fig. S6. Kaplan–Meier analyses for overall survival according to intratumoral β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression in patients of gastric cancer with differential human epidermal growth factor receptor 2 (HER2) classification.
Fig. S7. Kaplan–Meier analyses for overall survival according to intratumoral β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression in patients of gastric cancer with differential histological types.
Fig. S8. Kaplan–Meier analyses for overall survival according to intratumoral β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression in patients of gastric cancer with differential Lauren classification.
Acknowledgments
The authors would like to thank Haiying Zeng (Fudan University, Shanghai, China) for experimental technical assistance, and Dr. Jianguo Gu (Tohoku Pharmaceutical University, Miyagi, Japan) for the generous gift of plasmid. This work was supported by grants from the National Basic Research Program of China 973 Program (Grant Nos 2012CB822104, 2010CB912104), the State Key Project Specialized for Infectious Diseases of China (Grant Nos 2012ZX10002‐008, 2012ZX10002‐012), the National High‐Tech R&D 863 Program (Grant No. 2012AA020203), the National Natural Science Fund (Grant Nos 30930025, 31010103906, 31170766, 31100629, 31270863), and the Key Project of Science and Technology Commission of Shanghai Municipality (Grant Nos 09DZ1950101, 11411951000).
(Cancer Sci, doi: 10.1111/cas.12049, 2012)
References
- 1. Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet 2009; 374: 477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 3. Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 2010; 17: 3077–9. [DOI] [PubMed] [Google Scholar]
- 4. Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14: 113–23. [DOI] [PubMed] [Google Scholar]
- 5. Warneke VS, Behrens HM, Hartmann JT et al Cohort study based on the seventh edition of the TNM classification for gastric cancer: proposal of a new staging system. J Clin Oncol 2011; 29: 2364–71. [DOI] [PubMed] [Google Scholar]
- 6. Lim L, Michael M, Mann GB, Leong T. Adjuvant therapy in gastric cancer. J Clin Oncol 2005; 23: 6220–32. [DOI] [PubMed] [Google Scholar]
- 7. Raman R, Raguram S, Venkataraman G, Paulson JC, Sasisekharan R. Glycomics: an integrated systems approach to structure‐function relationships of glycans. Nat Methods 2005; 2: 817–24. [DOI] [PubMed] [Google Scholar]
- 8. Dube DH, Bertozzi CR. Glycans in cancer and inflammation–potential for therapeutics and diagnostics. Nat Rev Drug Discov 2005; 4: 477–88. [DOI] [PubMed] [Google Scholar]
- 9. Fernandes B, Sagman U, Auger M, Demetrio M, Dennis JW. Beta 1‐6 branched oligosaccharides as a marker of tumor progression in human breast and colon neoplasia. Cancer Res 1991; 51: 718–23. [PubMed] [Google Scholar]
- 10. Seelentag WK, Li WP, Schmitz SF et al Prognostic value of beta1,6‐branched oligosaccharides in human colorectal carcinoma. Cancer Res 1998; 58: 5559–64. [PubMed] [Google Scholar]
- 11. Murata K, Miyoshi E, Kameyama M et al Expression of N‐acetylglucosaminyltransferase V in colorectal cancer correlates with metastasis and poor prognosis. Clin Cancer Res 2000; 6: 1772–7. [PubMed] [Google Scholar]
- 12. Granovsky M, Fata J, Pawling J, Muller WJ, Khokha R, Dennis JW. Suppression of tumor growth and metastasis in Mgat5‐deficient mice. Nat Med 2000; 6: 306–12. [DOI] [PubMed] [Google Scholar]
- 13. Dosaka‐Akita H, Miyoshi E, Suzuki O, Itoh T, Katoh H, Taniguchi N. Expression of N‐acetylglucosaminyltransferase V is associated with prognosis and histology in non‐small cell lung cancers. Clin Cancer Res 2004; 10: 1773–9. [DOI] [PubMed] [Google Scholar]
- 14. Ishimura H, Takahashi T, Nakagawa H et al N‐acetylglucosaminyltransferase V and beta1‐6 branching N‐linked oligosaccharides are associated with good prognosis of patients with bladder cancer. Clin Cancer Res 2006; 12: 2506–11. [DOI] [PubMed] [Google Scholar]
- 15. Zhu XD, Zhang JB, Zhuang PY et al High expression of macrophage colony‐stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 2008; 26: 2707–16. [DOI] [PubMed] [Google Scholar]
- 16. Weichert W, Roske A, Gekeler V et al Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol 2008; 9: 139–48. [DOI] [PubMed] [Google Scholar]
- 17. McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 2005; 23: 9067–72. [DOI] [PubMed] [Google Scholar]
- 18. Bang YJ, van Cutsem E, Feyereislova A et al Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet 2010; 376: 687–97. [DOI] [PubMed] [Google Scholar]
- 19. Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta 1999; 1473: 21–34. [DOI] [PubMed] [Google Scholar]
- 20. Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 2004; 4: 45–60. [DOI] [PubMed] [Google Scholar]
- 21. Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer 2005; 5: 526–42. [DOI] [PubMed] [Google Scholar]
- 22. Tian H, Miyoshi E, Kawaguchi N et al The implication of N‐acetylglucosaminyltransferase V expression in gastric cancer. Pathobiology 2008; 75: 288–94. [DOI] [PubMed] [Google Scholar]
- 23. Perng GS, Shoreibah M, Margitich I, Pierce M, Fregien N. Expression of N‐acetylglucosaminyltransferase V mRNA in mammalian tissues and cell lines. Glycobiology 1994; 4: 867–71. [DOI] [PubMed] [Google Scholar]
- 24. Japanese Gastric Cancer Association . Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14: 101–12. [DOI] [PubMed] [Google Scholar]
- 25. Bang YJ, Kim YW, Yang HK et al Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open‐label, randomised controlled trial. Lancet 2012; 379: 315–21. [DOI] [PubMed] [Google Scholar]
- 26. Lee J, Lim do H, Kim S et al Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012; 30: 268–73. [DOI] [PubMed] [Google Scholar]
- 27. Yoshikawa T, Sasako M. Gastrointestinal cancer: adjuvant chemotherapy after D2 gastrectomy for gastric cancer. Nat Rev Clin Oncol 2012; 9: 192–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Relation between non‐tumor β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression and clinical characteristics in the training set and validation set.
Table S2. Univariate Cox regression analyses for overall survival in three independent sets.
Table S3. Multivariate Cox regression analyses for overall survival in three independent sets.
Table S4. Log–rank test on overall survival for non‐tumor β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression in the training set and validation set.
Table S5. Log–rank test on overall survival in three independent sets of gastric cancer subtypes.
Fig. S1. Kiel proposal of stage grouping and TNM classification of gastric cancer.
Fig. S2. β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) antibody specificity in human gastric cancer cells.
Fig. S3. Optimal cut‐off value of β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) immunoreactivity scoring was obtained by receiver operating characteristic analysis.
Fig. S4. Comparison of overall survival in gastric cancer patients according to non‐tumor β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression.
Fig. S5. Analysis of overall survival according to the expression of intratumoral β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) in patients with late‐stage gastric cancer.
Fig. S6. Kaplan–Meier analyses for overall survival according to intratumoral β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression in patients of gastric cancer with differential human epidermal growth factor receptor 2 (HER2) classification.
Fig. S7. Kaplan–Meier analyses for overall survival according to intratumoral β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression in patients of gastric cancer with differential histological types.
Fig. S8. Kaplan–Meier analyses for overall survival according to intratumoral β1, 6‐N‐acetylglucosaminyltransferase V (MGAT5) expression in patients of gastric cancer with differential Lauren classification.


