Abstract
The prognosis of patients with advanced diffuse‐type gastric cancer (GC), especially scirrhous gastric cancer (SGC) remains extremely poor. Peritoneal carcinomatosis is a frequent form of metastasis of SGC. With survival rates of patients with peritoneal metastasis at 3 and 5 years being only 9.8% and 0%, respectively, development of a new treatment is urgently crucial. For such development, the establishment of a therapeutic mouse model is required. Among the 11 GC cell lines we examined, HSC‐60 showed the most well‐preserved expression profiles of the Hedgehog and epithelial‐mesenchymal transition pathways found in primary SGCs. After six cycles of harvest of ascitic tumor cells and their orthotopic inoculation in scid mice, a highly metastatic subclone of HSC‐60, 60As6 was obtained, by means of which we successfully developed peritoneal metastasis model mice. The mice treated with small interfering (si) RNA targeting NEDD1, which encodes a gamma‐tubulin ring complex‐binding protein, by the atelocollagen‐mediated delivery system showed a significantly prolonged survival. Our mouse model could thus be useful for the development of a new therapeutic modality. Intraperitoneal administration of siRNAs of targeted genes such as NEDD1 could provide a new opportunity in the treatment of the peritoneal metastasis of SGC.
Gastric cancer (GC) is one of the leading causes of cancer‐related death worldwide.1, 2 Histopathological research has long suggested that gastric cancer is not a single disease and recognizes two major categories: intestinal and diffuse.3 Intestinal‐type GC develops through some sequential stages including Helicobacter pylori (H. pylori)‐associated gastritis, intestinal metaplasia (IM), and dysplasia. This type predominates in high‐risk geographic areas, such as East Asia, showing a correlation with the prevalence there of H. pylori infection among elderly people. Diffuse‐type GC, however, is more uniformly distributed geographically, is apparently unrelated to H. pylori prevalence and typically develops from H. pylori‐free, morphologically normal gastric mucosa without atrophic gastritis, or IM. Unlike the decreasing incidence of the intestinal‐type, the prevalence of the diffuse‐type is reportedly increasing worldwide.4 Although therapeutic results for GC have recently improved, the prognosis for patients with advanced diffuse‐type GC, especially scirrhous gastric cancer (SGC, Borrmann's type IV carcinoma or the linitis plastic type) remains extremely poor. The 5‐year overall survival rate of SGC is approximately 10%, and ranges from 18% to 29% even after curative surgery.5, 6, 7 Histopathologically, SGC does not form glands; instead, it causes diffuse infiltration of a broad region of the gastric wall rather than a well‐defined mass, resulting in a fibrous‐like thickening of the wall. Such pathological features make an early clinical diagnosis of SGC difficult, and in approximately half of the cases, by the time the diagnosis is made, peritoneal dissemination has, unfortunately, already occurred.5, 8 Peritoneal dissemination, known to be a frequent form of metastasis and recurrence of SGC, serves as a major factor determining patient prognosis.9 Currently, no effective therapy exists for this condition. For SGC patients with peritoneal metastasis, the survival rates at 3 and 5 years are only 9.8% and 0%, respectively, even if the patients received multidisciplinary treatment.5
It has been suggested that peritoneal dissemination is a consequence of free cancer cells that are shed from the serosa of the primary lesion and/or may leak out from the lymphatics to the peritoneal cavity; however, no detailed mechanism of peritoneal dissemination has been fully elucidated. In either situation, it is assumed that free cancer cells detached from a primary lesion must have a predilection for the peritoneum. Efficacious control of invisible free cancer cells in the peritoneal cavity should help suppress the progression of carcinomatous peritonitis, and could ultimately yield a survival benefit. Some investigators have reported good, but limited, outcomes with new treatment strategies for peritoneal dissemination, including systemic chemotherapy,10 intraperitoneal (i.p.) chemotherapy and/or hyperthermia,11 and peritonectomy.12 Therefore, to improve patient outcome, the development of a new therapeutic strategy for peritoneal dissemination of SGC is urgently crucial.
In this study, we developed peritoneal metastasis model mice of SGC and an atelocollagen‐mediated delivery system for i.p. administration of small interfering (si) RNA, and also reported that the i.p. delivery of an siRNA targeting NEDD1, which functions in the metaphase regulation of the cell cycle, was able to regress the tumor and prolong, without toxicity, the survival of the mice.
Materials and Methods
Tissue samples
Gastric cancer and non‐cancerous tissues were provided by the National Cancer Center Hospital (Tokyo, Japan) after obtaining informed consent from each patient and approval by the Center's Ethics Committee. Tissue specimens were immediately frozen with liquid nitrogen after surgical extraction, and stored at −80°C until use.
Cell lines and culture
A human scirrhous gastric cancer cell line, HSC‐60 was established by a collaborator using the procedure as described.13 A highly peritoneal‐seeding cell line, 60As6 was established from HSC‐60 using orthotopic tissue implantation into scid mice as briefly follows: the xenografted tumor of HSC‐60 cells was transplanted into the gastric wall of a scid mouse. We repeated six cycles of harvest of ascitic tumor cells and the orthotopic inoculation of these cells, in turn, into the animals to establish a highly metastatic 60As6 cell line. These two cell lines were maintained in an RPMI1640 medium supplemented with 10% FCS. In this study, we also used luciferase‐ or green fluorescence protein (GFP)‐expressing transfectants. Another 11 GC‐derived cell lines (HSC‐39, HSC‐43, HSC‐44, HSC‐58, HSC‐59, HSC‐60, KATOIII, MKN7, MKN28, MKN74, and HSC‐57) were also maintained in the same way. Of them, seven HSC cell lines were established by a collaborator using the procedure as described,13 and four other cell lines were obtained from American Type Culture Collection.
In vivo photon counting analysis
To establish transfectants expressing the luciferase gene, plasmid vectors carrying the firefly luciferase gene named pLuc/Neo and a transfection reagent, LipofectAMINE 2000 (Invitrogen, Carlsbad, CA, USA) were used in accordance with the manufacturer's instructions. Stable transfectants were selected in geneticin (500 μg/mL; Invitrogen) and bioluminescence was used to screen the transfected clones for luciferase gene expression using the IVIS system (Xenogen, Alameda, CA, USA). In vivo photon counting and optical imaging to detect luciferase activity in the mice were conducted on the IVIS system as described previously.14 Animal protocols were approved by the committee for Ethics of Animal Experimentation and were in accordance with the Guideline for Animal Experiments at the National Cancer Center.
siRNA preparation
The sequence of NEDD1 siRNA was 5′‐CGAAGUGUUAAUGUGAAUGtt‐3′ and 3′‐ttGCUUCACAAUUACACUUAC‐5′ (Ambion, Austin, TX, USA). Non‐specific control siRNA duplex and luciferase GL3 siRNA duplex were purchased from Dharmacon (Lafayette, CO, USA). ELK1 siRNA, 5′‐GCUGAGAGAGCAAGGCAAUtt‐3′and 5′‐AUUGCCUUGCUCUCUCAGCtt‐3′ (SI00300146, Qiagen, Valencia, CA, USA) and MSX2 siRNA, 5′‐CCAUUACCUAUAUGCUAAAt t‐3′ and 5′‐UUUAGCAUAUAGGUAAUGGtt‐3′(SI00038031, Qiagen) were used. For in vitro studies, 5 × 104 cells were seeded per 6‐well culture dish. When cells had grown to approximately 80% confluency, a mixture of 3 μg siRNA and 5 μL DharmaFECT (Dharmacon) was added to the medium in each dish.
Therapeutic studies with NEDD1 siRNA
Intraperitoneal (i.p.) injection of 60As6Luc cells resuspended in 1 mL PBS was conducted in 6‐week‐old female C.B17/Icr‐scid (scid/scid) mice, followed by i.p. inoculation of various siRNA/atelocollagen complexes. Atelocollagen is a highly purified type I collagen of calf dermis with pepsin treatment (Koken, Tokyo, Japan). The siRNA/atelocollagen complexes were prepared as follows: an equal volume of atelocollagen (pH 7.4) and an siRNA solution were mixed by rotation at 4°C for 20 min. Before i.p. inoculation, 10 μL of DharmaFECT1 was added to the complex. The final mixture was 1 mL containing 50 μg siRNA, 10 μL DharmaFECT1 and 0.5% atelocollagen. For obtaining delivery evidence of siRNA to cancer cells in the peritoneal cavity, we used a fluorescence‐labeled human siGLO LaminA/C Control siRNA (Thermo Fisher Scientific, Rockford, IL, USA).
Laser microdissection, RNA extraction and RT‐PCR
In the gastric corpus, the epithelium consists of three tubular units from surface to base: a pit region containing mucus‐secreting pit cells, an isthmus/neck region containing stem cells, and a gland region containing chief and parietal cells.15 We prepared each region (pit, neck, and gland) as follows: the cryostat sections (8 μm) of frozen tissues were microdissected with a Pixcell II LCM system (Arcturus Engineering, Mountain View, CA, USA). Total RNA was isolated by suspending the cells in an ISOGEN lysis buffer (Nippon Gene, Toyama, Japan) followed by precipitation with isopropanol. The mRNA was amplified by an efficient method of high‐fidelity mRNA amplification.16, 17 Other normal and gastric cancer tissues were provided by our hospital between 2003 and 2004 after obtaining informed consent from each patient and approval by the Institutional Ethics Committee. Tissue specimens were snap‐frozen in liquid nitrogen, and stored at −80°C until use. Total RNA was isolated by suspending the cells in an ISOGEN lysis buffer followed by precipitation with isopropanol. As described in our previous report,18 semi‐quantitative RT‐PCR within linear range by performing 25–35 cycles for IHH, DHH, SHH, GLI1, GLI2, PTCH, SMO, SIP1, TWIST2, ISL1, BMP4, FOXM1, FOXA2, FN1, EDNRA, and ACTB (β‐Actin) was carried out. For NEDD1, 5′‐TTCTGTCACTGCTGGAGTTG‐3′ and 5′‐TGTGTTGCCAGAAACTTCCC‐3′ were used as primers.
Western blot analysis
The proteins (20 μg) were separated on a 10% SDS‐polyacrylamide gel and transferred to an Immobilon‐P membrane (Millipore, Billerica, MA, USA). The blots were incubated overnight with mouse monoclonal anti‐human Nedd1 antibody (Abcam, Cambridge, MA, USA).
Statistical analysis
All data were expressed as the mean ± SE, and analyzed using the unpaired t‐test. Survival curves were calculated according to the Kaplan–Meier method. Differences between survival curves were examined with the log rank test. The accepted level of significance was P < 0.05. spss (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
Establishment of a highly metastatic cell line 60As6 from a parental cell line HSC‐60
We previously reported that the hedgehog signal is more active in diffuse‐type gastric cancer (GC) including scirrhous GC (SGC) than in the intestinal‐type GC and recently reported that crossroad between hedgehog and epithelial‐mesenchymal transition (EMT) signals is present in the diffuse‐type. 17, 18 Among 11 GC‐derived cell lines (HSC‐39, HSC‐43, HSC‐44, HSC‐58, HSC‐59, HSC‐60, KATOIII, MKN7, MKN28, MKN74, and HSC‐57), HSC‐60 was found to most‐closely mimic the diffuse‐type GC phenotype in mRNA expression of hedgehog‐ and EMT‐related genes.18 However, HSC‐60 cells often formed only a single tumor nodule in the peritoneal cavity and no ascites in scid mice despite intraperitoneal (i.p.) implantation of many cells (more than 1 × 106 cells) (data not shown). Therefore, we established a highly peritoneal‐seeding cell line, 60As6, from this parental cell line, HSC‐60, by six cycles of isolating ascitic tumor cells and orthotopic inoculation of these cells as described in our previous report,13 and we next obtained transfectants (HSC‐60Luc and 60As6Luc) containing the luciferase gene for in vivo imaging in animal experiments. Reverse transcription‐PCR showed that the expression of the abovementioned hedgehog and EMT signaling genes in HSC‐60 cells was maintained in the 60As6 cells (Fig. 1a). The doubling time of these two cell lines was comparable (30 h in HSC‐60 and 31 h in 60As6). The peritoneal dissemination and the survival rates of scid mice after i.p. implantation of HSC‐60 and 60As6 are shown (Fig. 1b–f). None of the HSC‐60‐tumor‐bearing mice developed ascites (Fig. 1b,c), and the median mice survival time was 167 days after implantation of 5 × 106 HSC‐60Luc cells (Fig. 1f). On the other hand, implantation of 5 × 106 60As6Luc cells resulted in the formation of remarkably bloody ascites approximately 14 days later (Fig. 1d), and the median survival time was 28 days (Fig. 1f). In the 60As6‐tumor‐bearing mice, peritoneal dissemination was often seen in the omentum, mesenterium, parietal peritoneum, diaphragm, and so on (Fig. 1e).
Figure 1.
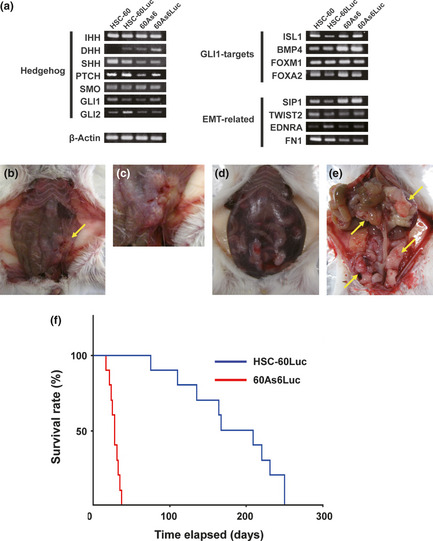
Characteristics of HSC‐60 and 60As6 cells. (a) mRNA expression of hedgehog‐ and epithelial‐mesenchymal transition (EMT)‐related gene in HSC‐60 and 60As6. Shown are results of reverse transcription‐polymerase chain reaction (RT‐PCR) of hedgehog ligands (IHH, DHH, and SHH), a receptor (PTCH), a modulator (SMO), two primary target transcriptional factors (GLI1 and GLI2), four authentic GLI1‐targets (ISL1, BMP4, FOXM1, and FOXA2), two EMT regulators (SIP1 and TWIST2), two EMT‐related molecules (FN1 and EDNRA), and a control (β‐Actin). (b, c) Macroscopic appearance of the peritoneal dissemination and survival of scid mice after intraperitoneal injection of HSC‐60Luc and 60As6Luc. A few peritoneal nodules are observed 15 weeks after i.p injection of HSC‐60Luc. Yellow arrow: tumor nodule. (d, e) Carcinomatous peritonitis forming multiple tumor nodules observed 2 weeks after i.p. injection of 60As6. Abdominal distension because of bloody ascites was evident. Yellow arrow: tumor nodule. (f) Survival of HSC‐60Luc‐ and 60As6Luc‐tumor‐bearing mice. Each median survival time is 28 days and 167 days, respectively. n = 10; P < 0.0001.
Development of an siRNA delivery system into peritoneal metastatic tumor cells
The atelocollagen‐mediated gene delivery system was originally developed by a collaborator.19 In mice, this delivery system has been reported to be useful for gene delivery into some body sites including metastatic tumors and also for systemic gene delivery;20 however, its application into a peritoneal metastatic tumor has not been reported. Previous studies indicated that a low concentration (0.05%) of atelocollagen was effective for systemic siRNA delivery, whereas a high concentration (0.5%) was useful for an intratumor siRNA delivery,20 and also indicated that a transfection reagent, DharmaFECT1 accelerated an atelocollagen‐mediated siRNA delivery.20 We first investigated an optimal atelocollagen concentration for an i.p. siRNA delivery into tumor cells by measuring luciferase activity. Those between a 50 μg luciferase GL3 siRNA/0.05%atelocollagen/DharmaFECT1 complex and a 50 μg luciferase GL3 siRNA/0.5%atelocollagen/DharmaFECT1 complex 48 h after i.p. injection were compared in scid mice that had 1 × 106 60As6Luc cells introduced into the peritoneal cavity. Both of the two complexes clearly reduced the luciferase activity compared with an untransfected control (Fig. 2a), and the 0.5% atelocollagen complex rather than the 0.05% complex reduced it significantly (Fig. 2a). However, a 50 μg luciferase GL3 siRNA/0.5%atelocollagen only complex did not inhibit luciferase activity (Fig. 2b). The reduction of the luciferase activity 24 h after injection of a 50 μg luciferase GL3 siRNA/0.5%atelocollagen/DharmaFECT1 complex was visualized in four scid mice (Fig. 2c, right), and the significant reduction of the photon counts was shown (Fig. 2c, left).
Figure 2.
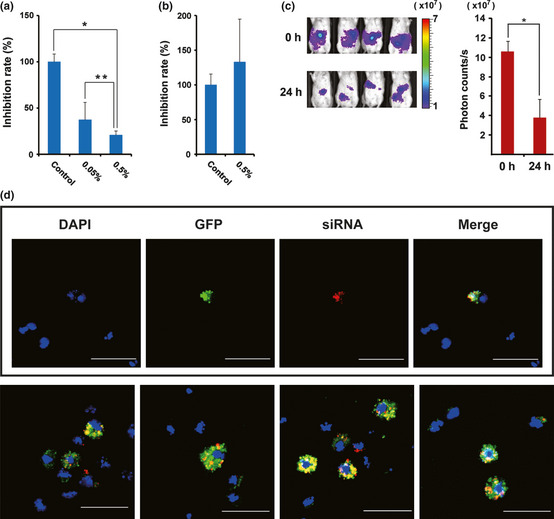
Evaluation of an atelocollagen‐mediated siRNA delivery system by measuring the luciferase activity after i.p injection of luciferase siRNA. (a) Inhibition rates of photon counts 48 h after injection are compared between 0.05% and 0.5% atelocollagen/luciferase siRNA/DharmaFECT1. *P = 0.006; **P = 0.012. (b) Inhibition rates of photon counts 48 h after injection are compared between 0.5% atelocollagen/control siRNA and 0.5% atelocollagen/luciferase siRNA. No significant inhibition is observed in a luciferase siRNA complex containing no DharmaFECT1. (c) Reduction of luciferase activity 24 h after injection with 0.5% atelocollagen/luciferase siRNA/DharmaFECT1 is visualized in four representative mice (left). Significance of the reduction is also shown (right). *P < 0.0001. Color bar indicates × 107 photon/s. (d) Delivery evidence of siRNA to cancer cells in the peritoneal cavity. Most green fluorescent protein (GFP)‐expressing 60As6 cells (green) incorporate fluorescence‐labeled siRNA (red). Bar, 50 μm.
By this method, we also obtained delivery evidence of fluorescence‐labeled siRNA to tumor cells in scid mice that had 1 × 106 GFP‐expressing 60As6 cells introduced into the peritoneal cavity. As shown in Figure 2(d), most GFP‐expressing 60As6 cells (89.6%, 69/77 cells in eight fields), which were recovered from the peritoneal cavity 72 h after injection, incorporated fluorescence‐labeled siRNA. These results demonstrated that siRNA was able to be delivered into peritoneal metastatic tumor cells by using both 0.5% atelocollagen and DharmaFECT1.
In vitro and in vivo effects of siRNA treatment of two diffuse‐type GC‐specific hedgehog targets, ELK1 and MSX2 on highly metastatic 60As6 cells
As mentioned in the first part of the Results, we previously reported hedgehog signal activation in diffuse‐type GC including a scirrhous‐type,17 and identified two cancer‐specific hedgehog targets, ELK1 and MSX2.18 Treatment of each siRNA of ELK1 and MSX2 induced growth inhibition (53% and 41% respectively), of HSC‐60 cells. Reverse transcription‐PCR confirmed that expression of ELK1 and MSX2 in HSC‐60 cells was maintained in 60As6 cells (Fig. 3a). Double transfection of these two siRNA strongly inhibited in vitro cell growth of 60As6 (Fig. 3b) as well as HSC‐60.18
Figure 3.
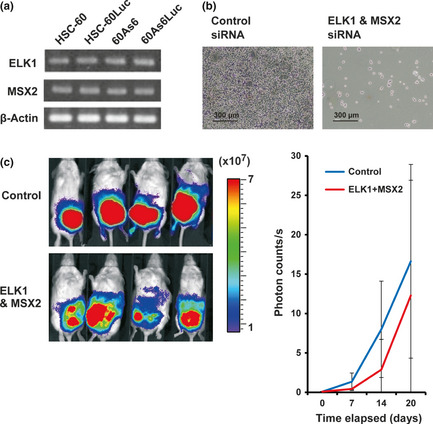
The expression of cancer‐specific hedgehog targets ELK1 and MSX2 in HSC‐60 and 60As6 and the effect of silencing of ELK1and MSX2 on in vitro and in vivo cell growth of 60As6. (a) RT‐PCR of ELK1 and MSX2 in HSC‐60 and 60As6. (b) Cell growth inhibition of 60As6 cells 2 days after double transfection of ELK1 and MSX2 siRNA. (c) Each of four mice treated with ELK1 and MSX2 siRNA or control siRNA is visualized 27 days after inoculation of 60As6Luc by the IVIS system (left). Color bar indicates × 107 photon/s. Results of the time course experiments on tumor growth inhibition by the double treatment are shown (right). Although small effects on the growth inhibition are observed, no significance is shown.
Before starting in vivo tumor growth inhibition studies, we accessed the tumor formation ability of 60As6 cells in scid mice. Tumor formation rates in serial i.p. injection of 1 × 104, 1 × 105, 5 × 105, 1 × 106, and 5 × 106 cells into 10–27 mice showed 0% (0/10), 76% (13/17), 100% (20/20), 100%(27/27, and 100% (15/15), respectively. By i.p. injection of more than 5 × 105 cells, all of the subject mice formed multiple tumors. In 1 × 106 or 5 × 106 cells, tumors were formed within 2 weeks, and the tumor‐bearing mice died rapidly (median survival time: approximately 30 days). These cases were thought to be quite different from peritoneal recurrence in humans, which is known to develop from occult or minimal tumor cells.21 On the other hand, by injection of 5 × 105 cells, tumors were formed within 4 weeks, and a median survival time was approximately 50 days (data not shown). To extend our in vitro studies (Fig. 3b), 10 scid mice were inoculated with 5 × 105 60As6Luc cells, and each five of those 10 mice was injected with a 25 μg ELK1 and 25 μg MSX2 siRNA/0.5% atelocollagen/DharmaFECT1 complex or a 0.5% atelocollagen/DharmaFECT1 complex as control five times every 3 days for 15 days. After exclusion of one pair of un‐inoculated mice, optical imaging of the luciferase activity by use of the IVIS system at 27 days after administration was shown (Fig. 3c, left). Results of the time course experiments (at 0, 7, 14, 20 days) of tumor growth inhibition were shown by the quantification of photon counts (Fig. 3c, right). Although small effects on tumor growth inhibition by the double treatment of ELK1 and MSX2 siRNAs were observed, no significance was shown. Accordingly, no significant difference on mouse survival was found (data not shown). Therefore we next searched for other powerful targets for prolonging survival by intraperitoneal delivery of a single siRNA.
NEDD1 siRNA inhibited in vitro cell growth of a highly metastatic 60As6 cell line
Taxanes, which bind microtubules and inhibit tumor cell division in the metaphase of the cell cycle, are anti‐tumor reagents for GC patients with peritoneal recurrence, because a significant pharmacokinetic advantage associated with i.p. delivery was predicted by their large bulky structure and known hepatic metabolism.22 However, this drug has many adverse reactions including bone marrow suppression, alopecia, and neuropathy.23 Therefore, we investigated target genes, which are involved in the metaphase regulation, from our previously reported GC‐related genes.18 In the previous study, we obtained gene expression profiles of 18 intestinal‐type GCs and 12 diffuse‐type GCs, and six non‐cancerous tissues, and approximately 60 genes were found to be expressed aberrantly in more than 80% of GCs. Among them, only the NEDD1 gene was known to be involved in the metaphase regulation. Therefore, we selected this gene as a new target for evaluating the therapeutic effect of an atelocollagen‐mediated siRNA delivery on the peritoneal metastasis model mice established here. The NEDD1 gene encodes a protein that binds to the gamma‐tubulin ring complex, a multiprotein complex at the centrosome and at the mitotic spindle that mediates the nucleation of microtubules.24 First, we showed NEDD1 mRNA expression in three regions (pit, neck, and gland) of normal gastric mucosa prepared by laser‐captured microdissection17, 18 in 18 primary gastric cancer tissues (Fig. 4a) and in 20 normal organs of the human body (Fig. 3b). NEDD1 mRNA was found to be highly expressed in all primary lesions of gastric cancer by RT‐PCR, while no or low expression was observed in normal organs except for the testis. The expression pattern of NEDD1 was similar to that of the testis‐tumor antigen gene. Next, we examined whether NEDD1 knockdown inhibits the growth of highly metastatic 60As6 cells in vitro. RT‐PCR and Western blot analyses revealed that Nedd1 protein expression was diminished efficiently by treatment of the NEDD1 siRNA (Fig. 4c, upper left). In accordance with a decrease of NEDD1 mRNA, 60As6 cell growth was eminently inhibited by treatment of NEDD1 siRNA compared with the control siRNA (Fig. 4c, right). Representative photos of the cells are also shown (Fig. 4c, lower left).
Figure 4.
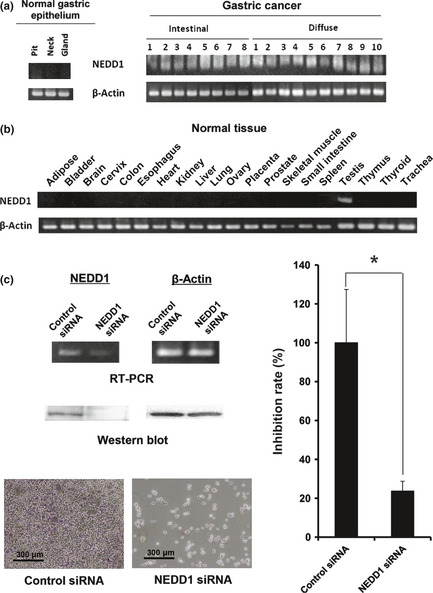
The expression and silencing of NEDD1. (a) NEDD1 mRNA expression in three regions of normal gastric mucosa (pit, neck, and gland) and 18 primary GCs containing eight intestinal‐type and 10 diffuse‐type. (b) NEDD1 mRNA expression in 20 other organs of the human body. (c) Reverse transcription‐polymerase chain reaction (RT‐PCR) and western blot results for NEDD1 gene silencing (upper left) and representative photos for growth inhibition (lower left) of 60As6 cells 2 days after treatment of NEDD1 siRNA are shown. The cell growth inhibition rate 5 days after siRNA transfection is also shown (*P = 0.029) (right).
In vivo inhibition of peritoneal metastasis in the mouse xenograft model by i.p. administration of NEDD1 siRNA
To extend our in vitro studies (Fig. 4), 12 scid mice were inoculated with 5 × 105 60As6Luc cells, and each of six of those 12 mice was injected with a 50 μg NEDD1siRNA/0.5% atelocollagen/DharmaFECT1 complex or a 0.5% atelocollagen/DharmaFECT1 complex as control five times every 3 days for 15 days. Optical imaging to detect luciferase activity in the mice was performed by using the IVIS system to evaluate tumor progression three times a week for 3 weeks. Quantitative photon‐counting analysis of disseminated 60As6 cells revealed effective and significant inhibition of the tumor growth in the mice treated with NEDD1 siRNA (Fig. 5).
Figure 5.
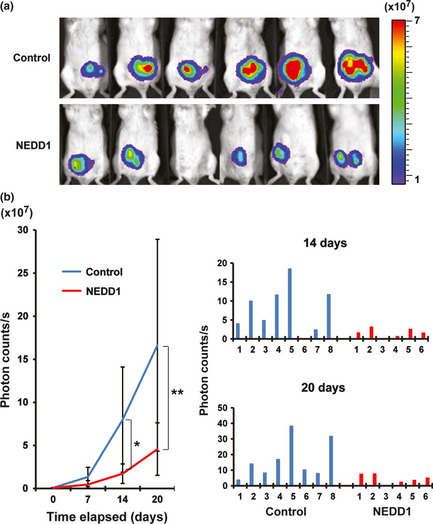
Inhibition of tumor cell growth in the peritoneal cavity by atelocollagen‐mediated NEDD1 siRNA delivery. (a) Each of six mice treated with NEDD1 siRNA or vehicle alone as control is visualized at 21 days after inoculation of 60As6Luc. Color bar indicates × 107 photon/s. (b) Results of quantitative photon‐counting analysis three times a week for 3 weeks are shown after inoculation of 60As6Luc with or without the NEDD1 siRNA treatment. This experiment was repeated three times, and similar results were observed (*P = 0.04; **P = 0.034) (left). Photon counts 14 and 20 days after inoculation in each mouse are also indicated (right), because results among animal experiments often are variable.
Evaluation of i.p. administration safety of NEDD1 siRNA and mice survival rates
To assess the safety of i.p. administration of a NEDD1siRNA/atelocollagen/DharmaFECT1 complex in liver and kidney function, we compared the activities of three enzymes (aspartate aminotransferase [AST], lactate dehydrogenase [LDH], alkaline phosphatase [ALP]) and the levels of blood urea nitrogen (BUN) and Cre in the serum of each group (no treatment, control siRNA, and NEDD1siRNA group; n = 9) 3 weeks after treatment. No significant toxicity was detected in the mice treated with NEDD1siRNA (Fig. 6). In addition, no difference in the activity or glossiness of hair was also observed among the three groups (data not shown). To yield survival benefits, 20 scid mice were inoculated with 1 × 105 60As6Luc cells and, 3 weeks after inoculation, each of 10 of those 20 mice was injected with NEDD1 siRNA or control siRNA eight times every 3 days for 22 days (Fig. 7a). The survival rates of these 20 mice are shown in Figure 7(b). Although NEDD1 siRNA administration stopped at 22 days after inoculation of 60As6Luc, mice treated with NEDD1 siRNA survived longer than the control mice with a significance (NEDD1 siRNA: the median survival time was 61 ± 2 days; control siRNA: the median survival time was 49 ± 6 days, P = 0.0115).
Figure 6.
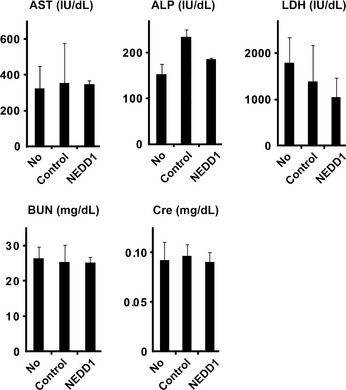
Evaluation of selected serum chemistries 3 weeks after i.p. administration of NEDD1 siRNA. No significant difference in the activity of aspartate aminotransferase (AST), lactate dehydrogenase (LDH) and alkaline phosphatase (ALP) and in levels of blood urea nitrogen (BUN) and Cre is observed among the no‐treatment group (No), control siRNA‐treated group (Control) and NEDD1 siRNA‐treated group (NEDD1) (n = 9).
Figure 7.
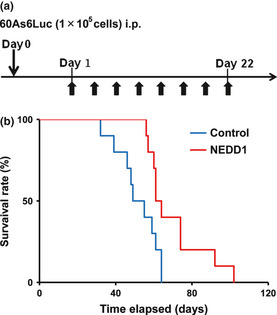
Effect on survival of mice treated with NEDD1 siRNA. (a) Schedule of i.p. administration of NEDD1 siRNA 1 day after inoculation of 1 × 105 60As6Luc cells. (b) The i.p. administration of NEDD1 siRNA significantly prolongs mice survival (P = 0.012).
Discussion
For gene therapy, one of the most dramatic events of the past 5 years in this field has been the discovery of RNA interference (RNAi). The success of cancer therapeutic use of RNAi relies on the development of safe and efficacious delivery systems that introduce siRNA and shRNA expression vectors into target tumor cells. However, such delivery systems into peritoneal metastatic tumor cells have not been established well. The atelocollagen‐mediated gene delivery system was originally developed for a adenovirus vector by a collaborator.19 An atelocollagen is a highly purified type I collagen of calf dermis with pepsin treatment, which allows nuclease resistance, prolonged release of genes and reduction of cellular immune responses. In mice, this delivery system has been reported to be useful for gene delivery into some body sites including metastatic tumors and also for systemic gene delivery.20 As mentioned in the introduction, diffuse‐type GCs including scirrhous GC frequently show peritoneal dissemination even if tumor cells are circulating systemically. Therefore, in this type of GCs, peritoneal metastasis control is urgently crucial for improving the quality of life and patient outcome. Here we provided peritoneal metastasis model mice and an effective delivery system for i.p. administration of siRNA. Figure 2 showed that the 0.5% atelocollagen/DharmaFECT1/siRNA complex rather than the 0.05% atelocollagen/DharmaFECT1/siRNA complex reduced luciferase activity and that the DharmaFECT1 free complex did not reduce it. To date, a collaborator usually uses DharmaFECT1 in the atelocollagen‐mediated systemic gene delivery by i.v. administration, because this reagent improves it (Takeshita F, unpublished observation, 2010).
In peritoneal metastasis model mice, the i.p. administration of NEDD1 siRNA was able to inhibit tumor growth and prolong survival even without any side effects (Figs 5, 7). If targets such as NEDD1 function in the cell cycle regulation, the slow gene release arising from protection from nucleases by atelocollagen may provide an advantage for long acting and for reducing the number of administrations. As shown in Figure 7, we administered the NEDD1 siRNA complex five times every 3 days for 15 days in this study; however, that number may possibly be reduced. In another report for intraperitoneal administration of siRNA targeting nuclear factor‐κB with only DharmaFECT1, the siRNA prolonged the survival of mice only by the administration of paclitaxel, whereas the siRNA/DharmaFECT1 complex alone could not succeed.25 Taken together, the atelocollagen/DharmaFECT1/siRNA complex is very useful for gene delivery to the peritoneal cavity.
In the mouse model used, the number of tumor cells (1 × 105 cells) implanted into the mouse peritoneal cavity was estimated to be still very large compared with the number of tumor cells in the peritoneal cavity in human GC patients with cytology positive, who often showed peritoneal metastasis within 2 years. Therefore, the present i.p. delivery system of siRNA has a great potential for treatment of such GC patients.
Although in vitro cell growth inhibition was observed by the double siRNA treatment of ELK1 and MSX2 (Fig. 3b), no significant difference on in vivo tumor growth and mouse survival was found (Fig. 3c and data not shown). Investigation of other hedgehog components (SMO, GLIs, ISL1, BMP4, FOXM1, and FOXA2) and EMT‐regulators (SIP1/ZEB2, TWIST2) remains for a future study, because cross‐talk between hedgehog and EMT signals is specific to diffuse‐type GC.18
Atelocollagen has also been reported to efficiently deliver microRNA.26 Recently, genome‐wide microRNA expression profiles of 353 GC samples have shown that some microRNAs including let‐7b, miR‐214, and miR‐433 are expressed aberrantly and correlate with tumorigenesis, progression, and prognosis of diffuse‐type GC.27 Thus, these microRNAs may be candidates for SGC therapy. In addition, transforming growth factor‐β (TGF‐β) has been reported to induce apoptosis of a subset of diffuse‐type GCs whose receptor is not inactivated.28, 29 Therefore, adenovirus‐mediated TGF‐β or the downstream targets such as Gasdermin/GasderminA delivery also have great potential for SGC therapy.
In conclusion, we developed a novel i.p. delivery system of siRNA to disseminated tumor cells in the peritoneal cavity that successfully prolongs the survival of model mice. The present mouse model is for an adjuvant therapy after surgical resection. The ability of atelocollagen/DharmaFECT complex is keeping siRNA from nucleases, leading slow gene release and reducing the amount of administration that results in effective eradication of residual tumor cells in the peritoneal cavity.
Thus, considering other potential targets of the diffuse‐type GCs, this system is a highly flexible therapeutic platform for the treatment of peritoneal dissemination.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported by of the National Institute of Biomedical Innovation (for the Advanced Research for Medical Products Mining Programme), The Ministry of Health, Labour and Welfare of Japan (for the Third Comprehensive 10‐Year Strategy for Cancer Control), and The Princess Takamatsu Cancer Research Fund. Drs T Fujita and T Nishimura are the recipients of a Research Resident Fellowship from the Foundation of Promotion of Cancer Research in Japan.
(Cancer Sci, doi: 10.1111/cas.12054, 2012)
References
- 1. Ries LAG, Eisner MP, Kosary CL et al SEER Cancer Statistics Review, 1973–1998, Bethesda, MD: National Cancer Institute, 2001. [Google Scholar]
- 2. Maxwell Parkin D. Global cancer statistics in the year 2000. Lancet Oncol 2001; 2: 533–43. [DOI] [PubMed] [Google Scholar]
- 3. Lauren P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. An attempt at a histo‐clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 4. Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006; 12: 354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakamura R, Saikawa Y, Wada N et al Retrospective analysis of prognosis for scirrhous‐type gastric cancer: one institution's experience. Int J Clin Oncol 2007; 12: 291–4. [DOI] [PubMed] [Google Scholar]
- 6. Maehara Y, Moriguchi S, Orita H et al Lower survival rate for patients with carcinoma of the stomach of Borrmann type IV after gastric resection. Surg Gynecol Obstet 1992; 175: 13–6. [PubMed] [Google Scholar]
- 7. Arveux P, Faivre J, Boutron MC et al Prognosis of gastric carcinoma after curative surgery: a population‐based study using multivariate crude and relative survival analysis. Dig Dis Sci 1992; 37: 757–63. [DOI] [PubMed] [Google Scholar]
- 8. Kunisaki C, Shimada H, Nomura M et al Therapeutic strategy for scirrhous type gastric cancer. Hepatogastroenterology 2005; 52: 314–8. [PubMed] [Google Scholar]
- 9. Kitamura K, Beppu R, Anai H et al Clinicopathologic study of patients with Borrmann type IV gastric carcinoma. J Surg Oncol 1995; 58: 112–7. [DOI] [PubMed] [Google Scholar]
- 10. Sugarbaker PH, Yonemura Y. Clinical pathway for the management of resectable gastric cancer with peritoneal seeding: best palliation with a ray of hope for cure. Oncology 2000; 58: 96–107. [DOI] [PubMed] [Google Scholar]
- 11. Fujimoto S, Takahashi M, Kobayashi K et al Relation between clinical and histologic outcome of intraperitoneal hyperthermic perfusion for patients with gastric cancer and peritoneal metastasis. Oncology 1993; 50: 338–43. [DOI] [PubMed] [Google Scholar]
- 12. Yonemura Y, Kawamura T, Bandou S, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005; 92: 370–5. [DOI] [PubMed] [Google Scholar]
- 13. Yanagihara K, Takigahira M, Tanaka H et al Development and biological analysis of peritoneal metastasis mouse models for human scirrhous stomach cancer. Cancer Sci 2005; 96: 323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yanagihara K, Takigahira M, Takeshita F et al A photon counting technique for quantitatively evaluating progression of peritoneal tumor dissemination. Cancer Res 2006; 66: 7532–9. [DOI] [PubMed] [Google Scholar]
- 15. Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. Anat Rec 1993; 236: 259–340. [DOI] [PubMed] [Google Scholar]
- 16. Aoyagi K, Tatsuta T, Nishigaki M et al A faithful method for PCR‐mediated global mRNA amplification and its integration into microarray analysis on laser‐captured cells. Biochem Biophys Res Commun 2003; 300: 915–20. [DOI] [PubMed] [Google Scholar]
- 17. Fukaya M, Isohata N, Ohta H et al Hedgehog signal activation in gastric pit cell and in diffuse‐type gastric cancer. Gastroenterology 2006; 131: 14–29. [DOI] [PubMed] [Google Scholar]
- 18. Ohta H, Aoyagi K, Fukaya M et al Cross talk between hedgehog and epithelial‐mesenchymal transition pathways in gastric pit cells and in diffuse‐type gastric cancer. Br J Cancer 2009; 100: 389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ochiya T, Takahama Y, Nagahara S et al New delivery system for plasmid DNA in vivo using atelocollagen as a carrier material: the minipellet. Nat Med 1999; 5: 707–10. [DOI] [PubMed] [Google Scholar]
- 20. Takeshita F, Minakuchi Y, Nagahara S et al Efficient delivery of small interfering RNA to bone‐metastatic tumors by using atelocollagen in vivo . Proc Natl Acad Sci USA 2005; 102: 12 177–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mori K, Suzuki T, Uozaki H et al Detection of minimal gastric cancer cells in peritoneal washings by focused microarray analysis with multiple markers: clinical implications. Ann Surg Oncol 2007; 14: 1694–702. [DOI] [PubMed] [Google Scholar]
- 22. Markman M, Brady MF, Spirtos NM, Hanjani P, Rubin SC. Phase II trial of intraperitoneal paclitaxel in carcinoma of the ovary, tube, and peritoneum: a gynecologic oncology group study. J Clin Oncol 1998; 16: 2620–4. [DOI] [PubMed] [Google Scholar]
- 23. Nishiyama M, Wada S. Docetaxel: its role in current and future treatments for advanced gastric cancer. Gastric Cancer 2009; 12: 132–41. [DOI] [PubMed] [Google Scholar]
- 24. Haren L, Remy MH, Bazin I, Callebaut I, Wright M, Merdes A. NEDD1‐dependent recruitment of the gamma‐tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J Cell Biol 2006; 172: 505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inoue M, Matsumoto S, Saito H, Tsujitani S, Ikeguchi M. Intraperitoneal administration of a small interfering RNA targeting nuclear factor‐kappa B with paclitaxel successfully prolongs the survival of xenograft model mice with peritoneal metastasis of gastric cancer. Int J Cancer 2008; 123: 2696–701. [DOI] [PubMed] [Google Scholar]
- 26. Takeshita F, Patrawala L, Osaki M et al Systemic delivery of synthetic microRNA‐16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell‐cycle genes. Mol Ther 2010; 18: 181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ueda T, Volinia S, Okumura H et al Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol 2010; 11: 136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yanagihara K, Tsumuraya M. Transforming growth factor β1 induces apoptotic cell death in cultured human gastric carcinoma cells. Cancer Res 1992; 52: 4042–5. [PubMed] [Google Scholar]
- 29. Saeki N, Kim DH, Usui T et al GASDERMIN, suppressed frequently in gastric cancer, is a target of LMO1 in TGF‐β‐dependent apoptotic signalling. Oncogene 2007; 26: 6488–98. [DOI] [PubMed] [Google Scholar]


