Abstract
Cyclin F, capable of forming Skp1‐Cul1‐F‐box protein ubiquitin ligase complex, is implicated in controlling centrosome duplication and preventing genome instability. Cyclin F oscillates during cell cycle with a similar pattern to cyclin A. However, its expression and significance in cancer remain obscure. In this study, we showed that cyclin F was noticeably decreased in 16 pairs of tissue samples of hepatocellular carcinoma (HCC) compared to paracarcinoma tissues, at both mRNA and protein levels. Immunohistochemical staining data revealed that in 71.8% (176/245) of HCC cases, cyclin F expression in tumor tissue was much lower than that in nontumorous tissue. Low cyclin F expression, defined by receiver operating characteristic curve analysis, was present in 69.0% of HCC patients. Low expression of cyclin F was significantly correlated with tumor size, clinical stage, serum alpha‐fetoprotein level and tumor multiplicity. Further study showed that cyclin F expression was reversely associated with tumor differentiation in HCC. Kaplan– Meier analysis indicated that low cyclin F expression was related to poor overall survival and recurrence‐free survival. The prognostic impact of cyclin F was further confirmed by stratified survival analysis. Importantly, multivariate analysis revealed that low cyclin F expression was an independent poor prognostic marker for overall survival. We conclude that cyclin F is downregulated in HCC and is a promising prognostic marker for patients suffering from this deadly disease.
Hepatocellular carcinoma (HCC) is the fifth most prevalently diagnosed malignancy in men worldwide and the second most frequent cause of cancer death, whereas in women, HCC is the seventh most prevalently diagnosed malignancy and the sixth most frequent cause of cancer death.1 Although approximately 50% of HCC cases and deaths occur in China,2 in three decades, the incidence has been increasing in economically developed regions, including Japan, Western Europe and the USA.3, 4 Studies on HCC etiology have revealed that hepatitis (HBV or HCV) is a major risk factor for hepatocarcinogenesis.5, 6 In view of the poor outcome of patients receiving HCC treatment, there has been increased interest in developing novel strategies for HCC therapy.7, 8 Discovery of biological markers useful for HCC diagnosis and prognosis prediction is important to clinical management.
In mammals, cyclins are essential regulators of cell cycle machinery, through their ability to interact with activate cyclin‐dependent kinases (CDK).9, 10 Cyclin F, originally identified as a cDNA affecting the temperature sensitivity of a Saccharomyces cerevisiae cdc4‐1 mutant,11 is the largest cyclin, with a molecular weight of 87 kD. Conserved with other cyclins, an extensive PEST‐rich region near the C‐terminus and a cyclin box region are presented in cyclin F.11 Displaying a very similar pattern to cyclin A, expression of cyclin F fluctuates during the cell cycle: accumulating in the S phase, peaking in the G2 phase and decreasing at mitosis.11 In contrast to other cyclins, cyclin F does not bind or activate any CDK.12 Instead, cyclin F has been demonstrated to bind to cyclin B1 to retain its nuclear localization.13 Bai et al.11 report that overexpression of cyclin F leads to a significant increase in cell population in the G2 phase. Furthermore, cyclin F controls centrosome duplication by facilitating the degradation of CP110.14 Similarly, Emanuele et al.15 provide evidence that cyclin F enhances the degradation of NuSAP1, which contributes to mitotic spindle organization. In D'Angiolella et al.16 cyclin F is demonstrated to regulate cellular dNTP pools and to maintain genome stability by interacting with ribonucleotide reductase family member 2 (RRM2). Despite its essential nature and role in cell cycle regulation, cyclin F expression and its significance in human cancer have never been studied.
In the present study, the expression of cyclin F in HCC was examined. The relationship between cyclin F expression and clinicopathological features was investigated. The role of cyclin F in HCC prognosis was accessed. Our results reveal that cyclin F is noticeably decreased in HCC and significantly correlated with clinical variables and prognosis of HCC patients.
Materials and methods
Patients and tissue specimens
All HCC specimens along with complete clinical and pathological data were obtained from 245 HCC patients who underwent surgical resection at Sun Yat‐sen University Cancer Center (SYSUCC), Guangzhou, China between January 1997 and December 2007. The cohort includes 217 (88.6%) men and 28 (11.4%) women. The mean age is 47.7 years, with ages ranging from 13.0 to 68.0 years. Postsurgical survival data are available for all patients. The mean follow‐up time is 32.8 months. Another 16 paired fresh resection HCC tissues and the corresponding adjacent liver tissues were collected for quantitative real‐time PCR and western blot analysis. None of the patients had received adjuvant therapies before surgery. The use of tissues for this study was approved by the Institute Research Medical Ethics Committee of SYSUCC.
Immunohistochemistry
Tissue microarray (TMA) consisting of 245 HCC and adjacent nontumorous liver tissues was constructed. Formalin‐fixed and paraffin‐embedded HCC sections were dewaxed in xylene and graded alcohols, hydrated, and washed in PBS. After pretreatment in a microwave oven, endogenous peroxidase was inhibited by 3% hydrogen peroxide in methanol for 20 min, followed by avidin–biotin blocking using a biotin‐blocking kit (DAKO, Darmstadt, Germany). Slides were then incubated with cyclin F antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight in a moist chamber at 4°C, washed in PBS and incubated with biotinylated goat anti‐rabbit/mouse antibodies. Slides were developed with DAB and counterstained with hematoxylin.
Quantitative real‐time PCR and western blot
Quantitative real‐time PCR (qRT‐PCR) and western blot analyses were performed as described in our previous study.17 Primers were designed as follows: cyclin F, forward: 5′‐CCCCGAAGATGTGCTCTTTCA‐3′ and reverse: 5′‐GCCTTCATTGTAGAGGTAGGC T‐3′; β‐actin, forward: 5′‐TGGCACCCAGCACAATGAA‐3′ and reverse: 5′‐CTAAGTCATAGTCCG CCTAGAAGCA‐3′.
Immunohistochemistry evaluation
Semi‐quantitative immunohistochemistry (IHC) detection was used to determine the cyclin F protein levels. We multiplied the percentage score by the staining intensity score. The percentage of positively‐stained cells were scored as “0” (0%), “1” (1–25%), “2” (26–50%), “3” (51–75%) and “4” (76%–100%). Intensity was scored as “0” (negative staining), “1” (weak staining), “2” (moderate staining) and “3” (strong staining). For each case, 1000 cells were randomly selected and scored. The scores were independently decided by two pathologists (Dr JP Yun and Dr MF Zhang).
Selection of cutoff score
Receiver operating characteristic (ROC) curve analysis was applied to determine the cutoff score for tumors with low cyclin F expression by using the 0,1‐criterion. In the immunohistochemical evaluation, the score with the shortest distance from the curve to the point with both maximum sensitivity and specificity, that is, the point (0.0, 1.0), was selected as the cutoff score, resulting in the largest number of tumors in any study correctly classified as having or not having the clinical outcome.18, 19 According to cyclin F score, the sensitivity and specificity for each outcome under study was plotted, thus generating various ROC curves. The score with both maximum sensitivity and specificity was selected as the cutoff value. Cases defined as having high cyclin F expression were those with scores below or equal to the cutoff value, while low cyclin F expression was associated with those with scores above the value. In order to perform ROC curve analysis, clinicopathological features were dichotomized: tumor multiplicity (single versus multiple), tumor size (<5 vs ≥5 cm), alpha‐fetoprotein level (AFP) (<20 vs ≥20 ng/mL), tumor differentiation (well–moderate versus poor–undifferentiated), stage (I + II vs III + IV), vascular invasion (yes versus no), relapse (yes versus no) and survival status (dead versus alive).
Statistical analysis
Receiver operating characteristic curve analysis was applied to determine the cutoff value for high expression of cyclin F according to the 0,1‐criterion, and the areas under the curve (AUC) were calculated. The Mann–Whitney U‐test was used for comparison between groups. The Wilcoxon matched pairs test was used to determine the significant difference in cyclin F expression in fresh HCC and normal liver tissues. The χ2‐test was performed to analyze the correlation between cyclin F expression and clinicopathological parameters. The Kaplan–Meier method (the log‐rank test) was used for survival analysis and univariate analysis. Independent analyses were performed according to the selected population: overall population and different morphological and pathological subgroups. The Cox proportional hazards regression model was used to identify the independent prognostic factors. Statistical analyses were performed using the spss 16.0 software (SPSS, Chicago, IL, USA). Statistical significance was initially set at P < 0.05.
Results
Expression of cyclin F in hepatocellular carcinoma tissue samples by quantitative real‐time PCR and western blot
To examine the expression of cyclin F, 16 pairs of HCC samples along with the corresponding paracarcinoma tissues were subjected to qRT‐PCR and western blot analyses. Results showed that in 75.0% (12/16) of cases, cyclin F mRNA levels in HCC were much lower than those in nontumorous tissues (Fig. 1a). In contrast, protein levels of cyclin F in HCC were noticeably downregulated, compared to those in adjacent nontumorous samples (Fig. 1c). Statistically significant change in cyclin F expression was indicated (Fig. 1b,d).
Figure 1.
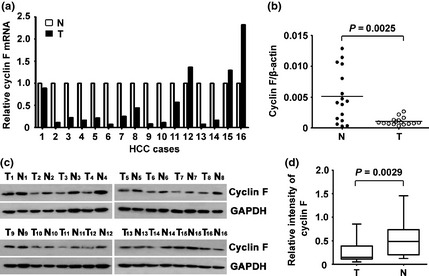
Expression of cyclin F was examined in hepatocellular carcinoma (HCC) tissue samples. (a) mRNA level of cyclin F in HCC (T) and corresponding adjacent liver tissue (N) was determined in 16 patients. Relative cyclin F mRNA is presented. (b) The Wilcoxon matched paired test indicated a significant alteration of cyclin F in tissue samples. (c) Expression of cyclin F protein in 16 paired HCC (T) and adjacent liver tissues (N) were examined by western blot. (d) Relative intensity of PLK4 normalized to GAPDH was calculated.
Determination of cutoff value for low cyclin F expression by receiver operating characteristic curve analysis
To define an optimal cutoff score for low cyclin F expression in HCC, the ROC curve was used according to the results of the IHC evaluation. The results revealed that the ROC curve for serum AFP had the closest distance from (0.0, 1.0), which maximizes both sensitivity and specificity for the outcome (Fig. 2). As a result, a score of 6.75 was chosen as the cutoff value for low cyclin F expression.
Figure 2.
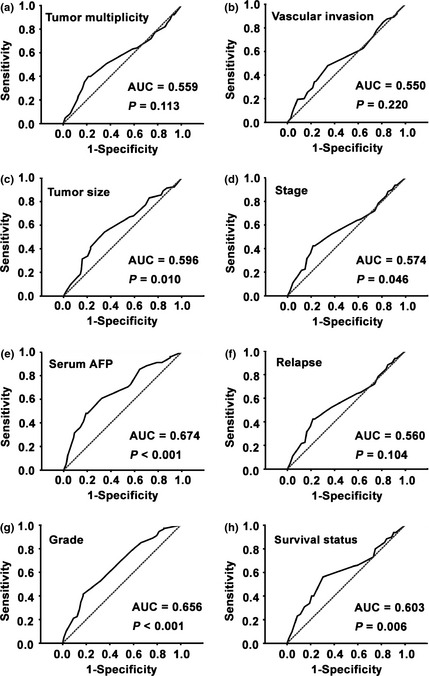
Cutoff value of low cyclin F expression in hepatocellular carcinoma (HCC) was determined by receiver operating characteristic curves. The sensitivity and 1‐specificity for several variables of HCC patients, including tumor multiplicity, tumor size, serum alpha‐fetoprotein level, pathological grade, clinical stage, vascular invasion, relapse and survival status, were plotted. AUC, area under curve.
Expression of cyclin F in hepatocellular carcinoma by immunohistochemistry
To further examine the expression of cyclin F in HCC, 245 paraffin‐embedded HCC samples were collected to construct TMA. As shown by the result of TMA‐based IHC, immunoreactivities of cyclin F were present in the cytoplasm in most of the cancer cells (Fig. 3a–c). In some cases, nuclear staining of cyclin F was also observed (Fig. 3b). Expression patterns of cyclin F in normal liver tissues are depicted in Figure 3(d,e). Furthermore, low cyclin F expression in tumor tissue was identified in 69.0% (169/245) of cases, according to the cutoff value defined by the ROC curve. Moreover, for 71.8% (176/245) of HCC patients, less cyclin F was expressed in tumor tissue. Statistically, cyclin F expression was significantly lower in tumor tissue (Fig. 3f).
Figure 3.
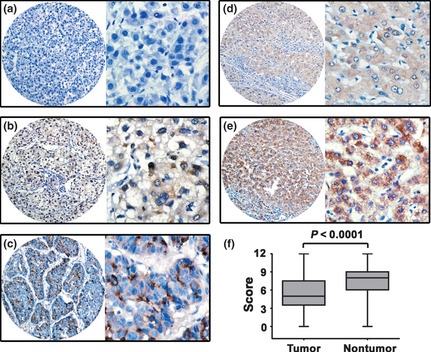
Expression of cyclin F was decreased in hepatocellular carcinoma (HCC) tissues by immunohistochemistry. Cyclin F was presented predominantly in cytoplasm within tumor and normal liver cells. The micrographs showed negative (a), weak (b) and strong (c) staining of cyclin F in HCC, as well as weak (d) and strong (e) staining of cyclin F in normal liver tissues. (Left panel: magnification ×100; right panel: magnification ×400.) (f) Reproducibility of the measurement in all 245 patients was calculated using the Wilcoxon matched paired test.
Correlation of cyclin F expression and clinical variables in hepatocellular carcinoma
To determine the clinical significance of cyclin F in HCC, the relationship between expression of cyclin F and clinicopathological parameters was analyzed. Significant associations were found with tumor size (P = 0.004), tumor differentiation (P < 0.001), clinical stage (P < 0.001), serum AFP level (P < 0.001) and tumor multiplicity (P = 0.002), indicating that HCC in patients with low cyclin F expression was frequently associated with large tumor size, high level of serum AFP, poor tumor differentiation, advanced clinical stage and multiple tumor number (Table 1).
Table 1.
Correlation between the clinicopathologic variables and cyclin F expression in HCC
| Variable | Cyclin F protein | ||||
|---|---|---|---|---|---|
| All cases | Low expression | High expression | χ2 | P‐value* | |
| Age (years)† | |||||
| <47.7 | 122 | 85 (69.7%) | 37 (30.3%) | 0.054 | 0.815 |
| ≥47.7 | 123 | 84 (68.3%) | 39 (31.7%) | ||
| Gender | |||||
| Female | 28 | 19 (67.9%) | 9 (32.1%) | 0.019 | 0.891 |
| Male | 217 | 150 (69.1%) | 67 (30.9%) | ||
| HBsAg | |||||
| Positive | 32 | 22 (68.8%) | 10 (31.3%) | 0.001 | 0.976 |
| Negative | 213 | 147 (69.0%) | 66 (31.0%) | ||
| AFP (ng/mL) | |||||
| <20 | 102 | 53 (52.0%) | 49 (48.0%) | 23.655 | 0.000 |
| ≥20 | 143 | 116 (81.1%) | 27 (18.9%) | ||
| Cirrhosis | |||||
| Yes | 177 | 45 (66.2%) | 23 (33.8%) | 0.346 | 0.557 |
| No | 68 | 124 (70.1%) | 53 (29.9%) | ||
| Tumor size (cm) | |||||
| <5 | 118 | 71 (60.2%) | 47 (39.8%) | 8.257 | 0.004 |
| ≥5 | 127 | 98 (77.2%) | 29 (22.8%) | ||
| Tumor multiplicity | |||||
| Single | 128 | 77 (60.2%) | 51 (39.8%) | 9.752 | 0.002 |
| Multiple | 117 | 92 (78.6%) | 25 (21.4%) | ||
| Differentiation | |||||
| Well–moderate | 147 | 88 (59.9%) | 59 (40.1%) | 14.271 | 0.000 |
| Poor–undifferentiation | 98 | 81 (82.7%) | 17 (17.3%) | ||
| Stage | |||||
| I–II | 111 | 64 (57.7%) | 47 (42.3%) | 12.158 | 0.000 |
| III–IV | 134 | 105 (78.4%) | 29 (21.6%) | ||
| Hepatic vein invasion | |||||
| Yes | 70 | 54 (77.1%) | 16 (22.9%) | 3.052 | 0.081 |
| No | 175 | 115 (65.7%) | 60 (34.3%) | ||
| Involucrum | |||||
| Complete | 40 | 27 (67.5%) | 13 (32.5%) | 0.049 | 0.825 |
| Incomplete or absent | 205 | 142 (69.3%) | 63 (30.7%) | ||
| Relapse | |||||
| Yes | 111 | 82 (73.9%) | 29 (26.1%) | 2.272 | 0.132 |
| No | 134 | 87 (64.9%) | 47 (35.1%) | ||
*χ2‐test. †Mean age. AFP, alpha‐fetoprotein; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma.
Significant connection between cyclin F expression in HCC and tumor differentiation was further confirmed in another cohort comprising 42 cases of HCC patients diagnosed from January 2012 to July 2012. Higher expression of cyclin F was observed in well‐differentiated HCC (Fig. 4a). The alteration of cyclin F expression was noticeably significant (Fig. 4b) and the percentage of cases with low cyclin F expression was markedly higher in poorly‐differentiated HCC than that in well‐differentiated HCC (Fig. 4c).
Figure 4.
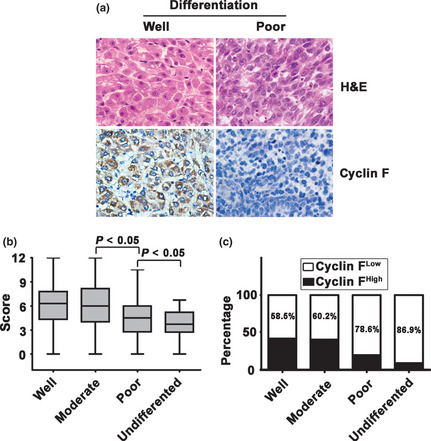
Expression of cyclin F was lower in hep‐atocellular carcinoma (HCC) with poor differentiation. (a) Representative micrographic images showed the cyclin F expression in well‐differentiated and poorly‐differentiated HCC. (b) The box plot indicates the decreased trend of cyclin F expression from well‐differentiated to poorly‐differentiated HCC. (c) Percentages of low cyclin F expression in differential HCC are indicated by histogram.
Correlation of cyclin F expression with survival of postoperative hepatocellular carcinoma patients
To determine the prognostic impact of cyclin F on survival of postsurgical HCC patients, Kaplan–Meier survival analysis was performed. Survival data were available for 245 patients. The mean survival period was 43.9 months for the patients with low cyclin F expression, whereas it was 61.1 months for patients with high levels of cyclin F expression. The results indicated that patients with low cyclin F expression were likely to survive for a shorter time than those with high cyclin F expression (P = 0.001) (Fig. 5a), and had a higher tendency of recurrence (P = 0.037) (Fig. 5b).
Figure 5.
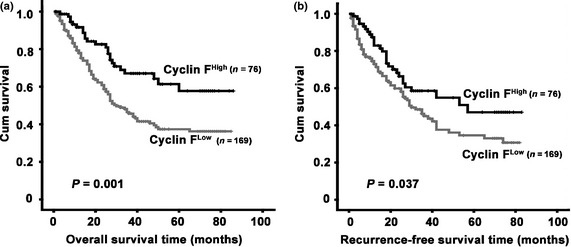
Low cyclin F expression associated with poor overall survival and recurrence‐free survival. Probabilities of overall survival (a) and recurrence‐free survival (b) of total 245 hepatocellular carcinoma patients were analyzed by Kaplan–Meier survival analysis (log‐rank test).
The prognostic effect of cyclin F was further confirmed by stratified survival analysis. Results showed that patients with low cyclin F expression lived a significant shorter life after surgical resection in nine subgroups of HCC patients (Fig. S1).
Univariate and multivariate analyses of prognostic variables in hepatocellular carcinoma patients
To evaluate the representativeness of our samples, univariate analyses were performed. Cyclin F, as well as tumor size, serum AFP level, tumor multiplicity, clinical stage, vascular invasion, tumor differentiation, involucrum and relapse were shown to be responsible for the outcome of HCC patients (Table 2).
Table 2.
Univariate analysis of cyclin F expression and clinicopathologic variables in HCC
| Variable | All cases | Overall survival (months) | Recurrence‐free survival (months) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Median | P‐value | Mean | Median | P‐value | ||
| Age (years)† | |||||||
| <47.7 | 122 | 52.2 | 50.0 | 0.249 | 46.0 | 42.0 | 0.137 |
| ≥47.7 | 123 | 46.0 | 36.0 | 41.0 | 30.0 | ||
| Gender | |||||||
| Female | 28 | 50.4 | 49.0 | 0.552 | 34.6 | 42.0 | 0.602 |
| Male | 217 | 49.0 | 40.0 | 45.0 | 35.0 | ||
| HBsAg | |||||||
| Positive | 32 | 50.5 | 40.0 | 0.233 | 43.6 | 42.0 | 0.425 |
| Negative | 213 | 40.5 | 30.0 | 46.8 | 35.0 | ||
| AFP (ng/mL) | |||||||
| <20 | 102 | 63.9 | NR | 0.000 | 53.6 | 65.0 | 0.000 |
| ≥20 | 143 | 38.7 | 27.0 | 36.1 | 25.0 | ||
| Cirrhosis | |||||||
| Yes | 177 | 48.6 | 38.0 | 0.576 | 41.9 | 30.0 | 0.350 |
| No | 68 | 51.1 | 48.0 | 49.1 | 42.0 | ||
| Tumor size (cm) | |||||||
| <5 | 118 | 57.9 | NR | 0.001 | 53.9 | NR | 0.000 |
| ≥5 | 127 | 42.0 | 27.0 | 35.9 | 25.0 | ||
| Tumor multiplicity | |||||||
| Single | 128 | 63.2 | NR | 0.000 | 49.9 | 42.0 | 0.009 |
| Multiple | 117 | 35.3 | 25.0 | 37.2 | 26.0 | ||
| Differentiation | |||||||
| Well–moderate | 147 | 52.5 | 60.0 | 0.033 | 49.1 | 42.0 | 0.012 |
| Poor–undifferentiation | 98 | 43.7 | 28.0 | 35.8 | 26.0 | ||
| Stage | |||||||
| I–II | 111 | 68.4 | NR | 0.000 | 61.0 | NR | 0.000 |
| III–IV | 134 | 34.2 | 23.0 | 31.5 | 22.0 | ||
| Hepatic vein invasion | |||||||
| Yes | 70 | 27.3 | 18.0 | 0.000 | 18.9 | 15.0 | 0.000 |
| No | 175 | 57.8 | NR | 57.5 | NR | ||
| Involucrum | |||||||
| Complete | 40 | 36.7 | 27.0 | 0.016 | 39.5 | 42.0 | 0.972 |
| Incomplete | 205 | 52.0 | 49.0 | 44.4 | 35.0 | ||
| Relapse | |||||||
| Yes | 111 | 36.6 | 26.0 | 0.000 | |||
| No | 134 | 60.2 | NR | ||||
| Cyclin F | |||||||
| Low expression | 169 | 43.9 | 30.0 | 0.001 | 40.5 | 30.0 | 0.037 |
| High expression | 76 | 61.1 | NR | 51.6 | 57.0 | ||
†Mean age. AFP, alpha‐fetoprotein; HbsAg, hepatitis B surface antigen; NR, not reached.
Multiple Cox regression analysis was conducted to determine the independent prognostic value of cyclin F. After adjusting for the prognostic factors established in the univariate analysis, a significant correlation between low cyclin F expression and worse overall survival (hazard ratio [HR] 0.987, P = 0.033) was observed (Table 3). However, cyclin F was revealed not to be an independent factor of recurrence‐free survival for HCC patients (Table S1).
Table 3.
Cox multivariate analyses of prognostic factors on overall survival
| Variable | β | SE | Hazard ratio (95%CI) | P‐value |
|---|---|---|---|---|
| Tumor multiplicity | 0.523 | 0.224 | 1.568 (0.841–2.365) | 0.078 |
| Tumor size | 0.043 | 0.208 | 1.110 (0.578–1.468) | 0.758 |
| Involucrum | 0.511 | 0.237 | 1.489 (1.058–2.589) | 0.024 |
| AFP | 0.768 | 0.256 | 2.356 (1.465–3.875) | 0.000 |
| Differentiation | −0.047 | 0.219 | 0.879 (0.545–1.498) | 0.807 |
| Hepatic vein invasion | 0.547 | 0.264 | 1.689 (1.012–2.754) | 0.015 |
| Stage | 0.568 | 0.375 | 1.768 (0.754–3.732) | 0.038 |
| Relapse | 0.363 | 0.264 | 1.456 (1.047–2.506) | 0.042 |
| cyclin F | −0.065 | 0.256 | 0.987 (0.569–1.698) | 0.033 |
AFP, alpha‐fetoprotein level; β, Regression coefficient; CI, confidence interval; SE, standard error.
Discussion
In D'Angiolella et al.14, 16 (2010, 2012) cyclin F is demonstrated to perform the important functions of controlling centrosome duplication and preventing genome instability through promoting the degradation of key proteins involved in such events (e.g. CP110 and RRM2). Given that abnormal centrosome duplication and chromosome aberration contribute to carcinogenesis,20, 21 and that the role of cyclin F in cancer remains elusive, we investigated the expression and prognostic value of cyclin F in a large cohort of primary HCC patients who had received curative surgical treatment.
Cyclins are capable of interacting with CDK to promote cell cycle progression, and are frequently upregulated in human cancers. For instance, cyclin B1, cyclin D1 and cyclin E have been shown to be overexpressed in breast cancer.22, 23, 24 However, in the present study, cyclin F was significantly downregulated in HCC. This could be explained by the functional nature of cyclin F. To date, no CDK substrate has been identified for cyclin F, indicating that cyclin F, in contrast to other cyclins, might not function as a regulator in the cell cycle. Furthermore, overexpression of cyclin F resulted in G2 phase arrest and limitation of centrosome duplication, which are considered tumor‐suppressing events.11, 14 Therefore, it is plausible that unlike other cyclin family proteins, cyclin F was dramatically decreased in HCC. In our study, HCC in patients with large‐size tumors and advanced stage was frequently associated with low cyclin F expression, which indicated that cyclin F might be capable of interfering with the progression of HCC. Although the underlying mechanism remains elusive, our preliminary data for MTT and colony formation revealed that overexpression of cyclin F in HCC cells resulted in inhibition of proliferation through induction of autophagy (data not shown). However, the detailed mechanism through which cyclin F inhibits cell growth requires further investigation.
Genomic abnormalities, in which cyclin F was involved,16 clearly play a major role in differentiation and carcinogenesis. Previous studies show that differentiation is tightly connected to carcinogenesis. For example, Leon et al.25 report that myc‐mediated carcinogenesis was partly a result of inhibition of differentiation. Wang et al.26 demonstrate that cell differentiation induced by FHL2 abolishes gastric and colon carcinogenesis. In our study, low cyclin F expression was more frequently observed in poorly‐differentiated HCC. In line with our data, Movsesyan et al.27 show that cyclin F is downregulated during the differentiation of PC12EY cells. The decrease of cyclin F in poorly‐differentiated HCC might result in the exacerbation of HCC because of the imbalance of homeostasis, which might subsequently contribute to the initiation and progression of HCC.
Under normal circumstances, cyclins have distinct patterns of subcellular localization. For example, cyclin A preferentially localizes in the nucleus,28 while cyclin B1 accumulates in cytoplasm and translocates to the nucleus.29 Cyclin F has been previously shown to localize in the nucleus.14, 16 However, cyclin F has been shown to partially localize in cytoplasm where it was interwoven with γ‐tubulin.14 In this study, cytoplasmic staining of cyclin F was primarily observed in most of the cases, while weakly nuclear staining was also found in some samples. In Weng et al.30 cyclin A is aberrantly detected in cytoplasm in most of the HBV‐related HCC samples. Wang and colleagues demonstrate that aberrant cyclin A expression led to centrosome overduplication and probably the subsequent hepatocarcinogenesis.30 Because cyclin F shares a close match in amino acid sequence and a very similar dynamic expression pattern with cyclin A,11 it is not surprising to find cyclin F localizing in cytoplasm in HCC cells.
Because of their critical role in cell cycle regulation, cyclin family proteins have been attracting increasing attention in regards to their significance in human cancers. Interestingly, altered expression of cyclin is usually of prognostic value in cancer. Caldon et al.31 report that cyclin E2 upregulation in breast cancer is associated with shorter distant metastasis‐free survival. Decreased expression of cyclin G1 and its association with poor survival in HCC is revealed in a study by Cui and colleagues.32 Akli et al.33 show that overexpression of low molecular weight cyclin E in bladder cancer predicts poor overall survival. In a study of 602 colon cancer cases, cyclin D1 overexpression was correlated with favorable overall survival,34 whereas Che et al.35 report that elevated expression of cyclin D1 in HCC is associated with poor survival. In our study, a decrease in cyclin F is associated with poor prognosis for HCC patients. Some of our findings support that cyclin F is an independent prognostic factor for overall survival of HCC patients. Expression of cyclin F is significantly correlated with tumor size, clinical stage, serum AFP level and tumor multiplicity, which are well‐established factors responsible for outcome of HCC. Furthermore, cyclin F expression is reversely associated with HCC differentiation which has been identified as an ideal predictor for HCC prognosis. In addition, because cyclin F is capable of limiting abnormal centrosome duplication, decrease in cyclin F might lead to a poor outcome.
In summary, our data reveal that cyclin F was frequently downregulated in HCC. Decrease in cyclin F was significantly correlated with tumor size, differentiation, clinical stage, serum AFP level and tumor multiplicity, suggesting that cyclin F might play a role in HCC initiation and progression. Low cyclin F expression unfavorably impacted the survival of HCC patients. Collectively, our study revealed that low cyclin F expression might be of immense importance for predicting the postsurgical overall survival of patients suffering from HCC.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting information
Table S1. Cox multivariate analyses of prognostic factors on recurrence‐free survival.
Fig. S1. Expression of cyclin F was connected with overall survival in subgroups of hepatocellular carcinoma patients. Kaplan–Meier survival analyses were performed in subgroups according to the factors that are attributed to outcome of hepatocellular carcinoma patients (log‐rank test). AFP, alpha‐fetoprotein; HBsAg, hepatitis B surface antigen.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81201717) and the China Postdoctoral Science Foundation (No. 2012M511867).
(Cancer Sci 2013; 104: 508–515)
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay JSH, Bray F, Forman D, Mathers CD, Parkin D. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide. IARC CancerBase No. 10. Lyon, France: International Agency for Research on Cancer; [Cited 17 Aug 2010.] Available from: http://globocaniarcfr 2010 Last accessed 8/17/2010. [Google Scholar]
- 3. Erichsen R, Jepsen P, Jacobsen J, Norgaard M, Vilstrup H, Sorensen HT. Time trends in incidence and prognosis of primary liver cancer and liver metastases of unknown origin in a Danish region, 1985–2004. Eur J Gastroenterol Hepatol 2008; 20: 104–10. [DOI] [PubMed] [Google Scholar]
- 4. Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology 2004; 127: S5–16. [DOI] [PubMed] [Google Scholar]
- 5. Parkin DM. The global health burden of infection‐associated cancers in the year 2002. Int J Cancer 2006; 118: 3030–44. [DOI] [PubMed] [Google Scholar]
- 6. Wong DK, Huang FY, Lai CL et al Occult hepatitis B infection and HBV replicative activity in patients with cryptogenic cause of hepatocellular carcinoma. Hepatology 2011; 54: 829–36. [DOI] [PubMed] [Google Scholar]
- 7. Marquardt JU, Galle PR, Teufel A. Molecular diagnosis and therapy of hepatocellular carcinoma (HCC): an emerging field for advanced technologies. J Hepatol 2012; 56: 267–75. [DOI] [PubMed] [Google Scholar]
- 8. Breuhahn K, Gores G, Schirmacher P. Strategies for hepatocellular carcinoma therapy and diagnostics: lessons learned from high throughput and profiling approaches. Hepatology 2011; 53: 2112–21. [DOI] [PubMed] [Google Scholar]
- 9. Suryadinata R, Sadowski M, Sarcevic B. Control of cell cycle progression by phosphorylation of cyclin‐dependent kinase (CDK) substrates. Biosci Rep 2010; 30: 243–55. [DOI] [PubMed] [Google Scholar]
- 10. Arellano M, Moreno S. Regulation of CDK/cyclin complexes during the cell cycle. Int J Biochem Cell Biol 1997; 29: 559–73. [DOI] [PubMed] [Google Scholar]
- 11. Bai C, Richman R, Elledge SJ. Human cyclin F. EMBO J 1994; 13: 6087–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fung TK, Siu WY, Yam CH, Lau A, Poon RY. Cyclin F is degraded during G2‐M by mechanisms fundamentally different from other cyclins. J Biol Chem 2002; 277: 35140–9. [DOI] [PubMed] [Google Scholar]
- 13. Kong M, Barnes EA, Ollendorff V, Donoghue DJ. Cyclin F regulates the nuclear localization of cyclin B1 through a cyclin–cyclin interaction. EMBO J 2000; 19: 1378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D'Angiolella V, Donato V, Vijayakumar S et al SCF(Cyclin F) controls centrosome homeostasis and mitotic fidelity through CP110 degradation. Nature 2010; 466: 138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Emanuele MJ, Elia AE, Xu Q et al Global identification of modular cullin‐RING ligase substrates. Cell 2011; 147: 459–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D'Angiolella V, Donato V, Forrester FM et al Cyclin F‐mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell 2012; 149: 1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu L, Zhang CZ, Cai M, Fu J, Chen GG, Yun J. Downregulation of polo‐like kinase 4 in hepatocellular carcinoma associates with poor prognosis. PLoS ONE 2012; 7: e41293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cai MY, Zhang B, He WP et al Decreased expression of PinX1 protein is correlated with tumor development and is a new independent poor prognostic factor in ovarian carcinoma. Cancer Sci 2010; 101: 1543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zlobec I, Steele R, Terracciano L, Jass JR, Lugli A. Selecting immunohistochemical cut‐off scores for novel biomarkers of progression and survival in colorectal cancer. J Clin Pathol 2007; 60: 1112–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dementyeva E, Nemec P, Kryukov F et al Centrosome amplification as a possible marker of mitotic disruptions and cellular carcinogenesis in multiple myeloma. Leuk Res 2010; 34: 1007–11. [DOI] [PubMed] [Google Scholar]
- 21. Shinmura K, Iwaizumi M, Igarashi H et al Induction of centrosome amplification and chromosome instability in p53‐deficient lung cancer cells exposed to benzo[a]pyrene diol epoxide (B[a]PDE). J Pathol 2008; 216: 365–74. [DOI] [PubMed] [Google Scholar]
- 22. Aaltonen K, Amini RM, Heikkila P et al High cyclin B1 expression is associated with poor survival in breast cancer. Br J Cancer 2009; 100: 1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aaltonen K, Amini RM, Landberg G et al Cyclin D1 expression is associated with poor prognostic features in estrogen receptor positive breast cancer. Breast Cancer Res Treat 2009; 113: 75–82. [DOI] [PubMed] [Google Scholar]
- 24. Scaltriti M, Eichhorn PJ, Cortes J et al Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci U S A 2011; 108: 3761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leon J, Ferrandiz N, Acosta JC, Delgado MD. Inhibition of cell differentiation: a critical mechanism for MYC‐mediated carcinogenesis? Cell Cycle 2009; 8: 1148–57. [DOI] [PubMed] [Google Scholar]
- 26. Wang J, Yang Y, Xia HH et al Suppression of FHL2 expression induces cell differentiation and inhibits gastric and colon carcinogenesis. Gastroenterology 2007; 132: 1066–76. [DOI] [PubMed] [Google Scholar]
- 27. Movsesyan V, Whalin M, Shibutani M, Katagiri Y, Broude E, Guroff G. Down‐regulation of cyclin F levels during nerve growth factor‐induced differentiation of PC12 cells. Exp Cell Res 1996; 227: 203–7. [DOI] [PubMed] [Google Scholar]
- 28. Pascreau G, Eckerdt F, Churchill ME, Maller JL. Discovery of a distinct domain in cyclin A sufficient for centrosomal localization independently of Cdk binding. Proc Natl Acad Sci U S A 2010; 107: 2932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fidalgo daSilva E, Ansari SB, Maimaiti J et al The tumor suppressor tuberin regulates mitotic onset through the cellular localization of cyclin B1. Cell Cycle 2011; 10: 3129–39. [DOI] [PubMed] [Google Scholar]
- 30. Weng L, Du J, Zhou Q et al Identification of cyclin B1 and Sec62 as biomarkers for recurrence in patients with HBV‐related hepatocellular carcinoma after surgical resection. Mol Cancer 2012; 11: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caldon CE, Sergio CM, Kang J et al Cyclin E2 overexpression is associated with endocrine resistance but not insensitivity to CDK2 inhibition in human breast cancer cells. Mol Cancer Ther 2012; 11: 1488–99. [DOI] [PubMed] [Google Scholar]
- 32. Cui X, Yu L, Wang Y et al The relationship between cyclin G1 and survival in patients treated surgically for HCC. Hepatogastroenterology 2012; 60. doi: 10.5754/hge12549 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33. Akli S, Zhang XQ, Bondaruk J et al Low molecular weight cyclin E is associated with p27‐resistant, high‐grade, high‐stage and invasive bladder cancer. Cell Cycle 2012; 11: 1468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ogino S, Nosho K, Irahara N et al A cohort study of cyclin D1 expression and prognosis in 602 colon cancer cases. Clin Cancer Res 2009; 15: 4431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Che Y, Ye F, Xu R et al Co‐expression of XIAP and cyclin D1 complex correlates with a poor prognosis in patients with hepatocellular carcinoma. Am J Pathol 2012; 180: 1798–807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cox multivariate analyses of prognostic factors on recurrence‐free survival.
Fig. S1. Expression of cyclin F was connected with overall survival in subgroups of hepatocellular carcinoma patients. Kaplan–Meier survival analyses were performed in subgroups according to the factors that are attributed to outcome of hepatocellular carcinoma patients (log‐rank test). AFP, alpha‐fetoprotein; HBsAg, hepatitis B surface antigen.


