Abstract
Platelet‐derived serotonin (5‐HT) is involved in liver regeneration. The liver is also the metastatic site for malignant enterochromaffin (EC) cell “carcinoid” (neuroendocrine) neoplasms, the principal cellular source of 5‐HT. We hypothesized that 5‐HT produced by metastatic EC cells played a role in the hepatic tumor‐microenvironment principally via 5‐HT 7 receptor‐mediated activation of hepatocyte IGF‐1 synthesis and secretion. Using isolated rat hepatocytes, we evaluated 5‐HT7 receptor expression (using PCR, sequencing and western blot). ELISA, cell transfection and western blots delineated 5‐HT‐mediated signaling pathways (pCREB, AKT and ERK). IGF‐1 synthesis/secretion was evaluated using QPCR and ELISA. IGF‐1 was tested on small intestinal neuroendocrine neoplasm proliferation, while IGF‐1 production and 5‐HT 7 expression were examined in an in vivo SCID metastasis model. Our results demonstrated evidence for a functional 5‐HT 7 receptor. 5‐HT activated cAMP/PKA activity, pCREB (130–205%, P < 0.05) and pERK/pAKT (1.2–1.75, P < 0.05). Signaling was reversed by the 5‐HT7 receptor antagonist SB269970. IGF‐1 significantly stimulated proliferation of two small intestinal neuroendocrine neoplasm cell lines (EC50: 7–70 pg/mL) and could be reversed by the small molecule inhibitor BMS‐754807. IGF‐1 and 5‐HT were elevated (40–300×) in peri‐tumoral hepatic tissue in nude mice, while 5‐HT 7 was increased fourfold compared to sham‐operated animals. We conclude that hepatocytes express a cAMP‐coupled 5‐HT7 receptor, which, at elevated 5‐HT concentrations that occur in liver metastases, signals via CREB/AKT and is linked to IGF‐1 synthesis and secretion. Because IGF‐1 regulates NEN proliferation, identification of a role for 5‐HT 7 in the hepatic metastatic tumor microenvironment suggests the potential for novel therapeutic strategies for amine‐producing mid‐gut tumors.
Serotonin, also known as 5‐hydroxytryptamine (5‐HT), is a biogenic amine produced by enterochromaffin (EC) cells of the gastrointestinal tract.1 In the blood, 5‐HT is carried by platelets2 and exported to various sites in the body, where it produces mitogenic effects.3, 4, 5, 6 Recently, 5‐HT has been considered a modifier of liver function7 due to the autonomic nervous system input8 as well as the blood flow within this organ.9 This amine is thought to regulate proliferation and function of a number of key resident liver cells, including hepatocytes, stellate cells and sinusoidal endothelial cells.10
5‐HT1A,1B,1D,1F,2A,2B,2C,3A,3B receptors are known to be expressed on hepatocytes.10, 11 While the 5‐HT1 family inhibits cAMP,12 5‐HT2 receptors signal through PLC and 5‐HT4,6,7 receptors stimulate PKA/cAMP. Neither the complete 5‐HT receptor profile nor the intracellular pathways associated with 5‐HT‐mediated stimulation of hepatocyte function are known.7 We hypothesized that PKA‐dependent as well as PKA‐independent cAMP effects and AKT signaling are linked to 5‐HT driven hepatocyte stimulation.13
Small intestinal (SI) neuroendocrine neoplasms (NEN) represent a group of tumors derived from 5‐HT producing EC cells; they have an approximate 2/100 000 incidence and an estimated prevalence of approximately 30 000 patients.14 All tumors produce 5‐HT in excess (approximately 1 μM), which is usually removed by hepatocyte‐mediated monoamine oxidase. However, once a tumor metastasizes to the liver, this physiological clearance is surmounted and patients exhibit an expansion of the 5‐HT pool size with striking increases in blood and platelet 5‐HT, an elevation of 5‐HIAA in urine14, 15 and the production of a number of symptoms (“carcinoid syndrome”) all effected by elevated 5‐HT.14, 15
Metastatic SI NEN cells are likely trapped within the hepatic trabecular structures and, in the process of tumor proliferation, enter the sinusoidal spaces, making direct contact with hepatocytes. In this hepatic tumor microenvironment, tumor 5‐HT reaches concentrations at least in the μM range: 1000‐fold higher than normal serum levels.16, 17 We postulate a direct cross‐talk between metastatic tumor cell and hepatocytes under these conditions, with co‐regulation of cell secretion and proliferation. IGF‐1 and HGF are crucial growth factors supporting progression of various cancers18, 19, 20, 21, 22, 23, 24; these are produced and secreted in hepatocytes25, 26 and, therefore, are of importance in the tumor cell:hepatocyte interaction. We postulated that a hepatocyte : tumor axis existed comprising growth factors and amines. Our aim was to examine the 5‐HT receptor profile in hepatocytes, determine the effects of 5‐HT on hepatocyte function (signal pathway activation, transcription and growth factor secretion) and evaluate evidence for a 5‐HT/IGF‐1 axis in an in vivo model of SI NEN metastasis.
Material and Methods
Experimental approach
Studies were performed on freshly isolated rat hepatocytes, two well‐characterized SI NEN cell lines 27 and a murine hepatic‐metastasis model (SCID/splenic H‐STS injection). For hepatocytes, the effects of 5‐HT at physiological (10−9 M) and elevated (circulating levels detectable in patients with “carcinoid syndrome” and denoted as “pathological”; 10−6 M) levels16, 17 on cell viability, PKA/cAMP/CREB, AKT and ERK1/2 (MAPK) signaling were evaluated. Growth factor transcription as well as growth factor secretion (IGF‐1, HGF) were determined in hepatocytes after 5‐HT administration. Antisense strategies were used to confirm 5‐HT7 receptor function. SI NEN cell line proliferation (in response to recombinant IGF‐1) was evaluated by both MTT uptake and Glomax‐based cytotoxicity assays (in the absence or presence of the small molecule IGF‐1R inhibitor BMS‐754807)28 as was IGF‐1R phosphorylation (western blot). IGF‐1, 5‐HT and 5‐HT7 expression were evaluated in vivo in the livers of sham‐operative and tumor‐bearing animals.
Chemicals and antibodies
The 5‐HT7 receptor antagonist SB269970 was from Tocris Bioscience (Ellisville, MO, USA), Methiothepin (5‐HT5A/B,6,7 antagonist) and Ketanserin (5‐HT2A/C antagonist) from Sigma Aldrich, PRX‐08066 (5‐HT2B antagonist) was a gift of EPIX Pharmaceutical (Lexington, MA, USA) and recombinant IGF‐1 from R&D Systems (Minneapolis, MN, USA). Primary antibodies, phosphorylated(p)‐AKT(Ser473), AKT, pERK1/2(Thr185, Tyr187), ERK1/2, pCREB(Ser133), CREB, pIGF‐1R(Tyr1361) and IGFIR, and HRP‐secondary antibodies were from Cell Signaling Technology (Danvers, MA, USA). 5‐HT7 (AB9405) was from Millipore (Billerica, MA, USA) and BMS‐754807 was from Chemietek (Indianapolis, IN, USA).
Rat hepatocytes
Normal rat hepatocyte isolation: Hepatocytes were isolated from rat liver by collagenase perfusion, as described previously,29 and maintained in Williams E medium supplemented with 10 mM Hepes buffer, 2 mM L‐glutamine, 1.8 g/L glucose, 1 μM dexamethasone, 4 mg/L insulin, 100 U/mL penicillin, 100 μg/mL streptomycin and 10 mg/L gentamycin.30 Preparations with >90% viable cells were used for experiments.
Hepatocyte culture
Freshly isolated primary rat hepatocytes were maintained in Williams E medium supplemented with HEPES (2.8 g/L), dextrose (1.8 g/L), l‐glutamine (20 mM), gentamycin (800 μg/L), dexamethason (400 μg/L), insulin, 10% FBS, penicillin (100 IU/mL), streptomycin (100 μg/mL) and amphotericin B (2.5 μg/mL). Cells were plated into collagen‐coated plates at a density of 3–4 × 104 cells/cm2, and after 24 h hepatocytes were coated with a second layer of rat tail collagen I (BD Biosciences, Bedford, MA, USA) in a “sandwich” configuration.31, 32 For experiments, cells were cultured under serum‐free conditions. All experiments were performed within 48 h of plating.
Hepatocyte “proliferation/viability”
Hepatocytes (4 × 104 cells/cm2) were plated in 96‐well plates (sandwich configuration) and stimulated with 5‐HT (10−6 M to 10−12 M: n = 6 wells/concentration). After 1 h, cell viability was evaluated using WST‐1 (Roche, Indianapolis, NJ, USA).33 5‐HT receptor antagonists were included (10−6 M).
PKA/cAMP signaling pathway analysis
After 30 min pre‐incubation with 5‐HT receptor antagonists (10−6 M), cultured hepatocytes (48 h) were stimulated with physiological (10−9 M) and “pathological” (10−6 M) 5‐HT for 0.5 h. PKA activity and cAMP levels were quantified using SuperArray ELISA kits (R&D Systems).34
pAKT/AKT signaling pathway analysis
After pre‐incubation with 5‐HT receptor antagonists (10−6 M), hepatocytes were stimulated with 5‐HT (10−9 M, 10−6 M) and pAKT/total AKT signal activity quantified using SuperArray CASE ELISA kits (SABiosciences, Frederick, MD, USA; ERK‐FE‐002).35
Protein extraction and western blot
Fresh isolated hepatocytes (4 × 104 cells/cm2) were maintained with a collagen layer in collagen‐coated 6‐well plates (Falcon, BD Biosciences) and treated with 5‐HT (10−9 M, 10−6 M) for 15 min to 8 h. Selective receptor antagonists were used as described. Whole cell lysates were prepared in ice‐cold lysis buffer (10× RIPA lysis buffer [Millipore, Billerica, MA, USA]) and, after centrifugation, the supernatant was transferred and quantified using the BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). After denaturation (SDS sample buffer), total protein lysates (20 μg) were separated on an SDS‐PAGE gel (10%) and transferred to a PVDF membrane (Bio‐Rad, Hercules, CA, USA; pore size: 0.45 mM). Following blocking, membranes were incubated with primary antibodies (Cell Signaling Technology, Danvers, MA, USA) overnight at 4°C and, after HRP‐secondary antibodies, immunodetection was performed using the Supersignal West Pico Luminol/Enhancer solution (Thermo Fisher Scientific, Rockford, IL, USA) and the blots were exposed on X‐OMAT‐AR films.36 The optical density of the appropriately sized bands was quantified using ImageJ software (NIH, Bethesda, MD, USA). The ratio between phosphorylated‐protein and total protein was calculated; total protein expression was reported relative to that of β‐actin (Sigma‐Aldrich, St. Louis, MO, USA).
Hepatocyte transfection with serum response element (SRE) vector
To confirm activation of the MAPK/ERK signaling pathway, hepatocytes were transfected using the pGL4.33 [luc2P/SRE/Hygro] Vector (Promega, Madison, WI, USA). Hepatocytes were plated as described and after 24 h, transfection was undertaken using Lipofectamin 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. After 18 h, media was changed and cells were stimulated with 5‐HT (10−9 M, 10−6 M) and the 5‐HT receptor antagonists for 1 and 4 h. Luminescence was measured using the Bright‐Glo Luciferase Assay System (GloMax Multi+ Detection System; Promega).
RNA isolation and reverse transcription
RNA was isolated from rat hepatocytes (1 × 106 cells, n = 6) using TRIzol (Invitrogen), cleaned (Qiagen, RNeasy kit; Qiagen, Valencia, CA, USA) and converted to cDNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Carlsbad, CA, USA).34, 37
Primer preparation and QT‐PCR
Primers for each subtype of 5‐HT receptors (1A,1B,1D,1E,1F,2A,2B,2C,3,4,5A,5B,6,7) were used as described.38 Appropriate bands were excised and prepared for sequencing (Wizard SV Gel and PCR Clean‐UP System [Promega]). Purified PCR products were sequenced (W.M. Keck Biotechnology Resource Laboratory, Yale University) using an automated Applied Biosystems 373A Stretch DNA Sequencer (Perkin‐Elmer, Norwalk, CT, USA) utilizing both the forward and reverse primers.37 Sequencing products were analyzed using Bioedit.39
5‐HT7 receptor knockdown
A 21‐mer oligonucleotide antisense corresponding to 1011–31 of the rat 5HT7 receptor (NM_022938.2) was designed to induce a steric obstacle for protein translation (Yale Medical School Keck Oligonucleotide Synthesis Facility).40 Control nucleotides were prepared with randomized sequence of matching nucleotides per protocol. In these experiments, isolated hepatocytes cells were exposed to oligonucleotides (antisensense: CACACTCTTCCACCTCCTTCT, or control: CTCCTTCTCAACCCCACTTTC, 150 pmol) prior to sandwiching, culture and study.
RT‐PCR analyses
RT‐PCR analyses were performed using Assays‐on Demand and the ABI 7900 Sequence Detection System with primer sets from Applied Biosystems; the presence of single bands for each primer sets was confirmed by PCR mix on gels. Data was normalized using the ∆∆CT approach and the rat Act1b was used as a housekeeping gene.
HGF and IGF‐1 secretion
Secretion levels of HGF and IGF‐1 were analyzed using commercially available ELISA assays (HGF: B‐Bridge International; IGF‐1: R&D Systems). Cells were seeded into 6‐well plates and stimulated with 5‐HT (10−9 M, 10−6 M) and the 5‐HT antagonists. After 1 h, growth factor levels were quantified in the supernatant according to the manufacturer's instructions.
Small intestinal neuroendocrine neoplasms and in vivo model
Small intestinal neuroendocrine neoplasm culture and proliferation
Two well‐characterized NEN cell lines (KRJ‐I, primary tumor; and H‐STS, hepatic metastasis41) were cultured as floating aggregates in Quantum 263 (PAA, Dartmouth, MA, USA) supplemented with 100 IU penicillin/mL and 100 μg streptomycin/mL.27 All experiments were performed without antibiotics at day 2 following sub‐culture. The proliferation of cell lines (5 × 104 cells/mL) was studied using MTT uptake or luminescent‐based protocols (live/dead, caspase 3/7 activation: Promega).42 in response to recombinant IGF‐1 (1–1000 pg/mL) with or without BMS‐754807 (10−9 to 10−5 M; 24 h).27
Western blot
Cells (1 × 106/mL) were stimulated with recombinant IGF‐1 (1 ng/mL) for 1 h, protein isolated and western blot performed using antibodies against pIGFR, IGFR and β‐actin.27
In vivo metastasis model
Intrasplenic injection of 10 × 106 (200 μL, 27G needle) H‐STS cells was undertaken in laparotomized, isoflurane‐anesthetized male or female SCID mice (6–8 weeks; approximately 20 g, Jackson Laboratories, Bar Harbor, ME, USA).43 The wound was closed in one layer with wound clips; the animals were kept until 6 weeks and then killed. Studies were approved by the Yale University Institutional Animal Care and Use Committee. Clinical standard staining was conducted for pathologist examination using H&E, or Chromgranin A or MKI67 ([MIB1], Dako, Glostrup, Denmark), which were detected using DAB (3,3′‐Diaminobenzidine). Normal liver (sham‐operated) and liver adjacent to the macroscopic tumor (<2 mm) were processed to protein. IGF‐1 and 5‐HT levels (Rocky Mountain Diagnostics, Colorado Springs, CO, USA).27 were measured by ELISA. Western blot was performed for IGF‐1 and 5‐HT7.
Statistical evaluation
All statistical analyses were performed using Microsoft Excel and Prism 5 (GraphPad Software, San Diego, CA, USA). Nonlinear regression analyses were used to evaluate half maximal effective (EC50) concentration. Cell viability assays were evaluated using two‐tailed Student's t‐tests; all other data were assessed using the two‐tailed Mann–Whitney tests.
Results
Rat hepatocytes
Evaluation of the 5‐HT receptor profile and effects of 5‐HT on viability
The 5‐HT receptor profile of normal rat hepatocytes was evaluated using QT‐PCR.38 Appropriate band sizes were identified for 5‐HT1A,1D,2A,2B,5D,6,7 (Fig. 1a). Sequence analysis confirmed expression of these receptors. The confirmatory results for 5‐HT7 (sequencing and analysis: BioEdit software)39 (Fig. 1a), western blot (Fig. 1b: band size of approximately 50 kDa) and immunohistochemistry (Fig. 1c: largely membrane expression with some intracellular staining) are shown. A dose‐dependent effect on cell viability was noted after treatment with 5‐HT (10−12 M to 106 M), with increases at both physiological and pathological levels (10−9 M to 10−6 M, EC50~10−9 M, P < 0.05; Fig. 1d). The combination of the 5‐HT2 antagonists PXR‐08066 and ketanserin (both 10−6 M) reversed effects of 5‐HT (10−6 M, P = 0.04), while no effect was noted using the 5‐HT7 receptor antagonists SB269970 and methiothepin (both 10−6 M; Fig. 1e).
Figure 1.
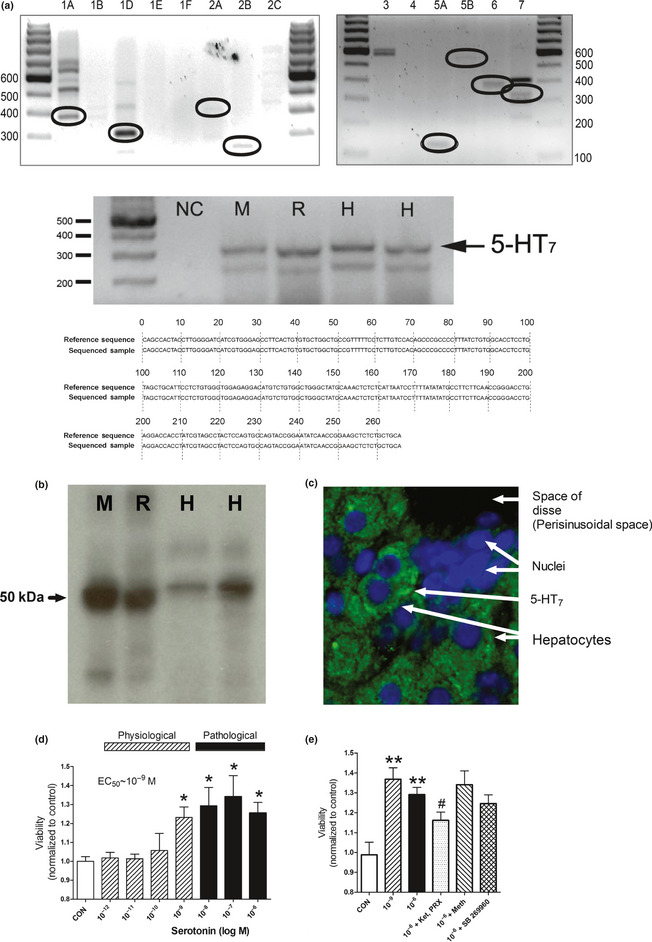
(Previous page) Identification of the 5‐HT 7 receptor and effect of 5‐HT on hepatocyte viability. A PCR analysis of hepatocytes identified transcripts of the correct band size for 5‐HT 1A,D,2A,B,5B,6,7. Analysis of 5‐HT 7 receptor confirmed expression by sequencing (a) (reference sequence: NM_022938.2), by western blot; size of approximately 50 kDA (b) and by immunohistochemistry (c). A dose‐dependent effect of 5‐HT was noted on isolated hepatocyte cell viability (mitochondrial activity assessed by WST‐1) with an EC 50~10−9 M (d). The increase in cell viability (1 h), was reversed by the combination of ketanserin and PRX‐08066 (e) but not by 5‐HT 5–7 antagonists indicating proliferation was mediated by 5‐HT 2 receptors. For western blot: M, mouse; R, rat; H, human. For immunohistochemistry: nuclei are blue (DAPI) and 5‐HT 7 is green (FITC‐labeled secondary antibody). This is a dual‐stained section from a rat liver. Mean ± SEM, n = 12, *P < 0.05. Ket, ketanserin; PRX, PRX‐08066; Meth, methiothepin.
Effects of 5‐HT on hepatocytes signaling pathways
2A. PKA activity and cAMP levels: PKA activity was induced in a dose‐dependent manner after 5‐HT (10−10 M to 10−6 M) administration (EC50~10−9 M, P < 0.05; Fig. 2a). A significant increase was noted at physiological (10−9 M, 178 ± 83%, P < 0.05) as well as “pathological” (10−6 M, 189 ± 94%, P < 0.05) levels. Effects of 5‐HT (10−6 M) were completely reversed by SB269970, methiothepin and the combination of PRX‐08066 and ketanserin (10−6 M; P < 0.05; Fig. 2b). A significant dose‐dependent increase in cAMP was noted after 5‐HT treatment (EC50~10−9 M, P < 0.05; Fig. 2c). cAMP levels were elevated at physiological (10−9 M, 172 ± 88%, P < 0.05) and “pathological” (10−6 M, 163 ± 51%, P < 0.05) levels. Effects of 5‐HT (10−6 M) were significantly reversed only by methiothepin and SB269970 (10−6 M; P < 0.05; Fig. 2d).
Figure 2.
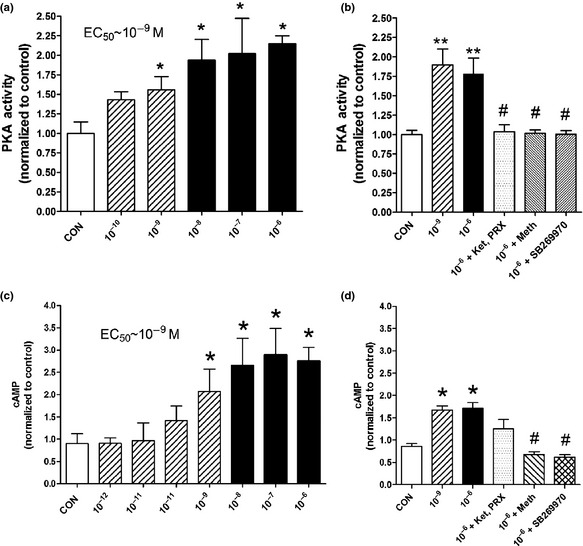
Effects of 5‐HT on PKA activity and cAMP levels in hepatocytes after 0.5 h treatment. A dose‐dependent increase in PKA activity was noted with an EC50~10−9 M (a) accompanied with increased cAMP levels (EC50~10−9 M) (b). Both effects were reversed by the 5‐HT7 inhibitors methiothepin and SB269970 (10−6 M) (2c,d). Mean ± SEM, n = 12 (three separate experiments), *P < 0.05, **P < 0.01 vs control, #P < 0.05 vs 10−6 M. Ket, ketanserin; PRX, PRX‐08066; Meth, methiothepin. Bars with stripes represent “physiological” 5‐HT concentrations and solid bars (black) represent “pathological” 5‐HT concentrations.
2B. pAKT/AKT activity: A significant increase in pAKT/AKT was noted after 5‐HT administration (10−9 M: 227 ± 73%, P < 0.05; 10−6 M: 369 ± 195%, P < 0.01) determined by ELISA assays (Fig. 3a). Using western blot analyses, a significant increase at physiological (114 ± 12%, P < 0.01) and “pathological” (137 ± 19%, P < 0.01) 5‐HT levels was noted (Fig. 3b). The increase in pAKT/AKT activity was significantly different between physiological and “pathological” concentrations (P < 0.05). pAKT/AKT activation was partially reversed by the combination of ketanserin and PRX‐08066 but completely reversed by SB269970 (10−6 M, P < 0.05; Fig. 3c,d).
Figure 3.
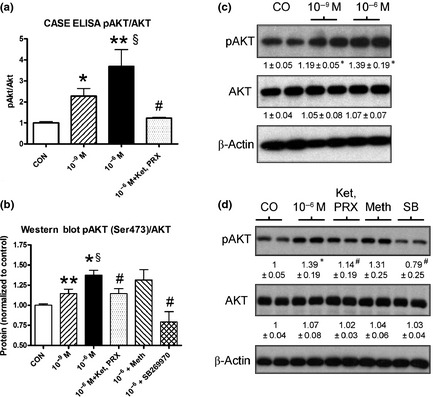
Effects of 5‐HT (10−6 M, 10−9 M) on AKT signaling in hepatocytes after 1 h of treatment. 5‐HT significantly increased pAKT/AKT ratio particularly at 10−6 M as assessed by CASE ELISA (a) and western blot analyses (b). Effects were significantly reversed by the 5‐HT7 inhibitors methiothepin and SB269970 (c,d). Mean ± SEM, n = 4 (three separate experiments), mean ± SEM; *P < 0.05 vs control; §P < 0.05 10−9 M vs 10−6 M 5‐HT; #P < 0.05 vs 10−6 M 5‐HT. Ket, ketanserin; PRX, PRX‐08066; Meth, methiothepin.
2C. pERK/ERK activity: A significant increase in pERK/ERK was noted after treatment with 10−9 M (131 ± 0.09%, P < 0.05) and 10−6 M (134 ± 0.13%, P < 0.05) 5‐HT. Effects of 5‐HT (10−6 M) were only reversed by methiothepin (10−6 M, P < 0.05). No inhibition was noted for SB269970 or the combination of ketanserin and PRX‐08066 (all 10−6 M). Transfection of hepatocytes with pGL4.33[luc2P/SRE/Hygro] confirmed activation of MAPK/ERK signaling at 10−6 M 5‐HT at 1 and 4 h (175 ± 69%, 334 ± 183%; Fig. 4a–c).
Figure 4.
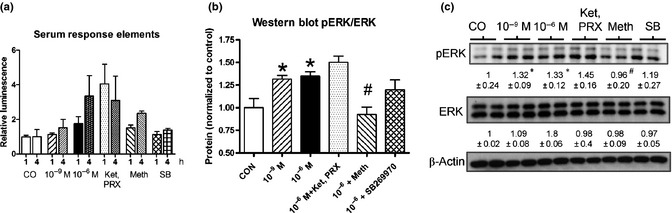
Effects of 5‐HT on ERK signaling in hepatocytes after 1 h of treatment. Luminescence‐based serum response elements vector expression (a) and western blot analyses (4b,c) identified intact ERK signaling pathways that could be activated by 5‐HT and inhibited by methiothepin. Mean ± SEM, n = 4 (three separate experiments), mean ± SEM; *P < 0.05 vs control; #P < 0.05 vs 10−6 M 5‐HT. Ket, ketanserin; PRX, PRX‐08066; Meth, methiothepin.
2D. CREB time response analyses: After administration of 10−9 M 5‐HT an increase of pCREB levels (SER133) was evident after 2 h (180 ± 45%) with a maximum effect at 4 h (184 ± 3.3%). At 5‐HT concentrations of 10−6 M, pCREB levels were elevated after 1 h (195 ± 46%), with a maximum effect at 8 h (271 ± 64%; Fig. 5a,c).
Figure 5.
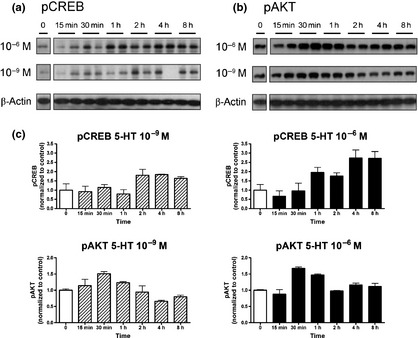
Time dependent effects of 5‐HT on CREB and AKT signaling. pCREB (a) and pAKT (b) levels determined by western blot analyses and the optical density measurements (c) measured using the ImageJ software. Early and prolonged effects were noted for 10−6 M 5‐HT. Mean ± SEM, n = 3 (two separate experiments).
2E. AKT time response analyses: A significant increase of pAKT protein (Ser473) levels was noted at 10−9 M as well as 10−6 M 5‐HT, with a maximum at 30 min (10−9 M, 151 ± 9%; 10−6 M, 167 ± 6% (Fig. 5b,c).
Effects of 5‐HT on secretion and production of growth factors
3A. IGF‐1 secretion and transcription: A significant increase of IGF‐1 secretion (128 ± 12%, P < 0.05) and IGF‐1 transcript levels (127 ± 24%, P < 0.01) was evident at 10−6 M 5‐HT compared to 10−9 M. Increased transcript as well as protein levels of IGF‐1 at 10−6 M 5‐HT were completely reversed by methiothepin and SB269970 (P < 0.05; Fig. 6a,c).
Figure 6.
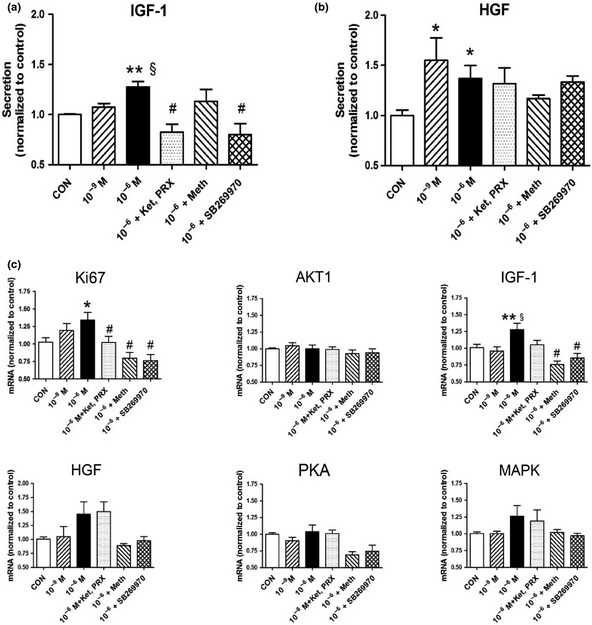
Growth factor and pathway targets in hepatocytes treated for 1 h. Secretion levels of IGF‐1 and HGF as well as transcript levels of Ki67,AKT1,IGF‐1,HGF,PKA and MAPK3 demonstrate 5‐HT stimulates IGF‐1 and HGF secretion as well as IGF‐1 and Ki67 synthesis. Effects were consistently reversed by SB269970. *P < 0.05, **P < 0.01 vs control, §P < 0.05 10−9 M vs 10−6 M 5‐HT, #P < 0.05 vs 10−6 M. Mean ± SEM, n = 6 (three separate experiments). Ket, ketanserin; PRX, PRX‐08066; Meth, methiothepin.
3B. HGF secretion and transcription: A significant increase of HGF was determined at both 10−9 M (148 ± 52%, P < 0.05) and 10−6 M (137 ± 43%, P < 0.05) 5‐HT. No significant inhibition was noted with the 5‐HT receptor antagonists. (Fig. 6b,c).
Effect of 5‐HT on Ki67, AKT, c‐MET, PKA and MAPK3 transcription
Transcript analyses demonstrated a significant increase of Ki67 levels at 10−9 M (124 ± 32%, P < 0.05) and 10−6 M (134 ± 42%, P < 0.05) 5‐HT (Fig. 6c). No effects on transcript levels of AKT1, PKA and MAPK3 were noted.
Mechanistic analysis of 5‐HT7 receptor expression in hepatocytes
We used an antisense approach to mechanistically confirm the pathways associated with 5‐HT7 signaling in isolated rat hepatocytes. 5‐HT7 antisense decreased transcription (approximately 50%; Fig. 7a) and antisense‐treated cells lost responsiveness to 5‐HT‐mediated PKA activation and cAMP production (Fig. 7b) as well as IGF‐1 secretion (Fig. 7c). These results confirm that signaling via this receptor (5‐HT7) via PKA/cAMP is a principle pathway involved in 5‐HT‐mediated IGF‐1 production from hepatocytes.
Figure 7.
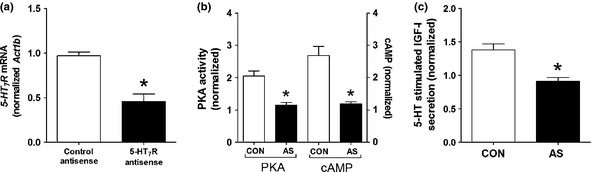
5‐HT7 knockdown and pathway activation in hepatocytes. Antisense oligonucleotides inhibited 5‐HT7 mRNA approximately 50% (a). PKA activity and cAMP production were significantly reduced in response to 5‐HT (10−6 M) stimulation in antisense‐treated cells (b). Secretion levels of IGF‐1, likewise, were significantly reduced by 5‐HT7R knockdown in 5‐HT stimulated cells (c). *P < 0.05 vs control. Mean ± SEM, n = 6. CON, control antisense; AS, antisense.
Small intestinal neuroendocrine neoplasm and in vivo model
IGF‐1 mediated signaling in NEN cell lines
6A. Effect of IGF‐1 on proliferation and IGF1R phosphorylation: A dose‐dependent effect of recombinant IGF‐1 treatment (1–1000 pg/mL) on proliferation was noted for both KRJ‐I (EC50~70 pg/mL) and H‐STS (EC50~7 pg/mL) cell lines (Fig. 8A). One hour after IGF1 administration (1000 pg/mL), both cell lines exhibited an increase in pIGF‐1R (pTYR1361) levels, indicating growth‐mediated signaling via this receptor (Fig. 8b). Pretreatment with BMS‐754807 (1 h, 10 μM) reversed IGF‐1‐mediated phosphorylation (Fig. 8b).
Figure 8.
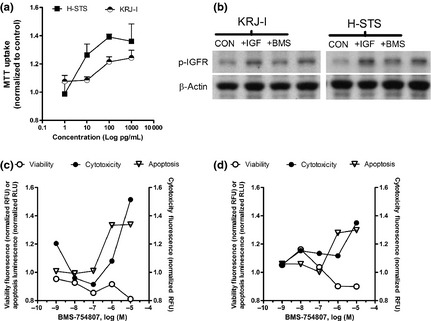
Examination of IGF‐1 effects on SI NEN cell lines. A dose dependent effect on proliferation was evident with EC50~7–100 pg/mL recombinant IGF‐1 (a). Effects of IGF‐1 (1000 pg/mL) on pIGF‐1R in each of the cell lines after 1 h treatment demonstrated upregulation of IGFR‐mediated signaling compared to untreated cells while pretreatment for 1 h with BMS‐754807 (10 μM) reversed phosphorylation (b). Pre‐treatment with BMS‐754807 reduced viability in both cell lines (c,d) and had both cytotoxic and apoptotic effects (estimated IC50 = 2 × 10−7 M). Mean ± SEM, n = 6 (three separate experiments). CON, control (unstimulated); IGF, IGF‐1 stimulated; BMS, BMS‐754807 treated.
6B. Effect of BMS‐754807 on proliferation: BMS‐754807 dose‐dependently inhibited live cells (cell viability), increasing dead cells (cytotoxicity) via apoptosis (caspase 3/7 activation; Fig. 8c,d). The estimated IC50 was 2 × 10−7 M, with an ICmax of 10 μM.
In vivo model of small intestinal neuroendocrine neoplasm metastasis
Liver metastasis developed in SCID mice intra‐splenically injected with H‐STS cells within 4–6 weeks. These H‐STS cell‐derived lesions have a typical NEN morphology, are CgA positive and are rapidly proliferating (Ki‐67 [Mib1 antibody]: 70–90%; Fig. 9a). Microdissection of “normal” liver adjacent to H‐STS cell tumors in the tumor‐bearing animals identified that protein levels of IGF‐1 (twofold, P < 0.05) and 5‐HT levels (300‐fold, P < 0.002) were all significantly increased compared to levels in the livers of sham‐operated animals (Fig. 9b,c). Western blot confirmed the increase in IGF‐1 as well as in 5‐HT7 which was elevated approximately fourfold compared to sham‐operated animals (Fig. 9d,e). Ki67 expression, likewise, was increased (approximately 15‐fold) in these livers compared to sham‐operated animals (Fig. 9f).
Figure 9.
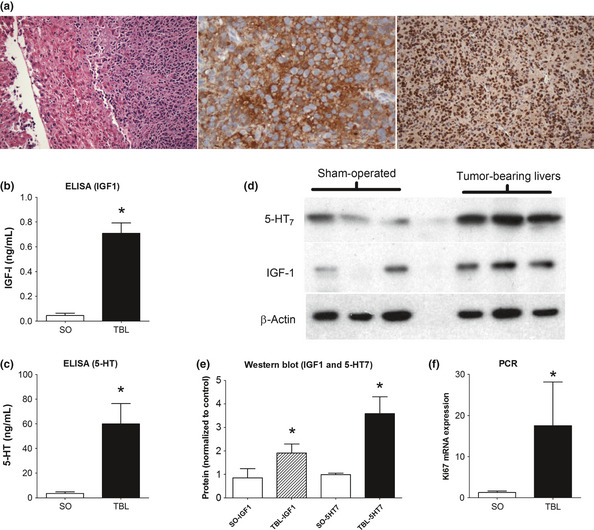
IGF‐1 and 5‐HT7 in a murine model of hepatic SI NEN metastasis. Nude mice injected splenically with H‐STS cells develop liver metastases within 4–6 weeks. Tumors demonstrate typical NEN morphology (H&E, left), are CgA positive (DAB‐brown stained cells, center) and are rapidly proliferating (Ki67: 90%, right) (a). IGF‐1 (b) and 5‐HT (c) were significantly increased in tumor‐bearing livers compared to sham‐operated animals. Western blot confirmed elevated 5‐HT7 and IGF‐1 in TBL animals (d); optical density (ImageJ software) confirmed over‐expression compared to SO mice (e). Ki67 mRNA expression was increased approximately 15‐fold compared to SO mice. SO, sham‐operated; TBL, tumor‐bearing livers; mean ± SEM, n = 6–9.
Discussion
Serotonin (5‐HT) is not only a neurotransmitter but also has a significant physiological role as a hormone in a number of gastrointestinal systems.44 An intestinal–hepatic axis linking serotonin producing EC cells with the liver remains a largely underexplored subject.7 Recent evidence supporting the role of 5‐HT in liver regeneration10 has strengthened the hypothesis that this amine plays a role in liver physiology. These physiological effects are generally considered to be mediated via the 5‐HT2 receptor family10 and are considered to reflect platelets as the aminergic source. The role of 5‐HT secreted by SI NEN that have metastasized to the liver (i.e. an exogenous cellular source of the amine) has not been investigated. We examined this at a number of levels, including measuring the effects of different 5‐HT concentrations on isolated hepatocytes as well as determining expression of the serotonin‐IGF‐1 axis in an in vivo liver metastasis model to identify potential therapeutic targets. Our data identify significant differences in signal pathway activation and growth factor synthesis and secretion at physiological (10−9 M) and “pathological” (10−6 M) 5‐HT levels, which suggests that high amine production by tumor cells (5‐HT reaches concentrations at least in the μM range: 1000 fold higher than normal serum levels)16, 17 may modify hepatocyte behavior (specifically the production of IGF‐1) in a fashion conducive to support local SI NEN cell proliferation. In addition, we demonstrate an intact 5‐HT7 receptor signaling pathway, which, together with IGF‐1R (on tumor cells) provides potential translational therapeutic targets. Our model proposes that 5‐HT secretion from SI NEN activates 5‐HT7 receptors on hepatocytes, leading to PKA/pCREB/AKT activation and IGF‐1 secretion, which, importantly, is only regulated at “pathological” concentrations of 5‐HT.
The liver is considered to express at least 5‐HT1A,B,D,F, 2A‐C and 3A‐B receptors,10 and although crude hepatocyte fractions are 5‐HT2 receptor positive,11 expression of 5‐HT5‐7 has not, to date, been examined.7 In our study, we screened rat hepatocyte preparations using primers developed by Chen et al.38 and confirmed expression of 5‐HT1A,D, 2A,B, 5D, 6 and 7 receptors in these cells. As we were specifically interested in 5‐HT7, we examined this in more detail. Our studies confirmed expression of this receptor on rat, mouse and human hepatocytes.
An analysis of the effects of 5‐HT on rat hepatocyte cell viability (measured by WST‐1 uptake and elevated Ki67 transcripts) identified that the 5‐HT2 receptors were principally responsible for mediating this phenomenon. The combination of the 5‐HT2 receptor antagonists, ketanserin and PRX‐08066, could reverse increases in WST‐1 and Ki67. In contrast, the 5‐HT5–6 receptor antagonist, methiothepin, as well as the 5‐HT7 antagonist, SB269970, did not have a significant inhibitory effect. These results confirm that hepatocyte proliferation or viability is principally 5‐HT2 driven.10 However, ERK activation (inhibited by methiothepin) and Ki67 transcription (inhibited by Ketanserin/PRX‐08066 and SB269970) suggest that hepatocyte proliferation is complex and may be regulated by 5‐HT at a number of levels.
5‐HT2 receptors principally signal via PLC while 5HT7 (and receptors 4,6) signal via cAMP/PKA.12 We measured a dose‐dependent increase in cAMP levels as well as PKA activity in cultured hepatocytes stimulated with either physiological or pathological 5‐HT concentrations. These effects were completely reversible by SB269970, confirming signaling through the 5‐HT7 receptor. Interestingly, the combination of ketanserin and PRX‐08066 also decreased PKA activity. As ketanserin is known to show an affinity for 5‐HT7 receptors,45, 46 the measured effect could be due to non‐specific antagonism of this receptor subtype.
Given prior observations that activated cAMP may also be associated with AKT as well as the ERK (MAPK) alterations in hepatocytes,13, 47, 48 we investigated the interaction with the AKT as well as the ERK (MAPK) pathway. A significant increase in pAKT/AKT at pathological and physiological 5‐HT concentrations was identified. Of note was the observation that the effect was significantly higher at pathological levels. We interpret this to indicate that AKT signaling in hepatocytes may be preferentially activated by 5‐HT secreting SI NEN metastases. In our studies, pAKT/AKT activity was completely reversed by SB269970, suggesting that AKT signaling is predominantly a 5‐HT7 receptor‐mediated effect via the PKA‐independent cAMP‐GEF/Rap pathway.49, 50, 51 A partial but not complete response was evident with Ketanserin/PRX‐08066, suggesting either antagonism at the 5‐HT7 receptor level or an effect via 5‐HT2 receptors.
While both protein kinases AKT and ERK are suggested downstream effectors of Rap, it is known that in hepatocytes, cAMP activates AKT and inhibits ERK,13, 52, 53, 54, 55, 56, 57, 58 effects that play an important role in hepatocyte survival.56, 59, 60, 61 The precise role of ERK in hepatocytes, however, is controversial. While some studies suggest that ERK activation has an anti‐apoptotic effect60, 62, 63 others indicate only a mild effect on hepatocyte survival.13, 53, 56, 64, 65 Cullen and colleagues13 demonstrate a decrease in pERK levels and an increase in pAKT levels in hepatocyte signaling through PKA independent cAMP activation. Our findings, in contrast, demonstrated a significant increase in pERK/ERK activation at both 5‐HT concentrations, an effect that was only reversible by methiothepin, which is known to have high affinity for 5‐HT6 and 7 receptors and moderate affinity for 5‐HT1, 2 and 5 receptors.45 Importantly, no effect was determined with the specific 5‐HT7 receptor antagonist SB269970, suggesting that ERK activation is not 5‐HT7 receptor‐linked. Interestingly, ERK activation was increased over time, suggesting that MAKP signaling was not only a direct consequence of 5‐HT but could also be a secondary effect, perhaps mediated via growth factor auto‐secretion of IGF‐1.66
We next investigated the time‐dependent effects of the two 5‐HT concentrations on pCREB (cAMP response element‐binding protein) as well as pAKT. Increases in pCREB were detected at both concentrations, but this was more evident in hepatocytes stimulated with pathological 5‐HT levels. Use of physiological 5‐HT levels resulted in an increase of pCREB after 2 h, with a maximum at 4 h (184 ± 3.3%); this effect was earlier (after 1 h), prolonged (maximum at 8 h) and increased (maximum 271 ± 64%) by pathological 5‐HT levels. Time course studies for pAKT identified an increase of protein activation at both concentrations (10−9 and 10−6 M), with a maximum after 30 min. This PKA‐independent response was earlier (30 min) compared to the PKA‐dependent CREB response (240 min). These findings suggest that 5‐HT signals in hepatocytes through both PKA‐dependent as well as PKA‐independent pathways; these are associated with differences in both time response and extent of effect.
CREB is known to regulate glucose homeostasis as well as growth factor‐dependent cell survival.67 A total of 155 target genes have been identified, including transcripts for cell survival and growth factor secretion.67 IGF‐1 gene transcription, in particular, is upregulated by CREB in osteoblasts and mesenchymal cells.68, 69, 70, 71 Under normal25, 26, 72, 73, 74 and pathological conditions (e.g. tumor development and progression of a variety of different cancers such as pancreatic carcinoma, colon carcinoma, esophageal carcinoma and non‐small cell lung cancer),1, 18, 19, 20, 21, 22, 23, 24 hepatocytes produce a variety of agents, including IGF‐1 and HGF. In our study, HFG secretion but not transcription was significantly elevated at both physiological and pathological 5‐HT concentrations, suggesting HGF release was amine‐regulatable. However, no significant difference was evident between 10−9 and 10−6 M 5‐HT. The HGF promoter region is characterized by a number of regulatory elements but is not regulated by cAMP.75 In contrast to HGF, both transcription and secretion of IGF‐1 was elevated by 5‐HT, an effect only noted at pathological 5‐HT concentrations. This was completely reversed by SB269970, suggesting that hepatocytes exposed to pathological 5‐HT levels produce and secrete IGF‐1 via 5‐HT7 receptor mediated pathways. IGF‐1 is a well‐known proliferative regulator of NEN;76, 77 our identification of pro‐proliferative effects for IGF‐1 on the two different SI NEN cell lines as well as activation (and reversal by BMS‐754807) of the IGF‐1R pathway confirm the link between the growth factor and these tumors.
We postulate that hepatocytes respond to elevated 5‐HT levels produced by specific SI NEN metastases (tumors that express TPH1 and synthesize this amine) with increased synthesis and secretion of IGF‐1 via the AKT pathway; a 5‐HT‐mediated paracrine production of IGF‐1 most likely supports tumor cell proliferation. Our in vivo model supports such an axis. IFG‐1 was increased in hepatocytes adjacent to the H‐STS cell tumors (a 5‐HT secreting tumor), while 5‐HT7 was upregulated in livers compared to sham‐operated animals. Hepatocytes also responded with upregulation of Ki67, suggesting a co‐proliferative drive.
These observations relating a metastasis to 5HT7 receptors on hepatocytes would be specific for tumors that synthesize and secrete serotonin; other neuroendocrine tumors that are non‐serotonergic, for example,rectal NEN, would not directly activate hepatocytes via this mechanism. It is, however, possible that metastases from other NEN or other sites, for example, colorectal adenocarcinoma, may indirectly regulate hepatocyte function through 5‐HT receptors. Such a mechanism would require tumor‐directed 5‐HT release from platelets as a cellular source.
In conclusion, we have confirmed a role for 5‐HT2 receptors in hepatocyte viability and demonstrated the existence of a functional 5‐HT7 receptor in isolated hepatocyte preparations. Furthermore, we have dissected the differences in pathway activation at physiological (10−9 M) versus pathological (10−6 M) 5‐HT levels on these cells. The former, which may be related to platelet‐derived circulating serotonin10 or neural effectors,8 was related to HGF production and hepatocyte viability. The latter was associated with increased levels of IGF‐1, which play a crucial role in regulating NEN proliferation. Overall, elevated 5‐HT levels, commensurate with metastatic SI NEN, are associated with increased levels of hepatocyte‐derived IGF‐1 synthesis and release. 5‐HT‐mediated perturbations in this growth factor, which plays a crucial supportive role in SI NEN proliferation, identify the significance of the hepatocyte‐NEN microenvironment in supporting metastatic neuroendocrine cell proliferation. Based on these observations, there is evidence to propose that 5‐HT7, AKT and the IGF‐1R may both be viable targets specifically for SI NEN liver metastasis therapy.
Disclosure Statement
The authors have no conflict of interest.
(Cancer Sci 2013; 104: 844–855)
References
- 1. Modlin IM, Kidd M, Pfragner R, Eick GN, Champaneria MC. The functional characterization of normal and neoplastic human enterochromaffin cells. J Clin Endocrinol Metab 2006; 91: 2340–8. [DOI] [PubMed] [Google Scholar]
- 2. George JN. Platelets. Lancet 2000; 355: 1531–9. [DOI] [PubMed] [Google Scholar]
- 3. Nemecek GM, Coughlin SR, Handley DA, Moskowitz MA. Stimulation of aortic smooth muscle cell mitogenesis by serotonin. Proc Natl Acad Sci USA 1986; 83: 674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seuwen K, Magnaldo I, Pouyssegur J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5‐HT1B receptors coupled to a Gi‐protein. Nature 1988; 335: 254–6. [DOI] [PubMed] [Google Scholar]
- 5. Grewal JS, Mukhin YV, Garnovskaya MN, Raymond JR, Greene EL. Serotonin 5‐HT2A receptor induces TGF‐beta1 expression in mesangial cells via ERK: proliferative and fibrotic signals. Am J Physiol 1999; 276: F922–30. [DOI] [PubMed] [Google Scholar]
- 6. Westbroek I, van der Plas A, de Rooij KE, Klein‐Nulend J, Nijweide PJ. Expression of serotonin receptors in bone. J Biol Chem 2001; 276: 28961–8. [DOI] [PubMed] [Google Scholar]
- 7. Ruddell RG, Mann DA, Ramm GA. The function of serotonin within the liver. J Hepatol 2008; 48: 666–75. [DOI] [PubMed] [Google Scholar]
- 8. Pyroja S, Joseph B, Paulose CS. Increased 5‐HT2C receptor binding in the brain stem and cerebral cortex during liver regeneration and hepatic neoplasia in rats. J Neurol Sci 2007; 254: 3–8. [DOI] [PubMed] [Google Scholar]
- 9. Cummings JL, Cilento EV, Reilly FD. Hepatic microvascular regulatory mechanisms. XII. Effects of 5‐HT2‐receptor blockade on serotonin‐induced intralobular hypoperfusion. Int J Microcirc Clin Exp 1993; 13: 99–112. [PubMed] [Google Scholar]
- 10. Lesurtel M, Graf R, Aleil B, et al Platelet‐derived serotonin mediates liver regeneration. Science 2006; 312: 104–7. [DOI] [PubMed] [Google Scholar]
- 11. Balasubramanian S, Paulose CS. Induction of DNA synthesis in primary cultures of rat hepatocytes by serotonin: possible involvement of serotonin S2 receptor. Hepatology 1998; 27: 62–6. [DOI] [PubMed] [Google Scholar]
- 12. Raymond JR, Mukhin YV, Gelasco A, et al Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther 2001; 92: 179–212. [DOI] [PubMed] [Google Scholar]
- 13. Cullen KA, McCool J, Anwer MS, Webster CR. Activation of cAMP‐guanine exchange factor confers PKA‐independent protection from hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol 2004; 287: G334–43. [DOI] [PubMed] [Google Scholar]
- 14. Modlin IM, Oberg K, Chung DC, et al Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008; 9: 61–72. [DOI] [PubMed] [Google Scholar]
- 15. Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology 2005; 128: 1717–51. [DOI] [PubMed] [Google Scholar]
- 16. Allen KR, Degg TJ, Anthoney DA, Fitzroy‐Smith D. Monitoring the treatment of carcinoid disease using blood serotonin and plasma 5‐hydroxyindoleacetic acid: three case examples. Ann Clin Biochem 2007; 44: 300–7. [DOI] [PubMed] [Google Scholar]
- 17. Woodard PK, Feldman JM, Paine SS, Baker ME. Midgut carcinoid tumors: CT findings and biochemical profiles. J Comput Assist Tomogr 1995; 19: 400–5. [PubMed] [Google Scholar]
- 18. Grugan KD, Miller CG, Yao Y, et al Fibroblast‐secreted hepatocyte growth factor plays a functional role in esophageal squamous cell carcinoma invasion. Proc Natl Acad Sci U S A 2010; 107: 11026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee C, Safdie FM, Raffaghello L, et al Reduced levels of IGF‐I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res 2010; 70: 1564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Navab R, Liu J, Seiden‐Long I, et al Co‐overexpression of Met and hepatocyte growth factor promotes systemic metastasis in NCI‐H460 non‐small cell lung carcinoma cells. Neoplasia 2009; 11: 1292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paduch R, Jakubowicz‐Gil J, Niedziela P. Hepatocyte growth factor (HGF), heat shock proteins (HSPs) and multidrug resistance protein (MRP) expression in co‐culture of colon tumor spheroids with normal cells after incubation with interleukin‐1beta (IL‐1beta) and/or camptothecin (CPT‐11). Indian J Exp Biol 2010; 48: 354–64. [PubMed] [Google Scholar]
- 22. Steffan JJ, Williams BC, Welbourne T, Cardelli JA. HGF‐induced invasion by prostate tumor cells requires anterograde lysosome trafficking and activity of Na+‐H+ exchangers. J Cell Sci 2010; 123: 1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu D, Matsuo Y, Ma J, et al Cancer cell‐derived IL‐1alpha promotes HGF secretion by stromal cells and enhances metastatic potential in pancreatic cancer cells. J Surg Oncol 2010; 102: 469–77. [DOI] [PubMed] [Google Scholar]
- 24. Zhang C, Hao L, Wang L, et al Elevated IGFIR expression regulating VEGF and VEGF‐C predicts lymph node metastasis in human colorectal cancer. BMC Cancer 2010; 10: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laviola L, Natalicchio A, Giorgino F. The IGF‐I signaling pathway. Curr Pharm Des 2007; 13: 663–9. [DOI] [PubMed] [Google Scholar]
- 26. Michalopoulos GK. Liver regeneration. J Cell Physiol 2007; 213: 286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Svejda B, Kidd M, Kazberouk A, Lawrence B, Pfragner R, Modlin IM. Limitations in small intestinal neuroendocrine tumor therapy by mTOR kinase inhibition reflect growth factor‐mediated PI3K feedback loop activation via ERK1/2 and AKT. Cancer 2011; 117: 4141–54. [DOI] [PubMed] [Google Scholar]
- 28. Carboni JM, Wittman M, Yang Z et al BMS‐754807, a small molecule inhibitor of insulin‐like growth factor‐1R/IR. Mol Cancer Ther 2009; 8: 3341–9. [DOI] [PubMed] [Google Scholar]
- 29. Boyer JL, Phillips JM, Graf J. Preparation and specific applications of isolated hepatocyte couplets. Methods Enzymol 1990; 192: 501–16. [DOI] [PubMed] [Google Scholar]
- 30. Quistorff B, Dich J, Grunnet N. Preparation of isolated rat liver hepatocytes. Methods Mol Biol 1990; 5: 151–60. [DOI] [PubMed] [Google Scholar]
- 31. Castell JV, Gomez‐Lechon MJ. Liver cell culture techniques. Methods Mol Biol 2009; 481: 35–46. [DOI] [PubMed] [Google Scholar]
- 32. LeCluyse EL. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci 2001; 13: 343–68. [DOI] [PubMed] [Google Scholar]
- 33. Svejda B, Kidd M, Giovinazzo F, et al The 5‐HT(2B) receptor plays a key regulatory role in both neuroendocrine tumor cell proliferation and the modulation of the fibroblast component of the neoplastic microenvironment. Cancer 2010; 116: 2902–12. [DOI] [PubMed] [Google Scholar]
- 34. Kidd M, Eick GN, Modlin IM, Pfragner R, Champaneria MC, Murren J. Further delineation of the continuous human neoplastic enterochromaffin cell line, KRJ‐I, and the inhibitory effects of lanreotide and rapamycin. J Mol Endocrinol 2007; 38: 181–92. [DOI] [PubMed] [Google Scholar]
- 35. Kidd M, Drozdov I, Joseph R, Pfragner R, Culler M, Modlin I. Differential cytotoxicity of novel somatostatin and dopamine chimeric compounds on bronchopulmonary and small intestinal neuroendocrine tumor cell lines. Cancer 2008; 113: 690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kidd M, Modlin IM, Pfragner R, et al Small bowel carcinoid (enterochromaffin cell) neoplasia exhibits transforming growth factor‐beta1‐mediated regulatory abnormalities including up‐regulation of C‐Myc and MTA1. Cancer 2007; 109: 2420–31. [DOI] [PubMed] [Google Scholar]
- 37. Kidd M, Eick G, Shapiro MD, Camp RL, Mane SM, Modlin IM. Microsatellite instability and gene mutations in transforming growth factor‐beta type II receptor are absent in small bowel carcinoid tumors. Cancer 2005; 103: 229–36. [DOI] [PubMed] [Google Scholar]
- 38. Chen JJ, Vasko MR, Wu X, et al Multiple subtypes of serotonin receptors are expressed in rat sensory neurons in culture. J Pharmacol Exp Ther 1998; 287: 1119–27. [PubMed] [Google Scholar]
- 39. Hall TA. BioEdit: a user‐friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 1999; 4: 95–8. [Google Scholar]
- 40. Lauffer JM, Tang LH, Zhang T, et al PACAP mediates the neural proliferative pathway of Mastomys enterochromaffin‐like cell transformation. Regul Pept 2001; 102: 157–64. [DOI] [PubMed] [Google Scholar]
- 41. Pfragner R, Behmel A, Hoger H, et al Establishment and characterization of three novel cell lines – P‐STS, L‐STS, H‐STS – derived from a human metastatic midgut carcinoid. Anticancer Res 2009; 29: 1951–61. [PubMed] [Google Scholar]
- 42. Svejda B, Aguiriano‐Moser V, Sturm S, et al Anticancer activity of novel plant extracts from Trailliaedoxa gracilis (W. W. Smith & Forrest) in human carcinoid KRJ‐I cells. Anticancer Res 2010; 30: 55–64. [PubMed] [Google Scholar]
- 43. Jackson LN, Chen LA, Larson SD, et al Development and characterization of a novel in vivo model of carcinoid syndrome. Clin Cancer Res 2009; 15: 2747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Veenstra‐VanderWeele J, Anderson GM, Cook EH Jr. Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharmacol 2000; 410: 165–81. [DOI] [PubMed] [Google Scholar]
- 45. Hoyer D, Clarke DE, Fozard JR, et al International Union of Pharmacology classification of receptors for 5‐hydroxytryptamine (Serotonin). Pharmacol Rev 1994; 46: 157–203. [PubMed] [Google Scholar]
- 46. Centurion D, Glusa E, Sanchez‐Lopez A, Valdivia LF, Saxena PR, Villalon CM. 5‐HT7, but not 5‐HT2B, receptors mediate hypotension in vagosympathectomized rats. Eur J Pharmacol 2004; 502: 239–42. [DOI] [PubMed] [Google Scholar]
- 47. Enserink JM, Christensen AE, de Rooij J, et al A novel Epac‐specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 2002; 4: 901–6. [DOI] [PubMed] [Google Scholar]
- 48. Kopperud R, Krakstad C, Selheim F, Doskeland SO. cAMP effector mechanisms. Novel twists for an ‘old’ signaling system. FEBS Lett 2003; 546: 121–6. [DOI] [PubMed] [Google Scholar]
- 49. Caron E. Cellular functions of the Rap1 GTP‐binding protein: a pattern emerges. J Cell Sci 2003; 116: 435–40. [DOI] [PubMed] [Google Scholar]
- 50. Kwon G, Pappan KL, Marshall CA, Schaffer JE, McDaniel ML. cAMP Dose‐dependently prevents palmitate‐induced apoptosis by both protein kinase A‐ and cAMP‐guanine nucleotide exchange factor‐dependent pathways in beta‐cells. J Biol Chem 2004; 279: 8938–45. [DOI] [PubMed] [Google Scholar]
- 51. Usechak P, Gates A, Webster CR. Activation of focal adhesion kinase and JNK contributes to the extracellular matrix and cAMP‐GEF mediated survival from bile acid induced apoptosis in rat hepatocytes. J Hepatol 2008; 49: 251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gines P, Li X, Brown SE, et al Inhibitory actions of cyclic adenosine monophosphate and pertussis toxin define two distinct epidermal growth factor‐regulated pathways leading to activation of mitogen‐activated protein kinase in rat hepatocytes. Hepatology 1996; 23: 1167–73. [DOI] [PubMed] [Google Scholar]
- 53. Graf D, Reinehr R, Kurz AK, Fischer R, Haussinger D. Inhibition of taurolithocholate 3‐sulfate‐induced apoptosis by cyclic AMP in rat hepatocytes involves protein kinase A‐dependent and ‐independent mechanisms. Arch Biochem Biophys 2003; 415: 34–42. [DOI] [PubMed] [Google Scholar]
- 54. Li J, Yang S, Billiar TR. Cyclic nucleotides suppress tumor necrosis factor alpha‐mediated apoptosis by inhibiting caspase activation and cytochrome c release in primary hepatocytes via a mechanism independent of Akt activation. J Biol Chem 2000; 275: 13026–34. [DOI] [PubMed] [Google Scholar]
- 55. Schliess F, Kurz AK, Haussinger D. Glucagon‐induced expression of the MAP kinase phosphatase MKP‐1 in rat hepatocytes. Gastroenterology 2000; 118: 929–36. [DOI] [PubMed] [Google Scholar]
- 56. Webster CR, Anwer MS. Cyclic adenosine monophosphate‐mediated protection against bile acid‐induced apoptosis in cultured rat hepatocytes. Hepatology 1998; 27: 1324–31. [DOI] [PubMed] [Google Scholar]
- 57. Webster CR, Anwer MS. Role of the PI3K/PKB signaling pathway in cAMP‐mediated translocation of rat liver Ntcp. Am J Physiol 1999; 277: G1165–72. [DOI] [PubMed] [Google Scholar]
- 58. Webster CR, Usechak P, Anwer MS. cAMP inhibits bile acid‐induced apoptosis by blocking caspase activation and cytochrome c release. Am J Physiol Gastrointest Liver Physiol 2002; 283: G727–38. [DOI] [PubMed] [Google Scholar]
- 59. Hatano E, Brenner DA. Akt protects mouse hepatocytes from TNF‐alpha‐ and Fas‐mediated apoptosis through NK‐kappa B activation. Am J Physiol Gastrointest Liver Physiol 2001; 281: G1357–68. [DOI] [PubMed] [Google Scholar]
- 60. Qiao L, Studer E, Leach K, et al Deoxycholic acid (DCA) causes ligand‐independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen‐activated protein kinase‐signaling module enhances DCA‐induced apoptosis. Mol Biol Cell 2001; 12: 2629–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Suzuki A, Hayashida M, Kawano H, Sugimoto K, Nakano T, Shiraki K. Hepatocyte growth factor promotes cell survival from fas‐mediated cell death in hepatocellular carcinoma cells via Akt activation and Fas‐death‐inducing signaling complex suppression. Hepatology 2000; 32: 796–802. [DOI] [PubMed] [Google Scholar]
- 62. Reinehr R, Haussinger D. Inhibition of bile salt‐induced apoptosis by cyclic AMP involves serine/threonine phosphorylation of CD95. Gastroenterology 2004; 126: 249–62. [DOI] [PubMed] [Google Scholar]
- 63. Roberts RA, James NH, Cosulich SC. The role of protein kinase B and mitogen‐activated protein kinase in epidermal growth factor and tumor necrosis factor alpha‐mediated rat hepatocyte survival and apoptosis. Hepatology 2000; 31: 420–7. [DOI] [PubMed] [Google Scholar]
- 64. Grambihler A, Higuchi H, Bronk SF, Gores GJ. cFLIP‐L inhibits p38 MAPK activation: an additional anti‐apoptotic mechanism in bile acid‐mediated apoptosis. J Biol Chem 2003; 278: 26831–7. [DOI] [PubMed] [Google Scholar]
- 65. Webster CR, Anwer MS. Phosphoinositide 3‐kinase, but not mitogen‐activated protein kinase, pathway is involved in hepatocyte growth factor‐mediated protection against bile acid‐induced apoptosis in cultured rat hepatocytes. Hepatology 2001; 33: 608–15. [DOI] [PubMed] [Google Scholar]
- 66. Desbois‐Mouthon C, Wendum D, Cadoret A, et al Hepatocyte proliferation during liver regeneration is impaired in mice with liver‐specific IGF‐1R knockout. FASEB J 2006; 20: 773–5. [DOI] [PubMed] [Google Scholar]
- 67. Mayr B, Montminy M. Transcriptional regulation by the phosphorylation‐dependent factor CREB. Nat Rev Mol Cell Biol 2001; 2: 599–609. [DOI] [PubMed] [Google Scholar]
- 68. Billiard J, Grewal SS, Lukaesko L, Stork PJ, Rotwein P. Hormonal control of insulin‐like growth factor I gene transcription in human osteoblasts: dual actions of cAMP‐dependent protein kinase on CCAAT/enhancer‐binding protein delta. J Biol Chem 2001; 276: 31238–46. [DOI] [PubMed] [Google Scholar]
- 69. Lambert HW, Lauder JM. Serotonin receptor agonists that increase cyclic AMP positively regulate IGF‐I in mouse mandibular mesenchymal cells. Dev Neurosci 1999; 21: 105–12. [DOI] [PubMed] [Google Scholar]
- 70. Lambert HW, Weiss ER, Lauder JM. Activation of 5‐HT receptors that stimulate the adenylyl cyclase pathway positively regulates IGF‐I in cultured craniofacial mesenchymal cells. Dev Neurosci 2001; 23: 70–7. [DOI] [PubMed] [Google Scholar]
- 71. Thomas MJ, Umayahara Y, Shu H, Centrella M, Rotwein P, McCarthy TL. Identification of the cAMP response element that controls transcriptional activation of the insulin‐like growth factor‐I gene by prostaglandin E2 in osteoblasts. J Biol Chem 1996; 271: 21835–41. [DOI] [PubMed] [Google Scholar]
- 72. Michalopoulos GK. Liver regeneration: alternative epithelial pathways. Int J Biochem Cell Biol 2011; 43: 173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 2010; 176: 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tenoutasse S, Van Vliet G, Ledru E, Deal C. IGF‐I transcript levels in whole‐liver tissue, in freshly isolated hepatocytes, and in cultured hepatocytes from lean and obese Zucker rats. Horm Res 2003; 59: 135–41. [DOI] [PubMed] [Google Scholar]
- 75. Liu Y, Michalopoulos GK, Zarnegar R. Structural and functional characterization of the mouse hepatocyte growth factor gene promoter. J Biol Chem 1994; 269: 4152–60. [PubMed] [Google Scholar]
- 76. Nilsson O, Wangberg B, Theodorsson E, Skottner A, Ahlman H. Presence of IGF‐I in human midgut carcinoid tumours: an autocrine regulator of carcinoid tumour growth? Int J Cancer 1992; 51: 195–203. [DOI] [PubMed] [Google Scholar]
- 77. von Wichert G, Jehle PM, Hoeflich A, et al Insulin‐like growth factor‐I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res 2000; 60: 4573–81. [PubMed] [Google Scholar]


