Abstract
Pancreatic cancer is an aggressive cancer with poor prognosis. Little is known about the immune response in the tumor microenvironment after chemotherapy for initially unresectable tumor. The purpose of this study was to investigate the immunological effects of chemoradiation therapy in the tumor microenvironment of pancreatic adenocarcinoma. Seventeen patients with pancreatic adenocarcinoma with and without preoperative chemoradiation therapy were retrospectively analyzed using immunohistochemical methods for HLA class I heavy chain, CD4+, CD8+, CD45RO + and Foxp3+ T cell infiltrations. Seven of the 17 study patients received preoperative chemoradiation therapy. There were no statistically significant differences in the number of CD4+ and CD8+ T cell infiltrations in the tumor microenvironment. However, the number of Foxp3+ T cell infiltrations was significantly lower in the neoadjuvant chemoradiation therapy group. The HLA class I expression status was the same between the two groups. In conclusion, preoperative chemoradiation therapy in pancreatic adenocarcinoma is useful for reducing regulatory T cell levels in combination with its direct cytotoxic effects.
Pancreatic carcinoma is a malignant disease with poor prognosis. It is the fourth leading cause of death worldwide.1 Surgery is the only curative treatment for localized pancreatic carcinoma;2, 3 however, clinical symptoms do not appear until the disease is in the advanced stage. Therefore, two‐thirds of all patients with pancreatic carcinoma have unresectable disease.4
Recently, some new anti‐cancer agents have been developed that are effective against pancreatic cancer. Administration of intensive neoadjuvant chemoradiation therapy with these drugs has sometimes enabled initially unresectable tumors (which were metastatic or abutted major arterial vessels) to become operable. We have been performing extensive surgery for patients with initially unresectable pancreatic disease; these patients have had good prognosis compared with the patients who underwent up‐front surgery first, before chemoradiation therapy.5
It has been confirmed that some anti‐cancer agents and irradiation have immunomodulatory effects in addition to their own cytotoxic effects.6, 7 The basic mechanisms underlying these immunomodulatory effects are mainly an increase in tumor antigenicity and control of regulatory T cells (Treg), myeloid‐derived suppressor cells (MDSC) and tumor associated macrophages (TAM).8, 9, 10 However, the immunomodulatory effects of chemoradiation therapy vary between types of cancer, and also sometimes depend on the host immune responses.
The aim of the present study was to investigate the immunological effects of chemoradiation therapy in the tumor microenvironment of pancreatic adenocarcinoma. We compared surgical specimens between the patients who underwent surgery before receiving chemoradiation therapy and those who received neoadjuvant chemoradiation therapy.
Materials and Methods
Patients
This study reviewed 17 patients with pancreatic carcinoma who underwent radical pancreatectomy with curative intent in the Hokkaido University Hospital Department of Gastroenterological Surgery II between 2006 and 2010.
Of the 17 patients who were reviewed, seven patients were pretreated by chemoradiation therapy for reasons of unresectability due to suspected invasion of major vessels or distant metastasis; the pretreatment was followed by adjuvant surgery (Adjuvant Surgery group: AS). The other 10 patients underwent surgery first without neoadjuvant treatment (Surgery First group: SF). The clinical typing of tumors was determined according to the TNM classification system of the International Union Against Cancer (UICC).11 The effects of chemoradiation therapy were clinically evaluated using the Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1.12 Histologic tumor response to chemoradiation therapy was assessed and graded according to an established score:13 tumor destruction up to 10%, grade 1; 10–50%, grade 2a; 50–90%, grade 2b; >90%, grade 3; and complete response, grade 4. All specimens were fixed in 10% formalin and embedded in paraffin wax. The thickest part of each tumor was selected for evaluation. Serial 4‐μm‐thick sections were obtained and examined by immunohistochemistry. Informed consent for immunohistochemical staining was obtained in accordance with the guidelines of the Hokkaido University Institutional Review Board authorization for this study.
Monoclonal antibodies
The mouse monoclonal primary antibody used was EMR8‐5, which recognizes the heavy chains of HLA‐A, HLA‐B, and HLA‐C (clone EMR8‐5; Hokudo, Sapporo, Japan) at a 1:1000 dilution in PBS; human CD4‐specific mAb and human CD8‐specific mAb (Histofine CD4 mouse IgG1 mAb and CD8, mouse IgG1κ mAb; Nichirei, Tokyo, Japan) at a 1:500 dilution in PBS; anti‐CD45RO antibody (UCH‐L1; Abcam, Cambridge, UK) at a 1:1000 dilution; and mouse anti‐human Foxp3 antibody (clone 246A/E7; Abcam) at a 1:40 dilution in antibody diluent (DakoCytomation, Glostrup, Denmark) to recognize Treg.
Immunohistochemical staining
Immunohistochemical reactions were carried out using the streptavidin‐biotin‐peroxidase method. As positive controls, normal adenoid tissue was used for CD4, CD8, CD45RO and Treg. Normal mucosal tissues were used for HLA class I heavy chains as internal positive controls. Sections were deparaffinized in xylene, washed in PBS (pH 7.4), and rehydrated in a graded series of ethanol solutions. Endogenous peroxidase activity was blocked by a 10‐min incubation with 3% hydrogen peroxide in methanol. After washing in PBS, specimens were saturated with 10% normal goat serum (Histofine SAB‐PO kit; Nichirei) for 5 min and then incubated at room temperature for 30 min with primary antibody.
In terms of antigen retrieval for all of the primary Abs, sections were floated on 1 mM EDTA buffer (pH 9.0) in a plastic container and then heated in a domestic pressure cooker for 3 min after reaching the maximum pressure. After washing in PBS, a biotinylated goat anti‐mouse immunoglobulin antibody (Histofine SAB‐PO kit; Nichirei) was applied for 60 min at room temperature. Immunohistochemical reactions were visualized with freshly prepared 3,3′‐diaminobenzidine tetrahydrochloride (Histofine SAB‐PO kit; Nichirei). Slides were counterstained with hematoxylin and mounted on coverslips.
Scoring of CD4+ T cells, CD8+ T cells, CD45RO and Treg infiltration
Immunohistochemistry testing and the evaluation of immune cells were performed according to a previous report.14 Briefly, the degree of immune cell infiltration was analyzed in more than 10 independent high‐power (×200) microscopic fields for each tissue sample. The five areas of most abundant distribution were selected. The numbers of CD4+ T cells, CD8+ T cells, and Treg cells were counted including mesenchymal stromal cells and the cancer nest. All the specimens were evaluated by two investigators blinded to the patients' clinical information under the instruction of a pathologist.
Scoring of HLA class I expression status
HLA class I heavy chain expression by tumor cells was scored using a scale of negative, heterogeneous, or positive, when the percentage of stained tumor cells was <25%, 25–75%, or >75%, respectively.
Statistical analysis
The χ2 test was used to assess the significance of the association of preoperative chemotherapy with clinicopathological parameters. The degree of T lymphocyte infiltration of CD4, CD8, CD45RO, Treg and the HLA class I expression pattern between the AS group and SF group were analyzed by the Mann–Whitney U‐test. In all tests, statistical significance was set at P < 0.05. All analyses were performed using statistical software (StatView, version 5.0; SAS Institute, Cary, NC, USA). Differences in survival times between patient subgroups were analyzed using the log‐rank statistic test. Survival probabilities were calculated using the Kaplan–Meier method.
Results
Clinicopathological features of enrolled patients
The characteristics of the 17 patients evaluated in this study are summarized in Table 1. There were no statistically significant differences in terms of the background characteristics between the AS group and SF group.
Table 1.
Background characteristics of the 17 patients evaluated in this study
| SF group (n = 10) | AS group (n = 7) | ||
|---|---|---|---|
| Age (years) | 71 (51–85) | 66 (43–68) | N.S. |
| Sex (male/female) | 4/6 | 2/5 | N.S. |
| Pathological UICC stage I/IIA/IIB/III | 5/1/3/1 | 0/1/3/3 | N.S. |
| Preoperative WBC (median, range, ×103/μL) | 4.9 (3.5–7.1) | 5.4 (2.5–7.9) | N.S. |
| Preoperative lymphocytes (median, range, ×102/μL) | 7.9 (7.0–1.3) | 1.5 (1.2–1.8) | P = 0.0025 |
AS, adjuvant surgery; N.S., not significant; SF, surgery first; UICC, Union for International Cancer Control; WBC, white blood cell count.
In the AS group, gemcitabine‐based chemotherapy was administered to all seven patients with varying intensity (800–1000 mg/body). Other combined agents included 5‐fluorouracil (FU) in arterial infusion for two patients and tegafur, gimeracil, oteracil, potassium (TS‐1) oral intake for four patients. One patient received radiation before surgery at the level of 45 Gy. The median treatment time before surgery was 11 months, ranging from 5 to 42 months. All of the chemotherapeutic treatment was withdrawn at least 4 weeks before surgery in principle.
In terms of the efficacy of the neoadjuvant chemoradiation therapy, four patients attained partial response and three patients showed stable disease clinically, based on the RECIST criteria. Pathological assessment showed Grade 1 in five patients, and Grade 2a and 2b in one patient, respectively. No statistically significant differences were observed between the two groups in terms of preoperative white blood cell count. However, the lymphocyte count in the peripheral blood was significantly more decreased in the AS group (Table 1).
In terms of preoperative clinical stage and overall survival, there were statistically no significant differences between the two groups.
Immunohistochemistry for immune‐related cells
CD4+ T cells, CD8+ T cells, CD45RO+ T cells and Foxp3+ Treg cells were detected within cancer cell nests or the stroma in contact with cancer cells. Representative photomicrographs of immunohistochemical staining of CD4, CD8, CD45RO and Treg are shown in Figure 1(a–d). The HLA class I expression pattern was classified into three groups; <25%, 25–75%, >75% (Fig. 2).
Figure 1.
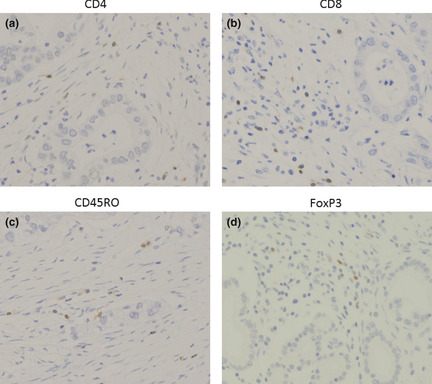
Representative immunohistochemical staining patterns of formalin fixed, paraffin embedded sections with CD 4 − , CD 8 − , CD 45 RO − and F oxp3 specific monoclonal antibodies, respectively (a–d). Original magnification, ×400 for all pictures.
Figure 2.
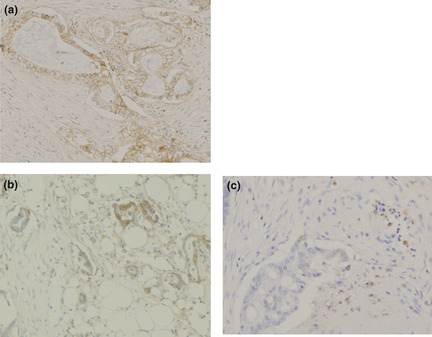
HLA class I expression pattern was classified into the following three groups, positive (a), heterogeneous (b), or negative (c), when the percentage of stained tumor cells was >75%, 25–75%, or <25%, respectively. Original magnification, ×400 for all pictures.
Degree of immune cell infiltration in the tumor microenvironment
We analyzed the infiltration status of CD4, CD8, CD45RO, Treg (Fig. 3a–d), and the expression pattern of HLA class I (Table 2) between the AS and SF groups. The number of CD4+ T cells and CD8+ T cells were statistically equal between these two groups.
Figure 3.
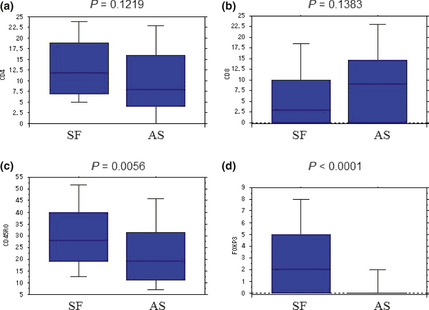
The degree of immune cell infiltration in the tumor microenvironment. We found no significant difference in the number of CD4+ and CD8+ T cells in the stroma and within the cancer nest between the two groups (a,b). The number of CD45RO + cells in the tumor microenvironment was significantly lower in the adjuvant surgery (AS) group (c). The number of Foxp3+ T cells was significantly lower in the AS group (d).
Table 2.
Comparison of HLA class I expression patterns between SF and AS groups
| HLA class I expression status | |||
|---|---|---|---|
| >75% | 25–75% | <25% | |
| SF group (n = 10) | 8 | 2 | 0 |
| AS group (n = 7) | 5 | 1 | 1 |
P = 0.8014 by Mann–Whitney U‐test. AS, adjuvant surgery; SF, surgery first.
The number of Treg, and also the Treg/CD4 ratio, was significantly higher in the SF group than the AS group (P < 0.0001). The number of CD45RO+ T cells was significantly lower in the AS group. We found no statistically significant difference in the HLA class I expression pattern between the two groups (Table 2).
Overall survival from the initial treatment
The median follow‐up period for all 17 patients was 35 months (range: 11–65). There was statistically no significant difference in overall survival between the AS and the SF group (P = 0.2388, Fig. 4).
Figure 4.
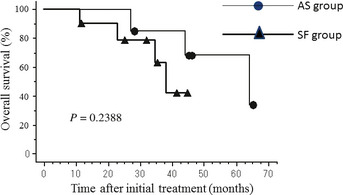
There was statistically no significant difference in overall survival between the adjuvant surgery (AS) group and the surgery first (SF) group.
Discussion
There have been growing evidences that chemotherapy can modulate the host immune response in a variety of cancers.15 Several types of evidence underlying this process have been proposed besides the cytotoxic effects of chemotherapy. First, is the association of calreticulin, one of the antigen‐processing components for HLA class I. It has been suggested that calreticulin increases the antigenicity of tumor antigens by releasing an “eat me” signal with the antigen‐presenting cells.6 Second, is the inhibitory effect of anti‐cancer agents on the immune suppressor cells, including Treg, MDSC and TAM.8, 9, 10 The last type of evidence is antigen spreading by tumor shrinkage and the formation of antigen antibody immune complex, stimulating activation of the complement pathway and inducing antibody‐dependent cellular cytotoxicity.16 Irradiation also mediates many immunological effects including upregulation of HLA class I molecules, tumor associated antigens, and adhesion molecules by endothelial cells, thereby boosting cytotoxic T‐cell activity.17
In the present study, we evaluated the effects of preoperative chemoradiation therapy on the tumor microenvironment of pancreatic carcinoma and analyzed whether the microenvironment became immunologically modified. In our previous study series, we demonstrated that CD8+ tumor‐infiltrating lymphocytes, together with CD4+ tumor‐infiltrating lymphocytes, improve the prognosis of patients with pancreatic adenocarcinoma,18 esophageal carcinoma14 and lung carcinoma.19 Furthermore, we indicated that neoadjuvant chemotherapy effectively induced CD4+ and CD8+ lymphocyte infiltration into the cancer microenvironment, together with HLA class I upregulation.20 However, the present data suggest that preoperative chemoradiation therapy did not induce CD4+ nor CD8+ lymphocyte infiltration compared with the specimens without preoperative therapy. This might be because of differences in the types and combinations of anti‐cancer agents or the dose intensities used in the preoperative clinical setting. Brode et al.9 reported that cyclophosphamide could modulate the host immune response, depending on the dose and timing of administration. Muranski et al.21 also suggested in their adoptive immunotherapy strategy for the metastatic melanoma that the host's immune system needs to be properly conditioned by lymphodepletion, in order to create an appropriate ‘lymphoid space’ that is devoid of regulatory mechanisms. Taken together with this evidence, our present data might be interpreted as the summed results of the balance of various factors including the type of reagents, cycles of chemotherapy, the depletion and the proliferation of the lymphocytes.
It is of interest that the numbers of CD4+ and CD8+ T cells were equal between the SF group and the AS group despite the number of preoperative lymphocytes becoming significantly decreased in the AS group. The reduction in the number of peripheral lymphocytes during the preoperative period might be the result of the bone marrow suppression. The correlation between the amount of bone marrow suppression and preoperative chemotherapy, duration of chemotherapy, number of tumor‐infiltrating lymphocytes (TIL), and patient survival rates should be investigated in a future prospective study. It might be possible that anti‐cancer agents improve the microenvironment, thereby inducing CD4 and CD8 T cell infiltration and also reducing the number of local Treg. In addition, bone marrow suppression during chemoradiation therapy might possibly boost proliferation in the host microenvironment, similar to the pretreatment regimen in adoptive immunotherapy.21 Regarding the clinical implication of the reduced regulatory T cells in the AS group, it might be contributing to the patients prognosis, because the overall survival of the AS group, which were initially considered unresectable disease, was equal to that of the SF group, which were all resectable.
In terms of the memory cells, it is of interest that CD45RO+ T cell infiltration was significantly lower in the AS group. Galon et al.22 reported that increased CD45RO infiltration was associated with good prognosis in human colorectal tumors. Although in Galon et al.'s study, increased CD45RO infiltration was associated with good prognosis in stage I–III according to the UICC‐TNM classification, there were no significant differences regarding CD45RO infiltration in stage IV. In agreement with these data, the patients in the AS group in our study initially had far advanced carcinoma, and there might be little contribution of the CD45RO infiltration to the patients survival in the AS group. Although we do not have data regarding the subgroup characteristics of the memory T cells, further fundamental study is warranted to investigate the possibility that the effecter memory T cell population decreases in the tumor microenvironment after chemotherapy.
The present study was limited by the retrospective nature of this investigation, the small number of the patients included and also the fact that lymphocyte infiltration was increased mainly by non‐specific inflammation induced by the chemotherapy; therefore, future prospective studies are needed to evaluate infiltrated lymphocytes for tumor specificity or antigen specificity. Further study is also needed in terms of the infiltration of other local immune cells, such as dendritic cells, TAM, MDSC and tumor‐specific lymphocytes. The purpose would be to clarify whether chemotherapeutic agents might help educate the immune system by destroying tumor cells, resulting in the release of internal tumor‐associated antigens, thereby inducing the formation of immune complexes and inducing specific immunity in combination with direct cytotoxic effects of the chemotherapy. The results of the present study help understand the complicated balance of immunological responses between the host and the tumor.
In conclusion, preoperative chemoradiation therapy in pancreatic adenocarcinoma is useful for reducing regulatory T cell levels in combination with the direct cytotoxic effects of the chemotherapeutic agents and radiation.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgment
The authors thank Hiraku Shida for his technical assistance in immunohistochemistry analysis.
(Cancer Sci 2013; 104: 531–535)
References
- 1. Ansari D, Chen B‐C, Dong L, Zhou M‐T, Andersson R. Pancreatic cancer: translational research aspects and clinical implications. World J Gastroenterol 2012; 18: 1417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conlon KC, Klimstra DS, Brennan MF. Long‐term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5‐year survivors. Ann Surg 1996; 223: 273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011; 378: 607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakao A, Takeda S, Inoue S et al Indications and techniques of extended resection for pancreatic cancer. World J Surg 2006; 30: 976–82. [DOI] [PubMed] [Google Scholar]
- 5. Kato K, Kondo S, Hirano S et al Adjuvant surgical therapy for patients with initially‐unresectable pancreatic cancer with long‐term favorable responses to chemotherapy. J Hepatobiliary Pancreat Sci 2011; 18: 712–6. [DOI] [PubMed] [Google Scholar]
- 6. Obeid M, Tesniere A, Ghiringhelli F et al Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13: 54–61. [DOI] [PubMed] [Google Scholar]
- 7. Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 2011; 8: 151–60. [DOI] [PubMed] [Google Scholar]
- 8. Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol 2008; 8: 59–73. [DOI] [PubMed] [Google Scholar]
- 9. Brode S, Cooke A. Immune‐potentiating effects of the chemotherapeutic drug cyclophosphamide. Crit Rev Immunol 2008; 28: 109–26. [DOI] [PubMed] [Google Scholar]
- 10. Yue ZQ, Liu YP, Ruan JS, Zhou L, Lu Y. Tumor‐associated macrophages: a novel potential target for cancer treatment. Chin Med J 2012; 125: 3305–11. [PubMed] [Google Scholar]
- 11. Sobin LH, Wittekind CH. International Union Against Cancer. TNM Classification of Malignant Tumors. NewYork: Wiley‐Liss, 2002; 60–4. [Google Scholar]
- 12. Therasse P, Arbuck SG, Eisenhauer EA et al New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 13. Evans DB, Rich TA, Byrd DR et al Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 1992; 127: 1335–9. [DOI] [PubMed] [Google Scholar]
- 14. Cho Y, Miyamoto M, Kato K et al CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res 2003; 63: 1555–9. [PubMed] [Google Scholar]
- 15. Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012; 11: 215–33. [DOI] [PubMed] [Google Scholar]
- 16. Noguchi T, Kato T, Wang L et al Intracellular tumor‐associated antigens represent effective targets for passive immunotherapy. Cancer Res 2012; 72: 1672–82. [DOI] [PubMed] [Google Scholar]
- 17. Rubner Y, Wunderlich R, Rühle PF et al How does ionizing irradiation contribute to the induction of anti‐tumor immunity? Front Oncol 2012; 2: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fukunaga A, Miyamoto M, Cho Y et al CD8+ tumor‐infiltrating lymphocytes together with CD4+ tumor‐infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 2004; 28: 26–31. [DOI] [PubMed] [Google Scholar]
- 19. Hiraoka K, Miyamoto M, Cho Y et al Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non‐small‐cell lung carcinoma. Br J Cancer 2006; 94: 275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsuchikawa T, Miyamoto M, Yamamura Y, Shichinohe T, Hirano S, Kondo S. The immunological impact of neoadjuvant chemotherapy on the tumor microenvironment of esophageal squamous cell carcinoma. Ann Surg Oncol 2012; 19: 1713–9. [DOI] [PubMed] [Google Scholar]
- 21. Muranski P, Boni A, Wrzesinski C et al Increased intensity lymphodepletion and adoptive immunotherapy – how far can we go? Nat Clin Pract Oncol 2006; 3: 668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galon J, Costes A, Sanchez‐Cabo F et al Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313: 1960–4. [DOI] [PubMed] [Google Scholar]


