Abstract
Cisplatin (CDDP) has been a key drug for chemotherapy in patients with head and neck squamous cell carcinoma. Nephrotoxicity is one of its adverse reactions that are dose limiting. To increase its antitumor effects and reduce such toxicity problems, polymeric micelles carrying CDDP (NC‐6004) have been developed. The present study was designed to evaluate the efficacy and safety of NC‐6004 for oral squamous cell carcinoma. In vitro antitumor activity was assayed in four oral squamous cell carcinoma cell lines. To investigate the antitumor and nephrotoxic effects of NC‐6004, nude mice bearing OSC‐19 were administered NC‐6004 or CDDP. The in vitro growth‐inhibitory effect of NC‐6004 was significantly less than that of CDDP. However, both NC‐6004 and CDDP showed equivalent antitumor effects in vivo. Mice with CDDP developed renal cell apoptosis; however, those injected with NC‐6004 were almost free of renal cell injury. Moreover, in an orthotopic tongue cancer model using OSC‐19, NC‐6004 reduced the rate of sentinel lymph node metastasis to lower than that with CDDP. In conclusion, considering the potential advantages in terms of noticeable antitumor activity, lymphatic drug delivery and reduced nephrotoxicity, NC‐6004 represents a significant structural improvement in the development of a platinum complex.
Head and neck cancer remains a significant public health problem and ranks in the six leading cancers by incidence worldwide, with an estimated 600 000 new cases every year.1 Cisplatin (cis‐dichlorodiammineplatinum; CDDP) has been demonstrated to be one of the most effective cytotoxic agents 2 and the CDDP‐based chemotherapy regimen has gained widespread use in patients with head and neck squamous cell carcinoma (HNSCC). However, its administration has been hindered by its adverse reactions, for example, nephrotoxicity, neurotoxicity, gastrointestinal toxicity, hematological toxicity and ototoxicity.3 Among these, the significant risk of nephrotoxicity frequently hinders the use of high doses to maximize its antineoplastic effects, which might be the cause of treatment failure.
To overcome these problems and improve the therapeutic effect of CDDP, we have been applying superselective supradose intra‐arterial CDDP infusion for advanced HNSCC.4 However, since this technique is more complicated than that of i.v. infusion of antitumor drugs, it is not prevalent in the chemotherapy scene. Recently, several kinds of nanoparticle therapeutic platforms, including liposomes, nanoparticles and polymeric micelles, have been developed based on the idea that the drug delivery system (DDS) can accumulate in the tumor selectively, with reduced distribution in normal tissues and minimized undesirable side‐effects.5, 6, 7 NC‐6004, which is a CDDP‐incorporating polymeric micellar nanoparticle, enhanced antitumor activity and reduced the nephrotoxicity and neurotoxicity of CDDP in gastric cancer.8, 9, 10 Poly(ethylene glycol)‐poly(glutamic acid) block copolymers (PEG‐P[Gu]) confer a stealth property to the formulation, which allows the micellar drug preparation to be less avidly taken up by the reticuloendothelial system and retained in the circulation for a longer period of time. This has been recognized as the enhanced permeability and retention (EPR) effect where extravasation of high‐molecular‐weight micelles through leaky tumor capillary fenestrations, which result from abnormalities in angiogenesis at the tumor site, lead to accumulation and retention for a long time.11 A prolonged circulation time and the ability of EPR lead to the accumulation of CDDP in tumor tissues. NC‐6004 has been previously shown to possess significant antitumor activity for human gastric cancer cells in a mouse model.10 However, the efficacy of NC‐6004 in head and neck cancer has never been studied.
In the present study, we analyzed the cytotoxicity of NC‐6004 using the MTS assay and apoptosis assay on oral squamous cell carcinoma cell lines. Antitumor and nephrotoxic effects of NC‐6004 were investigated and compared with those of CDDP in in vivo experiments. As frequent involvement of regional lymph nodes, especially the sentinel lymph node (SLN), is one of the outstanding features of oral SCC, we further evaluated the lymphatic distribution of NC‐6004 in order to develop a target therapy for cervical lymph node metastasis, which also has never been evaluated.
Materials and Methods
Materials
Human oral squamous cell carcinoma cell lines OSC‐19, OSC‐20, HSC‐3 and HSC‐4 were used for these experiments. OSC‐19 and OSC‐20 were maintained in Eagle's minimum essential medium supplemented with 10% fetal bovine serum. HSC‐3 and HSC‐4 were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum. Luciferase‐transfected OSC‐19 LN2‐Luc cells were kindly provided by Dr Jeffrey N. Myers (The University of Texas MD Anderson Cancer Center, Houston, TX, USA).12 The CDDP was obtained from Nippon Kayaku Co., LTD (Tokyo, Japan). NC‐6004 was supplied by NanoCarrier Co. Ltd (Chiba, Japan).9 The luciferase antibody was purchased from MBL (Nagoya, Japan). 6‐week‐old female BALB/c‐nu/nu mice were purchased from Charles River Japan Inc. (Kanagawa, Japan). All animal procedures were performed in compliance with the guidelines for the Ethical Committee of the Laboratory for Animal Experiments, Graduate School of Medical Science, Kanazawa University.
In vitro growth‐inhibition assay
Head and neck cancer cell lines were evaluated in the present study. A 3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium salt (MTS) assay was performed to assess the effect of cell proliferation using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega, WI, USA). Briefly, cells were seeded in 96‐well culture plates at a density of 2 × 103 cells/well. After 24 h incubation with 0.9% NaCl (control), a graded concentration of CDDP or NC‐6004 was added to each well and incubated for 48 or 72 h. Following 2 h incubation of MTS reagent, optical density was read with a Microplate Reader Manager (Molecular Device, Sunnyvale, CA, USA) at a wavelength of 490 nm. The IC50 values represented the drug concentrations that reduced the mean absorbance at 490 nm to 50% in the untreated control well.
Apoptosis assay
Before incubation with drugs, OSC‐19 cells were cultured in serum‐free medium for 24 h. Then, with a series of CDDP‐ or NC‐6004‐containing medium for 24 h, caspase‐3 activity was measured using the Caspase‐Glo 3/7 Assay (Promega, WI, USA) according to the manufacturer's instructions. Briefly, cells with CDDP or NC‐6004 were added with Caspase‐Glo 3/7 Reagent (promega) to each well of the 96‐well plate. The luminescence of each sample was measured with a Typhoon9200 imager (GE Healthcare UK Ltd, Amersham, UK).
Evaluation of antitumor activity
For this experiment, 1 × 105 OSC‐19 cells were injected s.c. on the dorsal skin of BALB/c‐nu/nu mice. When tumor diameters reached 3 mm, tumor‐bearing mice were allocated randomly to drug administration groups. There were three test groups as follows: control group; CDDP treatment; and NC‐6004 treatment. Drugs were injected i.v. via the tail vein each week, with three administrations in total. Mice were administered a single i.v. injection of 5% glucose solution (control), CDDP (5 mg/kg) or NC‐6004 (an equivalent dose of 5 mg/kg CDDP). The tumor volume was calculated using the formula: tumor volume (mm3) = a × b (where a is the longest tumor diameter and b is the shortest tumor diameter).
TUNEL assay
A TUNEL assay was performed for the detection and quantitation of kidney cell apoptosis, as previously described.13 The ApoAlert DNA Fragmentation Assay Kit obtained from Clontech (La Jolla, CA, USA) was used to assess apoptosis‐induced nuclear DNA fragmentation via a fluorescence assay. On day 28 after administration of the drug, the mice, in which the same experimental protocol described in “Evaluation of antitumor activity” was used, were killed. The kidney was removed and serum was collected. The kidney was fixed in 10% formalin solution. Paraffin sections were deparaffinized. TUNEL‐positive apoptotic cells were detected using visualization with a fluorescent microscope. The number of apoptotic cells per 100 at five fields in the tubule cells of the kidney was counted. In each blood sample, the plasma concentrations of creatinine were measured by SRL Laboratories (Tokyo, Japan).
Assessment of cervical lymph node metastasis
BALB/c‐nu/nu mice were anesthetized and 1 × 105 OSC‐19 LN2‐Luc cells were then injected submucosally into the right side of the tongue using a tuberculin syringe with a 30‐gauge hypodermic needle.14 Mice were administered a sublingual injection of 5% glucose solution (control), CDDP (5 mg/kg) or NC‐6004 (an equivalent dose of 5 mg/kg CDDP) each week around the implanted tumor, with three administrations in total. On day 21 after implantation of OSC‐19 LN2‐Luc cells, the mice were killed. Cervical lymph nodes were resected and histopathologically examined using HE staining and luciferase staining to confirm the presence or absence of tumors. The presence of lymphatic metastasis was compared among groups.
For the pharmacokinetic study, drug concentrations were measured in cervical lymph nodes and plasma. After the injection of CDDP or NC‐6004, samples were collected at 1, 24, 72 and 144 h.
Statistical analysis
Data were expressed as the mean ± SD. Differences between test groups were analyzed using the Student's t‐test. The spss statistical software version 18 (SPSS Japan Inc., Tokyo, Japan) was used. A value of P < 0.05 was considered to be significant.
Results
Sensitivity of oral cancer cells to CDDP
The cytotoxicity of NC‐6004 was evaluated in comparison to that of CDDP in oral carcinoma cell lines. The IC50 values, calculated from dose‐survival curves obtained after 48 and 72 h treatment from the MTS assay, were investigated (Table 1). The IC50 values for CDDP ranged from 16.5 to 28.3 μM at 48 h and 17.6 to 20.5 μM at 72 h. NC‐6004 showed a growth inhibitory potency that was lower than that of CDDP, with IC50 values ranging from 118.9 to 398.4 μM at 48 h and 113.9 to 119.3 μM at 72 h. The growth inhibitory effect of CDDP in vitro was seven‐ to 14‐fold more potent than that of NC‐6004.
Table 1.
The 50% inhibitory concentration (IC50) values of cisplatin (CDDP) and NC‐6004 (μM)
| Cell line | IC50 | |||
|---|---|---|---|---|
| 48 h | 72 h | |||
| CDDP | NC‐6004 | CDDP | NC‐6004 | |
| OSC‐19 | 28.3 | 129.3 | 17.6 | 117.0 |
| OSC‐20 | 22.4 | 398.4 | 18.4 | 113.9 |
| HSC‐3 | 11.2 | 110.5 | 10.2 | 71.7 |
| HSC‐4 | 16.5 | 118.9 | 20.5 | 119.3 |
Upregulation of caspases by cisplatin and NC‐6004
When OSC‐19 cells were exposed to increasing concentrations of CDDP, caspase‐3 and caspase‐7 were activated in a dose‐dependent manner. Neither CDDP nor NC‐6004 significantly influenced caspase activity at a concentration of 0.05 μg/μL. However, CDDP and NC‐6004 increased caspase activity at a concentration of 2.5 μg/μL by approximately 142–331% and 165–170%, respectively (Fig. 1). These data indicate that both drugs induced apoptosis in a dose‐dependent manner, which relied on caspase‐3 and caspase‐7 activation.
Figure 1.
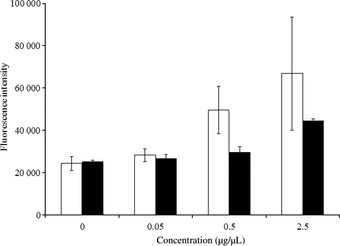
Caspase‐3 and caspase‐7 activity in OSC‐19 cells after 24 h exposure to cisplatin (CDDP) (□) or NC‐6004 (■). Caspase activity was measured using a fluorimetric assay. Values represent means ± SD from three independent experiments.
Antitumor activity of NC‐6004 on OSC‐19 xenograft
The antitumor activity of NC‐6004 or CDDP was evaluated in oral carcinoma‐bearing mice. All mice survived the experiment. In OSC‐19 xenografts, the tumor started to grow on day 14. On day 35, mice treated with either CDDP or NC‐6004 showed 4.9‐ or 6.6‐fold tumor growth inhibition relative to the control group, respectively (Fig. 2). There were no significant differences for the growth inhibitory effect between the two groups receiving chemotherapy.
Figure 2.
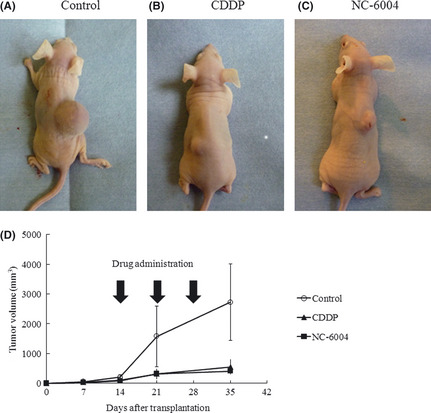
Effect of cisplatin (CDDP) or NC‐6004 in OSC‐19 mouse models. The OSC‐19 cell line was inoculated into the dorsal skin of nude mice. Photographs of representative mice and tumors treated with drugs are shown (A–C). (D) Reduced tumor volume treated with CDDP and NC‐6004 relative to the control group. Each data point is the mean value (±SD) of six primary tumors.
Decreased nephrotoxicity of NC‐6004 to CDDP in mice
Nude mice given an injection of CDDP or NC‐6004 at a dose of 10 mg/kg were used to evaluate the structural and functional consequences of CDDP‐induced nephrotoxicity. In the control group, no apoptosis in the tubule cells of the kidney was detected. Drug‐induced tubule cell apoptosis was recognized as TUNEL‐stained cells (Fig. 3a–c). Apoptotic cells from CDDP‐ or NC‐6004‐treated mice were counted and scored as structural damage. The number of apoptotic cells in NC‐6004‐treated mice was significantly lower than that in CDDP‐treated mice (P = 0.0085), and was similar to that in control mice (Fig. 3d). To examine functional damage, serum creatinine levels were measured. While the CDDP‐treated mice showed significantly higher serum creatinine than the control group, NC‐6004‐treated mice showed comparable levels of serum creatinine to the control (Fig. 3e). These results indicate that NC‐6004 is less toxic to the kidney than CDDP.
Figure 3.
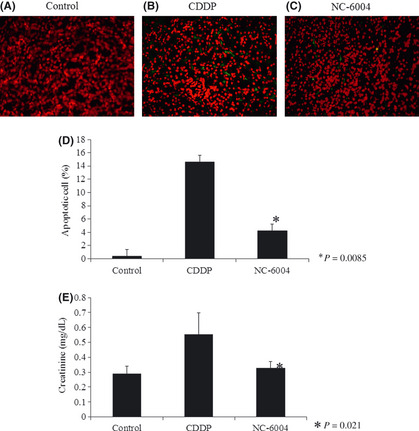
Low renal toxicity of NC‐6004. Mice were treated with cisplatin (CDDP) (10 mg/kg) or NC‐6004 (an equivalent dose of 10 mg/kg CDDP) and kidney sections were obtained on day 28 after administration of the drug. (A–C) Immunofluorescence staining of clusters of TUNEL‐positive nuclei (green) and DAPI (red). (D) The number of apoptotic cells per 100 were counted. Bars indicate standard deviations. *P = 0.0085 for CDDP versus NC‐6004. Each group included five mice. (E) Plasma concentration of creatinine (mg/dL) after treatment with the control, CDDP or NC‐6004. *P = 0.0021 for CDDP versus NC‐6004. Each group included five mice.
Efficacy of translymphatic chemotherapy and CDDP distribution in lymph nodes
To detect metastatic lymph nodes, an orthotopic xenograft mouse model of luciferase‐transfected OSC‐19 LN2‐Luc cells was made. Primary tongue tumors were detected at the cell‐inoculated site in all mice. Cervical lymph nodes were easily identified in OSC‐19‐inoculated mice and control mice as previously reported (Fig. 4a).14 As it was not possible to distinguish the nodes with metastasis from the ones without metastasis macroscopically, HE and luciferase staining was performed to reveal the presence of metastases microscopically (Fig. 4b). The incidence of lymphatic metastasis was significantly lower in the NC‐6004‐treated group (one of eight) than that of the controls (seven of eight) and the CDDP‐treated group (three of eight; P < 0.01; Fig. 4c). The time‐course of elemental platinum (Pt) concentrations in plasma and cervical lymph nodes after sublingual injection of CDDP or NC‐6004 was measured (Fig. 4d). Both plasma and lymph node concentrations after administration of NC‐6004 peaked at 24 h, representing remarkably prolonged blood and lymphatic circulation; however, Pt was cleared rapidly from circulation in the CDDP group. AUC0–144 h for plasma and lymph nodes in the NC‐6004 group were significantly higher than those in the CDDP group. Although at day 6 (144 h) the Pt levels in plasma were undetectable in both groups, lymph nodes of the NC‐6004 group still showed a high Pt concentration (1.15 ± 0.34 μg/g).
Figure 4.
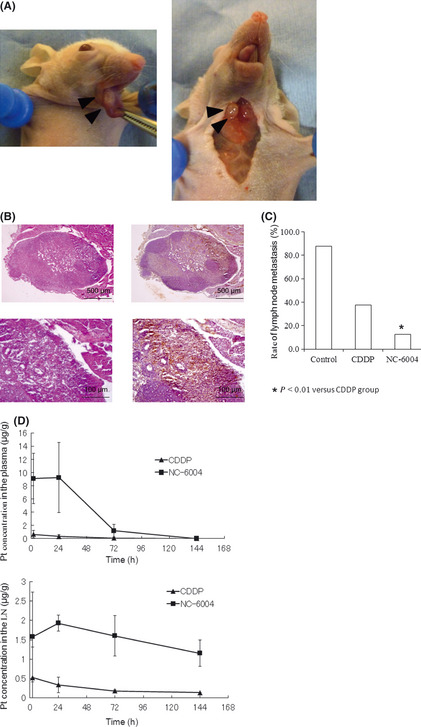
Translymphatic chemotherapy targeting lymph nodes. (A) Tongue tumor and cervical lymph node metastasis of an orthotopic xenograft model. OSC‐19 LN2‐Luc cells were inoculated to the right side of the tongue of nude mice. Mice were killed after 35 days. A primary tumor (left, arrowhead) and cervical lymph node (right, arrowhead) were seen in the tongue. (B) Photomicrograph of lymph node metastasis. HE stain (right) and luciferase stain (left). (C) The rate of lymphatic metastasis. Each group included eight mice. (D) Time‐course of elemental platinum (Pt) concentrations in plasma and cervical lymph nodes (LN) after sublingual injection of cisplatin (CDDP) (5 mg/kg) or NC‐6004 (an equivalent dose of 5 mg/kg CDDP).
Discussion
Cancer nanotechnology is a new field of interdisciplinary research aiming to enhance the methods of cancer diagnosis and treatment.15 From the standpoint of DDS targeting solid carcinoma, a variety of polymeric micelle‐based anticancer drugs have been developed to achieve high and selective accumulation at the tumor site.16, 17, 18 CDDP is a key drug in chemotherapy for malignancies such as lung, gastrointestinal, genitourinary and head and neck cancer.2 Studies have shown that NC‐6004 caused better selective accumulation of CDDP in tumors while lessening its distribution in normal tissue.9 NC‐6004, of which the drug‐loaded core is covered by a hydrophilic poly(ethylene glycol) shell layer, is not detected by macrophages, can be retained in the circulation for long periods, is redistributed in tissue and can extravagate preferentially to infiltrate solid tumors. In the present study, we have investigated the antitumor properties and nephrotoxic effects of NC‐6004 on human oral cancer cell lines. Moreover, a submucosal injection of NC‐6004 exhibited substantial therapeutic efficacy in inhibiting cervical lymphatic metastasis in an orthotopic tongue cancer model.
To characterize the cellular basis of NC‐6004 cytotoxicity relative to CDDP, we tested the two drugs in four different oral squamous carcinoma cell lines. The success of CDDP lies in its ability to arrest DNA synthesis, induce oxidative stress and activate apoptotic pathways in tumor cells.19 The growth‐inhibitory effect of NC‐6004 was significantly less than that of CDDP. The different effects between these two drugs lies in the slow release of CDDP in the presence of abundant chloride ions as NC‐6004 contains coordination bonds between the atoms of Pt in CDDP and the carboxylic group in the side chain,10 which is in line with previous studies.9, 10 The caspase cascade is activated when a CDDP stimulus induces the release of cytochrome c from mitochondria.20 This activation leads to an irreversible commitment to apoptotic cell death. We found that activation of caspase‐3 and caspase‐7 was induced in both CDDP‐ and NC‐6004‐treated OSC‐19 cells.
Nephrotoxicity is one of the most significant adverse effects of CDDP.21 CDDP accumulates in cells from all nephron segments, but is preferentially taken up by the highly susceptible proximal tubular epithelial cells, which bear the brunt of the damage. This nephrotoxicity limits the dose that can be administered and prevents the potential efficacy of CDDP.22 The size of NC‐6004 is approximately 30 nm in diameter, which is large enough to avoid renal secretion.9 The Cmax value for Pt concentrations in the kidney after NC‐6004 administration is much lower than that of free CDDP administration.9 In the present study, the number of apoptotic renal cells in NC‐6004‐treated mice was significantly lower than that in CDDP‐treated mice by approximately 66% (CDDP, 14.2%; NC‐6004, 4.2%). Reductions in nephrotoxicity of NC‐6004 might allow patients to undergo therapy without hospitalization for hydration and the treatment of CDDP‐related toxicities.23
We evaluated the in vivo antitumor activity of NC‐6004 in mice. Reductions in tumor size after administration of NC‐6004 or CDDP were approximately 14–20%, respectively, and were not significantly different from each other. The data obtained could be interpreted as these micelles having efficacy against oral squamous cell carcinoma cell lines similar to that of CDDP, but with much less renal toxicity toward the host.
The presence of cervical lymph node metastasis is an indicator of poor prognosis in patients with HNSCC.24, 25, 26, 27 Recently, the SLN has been highlighted as the lymph node that first receives lymphatic drainage from the primary site of a tumor.28 To control lymph node metastasis, especially SLN micrometastasis in the early stages, drugs need to be delivered in tumoricidal concentrations from the site of application. The drug is highly selective depending on the size of molecule to access the lymphatic system, which was reported as the “blood–lymph barrier”. The blood–lymph barrier makes conventional anticancer agents fail to effectively enter the lymphatic system.29 The present study showed that sublingual injection of NC‐6004 in an orthotopic tongue cancer mouse model significantly reduced lymphatic tumor metastasis. Moreover, a high concentration of Pt in cervical lymph nodes was maintained for at least 24 h after administration of NC‐6004, whereas CDDP was not delivered to lymph nodes, which is attributable to the lymphatic drug delivery of NC‐6004 from the primary tumor. These results suggest that this new drug‐delivery system and the accumulation of micelles in lymph nodes are feasible for local chemotherapy targeting SLN in patients with occult lymphatic metastasis or micrometastasis.
We did not examine Pt concentrations in cervical lymph nodes with intravenous infusion of NC‐6004 and CDDP. However, Pt concentrations in the SNL were higher in the NC‐6004‐injected group than CDDP in topically infused groups, indicating that NC‐6004 is more suitable for a lymphatic delivery system. These results suggest that such polymeric micelled‐drugs, which accumulate in higher concentrations around the primary tumor than NC‐6004, might be more suitable for the control of lymphatic disease in HNSCC.
The present study demonstrated the superior safety and antitumor efficacy of NC‐6004 against head and neck cancer cells over that of CDDP. Considering the potential advantages in terms of noticeable antitumor activity, lymphatic drug delivery and reduced nephrotoxicity, NC‐6004 represents a significant structural improvement in the development of a platinum complex. NC‐6004 has progressed to a phase I clinical trial in the UK,23 and phase I/II trials are currently underway in East Asia.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported by JSPS KAKENHI grant numbers 22791579 and 2365972, and a Grant for Clinical Cancer Research from the Ministry of Health, Labour, and Welfare of Japan.
(Cancer Sci, doi: 10.1111/cas.12079, 2013)
References
- 1. Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011; 11: 9–22. [DOI] [PubMed] [Google Scholar]
- 2. Boulikas T, Vougiouka M. Recent clinical trials using cisplatin, carboplatin and their combination chemotherapy drugs (review). Oncol Rep 2004; 11: 559–95. [PubMed] [Google Scholar]
- 3. Pinzani V, Bressolle F, Haug IJ, Galtier M, Blayac JP, Balmes P. Cisplatin‐induced renal toxicity and toxicity‐modulating strategies: a review. Cancer Chemother Pharmacol 1994; 35: 1–9. [DOI] [PubMed] [Google Scholar]
- 4. Yoshizaki T, Wakisaka N, Murono S et al Intra‐arterial chemotherapy less intensive than RADPLAT with concurrent radiotherapy for resectable advanced head and neck squamous cell carcinoma: a prospective study. Ann Otol Rhinol Laryngol 2007; 116: 754–61. [DOI] [PubMed] [Google Scholar]
- 5. Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discovery 2008; 7: 771–82. [DOI] [PubMed] [Google Scholar]
- 6. Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discovery 2005; 4: 145–60. [DOI] [PubMed] [Google Scholar]
- 7. Nishiyama N, Kataoka K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Ther 2006; 112: 630–48. [DOI] [PubMed] [Google Scholar]
- 8. Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 2001; 47: 113–31. [DOI] [PubMed] [Google Scholar]
- 9. Nishiyama N, Okazaki S, Cabral H et al Novel cisplatin‐incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res 2003; 63: 8977–83. [PubMed] [Google Scholar]
- 10. Uchino H, Matsumura Y, Negishi T et al Cisplatin‐incorporating polymeric micelles (NC‐6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br J Cancer 2005; 93: 678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maeda H, Sawa T, Konno T. Mechanism of tumor‐targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release 2001; 74: 47–61. [DOI] [PubMed] [Google Scholar]
- 12. Sano D, Myers JN. Xenograft models of head and neck cancers. Head Neck Oncol 2009; 1: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int 2003; 63: 1714–24. [DOI] [PubMed] [Google Scholar]
- 14. Maekawa K, Sato H, Furukawa M, Yoshizaki T. Inhibition of cervical lymph node metastasis by marimastat (BB‐2516) in an orthotopic oral squamous cell carcinoma implantation model. Clin Exp Metastasis 2002; 19: 513–8. [DOI] [PubMed] [Google Scholar]
- 15. Manchun S, Dass CR, Sriamornsak P. Targeted therapy for cancer using pH‐responsive nanocarrier systems. Life Sci 2012; 90: 381–7. [DOI] [PubMed] [Google Scholar]
- 16. Yokoyama M, Miyauchi M, Yamada N et al Characterization and anticancer activity of the micelle‐forming polymeric anticancer drug adriamycin‐conjugated poly(ethylene glycol)‐poly(aspartic acid) block copolymer. Cancer Res 1990; 50: 1693–700. [PubMed] [Google Scholar]
- 17. Yokoyama M, Okano T, Sakurai Y, Ekimoto H, Shibazaki C, Kataoka K. Toxicity and antitumor activity against solid tumors of micelle‐forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res 1991; 51: 3229–36. [PubMed] [Google Scholar]
- 18. Matsumura Y. Polymeric micellar delivery systems in oncology. Jpn J Clin Oncol 2008; 38: 793–802. [DOI] [PubMed] [Google Scholar]
- 19. Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level. (Review). Oncol Rep 2003; 10: 1663–82. [PubMed] [Google Scholar]
- 20. Alnemri ES. Mammalian cell death proteases: a family of highly conserved aspartate specific cysteine proteases. J Cell Biochem 1997; 64: 33–42. [DOI] [PubMed] [Google Scholar]
- 21. Safirstein R, Winston J, Goldstein M, Moel D, Dikman S, Guttenplan J. Cisplatin nephrotoxicity. Am J Kidney Dis 1986; 8: 356–67. [DOI] [PubMed] [Google Scholar]
- 22. Townsend DM, Deng M, Zhang L, Lapus MG, Hanigan MH. Metabolism of cisplatin to a nephrotoxin in proximal tubule cells. J Am Soc Nephrol 2003; 14: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Plummer R, Wilson RH, Calvert H et al A Phase I clinical study of cisplatin‐incorporated polymeric micelles (NC‐6004) in patients with solid tumours. Br J Cancer 2011; 104: 593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer 1993; 71: 452–6. [DOI] [PubMed] [Google Scholar]
- 25. Layland MK, Sessions DG, Lenox J. The influence of lymph node metastasis in the treatment of squamous cell carcinoma of the oral cavity, oropharynx, larynx, and hypopharynx: N0 versus N+. Laryngoscope 2005; 115: 629–39. [DOI] [PubMed] [Google Scholar]
- 26. Yoshizaki T, Maruyama Y, Sato H, Furukawa M. Expression of tissue inhibitor of matrix metalloproteinase‐2 correlates with activation of matrix metalloproteinase‐2 and predicts poor prognosis in tongue squamous cell carcinoma. Int J Cancer 2001; 95: 44–50. [DOI] [PubMed] [Google Scholar]
- 27. Wakisaka N, Hirota K, Kondo S et al Induction of lymphangiogenesis through vascular endothelial growth factor‐C/vascular endothelial growth factor receptor 3 axis and its correlation with lymph node metastasis in nasopharyngeal carcinoma. Oral Oncol 2012; 48: 703–8. [DOI] [PubMed] [Google Scholar]
- 28. Morton DL, Wen DR, Wong JH et al Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992; 127: 392–9. [DOI] [PubMed] [Google Scholar]
- 29. Grotte G, Knutson RC, Bollman JL. The diffusion of dextrans of different molecular sizes to lymph and urine. J Lab Clin Med 1951; 38: 577–82. [PubMed] [Google Scholar]


