Abstract
The oral fluoropyrimidine, S‐1, combined with or without gemcitabine is considered to be a promising agent for treating advanced biliary tract cancer; gemcitabine plus cisplatin is the current standard regimen. This randomized phase II trial was designed to evaluate the safety and efficacy of two regimens: gemcitabine plus S‐1 (GS) (gemcitabine: 1000 mg/m2, day 1 and day 8; S‐1: 60 mg/m2, twice daily on days 1–14, repeated every 3 weeks); and S‐1 (80 mg/m2, days 1–28, given orally twice daily for 4 weeks, followed by a 2‐week rest, repeated every 6 weeks). The regimen with a higher 1‐year survival would be selected for a subsequent phase III trial. Between February 2009 and April 2010, 101 patients were randomized. For the GS (n = 51) and S‐1 (n = 50) arms, the 1‐year survival was 52.9% (95% confidence interval, 38.5–65.5) and 40.0% (95% confidence interval, 26.5–53.1), and the median survival times were 12.5 and 9.0 months, respectively. Grade 3/4 hematological toxicities were more frequent in the GS arm (leucocytes 29.4%, neutrophils 60.8%, hemoglobin 11.8%, platelets 11.8%) than in the S‐1 arm (leucocytes 2.0%, neutrophils 4.0%, hemoglobin 4.0%, platelets 4.0%). Although two treatment‐related deaths occurred in the GS arm, all other grade 3/4 non‐hematological toxicities were reversible. In conclusion, GS was considered to be more promising and was selected as the test regimen for a subsequent phase III trial comparing GS with gemcitabine plus cisplatin combination therapy. This study was registered at the UMIN Clinical Trials Registry as UMIN 000001685 (http://www.umin.ac.jp/ctr/index.htm).
Biliary tract cancer (BTC) includes carcinomas of the intrahepatic bile duct (IHBD), extrahepatic bile duct (EHBD), gallbladder (GB), and ampulla of Vater (AV). Biliary tract cancer is relatively rare, but high incidence rates have been reported in eastern Asia. Recently, a rising tendency of BTC incidence, especially in IHBD, was reported in Europe and North America.1, 2, 3, 4 For BTC, curative surgical resection offers the only chance for cure; however, most patients are initially diagnosed with unresectable disease. Even after curative surgery, many patients subsequently develop recurrence.5 For unresectable or recurrent BTC, gemcitabine, platinum analogues, and fluoropyrimidine have been considered the key drugs for treatment.5, 6
Until recently, gemcitabine alone was regarded as the standard regimen for the treatment of advanced BTC. However, gemcitabine plus cisplatin combination therapy (GC) has become the new standard regimen based on the results of the ABC‐02 trial,7 which showed superiority in overall survival of patients treated with GC versus gemcitabine alone. A randomized phase II trial (BT22 trial) was carried out in Japan to evaluate both gemcitabine alone and GC in BTC patients.8 Its outcome was similar to that of the ABC‐02 study. From the results of these two clinical studies, GC therapy has been accepted as the standard therapy in Japan.
In a phase II trial for BTC, S‐1 monotherapy showed promising results with a response rate (35%) and median survival time (9.4 months) associated with mild toxicity.9 Therefore, S‐1 is considered a promising agent for the treatment of advanced BTC. Kanai et al. and Sasaki et al. reported two phase II studies of gemcitabine plus S‐1 combination therapy (GS) for advanced BTC. In their reports, GS also showed promising response rates (30% and 34%, respectively) and median survival times (12.7 and 11.6 months, respectively).10, 11
Based on these observations, we planned the present randomized phase II trial. The aim of this study was to evaluate the safety and efficacy of the two regimens and to determine which regimen would be more promising as a test arm regimen in a subsequent phase III trial.
Materials and Methods
Patients
The eligibility criteria for inclusion were as follows: clinical diagnosis of BTC including carcinomas of IHBD, EHBD, GB, and AV; unresectable or recurrent disease; histologically proven adenocarcinoma or adenosquamous carcinoma for patients with EHBD, GB, or AV carcinomas, or adenocarcinoma for IHBD carcinomas; absence of central nervous system metastasis; absence of moderate or severe ascites and/or pleural effusion; no previous chemotherapy or radiotherapy for BTC or other malignancies; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; sufficient oral intake; age 20–79 years; preserved organ functions such as leucocyte count ≥3000/mm3; neutrophil count ≥1500/mm3; hemoglobin level ≥9.0 g/100 mL; platelet count ≥100 000/mm3; aspartate aminotransferase (AST) and alanine aminotransferase (ALT) concentrations ≤100 IU/L (≤150 IU/L in patients with biliary drainage); total bilirubin level ≤2 mg/dL (≤3 mg/dL in patients with biliary drainage); creatinine concentration ≤1.2 mg/dL; and written informed consent. Patients with interstitial pneumonia, lung fibrosis, or watery diarrhea were excluded.
This study was approved by the Japan Clinical Oncology Group (JCOG) Protocol Review Committee and the institutional review board of each participating institution (see Appendix 1). The JCOG Data and Safety Monitoring Committee (DSMC), which is a standing committee, monitored patient safety, adverse events, and the progress of the trial.
Randomization and masking
We sent the information of each patient to the JCOG Data Center by fax or telephone. The data managers checked the eligibility, completed registration if appropriate, and randomly allocated the patient to either the GS or S‐1 treatment group, using a minimization method with an algorithm (concealed to the investigator) to balance the following stratification factors: institution; primary site (GB/others); and clinical stage (II or III/IV or recurrent). The treatment allocation was then communicated to the investigator by fax or telephone. The treatment allocation was not masked from the investigators or the patients. All the data in the case report forms were sent to the JCOG Data Center and were checked by the central data managers.
Treatment
The dose and schedule of the GS arm was planned based on those adopted in the randomized phase III study of pancreatic carcinoma carried out in Japan and Taiwan.12 For the GS arm, 1000 mg/m2 gemcitabine was infused on days 1 and 8, and 30 mg/m2 S‐1 (60 mg/day for a body surface area [BSA] <1.25 m2; 80 mg/day for 1.25 ≤ BSA < 1.50 m2; and 100 mg/day for BSA ≥1.50 m2) was given orally twice daily from days 1 to 14, repeated every 3 weeks. For the S‐1 monotherapy arm, 40 mg/m2 S‐1 (80 mg/day for BSA <1.25 m2, 100 mg/day for 1.25 ≤ BSA < 1.50 m2, and 120 mg/day for BSA ≥1.50 m2) was given orally twice daily for 4 weeks, followed by a 2‐week rest, repeated every 6 weeks.
If the patients showed a leucocyte count <2500/mm3, neutrophil count <1000/mm3, platelet count <75 000/mm3, total bilirubin level >3.0 mg/dL, AST and ALT levels >150 IU/L, creatinine level >1.2 mg/dL, diarrhea ≥ grade 2, mucositis in the oral cavity ≥ grade 2, or a rash ≥ grade 3, the initiation of the next cycle was postponed until recovery from those conditions, in both treatment arms. During the cycle, if patients in the GS arm showed a leucocyte count <2000/mm3 or neutrophil count <1000/mm3, platelet count <70 000/mm3, creatinine level ≥1.5 mg/dL, total bilirubin level ≥3.1 mg/dL, diarrhea ≥ grade 2, mucositis in the oral cavity ≥ grade 2, or rash ≥ grade 3, gemcitabine was not given on day 8 and S‐1 treatment was suspended. The dose of gemcitabine was reduced to 800 mg/m2 when patients experienced: (i) grade 4 leucopenia or neutropenia; (ii) febrile neutropenia or infection with grade 3 leucopenia or neutropenia; (iii) grade 4 thrombocytopenia or grade 3 thrombocytopenia requiring transfusion; (iv) grade 3 rash; or (v) grade 3 or grade 4 non‐hematological adverse reaction. If patients experienced: (vi) creatinine level ≥1.5 mg/dL; (vii) diarrhea ≥ grade 3; (viii) mucositis in the oral cavity ≥ grade 3; or (ix) rash ≥ grade 3, the dose of S‐1 was reduced by 20 mg/day in the subsequent cycle. For the S‐1 monotherapy arm, when patients experienced any of the above (i–ix), the dose of S‐1 was reduced by 20 mg/day in the subsequent cycle. The treatment was discontinued if disease progression was diagnosed clinically or detected by imaging, if a serious adverse event occurred, if a treatment cycle was delayed because of an adverse event longer than 42 days after the final anticancer drug administration in the previous cycle, if subsequent dose reduction was required after a second reduction, if the patient refused treatment, or if the treating doctor judged to discontinue the protocol treatment for other reasons.
Physical examination and laboratory tests were repeated at least on days 1 and 8 of each cycle in the GS arm and at least once every 2 weeks in the S‐1 monotherapy arm. All adverse events were evaluated according to the Common Terminology Criteria for Adverse Events, version 3.0. The JCOG DSMC reviewed the serious adverse events and assessed the attribution of adverse events to the treatment in order to judge whether the study was to be continued or to be modified. The tumor response was assessed every 6 weeks according to RECIST (version 1.0). The response rates were calculated without confirmation.
The primary end‐point was 1‐year survival. The secondary end‐points were progression‐free survival, the response rate in patients with measurable lesions, the incidence of adverse events, and that of serious adverse events. Serious adverse events were defined as death within 30 days after treatment, treatment‐related death (TRD) beyond 30 days after treatment, grade 4 non‐hematological toxicities. The overall survival was calculated from the date of randomization to the date of death or censored on date of last contact for surviving patients, and the progression‐free survival was counted to the date on which disease progression or death was detected, or was censored on the last date when progression‐free status was confirmed. The dose intensity (DI) was defined as the total amount of drug actually given per 1 week (mg/m2/week) during eight cycles (GS arm) or four cycles (S‐1 monotherapy arm) from the start of chemotherapy.
Statistical analysis
This study adopted selection design,13 in that the regimen with a higher 1‐year survival would be selected. The sample size was determined as follows using Simon's selection design. We assumed that the 1‐year survival for one regimen would be 30% and that for the other regimen would be higher than 40%. In this situation, the sample size ensuring a probability of at least 85% for correct selection of the more effective regimen was 98 patients, with 49 patients per arm. Considering the likelihood of some ineligible patients being enrolled, the total number of patients was set at 100. Overall survival and progression‐free survival were estimated by using the Kaplan–Meier method, and curves were compared using an unstratified log–rank test. Hazard ratios of treatment effects were estimated by using the unstratified Cox regression model. We carried out all the analyses based on an intention‐to‐treat using sas version 9.2 (SAS Institute, Cary, NC, USA). Unless otherwise specified, two‐sided P‐values for superiority were used.
Role of the funding source
The sponsor of the study was the Ministry of Health, Labour and Welfare, Japan, which had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit the report for publication.
Results
From February 2009 to April 2010, 101 patients with BTC (GB, n = 38; IHBD, n = 35; EHBD, n = 20; AV, n = 8) were enrolled. Fifty‐one patients were allocated to the GS arm and 50 patients were allocated to the S‐1 arm (Fig. 1). The baseline characteristics were well balanced between the treatment groups (Table 1). All the individuals had an ECOG PS of 0 or 1. Eighty‐nine percent (90/101) of the participants had at least one measurable lesion. Fifteen patients had unresectable stage II or III (locally advanced) disease, 61 patients had stage IV (metastatic) disease, and 25 patients had recurrent disease after curative resection.
Figure 1.
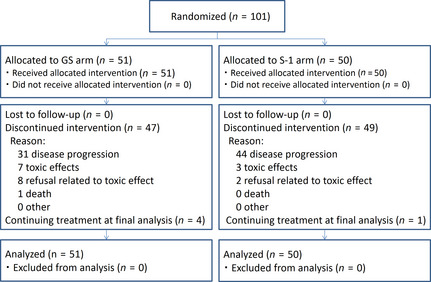
CONSORT diagram showing progress of the randomized phase II study of gemcitabine plus S‐1 combination therapy (GS) versus S‐1 alone in patients with advanced biliary tract cancer.
Table 1.
Characteristics of patients with advanced biliary tract cancer who participated in the randomized phase II study of gemcitabine plus S‐1 combination therapy (GS) versus S‐1 alone
| GS (n = 51) | S‐1 (n = 50) | Total | |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 66.0 (39–78) | 62.5 (49–79) | |
| Gender, n (%) | |||
| Male | 27 (52.9) | 28 (56.0) | 55 |
| Female | 24 (47.1) | 22 (44.0) | 46 |
| PS, n (%) | |||
| 0 | 39 (76.5) | 37 (74.0) | 76 |
| 1 | 12 (23.5) | 13 (26.0) | 25 |
| Target lesion, n (%) | |||
| Present | 44 (86.3) | 46 (92.0) | 90 |
| Absent | 7 (13.7) | 4 (8.0) | 11 |
| Primary tumor, n (%) | |||
| IHBD | 20 (39.2) | 15 (30.0) | 35 |
| EHBD | 9 (17.6) | 11 (22.0) | 20 |
| GB | 19 (37.3) | 19 (38.0) | 38 |
| AV | 3 (5.9) | 5 (10.0) | 8 |
| Stage, n (%) | |||
| II or III | 8 (15.7) | 7 (14.0) | 15 |
| IV | 29 (56.9) | 32 (64.0) | 61 |
| Recurrent | 14 (27.5) | 11 (22.0) | 25 |
| Biliary drainage, n (%) | |||
| − | 32 (62.7) | 31 (62.0) | 63 |
| + | 19 (37.3) | 19 (38.0) | 38 |
AV, ampulla of Vater; EHBD, extrahepatic biliary duct; GB, gallbladder; IHBD, intrahepatic biliary duct; PS, performance status.
Drug exposure and duration of treatments
At the data cut‐off point (April 2011; median follow‐up time for all randomized patients, 10.6 months), four patients in the GS arm and one patient in the S‐1 arm were still receiving the protocol treatment. Among the other patients, the median number of cycles of GS given was 10 (range, 1–34; interquartile range, 3–14) and that of S‐1 was 3 (range, 1–9; interquartile range, 1–4). At the data cut‐off point, 95% (96/101) of the patients terminated the protocol treatment. The protocol treatment was terminated because of disease progression in 61% (31/51) of the patients in the GS arm and 88% (44/50) in the S‐1 arm. Termination because of adverse events was observed in 29.4% (15/51) in the GS arm and 10.0% (5/50) in the S‐1 arm (Fig. 1). The median DI of gemcitabine and S‐1 was 641.5 mg/m2/week (96.2% of planned DI) and 258.8 mg/m2/week (92.4% of planned DI) in the GS arm, and the median dose intensity of S‐1 was 358.3 mg/m2/week (96.0% of planned DI) in the S‐1 arm.
Safety
Table 2 shows the adverse events recorded within 6 months after randomization. For patients assigned to the GS arm, grade 3 or 4 leucopenia (29.4%) and neutropenia (60.8%) were observed, whereas those toxicities were infrequent (2.0%, 4.0%, respectively) in the S‐1 arm. Common grade 1–4 adverse events were liver dysfunction (AST, ALT, ALP, and total bilirubin), fatigue, and anorexia in both arms, whereas grade 3 or 4 symptomatic toxicities were infrequent in both arms. One patient in the GS arm died as a result of pneumonitis 13 days after the last dose of the study drug (S‐1), and another patient in the GS arm died from myocardial infarction the day after the last dose of the study drug (S‐1), and these were judged to be TRD. Reported serious adverse events are: TRD in the above two patients and grade 4 hyponatremia in one patient (GS arm); grade 4 acute myocardial infarction in one patient (S‐1 arm); and grade 4 AST elevation in one patient (S‐1 arm).
Table 2.
Adverse events (CTCAE version 3.0) recorded in patients with advanced biliary tract cancer within 6 months after randomization of gemcitabine plus S‐1 combination therapy (GS) or S‐1 alone
| GS (n = 51) | S‐1 (n = 50) | |||||
|---|---|---|---|---|---|---|
| G3 (%) | G4 (%) | All grades (%) | G3 (%) | G4 (%) | All grades (%) | |
| Leucocytes | 29.4 | 0.0 | 90.2 | 2.0 | 0.0 | 40.0 |
| Hemoglobin | 9.8 | 2.0 | 82.4 | 4.0 | 0.0 | 66.0 |
| Platelets | 5.9 | 5.9 | 51.0 | 0.0 | 4.0 | 22.0 |
| Neutrophils | 43.1 | 17.6 | 88.2 | 4.0 | 0.0 | 40.0 |
| Bilirubin | 9.8 | 0.0 | 52.9 | 14.0 | 0.0 | 64.0 |
| ALP | 7.8 | 0.0 | 70.6 | 12.0 | 2.0 | 76.0 |
| AST | 11.8 | 0.0 | 72.5 | 12.0 | 2.0 | 70.0 |
| ALT | 13.7 | 0.0 | 64.7 | 12.0 | 0.0 | 62.0 |
| Creatinine | 0.0 | 0.0 | 29.4 | 0.0 | 0.0 | 12.0 |
| Fatigue | 7.8 | 0.0 | 56.9 | 4.0 | 0.0 | 62.0 |
| Anorexia | 7.8 | 0.0 | 51.0 | 6.0 | 0.0 | 60.0 |
| Nausea | 2.0 | 0.0 | 35.3 | 4.0 | 0.0 | 52.0 |
| Vomiting | 2.0 | 0.0 | 13.7 | 0.0 | 0.0 | 28.0 |
| Rash | 9.8 | 0.0 | 39.2 | 2.0 | 0.0 | 16.0 |
| Fever | 0.0 | 0.0 | 39.2 | 2.0 | 0.0 | 26.0 |
| Mucositis (oral cavity) | 5.9 | 0.0 | 25.5 | 0.0 | 0.0 | 18.0 |
| Cheilitis | 0.0 | – | 15.7 | 0.0 | – | 16.0 |
| Hyperpigmentation | – | – | 23.5 | – | – | 32.0 |
| Taste alteration | – | – | 15.7 | – | – | 18.0 |
| Diarrhea | 2.0 | 0.0 | 19.6 | 6.0 | 0.0 | 34.0 |
| Constipation | 0.0 | 0.0 | 31.4 | 0.0 | 0.0 | 12.0 |
| Alopesia | – | – | 13.7 | – | – | 2.0 |
| Pruritus | 0.0 | – | 11.8 | 0.0 | – | 8.0 |
| Infection with normal ANC | 7.8 | 0.0 | 19.6 | 10.0 | 2.0 | 12.0 |
| Infection with grade 3 or 4 ANC | 4.0 | 0.0 | 7.8 | 0.0 | 0.0 | 0.0 |
| Febrile neutropenia | 2.0 | 0.0 | 2.0 | 0.0 | 0.0 | 0.0 |
Events with a frequency of more than 10.0% or high‐grade events (grades 3,4) are listed. –, not applicable; ALP, alkaline phosphatase; AST, aspartate amino transferase; ALT, alanine aminotransferase; ANC, absolute neutrophil count; CTCAE, Common Terminology Criteria for Adverse Events.
Efficacy
At the data cut‐off point, 74 (73.3%) deaths had been recorded. The median survival time of the patients assigned to the GS arm was 12.5 (95% confidence interval [CI], 9.0–15.4) months, whereas that of the patients assigned to the S‐1 arm was 9.0 (95% CI, 7.3–12.7) months (hazard ratio, 0.859; 95% CI, 0.543–1.360; P = 0.52). One‐year survival was 52.9% in the GS arm and 40.0% in the S‐1 arm (Fig. 2). In the subgroup analysis, the survival of the patients with GB cancer tended to be worse than that of the patients with non‐GB cancer in both arms. The survival of the patients with recurrent disease tended to be better than that for the patients with stage II/III or IV disease in both treatment arms (Table 3).
Figure 2.
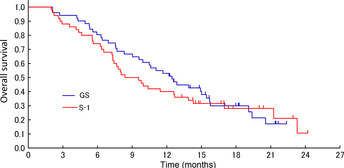
Kaplan–Meier curves for overall survival in the randomized phase II study of gemcitabine plus S‐1 combination therapy (GS) versus S‐1 alone in patients with advanced biliary tract cancer.
Table 3.
Median survival time by stratification factor in patients with advanced biliary tract cancer treated with gemcitabine plus S‐1 combination therapy (GS) or S‐1 alone
| GS | S‐1 | Total | |
|---|---|---|---|
| Tumor site | |||
| GB (n = 38) | 11.7 (6.3–13.9) | 6.5 (3.6–8.0) | 7.9 (6.3–11.1) |
| Non‐GB (n = 63) | 15.0 (7.7–20.6) | 14.3 (8.0–23.3) | 15.0 (9.9–20.6) |
| Stage | |||
| Stage II, III (n = 15) | 13.0 (5.9–NE) | 14.3 (2.1–NE) | 13.9 (7.3–NE) |
| Stage IV (n = 61) | 10.6 (6.3–15.4) | 7.5 (5.6–10.0) | 8.0 (6.9–10.6) |
| Recurrence (n = 25) | 19.1 (7.6–NE) | 17.0 (6.2–21.3) | 17.0 (11.7–21.3) |
Data are shown as the median (95% confidence interval). GB, gallbladder; NE, not estimable.
Median progression‐free survival was 7.1 (95% CI, 5.7–8.6) months in the GS arm and 4.2 (95% CI, 2.5–5.0) months in the S‐1 arm (Fig. 3; hazard ratio, 0.437; 95% CI, 0.286–0.669; P < 0.0001).
Figure 3.
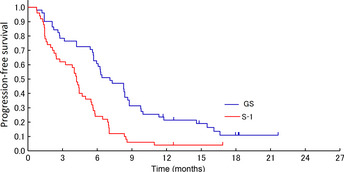
Kaplan–Meier curves for progression‐free survival in the randomized phase II study of gemcitabine plus S‐1 combination therapy (GS) versus S‐1 alone in patients with advanced biliary tract cancer.
Among the patients with measurable lesions, the response rates were 36.4% (16/44) in the GS arm and 17.4% (8/46) in the S‐1 arm. The response rates for the patients with GB carcinomas were 12.5% (2/16) in the GS arm and 16.7% (3/18) in the S‐1 arm. The response rates for patients with non‐GB carcinomas were 50% (14/28) in the GS arm and 17.9% (5/28) in the S‐1 arm.
Twenty‐five patients (49%) in the GS arm received second‐line chemotherapy containing S‐1 monotherapy (seven patients), gemcitabine monotherapy (six patients), GC therapy (seven patients), and other regimen (five patients). In the S‐1 arm, 39 (78%) patients received second‐line chemotherapy containing gemcitabine monotherapy (33 patients), GC therapy (one patient), GS therapy (one patient) and other regimen (four patients).
Discussion
The aim of this randomized phase II trial was to select the test arm regimen for a subsequent phase III trial. The frequency of toxicity was expected to be higher in the GS arm than in the S‐1 arm, but we expected that the frequency of serious adverse events would be almost equivalent. Therefore, we decided to select the more promising regimen based on efficacy, namely, 1‐year survival, as long as the levels of severe toxicity did not differ markedly between the two arms. In this study, the GS arm showed a higher 1‐year survival than the S‐1 arm. Furthermore, other measures of efficacy in the GS arm, such as the response rate, overall survival, and progression‐free survival, were also better than those obtained in the S‐1 arm.
Although hematological toxicities tended to be more frequent in the GS arm than in the S‐1 arm, most toxicities in both arms were tolerable and reversible. Serious adverse events occurred in 7.8% in the GS arm and in 6.0% in the S‐1 arm, and two patients in the GS arm experienced TRDs. The frequency of serious adverse events was almost equivalent, as expected. However, these findings should be noted carefully in subsequent phase III trials.
In this study, we stratified the patients into those with GB cancer and those with other BTCs. Gallbladder cancer has been recognized to have a poorer survival outcome.8, 14, 15, 16, 17 As shown in Table 3, the patients with GB cancer had a shorter survival than those with other BTCs, consistent with the findings of previous reports. Importantly, despite the dismal clinical outcomes of GB cancer patients, the median survival times of the patients with GB cancer was much better in the GS arm (11.7 months) than in the S‐1 arm (6.5 months) in the present trial as well, concurring with previous reports.8, 14, 15, 16, 17
Another stratification factor used in this study was the clinical stage (II or III versus IV or recurrent). Locally advanced or metastatic cancer, the stratification factor used in the ABC‐01 and ABC‐02 studies,7, 18 has been shown to affect the OS of patients with advanced BTC.19 However, there is no consensus as to whether recurrent disease should be classified as locally advanced disease or metastatic disease or should be an isolated disease entity. In this study, the patients with recurrent disease had more favorable overall survival than those with stage IV disease and even those with stage II/III. However, because of the limited number of patients in these subgroup analyses, the results should be viewed with caution.
When compared to the GC regimen (the current standard) in the BT22 study, the incidence of symptomatic gastrointestinal toxicities such as nausea (68.3% vs 35.3%), vomiting (48.8% vs 13.7%), and appetite loss (80.5% vs 51%) were lower for the GS regimen. Similar favorable gastrointestinal toxicity profiles were also observed in two previous phase II studies of GS regimens (Table 4), although the treatment schedule and dosage were different from those in the current study.10, 11 Additionally, although the GC regimen requires an infusion time including hydration before and after cisplatin administration (typically over 3 h), the GS regimen requires a 30‐min infusion only for gemcitabine administration. Therefore, the GS regimen may be a more convenient regimen for patients, compared with the GC regimen. Additionally, the median survival (12.5 vs 11.2 months), progression‐free survival (7.1 vs 5.8 months), and response rate (36.4% vs 19.5%) of the GS arm in the current study were better than those of the GC arm in the BT22 study, and similar efficacy was also observed in the abovementioned two previous phase II studies.10, 11 However, such cross‐study comparisons have limitations because meaningful selection biases may affect those results.
Table 4.
Clinical trials of gemcitabine plus S‐1 (GS) therapy or gemcitabine plus cisplatin (GC) combination therapy for patients with advanced biliary tract cancer
| GS therapy | Gemcitabine (mg/m2) | S‐1 (mg/m2/day) | Cycle (days) | No. of patients | RR (%) | Median PFS (TTP), months | Median OS (months) | Grade 3/4 Neutropenia (%) | Grade 1–4 Nausea (%) | Grade 1–4 Vomiting (%) | Grade 1–4 Anorexia (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sasaki et al. | 1000 (days 1, 15) | 80 (days 1–14) | 28 | 35 | 34 | 5.9 | 11.6 | 34.0 | 26.0 | 9.0 | 23.0 |
| Kanai et al. | 1000 (days 1, 8) | 60 (days 1–14) | 21 | 25 | 30 | NA | 12.7 | 56.0 | 20.0 | 4.0 | 28.0 |
| Current study | 1000 (days 1, 8) | 60 (days 1–14) | 21 | 51 | 36 | 7.1 | 12.5 | 60.8 | 35.3 | 13.7 | 51.0 |
| GC therapy | Gemcitabine (mg/m2) | Cisplatin (mg/m2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BT22 (GC regimen) | 1000 (days 1, 8) | 25 (days 1, 8) | 21 | 42 | 20 | 5.8 | 11.2 | 56.1 | 68.3 | 48.8 | 80.5 |
| ABC‐02 (GC regimen) | 1000 (days 1, 8) | 25 (days 1, 8) | 21 | 204 | 26 | 8.0 | 11.7 | 25.3 | NA | NA | NA |
NA, not available; OS, overall survival; PFS, progression‐free survival; RR, response rate; TTP, time to progression.
In summary, the 1‐year survival, which was the primary end‐point of the present study, was superior in the GS arm than in the S‐1 arm. The overall survival, progression‐free survival, and response rate were also superior in the GS arm than in the S‐1 arm, and most of the toxicities in both arms were tolerable and reversible. We concluded that the GS regimen would be more promising for a subsequent phase III trial. Our new phase III trial comparing the GS and GC regimens (JCOG1113) is now ongoing.
Disclosure Statement
Takuji Okusaka has received Honoraria from Lilly and Taiho Pharmaceutical, and Research Funding from Lilly, Taiho Pharmaceutical, and Yakult. Junji Furuse has received Honoraria and Research Funding from Lilly and Taiho Pharmaceutical. The other authors have no conflict of interest.
Acknowledgments
The authors are grateful to the members of the Japan Clinical Oncology Group's Data Center and Operations Office for their support in preparing the manuscript (Dr Kenichi Nakamura, Dr Hiroshi Katayama, Ms Aya Kimura), statistical analysis (Mr. Taro Shibata), data management (Ms Ayaka Nakano), and oversight of the study management (Dr Haruhiko Fukuda). This study was supported by the National Cancer Center Research and Development Fund (23‐A‐16 and 23‐A‐22), Grants‐in‐Aid for Cancer Research (20S‐3, 20S‐6), and Health and Labour Sciences Research Grant for Clinical Cancer Research (19‐022, 22‐013), from the Ministry of Health, Labour and Welfare of Japan.
Appendix 1.
Participating Institutions
Kanagawa Cancer Center, Kanagawa, Japan
National Cancer Center Hospital, Tokyo, Japan
National Cancer Center Hospital East, Chiba, Japan
Tochigi Cancer Center, Tochigi, Japan
Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan
Saitama Cancer Center, Saitama, Japan
Shizuoka Cancer Center, Shizuoka, Japan
National Kyushu Cancer Center, Fukuoka, Japan
Jichi Medical University, Tochigi, Japan
Chiba Cancer Center, Chiba, Japan
Aichi Cancer Center Hospital, Aichi, Japan
Osaka National Hospital, Osaka, Japan
Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka, Japan
National Hospital Organization Shikoku Cancer Center, Ehime, Japan
Sapporo‐Kosei General Hospital, Hokkaido, Japan
Kyorin University School of Medicine, Tokyo, Japan
Yokohama City University Medical Center, Kanagawa, Japan
Kansai Medical University Hirakata Hospital, Osaka, Japan
Kyushu University Hospital, Fukuoka, Japan
(Cancer Sci, doi: 10.1111/cas.12218, 2013)
References
- 1. McGlynn KA, Tarone RE, El‐Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev 2006; 15: 1198–203. [DOI] [PubMed] [Google Scholar]
- 2. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001; 33: 1353–7. [DOI] [PubMed] [Google Scholar]
- 3. Shaib Y, El‐Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004; 24: 115–25. [DOI] [PubMed] [Google Scholar]
- 4. West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971–2001. Br J Cancer 2006; 94: 1751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Furuse J, Takada T, Miyazaki M et al Guidelines for chemotherapy of biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg 2008; 15: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist 2008; 13: 415–23. [DOI] [PubMed] [Google Scholar]
- 7. Valle J, Wasan H, Palmer DH et al Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010; 362: 1273–81. [DOI] [PubMed] [Google Scholar]
- 8. Okusaka T, Nakachi K, Fukutomi A et al Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010; 103: 469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furuse J, Okusaka T, Boku N et al S‐1 monotherapy as first‐line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 2008; 62: 849–55. [DOI] [PubMed] [Google Scholar]
- 10. Kanai M, Yoshimura K, Tsumura T et al A multi‐institution phase II study of gemcitabine/S‐1 combination chemotherapy for patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 2011; 67: 1429–34. [DOI] [PubMed] [Google Scholar]
- 11. Sasaki T, Isayama H, Nakai Y et al Multicenter, phase II study of gemcitabine and S‐1 combination chemotherapy in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 2010; 65: 1101–7. [DOI] [PubMed] [Google Scholar]
- 12. Ioka T, Ikeda M, Ohkawa S et al Randomized phase III study of gemcitabine plus S‐1 (GS) versus S‐1 versus gemcitabine (GEM) in unresectable advanced pancreatic cancer (PC) in Japan and Taiwan: GEST study. J Clin Oncol 2011; 29(Suppl.): abstr 4007. [Google Scholar]
- 13. Simon R, Wittes RE, Ellenberg SS. Randomized phase II clinical trials. Cancer Treat Rep 1985; 69: 1375–81. [PubMed] [Google Scholar]
- 14. Doval DC, Sekhon JS, Gupta SK et al A phase II study of gemcitabine and cisplatin in chemotherapy‐naive, unresectable gall bladder cancer. Br J Cancer 2004; 90: 1516–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 2007; 96: 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim ST, Park JO, Lee J et al A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer 2006; 106: 1339–46. [DOI] [PubMed] [Google Scholar]
- 17. Wagner AD, Buechner‐Steudel P, Moehler M et al Gemcitabine, oxaliplatin and 5‐FU in advanced bile duct and gallbladder carcinoma: two parallel, multicentre phase‐II trials. Br J Cancer 2009; 101: 1846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valle JW, Wasan H, Johnson P et al Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study ‐ The UK ABC‐01 Study. Br J Cancer 2009; 101: 621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park I, Lee JL, Ryu MH et al Prognostic factors and predictive model in patients with advanced biliary tract adenocarcinoma receiving first‐line palliative chemotherapy. Cancer 2009; 115: 4148–55. [DOI] [PubMed] [Google Scholar]


