Abstract
Identification of new nasopharyngeal carcinoma (NPC) biomarkers is of great clinical value for the diagnosis and treatment of NPC. HOTAIR, a cancer‐related long non‐coding RNA, was tested and its prognostic value for NPC was evaluated. As determined using in situ hybridization (ISH), 91 of 160 (56.87%) paraffin‐embedded NPC biopsies showed high expression levels of HOTAIR (staining index score ≥ 6). HOTAIR was upregulated in tumors with a large size (P = 0.021), more advanced clinical stage (P = 0.012) and increased lymph node tumor burden (P = 0.005). Quantified using real‐time PCR,HOTAIR expression levels in fresh tissue and paraffin‐embedded samples were 5.2 ~ 48.4‐fold higher compared with non‐cancer tissue samples. Moreover, HOTAIR expression levels increased with clinical stage progression, which was consistent with ISH findings in the paraffin‐embedded tissue. Most importantly, NPC patients with higher HOTAIR levels had a poor prognosis for overall survival using univariate and multivariate analysis. In addition, HOTAIR mediated the migration, invasion and proliferation of NPC cells in vitro. HOTAIR is a potential biomarker for the prognosis of NPC, and dysregulation of HOTAIR might play an important role in NPC progression.
Nasopharyngeal carcinoma (NPC) is a rare tumor that arises from the nasopharynx epithelium. Nasopharyngeal carcinoma occurs around the world;1 however, it is vastly more common in certain regions of Southeast Asia than elsewhere. The incidence of NPC in South China, especially in the Cantonese region around Guangzhou, is approximately 100‐fold higher compared with Europe and North America,1 with viral, environmental and genetic factors implicated in its etiology.2, 3, 4, 5 Although treatment with radiotherapy temporarily controls the primary tumor, frequent tumor recurrence and distant metastases remain major obstacles for long‐term patient survival.6, 7 Therefore, identifying more accurate predictive biomarkers is of great clinical value to further understand NPC cell biology and develop novel therapeutic strategies.
The discovery of numerous non‐coding RNA (ncRNA) transcripts in human cells has dramatically altered our understanding of the biology of normal and malignant cells. A large class of small ncRNA, microRNA, has been characterized as oncogenes or tumor suppressors through post‐transcriptional regulation of protein expression. However, the findings that long non‐coding RNA (lncRNA) more than 200 nt in length are differentially expressed in cancer cells and bind to various regulatory proteins have increased the complexity of ncRNA involvement in tumor biology regulation.8, 9, 10 Dysregulated lncRNA expression levels characterize the entire spectrum of disease,10 and aberrant lncRNA function drives cancer through the disruption of cell processes, typically by facilitating transcriptional regulation.11 This process can occur through the targeting of either genomically local (cis‐regulation) or genomically distant (trans‐regulation) genes. Of the cis‐regulatory lncRNA (imprinting lncRNA), H19 has been most extensively studied in cancer.12, 13, 14 In model systems, silencing H19 expression impaired cell growth and clonogenicity in lung cancer cell lines in vitro 14 and decreased xenograft tumor growth of Hep3B hepatocellular carcinoma cells in vivo.12 Other cis‐regulatory lncRNA, such as HOTAIRMI and HOTTIP, play important roles in differentiation status of cancer cells in leukemias.15 Similar to most cis‐acting lncRNA, trans‐regulatory lncRNA typically facilitate the epigenetic regulation of gene expression. In cancer, trans‐regulatory lncRNA gained widespread attention through the characterization of HOTAIR. HOTAIR is located in the HoxC cluster and was found to regulate the HoxD cluster genes through a trans‐regulatory mechanism.16 Recently, upregulated expression of HOTAIR was observed in numerous solid tumors, including breast cancer,17 hepatocelluar carcinoma18 and colon cancer.19 In breast cancer, overexpression of HOTAIR facilitates aberrant polycomb repressive complex (PRC2) function by increasing PRC2 recruitment to the genomic positions of target genes and mediates the epigenetic repression of PRC2 target genes.17 Clinically, overexpression of HOTAIR is an independent predictor of overall survival and progression‐free survival for several cancers.17, 18 Thus, lncRNA represent a novel but poorly characterized aspect of cancer biology. While a biological understanding of HOTAIR in breast and hepatocellular carcinomas has progressed, increased understanding of the functional role of HOTAIR in other human cancers, such as NPC, is needed.
In the present study, we examined HOTAIR expression in normal and malignant human nasopharyngeal tissue and cell lines. Our results demonstrated that high HOTAIR expression is associated with NPC progression and predicts a poor patient prognosis. In addition, we studied how HOTAIR influences the invasiveness of NPC cells in vitro.
Matherials and Methods
Patient and tissue specimens
A total of 160 paraffin‐embedded NPC samples from Sun Yat‐Sen Memorial Hospital from June 2005 to June 2012 were examined in the present study. Fresh tumor samples of primary NPC and non‐NPC tissues were obtained from biopsies from the Department of Otolaryngology/Head and Neck, Sun Yat‐Sen Memorial Hospital, as approved by the Research Ethics Board at Sun Yat‐Sen Memorial Hospital. Written informed consent was obtained from all patients. No patients had received therapy prior to biopsy. The disease stages of the patients were classified according to American Joint Committee on Cancer (AJCC). The clinical information of the samples is described in detail in Supporting Information Table S1.
Quantitative PCR assay
Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) or a RNeasy FFPE Kit (QIAGEN, Hilden, Germany). cDNA was obtained using reverse transcription of total RNA with a TaqMan Reverse Transcription Kit (Applied Biosystems Inc., Carlsbad, CA, USA). The primer sequences of HOTAIR were as follows: forward, 5′‐GGTAGAAAAAGCAACCACGAAGC‐3′; and reverse, 5′‐ACATAAACCTCTGTCTGTGAGTGCC‐3′. Amplification and analysis were performed on the Roche LightCycler480 (Roche, Basel, Switzerland).
In situ hybridization and data analyses
HOTAIR expression was examined using in situ hybridization (ISH) in NPC and non‐NPC paraffin‐embedded sections. Briefly, after dewaxing and rehydration, the samples were digested with proteinase K, fixed in 4% paraformaldehyde, hybridized with the 5′digoxin‐labeled locked nucleic acid (LNA)‐modified HOTAIR probe (Exiqon, Vedbaek, Denmark) at 55°C overnight, and subsequently incubated overnight at 4°C with anti‐digoxin monoclonal antibody (Roche). After staining with nitro blue tetrazolium/5‐bromo‐4‐chloro‐3‐indolylphosphate, the sections were mounted and observed. Positive expression of HOTAIR (in blue) was primarily detected in the cytoplasm. The staining scores were determined based on both the intensity and proportion of HOTAIR‐positive cells in 10 random fields under a × 40 objective. The proportion of positively stained tumor cells was graded as follows: 0, no positive cells; 1, <10%; 2, 10–50%; and 3, >50%. The cells at each staining intensity were recorded on a scale of 0 (no staining), 1 (light blue), 2 (blue) and 3 (dark blue). The staining index (SI) was calculated as follows: SI = staining intensity × proportion of positively stained cells. Using this method, the expression of HOTAIR was evaluated using SI and scored as 0, 1, 2, 3, 4, 6 or 9. A SI score of 6 was used as a cut‐off value based on the distribution of frequency of SI score for HOTAIR expression and a measurement of heterogeneity with the log‐rank test statistic with respect to overall survival, and the expression levels of HOTAIR were defined as high (SI ≥ 6) or low (SI < 6). In addition, ISH signals for HOTAIR expression were quantified in the form of mean optical density (MOD) using the AxioVision Rel.4.6 computerized image analysis system assisted with the automatic measurement program (Carl Zeiss, Oberkochen, Germany), as previously reported.20
Statistical analyses
Statistical analyses were performed using SPSS version 16.0 (SPSS, Chicago, IL, USA). A Chi‐squared test was used to analyze the relationship between HOTAIR expression levels and the clinicopathological characteristics. A one‐way anova was used to compare the HOTAIR expression levels between the NPC tumors of different clinical stages. Survival curves were plotted using the Kaplan–Meier method and compared using the log‐rank test. The survival data were evaluated using univariate and multivariate Cox regression analyses. Receiver operating characteristic curves (ROC) were used to evaluate the average values of sensitivity and specificity of HOTAIR. P < 0.05 was considered significant.
Results
Correlation of HOTAIR expression with clinicopathological features in nasopharyngeal carcinoma
To correlate HOTAIR expression with NPC progression in the clinic, we used ISH to localize HOTAIR expression in nasopharyngeal tissue. A total of 160 paired paraffin‐embedded non‐cancer and NPC tissues were analyzed for HOTAIR expression. Specific HOTAIR staining was observed scattered in the cytoplasm of carcinoma cells in 149 of 160 cases, whereas no staining was observed in normal nasopharyngeal epithelia. However, staining was occasionally identified in nasopharyngeal cells in 14 of 41 cases of metaplasia with atypical hyperplasia (Fig. 1A). Furthermore, HOTAIR expression levels were compared in NPC samples in different stages. As shown in Figure 1B and S1, a quantitative analysis showed that HOTAIR staining, determined using MOD, in TNM stage I to IV tumors was significantly higher than in normal nasopharyngeal tissue; the MOD of HOTAIR staining were also significantly different between various clinical stages (P < 0.01; Fig. S1).
Figure 1.
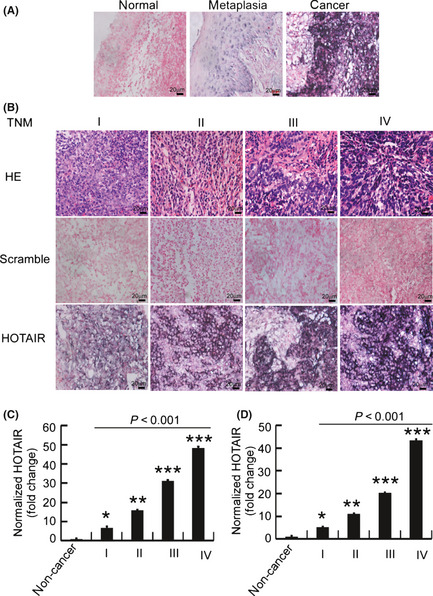
High levels of HOTAIR are correlated with nasopharyngeal carcinoma (NPC) progression. (A) Representative images (×400) of in situ hybridization for HOTAIR in normal nasopharyngeal tissue, metaplasia with atypical hyperplasia and NPC tissue. (B) Representative images (×400) of H&E and HOTAIR staining in paraffin‐embedded NPC tissue of different clinical stages. Scramble RNA was used as a negative control. (C,D) Quantification of HOTAIR levels was performed using real‐time PCR of paraffin‐embedded samples (C) and fresh frozen tissue (D). *P < 0.05, **P < 0.01 and ***P < 0.001 compared with non‐cancerous tissue. Bar, 20 μm.
Next we correlated HOTAIR expression levels with the clinicopathological status of patients with nasopharyngeal carcinoma (Table 1). The expression levels of HOTAIR were upregulated in tumors with a higher tumor burden, as defined by a larger tumor size (P = 0.021, Table 1), more advanced clinical staging (P = 0.012; Table 1) and increased lymph node tumor burden (P = 0.005; Table 1), as well as the status of distant metastasis (P = 0.023; Table 1). However, there was no significant correlation between the expression levels of HOTAIR and age, gender or histological classification. To further evaluate whether HOTAIR upregulation was linked to NPC clinical progression, HOTAIR levels were quantified using real‐time PCR in 160 paired paraffin‐embedded non‐cancer and NPC specimens (Fig. 1C) and 20 fresh frozen non‐cancer nasopharyngeal tissue, as well as 20 NPC tissues with different TNM stages (Fig. 1D). HOTAIR expression levels were 5.2~48.4‐fold higher compared with non‐cancer paraffin‐embedded samples (Fig. 1C) or fresh frozen tissue (Fig. 1D). More importantly, HOTAIR expression levels increased with clinical stage progression (Fig. 1C,D), which was consistent with the ISH findings in paraffin‐embedded tissue. These data imply that upregulated HOTAIR in cancer cells correlates with NPC progression.
Table 1.
Correlation between the clinicopathological features and expression of HOTAIR
| Characteristics | HOTAIR in situ hybridization (%) | P | |
|---|---|---|---|
| SI < 6 | SI ≥ 6 | ||
| Age (years) | |||
| <46 | 36 (50.7) | 35 (49.3) | 0.495 |
| ≥46 | 33 (37.1) | 56 (62.9) | |
| Gender | |||
| Male | 44 (40.4) | 65 (59.6) | 0.303 |
| Female | 25 (49.0) | 26 (51.0) | |
| Histological classification | |||
| WHO type I | 3 (27.3) | 8 (72.7) | 0.532 |
| WHO type II | 11 (42.3) | 15 (57.7) | |
| WHO type III | 55 (44.7) | 68 (55.3) | |
| Clinical stage | |||
| I–II | 27 (58.7) | 19 (41.3) | 0.012 |
| III–IV | 42 (36.8) | 72 (63.2) | |
| T classification | |||
| T1–T2 | 43 (51.8) | 40 (48.2) | 0.021 |
| T3–T4 | 26 (33.8) | 51 (66.2) | |
| N classification | |||
| N0 | 11 (42.3) | 15 (57.7) | 0.005 |
| N1 | 32 (60.4) | 21 (39.6) | |
| N2–N3 | 26 (32.1) | 55 (67.9) | |
| Metastasis | |||
| Yes | 56 (48.7) | 59 (51.3) | 0.023 |
| No | 13 (28.9) | 32 (71.1) | |
N, lymph node; T, tumor; WHO, World Health Organization.
High expression levels of HOTAIR are correlated with poor prognosis in NPC patients
Tumor recurrence and distant metastases are responsible for the poor survival of NPC patients. Therefore, next we analyzed the prognostic value of HOTAIR expression levels in 160 cancer patients using Kaplan–Meier analysis and the log‐rank test. Among the 160 patients, 82 of 91 with high HOTAIR expression levels developed local recurrence (50 cases) and/or distant recurrence (32 cases), whereas 38 of 61 with low HOTAIR expression levels developed local recurrence (28 cases) and/or distant recurrence (10 cases). Using local recurrence as the end‐point and a cut‐off SI ≥ 6 for HOTAIR expression, a survival curve with a median follow‐up period of 69 months demonstrated that patients with low HOTAIR expression had a longer local recurrence‐free survival (LRFS) than those with high HOTAIR expression (P = 0.024; Fig. 2A). Similarly, the distant metastasis‐free survival (DMFS) of patients with low HOTAIR expression was 83.6% at 82 months but only 64.8% in those with high HOTAIR expression (P = 0.008; Fig. 2B). Overall, patients with high HOTAIR expression had worse disease‐free survival (DFS) than those with low HOTAIR expression (P = 0.017; Fig. 2C). More importantly, the high HOTAIR‐expression group had shorter overall survival than the low‐expression group (P = 0.006; Fig. 2D).
Figure 2.
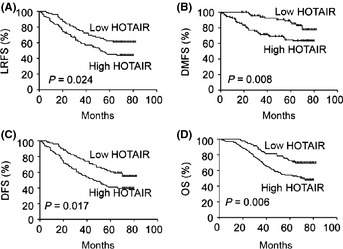
High HOTAIR levels are correlated with poor survival in nasopharyngeal carcinoma (NPC) patients. Kaplan–Meier survival curve of NPC patients with low (staining index [SI] <6) and high HOTAIR (SI ≥ 6) levels, with a median follow‐up period of 69 months. (A) Local recurrence‐free survival (LRFS). (B) Distant metastasis‐free survival (DMFS). (C) Disease‐free survival (DFS). (D) Overall survival (OS).
Using univariate analysis (Cox's proportional hazards model), the variables clinical stage (P < 0.001), lymph node classification (P < 0.001) and HOTAIR expression level (P < 0.01) were found to be significantly associated with prognosis (Table 2). Multivariate analyses were further used to determine whether HOTAIR expression levels were an independent prognostic factor of clinical outcomes. According to our results, clinical stage, lymph node (N) classification and HOTAIR expression levels showed significant prognostic effects on overall survival, independent of various clinical variables including age, gender and histological classification. Thus, our findings indicate that HOTAIR expression levels were significantly correlated with NPC patient prognosis.
Table 2.
Univariate and multivariate analysis of different prognostic variables in patients with nasopharyngeal carcinoma using Cox regression analysis
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| No. patients | P | Regression coefficient (SE) | P | Relative risk (95% CI) | |
| Histological classification | |||||
| WHO type I | 11 | 0.936 | −0.066 (0.199) | 0.990 | 1.003 (0.680–1.478) |
| WHO type II | 26 | ||||
| WHO type III | 123 | ||||
| Clinical stage | |||||
| I–II | 46 | 0.000 | 1.283 (0.358) | 0.004 | 2.631 (1.278–5.417) |
| III–IV | 114 | ||||
| N classification | |||||
| N0 | 26 | 0.000 | 0.897 (0.240) | 0.003 | 2.042 (1.273–3.274) |
| N1–N2 | 115 | ||||
| N3 | 19 | ||||
| HOTAIR | |||||
| SI < 6 | 69 | 0.008 | 0.702 (0.263) | 0.012 | 1.902 (1.132–3.194) |
| SI ≥ 6 | 91 | ||||
CI, confidence interval; N, lymph node; SE, standard error; WHO, World Health Organization.
HOTAIR is an indicator for predicting the prognosis of advanced‐stage NPC patients
Moreover, the prognostic value of HOTAIR expression in selective patient subgroups stratified by clinical stage, tumor size and lymph node status was evaluated. In light of our analysis, there was no difference between high and low HOTAIR‐expression groups in NPC patients with an early stage (Fig. 3A, left) or small tumor (T1–T2; Fig. 3B, left) or without lymph node metastasis (N0 classification; Fig. 3C, left). However, patients with tumors exhibiting high HOTAIR expression had significantly shorter overall survival compared with those with low expression of HOTAIR in the T3–T4 subgroup (n = 77; P = 0.001; Fig. 3B, right) or N1–N2 subgroup (n = 106; P = 0.014; Fig. 3C, middle). There was a trend toward shorter overall survival times of patients with high HOTAIR expression in the clinical stage III–IV (n = 114; P = 0.059; Fig. 3A, right) and N3 subgroups (n = 54; P = 0.144; Fig. 3C, right), although there were no significant differences between patients with low or high HOTAIR expression levels.
Figure 3.
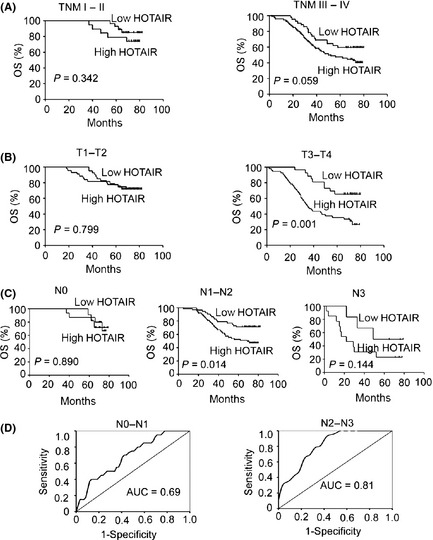
HOTAIR shows better prognostic value in more advanced nasopharyngeal carcinoma (NPC) patients. Kaplan–Meier analysis showing the overall survival (OS) of NPC patients with low (staining index [SI] < 6) and high (SI ≥ 6) HOTAIR levels categorized according to TNM stage (A), tumor size (B) and status of lymph nodes (C). The P‐value was calculated using the log‐rank test. (D) The receiver operating characteristic curves were conducted to evaluate the average value of sensitivity and specificity of HOTAIR for selected NPC patients stratified by lymph node (N) classification. AUC, area under the curve.
Next we constructed ROC curves to evaluate the average sensitivity and specificity of HOTAIR for NPC patients. The ROC curve analysis showed that HOTAIR performed better in the N2–N3 subgroups (area under the curve [AUC] = 0.814; 95% confidence interval [CI], 0.717–0.911; Fig. 3D, right) than the N0–N1 groups (AUC = 0.688; 95% CI, 0.560–0.816; Fig. 3D, left). Similarly, HOTAIR showed slightly better sensitivity and specificity for the III–IV subgroups (AUC = 0.755; 95% CI, 0.668–0.843; Fig. S2B) than the I–II groups (AUC = 0.724; 95% CI, 0.556–0.891; Fig. S2A). Together, these results suggest that HOTAIR might better serve as a prognostic marker for predicting the prognosis of advanced‐stage NPC patients.
HOTAIR mediates the migration and invasion of NPC cells
To further investigate the functional role of HOTAIR in NPC cells, we examined the expression levels of HOTAIR in normal, immortalized NPEC and several paired NPC cell lines with different invasive potentials. The corresponding materials and methods, including cell culture and treatment, boyden chamber assay, cell viability and proliferation are described in detail in the Method S1. Compared with NPEC, the NPC cells had upregulated HOTAIR levels (Fig. 4A). Consistent with increased capacity for invasion, the NPC cell lines with high invasive potential (5–8F, S18 and CNE2 cells) had increased HOTAIR expression levels compared with the paired lines with low invasive potential (6–10B, S26 and CNE1 cells). The HOTAIR expression levels in 5–8F, S18 and CNE2 cells increased 2.7‐fold (P < 0.001), 8.55‐fold (P < 0.001) and 4.47‐fold (P < 0.001), respectively (Fig. 4A).
Figure 4.
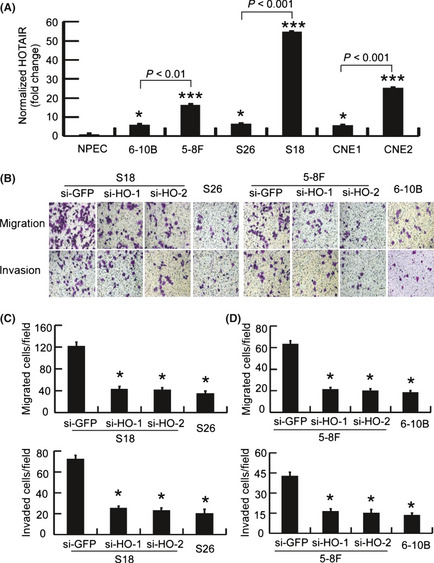
HOTAIR mediates nasopharyngeal carcinoma (NPC) cell migration and invasion. (A) The expression levels of HOTAIR were determined using real‐time PCR in NPEC and paired NPC cell lines with high (5–8F, S18 and CEN2) and low invasive potential (6–10B, S26 and CNE1). *P < 0.05 and ***P < 0.001 compared with nasopharyngeal epithelial cell line (NPEC). (B) Representative images are shown of a Boyden chamber assay for migrated and invaded NPC cells treated with si‐GFP or si‐HOTAIR (si‐HO). (C,D) The number of migrated and invaded NPC cells treated as in (B). *P < 0.01 compared with NPC cells treated with si‐GFP.
Because HOTAIR is upregulated in NPC cells exhibiting high invasion, we further investigated whether HOTAIR mediates NPC cell migration and invasion using Boyden chamber assays with or without Matrigel coating on the inserts. Transfecting S18 and 5–8F cells with siRNA against HOTAIR reduced the expression levels to levels similar to the paired S26 and 6–10B cells (Fig. S3). Transfection with HOTAIR siRNA, but not si‐GFP, decreased the number of migrating S18 cells and 5–8F cells by approximately 63% (P < 0.01; Fig. 4B,C) and 59.4% (P < 0.01; Fig. 4B,D), respectively. In agreement, knocking down HOTAIR expression significantly abrogated invasion in S18 cells by 61% (P < 0.01; Fig. 4B,C) and 5–8F cells by 58% (P < 0.01; Fig. 4B,D). These data suggest that HOTAIR plays a critical role in NPC cell migration and invasion.
It has been reported that HOTAIR mediates proliferation in breast cancer cells.17 Therefore, the effect of HOTAIR on the proliferation of NPC cells was also investigated using cell viability and clonogenic assays. Cancer cell growth was dramatically suppressed after knocking down HOTAIR expression (Fig. S4A,B), indicating that HOTAIR might promote NPC cell proliferation. These data suggest that HOTAIR contributes to NPC development through involvement in diverse cellular processes.
Discussion
Nasopharyngeal carcinoma is one of the most common cancers in southern China and Southeast Asia.1 This remarkable racial and geographic distribution of NPC indicates that the development of this cancer might be related to genetic factors. Identification of new NPC biomarkers will be helpful for the diagnosis and treatment of NPC and might provide new insight into its pathogenesis. In the present study, we found that clinically, high expression levels of HOTAIR, a cancer‐related lncRNA, correlate with NPC progression. HOTAIR is an indicator for predicting the prognosis of advanced‐stage NPC patients. In addition, HOTAIR contributes to the malignant character of NPC cells through involvement in diverse cellular processes, including migration, invasion and proliferation.
Recently, the roles for lncRNA as drivers of tumor suppressive and oncogenic functions have appeared in prevalent cancer types. It has been demonstrated that HOTAIR, a transregulatory lncRNA, is upregulated in breast cancer, hepatocellular carcinoma and colorectal cancer.17, 18, 19 The HOTAIR expression levels assessed were found to be higher in cancerous tissue than in the corresponding non‐cancerous tissue in primary breast tumors17 and colorectal cancers,19 and upregulated HOTAIR has also been detected in hepatocellular carcinoma (HCC) compared with the adjacent non‐tumorous tissue in HCC patients.18, 21 Similarly, the present study showed that higher levels of HOTAIR staining were observed in NPC tissue than in non‐cancerous tissue; furthermore, the expression levels of HOTAIR were upregulated in samples with a higher tumor burden, including larger tumor size, advanced clinical staging and increased tumor burden of lymph nodes, as well as the presence of distant metastases. Therefore, HOTAIR is crucial for the oncogenesis and development of NPC.
Dysregulated expression of lncRNA in cancer marks the spectrum of disease progression22 and might serve as an independent predictor for patient outcome.17 HOTAIR is one of the few biologically well‐studied lncRNA. Previous studies have demonstrated that high HOTAIR expression strongly correlates with the presence of liver metastasis.19 Similarly, overexpression of HOTAIR is linked to earlier recurrence in HCC patients who underwent surgical resection.18 Tumor recurrence and distant metastases are also responsible for poor survival of NPC patients. Here, the present study demonstrated that high expression levels of HOTAIR correlate with a poor LRFS, DMFS, DFS and overall survival in NPC patients. Multivariate analyses revealed that HOTAIR expression levels were a powerful independent prognostic factor of clinical outcomes, suggesting that HOTAIR could be a candidate biomarker for predicting tumor recurrence in NPC patients.
To date, in addition to tumor size and status of lymph nodes, molecular markers are considered important factors in predicting treatment and prognosis. Heterogeneous ribonucleoprotein K and thymidine phosphorylase are therapeutic markers for NPC.23 Epstein‐Barr virus (EBV) is widely used for screening and early diagnosis of NPC.2 Centromere protein H might function as a prognostic marker of NPC at earlier clinical stages.24 Interestingly, the prognostic value of HOTAIR expression was improved for advanced‐stage NPC patients. According to our data, NPC patients with tumors exhibiting high HOTAIR expression had significantly shorter overall survival compared with patients with low expression of HOTAIR in the subgroup with large tumors and high tumor burden in the lymph nodes. The sensitivity and specificity of HOTAIR for NPC patients was slightly improved for the III–IV and N2–N3 subgroups. These results suggest that HOTAIR might serve as a better prognostic marker for predicting the prognosis of advanced‐stage NPC patients, which differs from many biomarkers that are usually used for early stage NPC.
Emerging evidence suggests that lncRNA constitute an important component of tumor biology. Suppression of HOTAIR in liver cancer cells reduces cell viability and invasion, sensitizes TNF‐α‐induced apoptosis and increases chemotherapeutic sensitivity.18 In breast cancer, overexpression of HOTAIR increases lung metastases in mice.17 Upregulation of HOTAIR drives the malignant character of gastrointestinal stromal tumors and promotes cell invasiveness by altering the expression of reported HOTAIR‐targeted genes.25 Similarly, our data demonstrate that HOTAIR is upregulated in NPC cell lines with high invasive potential and the capacity for migration, invasion and proliferation was suppressed after knocking down HOTAIR expression. However, further studies are required to investigate the potential mechanisms of HOTAIR involvement in the development of NPC. The turnover of HOTAIR is not well understood and one study reported that depletion of HOTAIR induced a significant change in the gene expression profile, suggesting that HOTAIR might regulate a spectrum of genes by mechanisms other than directly interacting with histone modification complexes.25
The above findings not only suggest a useful candidate molecular marker for NPC and an indicator for advanced‐stage NPC but also provide new insights into the role of lncRNA in cancer biology. In addition to the prognostic value for NPC, HOTAIR combined with other biomarkers might be useful to stratify patients for individual therapies.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- AJCC
American Joint Committee on Cancer
- AUC
Area under the curve
- CI
Confidence interval
- DFS
Disease‐free survival
- DMFS
Distant metastasis‐free survival
- EBV
Epstein‐barr virus
- HCC
Hepatocellular carcinoma
- LNA
Locked nucleic acid
- LRFS
Local recurrence‐free survival
- MOD
Mean optical density
- NPC
Nasopharyngeal carcinoma
- ROC
Receiver operating characteristic
- SI
Staining index
- WHO
World health organization
Supporting information
Fig. S1. Expression of HOTAIR was quantified using the mean optical density (MOD) in non‐cancerous tissues and nasopharyngeal carcinoma (NPC) tissues of different clinical stages.
Fig. S2. Receiver operating characteristic curves were conducted to evaluate the average value of sensitivity and specificity of HOTAIR for selected nasopharyngeal carcinoma patients stratified by TNM stage.
Fig. S3. Expression of HOTAIR was determined using real‐time PCR in paired NPC cells treated with si‐GFP or si‐HOTAIR.
Fig. S4. Effect of HOTAIR on nasopharyngeal carcinoma cell proliferation.
Table. S1. Clinicopathological characteristics of patient samples and expression of HOTAIR in nasopharyngeal carcinoma.
Methods. S1. Including: cell culture and treatment; Boyden chamber assay; and cell viability and proliferation.
Acknowledgments
This work was supported by grants from 973 (2010CB912800, 2011CB504203) Projects from Ministry of Science and Technology of China, the Natural Science Foundation of China (81230060, 81261140373, 81272893, 81172524), National S&T Major Special Project on New Drug Innovation of China (No. 2011ZX09102‐010‐02) and Science Foundation of Guangdong Province (S2012030006287). Guangdong Province Grants (2012B031800035, 2012J2200092), Sun Yat‐sen University Training Project (11ykpy28, 11ykzd12).
(Cancer Sci 2013; 104: 458–464)
References
- 1. Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol 2002; 12: 421–9. [DOI] [PubMed] [Google Scholar]
- 2. Thompson MP, Kurzrock R. Epstein–Barr virus and cancer. Clin Cancer Res 2004; 10: 803–21. [DOI] [PubMed] [Google Scholar]
- 3. Xiong S, Wang Q, Zheng L, Gao F, Li J. Identification of candidate molecular markers of nasopharyngeal carcinoma by tissue microarray and in situ hybridization. Med Oncol 2011; 28(Suppl. 1): S341–8. [DOI] [PubMed] [Google Scholar]
- 4. Morrison JA, Gulley ML, Pathmanathan R, Raab‐Traub N. Differential signaling pathways are activated in the Epstein–Barr virus‐associated malignancies nasopharyngeal carcinoma and Hodgkin lymphoma. Cancer Res 2004; 64: 5251–60. [DOI] [PubMed] [Google Scholar]
- 5. Chou J, Lin YC, Kim J et al Nasopharyngeal carcinoma–review of the molecular mechanisms of tumorigenesis. Head Neck 2008; 30: 946–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee AW, Poon YF, Foo W et al Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys 1992; 23: 261–70. [DOI] [PubMed] [Google Scholar]
- 7. Vokes EE, Liebowitz DN, Weichselbaum RR. Nasopharyngeal carcinoma. Lancet 1997; 350: 1087–91. [DOI] [PubMed] [Google Scholar]
- 8. Gibb EA, Brown CJ, Lam WL. The functional role of long non‐coding RNA in human carcinomas. Mol Cancer 2011; 10: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huarte M, Rinn JL. Large non‐coding RNAs: missing links in cancer? Hum Mol Genet 2010; 19: R152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prensner JR, Iyer MK, Balbin OA et al Transcriptome sequencing across a prostate cancer cohort identifies PCAT‐1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol 2011; 29: 742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov 2011; 1: 391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matouk IJ, DeGroot N, Mezan S et al The H19 non‐coding RNA is essential for human tumor growth. PLoS One 2007; 2: e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lottin S, Adriaenssens E, Dupressoir T et al Overexpression of an ectopic H19 gene enhances the tumorigenic properties of breast cancer cells. Carcinogenesis 2002; 23: 1885–95. [DOI] [PubMed] [Google Scholar]
- 14. Barsyte‐Lovejoy D, Lau SK, Boutros PC et al The c‐Myc oncogene directly induces the H19 noncoding RNA by allele‐specific binding to potentiate tumorigenesis. Cancer Res 2006; 66: 5330–7. [DOI] [PubMed] [Google Scholar]
- 15. Zhang X, Lian Z, Padden C et al A myelopoiesis‐associated regulatory intergenic noncoding RNA transcript within the human HOXA cluster. Blood 2009; 113: 2526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rinn JL, Kertesz M, Wang JK et al Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 2007; 129: 1311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta RA, Shah N, Wang KC et al Long non‐coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010; 464: 1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Z, Zhou L, Wu LM et al Overexpression of long non‐coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol 2011; 18: 1243–50. [DOI] [PubMed] [Google Scholar]
- 19. Kogo R, Shimamura T, Mimori K et al Long noncoding RNA HOTAIR regulates polycomb‐dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res 2011; 71: 6320–6. [DOI] [PubMed] [Google Scholar]
- 20. Jiang L, Mao P, Song L et al. miR‐182 as a prognostic marker for glioma progression patient survival. Am J Pathol 2010; 177: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geng YJ, Xie SL, Li Q, Ma J, Wang GY. Large intervening non‐coding RNA HOTAIR is associated with hepatocellular carcinoma progression. J Int Med Res 2011; 39: 2119–28. [DOI] [PubMed] [Google Scholar]
- 22. Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non‐coding RNAs and cancer: a new frontier of translational research? Oncogene 2012; 31: 4577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen LC, Hsueh C, Tsang NM et al Heterogeneous ribonucleoprotein k and thymidine phosphorylase are independent prognostic and therapeutic markers for nasopharyngeal carcinoma. Clin Cancer Res 2008; 14: 3807–13. [DOI] [PubMed] [Google Scholar]
- 24. Liao WT, Song LB, Zhang HZ et al Centromere protein H is a novel prognostic marker for nasopharyngeal carcinoma progression and overall patient survival. Clin Cancer Res 2007; 13: 508–14. [DOI] [PubMed] [Google Scholar]
- 25. Niinuma T, Suzuki H, Nojima M et al Upregulation of miR‐196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res 2012; 72: 1126–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Expression of HOTAIR was quantified using the mean optical density (MOD) in non‐cancerous tissues and nasopharyngeal carcinoma (NPC) tissues of different clinical stages.
Fig. S2. Receiver operating characteristic curves were conducted to evaluate the average value of sensitivity and specificity of HOTAIR for selected nasopharyngeal carcinoma patients stratified by TNM stage.
Fig. S3. Expression of HOTAIR was determined using real‐time PCR in paired NPC cells treated with si‐GFP or si‐HOTAIR.
Fig. S4. Effect of HOTAIR on nasopharyngeal carcinoma cell proliferation.
Table. S1. Clinicopathological characteristics of patient samples and expression of HOTAIR in nasopharyngeal carcinoma.
Methods. S1. Including: cell culture and treatment; Boyden chamber assay; and cell viability and proliferation.


