Abstract
This phase I/II study was conducted to evaluate the safety and efficacy of bortezomib‐melphalan‐prednisolone in Japanese patients with previously untreated multiple myeloma who are ineligible for hematopoietic stem cell transplantation. One hundred and one patients were enrolled, and 99 patients received up to nine 6‐week cycles of bortezomib (0.7/1.0/1.3 mg/m2) on days 1, 4, 8, 11, 22, 25, 29, and 32 in cycles 1–4 and on days 1, 8, 22, and 29 in cycles 5–9, with melphalan (9 mg/m2) and prednisolone (60 mg/m2) on days 1–4 of each cycle. The recommended dose was determined in the phase I portion, and the overall response rate and safety of bortezomib‐melphalan‐prednisolone at the recommended dose were assessed in the phase II portion. The recommended dose of bortezomib was determined to be 1.3 mg/m2. Grade 3 or higher non‐hematological adverse events included diarrhea (12%) and peripheral neuropathy (10%); grade 4 hematological adverse events included lymphopenia (41%), neutropenia (30%), and thrombocytopenia (22%). Eleven patients had lung injury associated with bortezomib; two had grade 3 disease, and the other nine had grade 1 or 2 disease. Of the 86 patients treated with 1.3‐mg/m2 bortezomib in phases I and II, the median number of treatment cycles was 4.5, and the overall response rate was 70% (95% confidence interval: 59–79%). Bortezomib‐melphalan‐prednisolone with 1.3‐mg/m2 bortezomib was considered to be tolerable and effective in Japanese patients with previously untreated multiple myeloma. However, further investigation is needed to refine the administration schedule.
High‐dose therapy with autologous hematopoietic stem‐cell transplantation (HDT‐HSCT) has become the preferred treatment for patients with untreated multiple myeloma (MM) who are younger than 65 years old,1, 2, 3 but it is not usually indicated for older patients or patients with clinically significant coexisting diseases. For patients with newly diagnosed MM who are ineligible for HDT‐HSCT, therapy with melphalan plus prednisone (MP) has been the standard of care for more than 30 years.4, 5 A recent study showed that long‐term survival in younger patients with MM has improved significantly over the past few years while only limited improvement has been achieved in elderly patients.6 Therefore, there is a great need for improved treatment options for older patients ineligible for HDT‐HSCT.
Bortezomib, a proteasome inhibitor, was approved in the USA in 2005 for treatment of MM in patients who have received at least one prior therapy; approval was based on results from the phase III Assessment of Proteasome Inhibition for Extending Remissions study, which showed bortezomib's superiority over high‐dose dexamethasone in patients with relapsed MM.7, 8 Thereafter, the international phase III Velcade as Initial Standard Therapy in Multiple Myeloma (VISTA) trial demonstrated that bortezomib plus melphalan and prednisone (VMP) was superior to MP in all efficacy measurements, including time to disease progression, response rates, and overall survival in previously untreated patients with MM ineligible for HDT‐HSCT.9, 10 Based on these results, bortezomib was approved for treatment of patients with newly diagnosed MM in the USA. VMP combination therapy has become a recommended treatment choice for patients with untreated MM who are ineligible for HDT‐HSCT.11, 12
An earlier phase I/II study showed that bortezomib, as a single agent, is tolerable and effective in Japanese patients with relapsed or refractory MM,13 and bortezomib was approved for relapsed or refractory MM in Japan in 2006. However, the efficacy and safety of VMP in Japanese patients with newly diagnosed MM have not yet been systematically investigated. In addition, it has been suggested that Japanese patients might have a higher risk of developing lung disorders, including interstitial lung disease, when treated with bortezomib.13, 14, 15 Here, we conducted a phase I/II study in order to evaluate the efficacy, pharmacokinetics, and safety, especially in terms of the incidence of lung injury, of VMP in Japanese patients with previously untreated MM who are ineligible for HDT‐HSCT.
Materials and Methods
Eligibility
Study patients were required to be 20 years of age or older, have previously untreated measurable MM, and be ineligible for HDT‐HSCT. Measurable disease was defined as the presence of quantifiable monoclonal protein in serum or urine or measurable soft‐tissue plasmacytomas. Patients needed a Karnofsky performance status of 60 or higher and adequate bone marrow, liver, and renal function (neutrophils ≥ 1000/mm3, platelets ≥ 75, 000/mm3, hemoglobin ≥ 8 g/dL, total bilirubin ≤ 1.5 × upper limit of normal, aspartate aminotransferase and alanine aminotransferase ≤ 2.5 × upper limit of normal, and serum creatinine ≤ 2.0 mg/dL). Patients with plasma cell leukemia, smoldering myeloma, or monoclonal gammopathy of undetermined significance were excluded from this study. In order to ensure patient safety, the following patients were also excluded: patients who were clinically diagnosed with pneumonitis or pulmonary fibrosis or who had abnormal interstitial shadows bilaterally on chest computed tomography; patients with poorly controlled infection; patients with left ventricular ejection fraction below 55%; and patients with poorly controlled concurrent disease.
This study was conducted in compliance with the Guideline for Good Clinical Practice and the Declaration of Helsinki, and the study protocol was approved by local institutional review boards. A list of all participating institutions and investigators is listed in the Acknowledgements. All patients provided written informed consent.
Study design
This was a phase I/II, multicenter, dose‐escalating, non‐randomized, open‐label study. To determine a recommended dose (RD) for bortezomib in combination with MP in Japanese patients with newly diagnosed MM, the phase I portion tested three different dose levels: 0.7, 1.0, and 1.3 mg/m2. Dose escalation did not exceed 1.3‐mg/m2, as this is the global RD for bortezomib for VMP therapy.9 Six patients were enrolled in each cohort, and the RD was determined as the maximum dose level at which the incidence of dose‐limiting toxicity (DLT) was lower than 50%. In the phase II portion, the safety and efficacy of bortezomib combined with MP at the RD determined in the phase I portion were assessed.
Dose‐limiting toxicity definition
Dose‐limiting toxicity (DLT) was defined as those toxicities in Cycle 1 that were at least possibly related to bortezomib and that met one of the following criteria: grade 3 or higher peripheral neuropathy or neuropathic pain that persisted for more than 3 weeks after the suspension of bortezomib; grade 3 or higher nausea or vomiting that occurred despite prophylactic treatment with antiemetics; other grade 3 or higher non‐hematological toxicity; grade 4 neutropenia persisting for more than 5 days; grade 4 thrombocytopenia persisting for more than 5 days; and hemoglobin < 6.5 g/dL. Any adverse events that resulted in skipping at least three out of eight doses of bortezomib in Cycle 1 were regarded as DLT. Grade 3 abnormal laboratory findings attributable to tumor lysis syndrome and grade 3 non‐hematological toxicities controllable with supportive therapy were not considered DLT.
Drug administration
Patients received up to nine 6‐week cycles of bortezomib at doses ranging from 0.7 to 1.3 mg/m2 by intravenous bolus on days 1, 4, 8, 11, 22, 25, 29, and 32 during cycles 1–4 and on days 1, 8, 22, and 29 during cycles 5–9. Melphalan (9 mg/m2) and prednisolone (60 mg/m2) were administered on days 1–4 of each cycle.
Efficacy and safety assessment
The primary endpoint of the phase II portion was the best overall response rate after nine cycles of treatment, based on central evaluation by the Efficacy and Safety Evaluation Committee in accordance with the criteria described by the European Group for Blood and Marrow Transplantation (Barcelona, Spain).16 Both an exact 90% confidence interval (CI) and a 95% CI for the overall response rate were calculated. Secondary endpoints were time to response and duration of response, both of which were estimated by the Kaplan–Meier method. These analyses were performed based on the full analysis set, which was defined as all subjects excluding those who did not meet major criteria for enrollment and those who had not received any treatment with the investigational product.
The safety evaluation included analysis of the type and incidence of adverse events. All adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 13.0; toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Because lung injury associated with bortezomib has previously been classified into three groups (interstitial pneumonia, vascular hyperpermeability, and hypoxia),15 we collected data for all adverse events judged to be pneumonitis, lung disorder, interstitial lung disease, hypoxia, oxygen saturation decreased, non‐cardiogenic pulmonary edema, and capillary leak syndrome (excluding hypoxia and oxygen saturation decreased for which other definitive causes were identified). The final judgment on lung disorders was made by the Lung Disorders Third Party Assessment Committee (Tokyo, Japan), which was independent from the study organization, in order to ensure accurate and uniform diagnoses throughout the study.
Pharmacokinetics
Plasma concentrations of bortezomib, melphalan, and prednisolone were determined in patients registered in the phase I portion. Pharmacokinetic (PK) parameters were collected for bortezomib under two different conditions: (i) in combination with MP (on Cycle 2/days 4–7); and (ii) alone (Cycle 1/days 25–28). PK assessments for the individual drugs melphalan and prednisolone in combination with bortezomib were also conducted (Cycle 2/Days 4–5). Plasma concentrations of bortezomib were determined using liquid chromatography‐tandem mass spectrometry, and plasma concentrations of melphalan and prednisolone were determined using high‐performance liquid chromatography.
Results
Patients and protocol treatment
This study was performed between August 2008 and May 2011 at 36 sites in Japan. One hundred and one patients were registered in this study, and 99 of them received at least one dose of the study drug. Patient characteristics are shown in Table 1. More than 90% of patients were 65 years of age or older, and approximately two‐thirds of all patients had a Karnofsky performance status of 90 or higher. All but one patient had secretory‐type myeloma; 65 patients had IgG‐type myeloma (65.7%), 25 had IgA‐type (25.3%), and 12 had Bence Jones‐type (12.1%). Physical examination, hematological examination, and pulse oximeter tests were required on days when bortezomib was administered. Electrocardiogram and serum chemistry tests were done twice a cycle, and chest X‐ray was required at the end of every cycle. In the phase I portion, no DLT was observed at the 0.7‐ and 1.0‐mg/m2 dose levels. One out of six patients in the 1.3‐mg/m2 dose cohort had two adverse events judged to be DLT, grade 3 infectious colitis and grade 3 ileus, from which the patient recovered. Thus, the RD for the subsequent phase II portion was determined to be 1.3 mg/m2, which is also the RD for bortezomib in single‐agent use.13
Table 1.
Patient characteristics
| 0.7 mg/m2 (n = 6) | 1.0 mg/m2 (n = 6) | 1.3 mg/m2 (n = 87) | All (n = 99) | |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Men | 3 (50.0) | 3 (50.0) | 36 (41.4) | 42 (42.4) |
| Women | 3 (50.0) | 3 (50.0) | 51 (58.6) | 57 (57.6) |
| Age (years), n (%) | ||||
| <65 | 1 (16.7) | 1 (16.7) | 3 (3.4) | 5 (5.1) |
| 65–74 | 2 (33.3) | 5 (83.3) | 60 (69.0) | 67 (67.7) |
| 75≦ | 3 (50.0) | 0 (0.0) | 24 (27.6) | 27 (27.3) |
| Median (year) | 75 | 71 | 71 | 72 |
| Range | 57– 82 | 57–74 | 48–84 | 48–84 |
| KPS, n (%) | ||||
| 60–70 | 0 (0.0) | 0 (0.0) | 19 (21.8) | 19 (19.2) |
| 80 | 1 (16.7) | 0 (0.0) | 13 (14.9) | 14 (14.1) |
| 90≦ | 5 (83.3) | 6 (100) | 55 (63.2) | 66 (66.7) |
| Type of myeloma, n (%) | ||||
| IgG | 4 (66.7) | 3 (50.0) | 58 (66.7) | 65 (65.7) |
| IgA | 2 (33.3) | 2 (33.3) | 21 (24.1) | 25 (25.3) |
| IgD | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bence Jones | 0 (0.0) | 1 (16.7) | 11 (12.6) | 12 (12.1) |
| Non‐secretory | 0 (0.0) | 0 (0.0) | 1 (1.1) | 1 (1.0) |
| ISS stage, n (%) | ||||
| I | 1 (16.7) | 4 (66.7) | 16 (18.4) | 21 (21.2) |
| II | 3 (50.0) | 2 (33.3) | 47 (54.0) | 52 (52.5) |
| III | 2 (33.3) | 0 (0.0) | 24 (27.6) | 26 (26.3) |
ISS, international staging system; KPS, Karnofsky performance status.
A total of 87 patients, including six patients in the phase I portion, received VMP therapy with bortezomib at 1.3 mg/m2; these 87 patients received a median of five treatment cycles at the 1.3‐mg/m2 dose level. Excluding one patient who did not meet the eligibility criteria, the median number of treatment cycles in the remaining 86 patients who received the 1.3‐mg/m2 dose was 4.5. Treatment continuity was compared with the global phase III VISTA study,9, 10 and data for the number of patients who entered each cycle in the VISTA study and in the 1.3‐mg/m2 cohort of the current study are shown in Table 2. Overall, there were fewer patients who started the next treatment cycle in the current study than in the VISTA study.
Table 2.
Number of patients who entered each cycle and comparison with the VISTA study
| Cycle no. | 1.3 mg/m2 dose level in the current study (n = 87) n (%) | The global VISTA study (n = 340) n (%) |
|---|---|---|
| 1 | 87 (100) | 340 (100) |
| 2 | 73 (84) | 305 (90) |
| 3 | 63 (72) | 284 (83) |
| 4 | 50 (57) | 266 (78) |
| 5 | 44 (51) | 249 (73) |
| 6 | 39 (45) | 236 (69) |
| 7 | 37 (43) | 224 (65) |
| 8 | 33 (38) | 213 (62) |
| 9 | 28 (32) | 202 (59) |
VISTA, Velcade as Initial Standard Therapy in Multiple Myeloma.
Efficacy
Ninety‐eight patients were evaluable for efficacy; this excludes one patient who did not meet the eligibility criteria. Response was observed in 71 out of 98 evaluable patients (72%; 95% CI: 63–81%) and in 60 out of 86 patients at the 1.3‐mg/m2 dose level, including patients from the phase I portion (70%; 95% CI: 59–79%). Details regarding responses to treatment are shown in Table 3. Median time to first response at the 1.3‐mg/m2 dose level was 51 days (95% CI: 43–82 days), and median duration of response was not reached (median follow‐up: 251.5 days).
Table 3.
Best response to treatment
| Response | 0.7 mg/m2 (n = 6) n (%) | 1.0 mg/m2 (n = 6) n (%) | 1.3 mg/m2 (n = 86) n (%) | All (n = 98) n (%) |
|---|---|---|---|---|
| CR | 0 (0.0) | 3 (50.0) | 17 (19.8) | 20 (20.4) |
| PR | 6 (100.0) | 2 (33.3) | 43 (50.0) | 51 (52.0) |
| MR | 0 (0.0) | 1 (16.7) | 7 (8.1) | 8 (8.2) |
| NC | 0 (0.0) | 0 (0.0) | 14 (16.3) | 14 (14.3) |
| PD | 0 (0.0) | 0 (0.0) | 3 (3.5) | 3 (3.1) |
| NE | 0 (0.0) | 0 (0.0) | 2 (2.3) | 2 (2.0) |
| RR | 6 (100.0) | 5 (83.3) | 60 (69.8) | 71 (72.4) |
| 90% CI for RR | 60.7–100.0% | 41.8–99.1% | 60.6–77.9% | 64.1–79.8% |
| 95% CI for RR | 54.1–100.0% | 35.9–99.6% | 58.9–79.2% | 62.5–81.0% |
CI, confidence interval; CR, complete response; MR, minimal response; NC, no change; NE, not evaluable; PD, progressive disease; PR, partial response; RR, response rate.
The response rate by treatment cycle is shown in Table 4. Among the 60 subjects treated at 1.3‐mg/m2 bortezomib who achieved response to therapy, 45 subjects (75.0%) achieved first response during Cycle 1. Best response was achieved within four cycles in 52 subjects, with the remaining eight subjects achieving best response after four cycles of treatment. These 8 subjects achieved PR within 4 cycles of treatment but, notably, went on to achieve CR after 4 cycles. These data suggest that the majority of subjects who achieved response did so within four cycles of treatment, although there also appeared to be a benefit to continuing treatment as long as possible.
Table 4.
Response rate by treatment cycle in patients receiving 1.3‐mg/m2 bortezomib
| Cycle | First response (n = 60)a | Best response (n = 60)a | ||||
|---|---|---|---|---|---|---|
| CR + PR (%) | CR (%) | PR (%) | CR + PR (%) | CR (%) | PR (%) | |
| 1 | 45 (75.0) | 0 (0.0) | 45 (75.0) | 33 (55.0) | 0 (0.0) | 33 (55.0) |
| ≤ 4 | 60 (100.0) | 0 (0.0) | 60 (100.0) | 52 (86.7) | 9 (15.0) | 43 (71.7) |
| > 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (13.3) | 8 (13.3) | 0 (0.0) |
Includes subjects enrolled in phase I. CR, complete response; PR, partial response.
Adverse events
The safety analysis dataset consisted of all 99 patients who received at least one dose of the study drug. All 99 patients encountered at least one adverse event, and 98 patients had at least one grade 3 or higher adverse event. Adverse events observed in ≥ 20% of patients are shown in Table 5. The most commonly observed adverse events were hematological toxicities, including leukopenia, neutropenia, thrombocytopenia, and anemia. Peripheral neuropathy, diarrhea, nausea, and anorexia were also commonly seen.
Table 5.
Adverse eventsa (safety population: n = 99)
| Dose (mg/m2) Patients Grade | 0.7 (n = 6) | 1 (n = 6) | 1.3 (n = 87) | All (n = 99) | Total | % | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | 1/2 | 3/4 | |||
| Blood and lymphatic system disorders | ||||||||||
| Leukopenia | 3 | 3 | 2 | 4 | 21 | 65 | 26 | 72 | 98 | 99 |
| Lymphopenia | 0 | 6 | 1 | 5 | 4 | 82 | 5 | 93 | 98 | 99 |
| Thrombocytopenia | 3 | 3 | 5 | 1 | 37 | 49 | 45 | 53 | 98 | 99 |
| Neutropenia | 2 | 4 | 0 | 6 | 17 | 67 | 19 | 77 | 96 | 97 |
| Anemia | 0 | 2 | 3 | 1 | 26 | 35 | 29 | 38 | 67 | 68 |
| Leukocytosis | 3 | 0 | 2 | 0 | 44 | 0 | 49 | 0 | 49 | 50 |
| Metabolism and nutrition disorders | ||||||||||
| Decreased appetite | 5 | 0 | 2 | 0 | 43 | 7 | 50 | 7 | 57 | 58 |
| Hyponatremia | 3 | 0 | 3 | 0 | 30 | 15 | 36 | 15 | 51 | 52 |
| Hyperglycemia | 1 | 0 | 2 | 0 | 39 | 4 | 42 | 4 | 46 | 47 |
| Hypokalemia | 1 | 0 | 0 | 0 | 33 | 9 | 34 | 9 | 43 | 43 |
| Hypophosphatemia | 0 | 1 | 1 | 1 | 22 | 18 | 23 | 20 | 43 | 43 |
| Hyperkalemia | 2 | 0 | 2 | 0 | 28 | 6 | 32 | 6 | 38 | 38 |
| Hypoalbuminemia | 3 | 0 | 4 | 0 | 31 | 0 | 38 | 0 | 38 | 38 |
| Hypocalcemia | 0 | 0 | 1 | 0 | 33 | 2 | 34 | 2 | 36 | 36 |
| Hyperchloremia | 0 | 0 | 0 | 0 | 21 | 0 | 21 | 0 | 21 | 21 |
| Psychiatric disorders | ||||||||||
| Insomnia | 1 | 0 | 2 | 0 | 23 | 0 | 26 | 0 | 26 | 26 |
| Nervous system disorders | ||||||||||
| Peripheral neuropathyb | 3 | 0 | 2 | 1 | 50 | 9 | 55 | 10 | 65 | 66 |
| Gastrointestinal disorders | ||||||||||
| Diarrhea | 2 | 0 | 2 | 1 | 47 | 11 | 51 | 12 | 63 | 64 |
| Constipation | 4 | 0 | 2 | 0 | 44 | 2 | 50 | 2 | 52 | 53 |
| Nausea | 1 | 0 | 2 | 0 | 46 | 3 | 49 | 3 | 52 | 53 |
| Vomiting | 0 | 0 | 1 | 0 | 34 | 2 | 35 | 2 | 37 | 37 |
| Stomatitis | 2 | 0 | 2 | 0 | 18 | 0 | 22 | 0 | 22 | 22 |
| Hepatobiliary disorders | ||||||||||
| Hepatic function abnormal | 3 | 0 | 1 | 0 | 38 | 6 | 42 | 6 | 48 | 49 |
| Skin and subcutaneous tissue disorders | ||||||||||
| Rash | 2 | 0 | 4 | 0 | 53 | 5 | 59 | 5 | 64 | 65 |
| General disorders and administration site conditions | ||||||||||
| Malaise | 2 | 0 | 3 | 0 | 36 | 1 | 41 | 1 | 42 | 42 |
| Pyrexia | 2 | 0 | 2 | 0 | 30 | 0 | 34 | 0 | 34 | 34 |
| Fatigue | 0 | 0 | 1 | 0 | 21 | 2 | 22 | 2 | 24 | 24 |
| Edema | 1 | 0 | 1 | 0 | 19 | 0 | 21 | 0 | 21 | 21 |
| Investigations | ||||||||||
| C‐reactive protein increased | 2 | 0 | 3 | 0 | 56 | 1 | 61 | 1 | 62 | 63 |
| Blood lactate dehydrogenase increased | 0 | 0 | 4 | 0 | 49 | 1 | 53 | 1 | 54 | 55 |
| Weight decreased | 2 | 0 | 1 | 0 | 45 | 4 | 48 | 4 | 52 | 53 |
| Blood alkaline phosphatase increased | 4 | 0 | 1 | 0 | 37 | 3 | 42 | 3 | 45 | 46 |
| Alanine aminotransferase increased | 0 | 0 | 1 | 0 | 27 | 0 | 28 | 0 | 28 | 28 |
| Aspartate aminotransferase increased | 0 | 0 | 2 | 0 | 21 | 1 | 23 | 1 | 24 | 24 |
| Weight increased | 1 | 0 | 1 | 0 | 20 | 0 | 22 | 0 | 22 | 22 |
Listed adverse events were reported in at least 20% of patients.
Includes peripheral sensory neuropathy and peripheral motor neuropathy.
The most commonly observed grade 3 or higher non‐hematological adverse events were hypophosphatemia (20%), hyponatremia (15%), diarrhea (12%), and peripheral neuropathy (10%). Peripheral neuropathy was the most common cause of discontinuation of study treatment. Grade 4 hematological adverse events included lymphopenia (41%), neutropenia (30%), and thrombocytopenia (22%). Seventeen patients (17%) received platelet transfusion. Neutropenic infection was seen in 11 patients (11%), and febrile neutropenia in one patient (1%). Seven patients developed herpes zoster, including one patient who developed disseminated herpes zoster. The incidence of herpes zoster in patients who received prophylaxis with oral antiviral agents (7%, 5 out of 70 patients) was similar to that in patients who did not receive prophylaxis (7%, 2 out of 29 patients). However, no patients who received prophylaxis developed disseminated herpes zoster, while one patient who did not receive prophylaxis developed disseminated herpes zoster.
The Lung Disorders Third Party Assessment Committee confirmed that 11 patients had lung injuries that were at least possibly related to bortezomib; characteristics of these patients are shown in Table 6. The majority of these events was grade 1 or 2 and did not require any treatment. Onset was generally within the first or second cycle of treatment, although there were events reported as late as Cycle 4. Two out of the 11 patients experienced grade 3 lung disorders, for which they received steroid pulse or oxygen administration. Most patients recovered from these adverse events, but recovery was not reported in 4 of the 11 patients. Baseline variability in Karnofsky performance status was low as a result of the entry criteria, and only one patient who developed interstitial lung disease had a prior history of lung disease. It is of note, however, that nearly one‐third of the patients who experienced lung injury had a prior history of smoking, and screening levels of Krebs von den Lungen‐6 were also elevated in many of the subjects who were diagnosed with lung disorders associated with bortezomib.
Table 6.
Characteristics of patients diagnosed with lung disorders associated with bortezomib
| Gender | Age (years) | Type of myeloma | M protein (g/dL) | ISS stage | KPS | Plasma cell (%) | Smoking | Past history of lung disease | KL‐6 | Dose level (mg/m2) | Diagnosis of adverse events by investigators | Diagnosis of adverse events by the committee | Grade | Cycle | Study drugs | Treatment for lung injury | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Woman | 70s | IgG‐λ | 6.6 | II | 100 | 25.4 | No | None | 148 | 0.7 | Pneumonitis suspected | ILD | 1 | 4 | Discontinued | No | Not recovered |
| Man | 60s | IgG‐κ | 2.8 | III | 100 | 54 | Yes | None | 180 | 0.7 | Interstitial pneumonitis | ILD | 2 | 3 | Discontinued | No | Recovered |
| Woman | 70s | IgG‐κ | 4.4 | II | 90 | 41.8 | No | None | 207 | 1.3 | SpO2 decreased | Oxygen saturation decreased | 1 | 2 | Continued | No | Recovered |
| Man | 70s | IgG‐κ | 4.1 | I | 80 | 17.5 | Yes | Effort dyspnea | 229 | 1.3 | Hypoxia | ILD | 1 | 1 | Continued | No | Recovered |
| Man | 70s | IgG‐κ | 5.4 | I | 90 | 34.4 | Yes | None | 169 | 1.3 | Hypoxia | Hypoxia | 2 | 1 | Discontinued | No | Recovered |
| Woman | 70s | IgA‐κ | 0.8 | II | 100 | 50a | No | None | 310 | 1.3 | Interstitial pneumonitis | ILD | 1 | 3 | Discontinued | No | Not recovered |
| Woman | 60s | IgA‐κ | 3.4 | II | 80 | 20.4 | No | None | 68 | 1.3 | Pneumonitis suspected | Hypoxia | 3 | 1 | Dose reduction after transient cessation | Steroid pulse | Recovered |
| Pneumonitis suspected | Hypoxia | 3 | 2 | Dose reduction after transient cessation | Steroid pulse | Recovered | |||||||||||
| Hypoxia | Hypoxia | 2 | 2 | Continued | No | Recovered | |||||||||||
| Woman | 60s | IgG‐κ | 6.8 | II | 90 | 12.5 | No | None | 199 | 1.3 | Hypoxia | Hypoxia | 3 | 1 | Transient cessation | Oxygen administration | Recovered |
| Woman | 70s | BJP | 6.2 g/day | III | 90 | 30 | No | None | 151 | 1.3 | Pneumonitis | ILD | 1 | 1 | Continued | No | Not recovered |
| Woman | 80s | IgG‐λ | 3.8 | II | 90 | 14.2 | No | None | 311 | 1.3 | Hypoxia | ILD | 1 | 1 | Transient cessation | No | Recovered |
| Woman | 80s | IgG‐λ/BJP | 6.6 | II | 90 | 41.8 | No | None | 398 | 1.3 | Pneumonitis suspected | ILD | 1 | 2 | Continued | No | Not recovered |
Indicates data from bone marrow biopsy (other data from bone marrow aspiration). BJP, Bence Jones protein; ILD, interstitial lung disease; ISS, international staging system; KL‐6, Krebs von den Lungen‐6; KPS, Karnofsky performance status; M protein, monoclonal protein.
Pharmacokinetics
Pharmacokinetic analyses were conducted in a total of 16 patients in the phase I portion. Their mean plasma bortezomib concentration–time profiles with and without MP are shown in Figure 1. The PK parameters for bortezomib, using non‐compartmental analysis, are shown in Table 7, and the PK parameters for melphalan and prednisolone are presented in Table 8.
Figure 1.
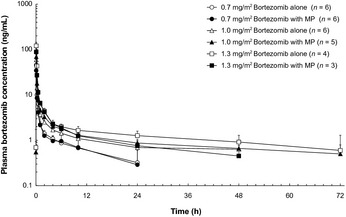
Plasma bortezomib concentrations with and without melphalan plus prednisone (MP) (mean ± SD). [Correction added on 18 July 2013, after first online publication: Concentrations of plasma bortezomib should not be extended to 0.1 ng/mL at 48 h and 72 h in the first online publication. The error is rectified in this version of the article.]
Table 7.
Pharmacokinetic parameters of bortezomib administered with or without melphalan and prednisolone
| PK parameter | Administration | 0.7 mg/m2 (n = 6) | 1.0 mg/m2 (n = 5–6)a | 1.3 mg/m2 (n = 3–4)b | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| C 0.08h (ng/mL) | Bortezomib alone | 45.43 | 10.090 | 59.42 | 18.89 | 120.30 | 24.53 |
| Bortezomib with MP | 34.40 | 5.799 | 69.50 | 19.46 | 88.87 | 19.57 | |
| AUC last (ng·h/mL) | Bortezomib alone | 28.82 | 14.640 | 62.56 | 24.80 | 115.00 | 28.67 |
| Bortezomib with MP | 26.69 | 12.870 | 82.77 | 13.83 | 75.59 | 20.43 | |
n = 6 for bortezomib alone; n = 5 for VMP.
n = 4 for bortezomib alone; n = 3 for VMP.
AUC last, area under the plasma bortezomib concentration–time curve from time zero to the last quantifiable time; C 0.08h, plasma bortezomib concentration at 0.08 h after administration; PK, pharmacokinetic; VMP, bortezomib‐melphalan‐prednisolone.
Table 8.
Pharmacokinetic parameters of melphalan and prednisolone administered as part of VMP therapy
| PK parameter | Melphalan (n = 11) | Prednisolone (n = 14) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| C max (ng/mL) | 100.200 | 49.5150 | 1131.000 | 223.9200 |
| t max (h) | 1.513 | 0.6049 | 2.503 | 1.2429 |
| AUC last (ng·h/mL) | 185.600 | 84.9200 | 7643.700 | 2304.7000 |
AUC last, area under the plasma concentration‐time curve from time zero to the last quantifiable time; C max, maximum plasma concentration; PK, pharmacokinetic; t max, time to reach the maximum plasma concentration.
The maximum plasma concentration for bortezomib was observed 0.08 h after administration at all three dose levels, both when bortezomib was administered in combination with MP and alone. Although the study population from which these results were obtained was small, the PK of bortezomib did not appear to be markedly different when given alone or with MP. Thus, administration in combination with MP did not appear to significantly alter the PK of bortezomib.
Discussion
This phase I/II multicenter study was conducted to evaluate the efficacy and safety of VMP therapy in Japanese patients with newly diagnosed MM who are ineligible for HDT‐HSCT. Based on the incidence of DLT in the phase I portion, the RD of bortezomib in VMP for the subsequent phase II portion was determined to be 1.3 mg/m2, which was the same dose adopted in the global VISTA study.9, 10 A maximum tolerated dose was not reached. Our current study included PK analyses in the phase I portion and showed that there were no apparent differences between PK parameters for bortezomib with or without MP, which suggests that melphalan and prednisolone have only limited impact on the PK of bortezomib. These PK parameters were also similar to those seen in the global VISTA study, suggesting that the PK profile of bortezomib is similar across patient backgrounds (Japanese versus non‐Japanese).
Hematological toxicities, gastrointestinal toxicities, and peripheral neuropathies were the most commonly observed adverse events in our study, and these events were also frequently seen in the global VISTA study.9, 10 The frequencies of these adverse events seem to be higher in our study than in the VISTA study: 97% vs 49% for neutropenia, 99% vs 52% for thrombocytopenia, and 64% vs 46% for diarrhea. These differences in frequencies, however, might be explained partially by the differences in data collection methods between the two studies. In our study, all adverse events were counted and listed while in the VISTA study only data from adverse events requiring countermeasures were collected. In addition, there were no apparent differences between our study and the VISTA study in terms of the frequency of adverse events that led to discontinuation of the study: 39% (34/87 patients at the 1.3‐mg/m2 dose level) in this study versus 32% (108/340 patients) in the VISTA study. In our study, the most common adverse event that led to discontinuation of the study was peripheral neuropathy, and only three patients discontinued the study because of hematological toxicities, which suggests that those hematological toxicities were clinically manageable in most patients. Other toxicities that resulted in discontinuation in two or more subjects include neutropenia, renal impairment, and weight decreased, which can be expected in this population, to a certain extent, and as a result of bortezomib administration.
Hematological toxicities are commonly observed with both MP and bortezomib. With respect to the dose of MP, neutropenia resulting in dose reductions of MP occurred in 5.1% of all subjects (n = 5), thrombocytopenia resulting in dose reductions of MP occurred in only 2.0% (n = 2), and febrile neutropenia and leukopenia resulting in dose reductions of MP each occurred in 1.0% (n = 1). Therefore, we conclude that these data show that the dosage of MP was suitable and that the adverse events arising from treatment with MP were generally manageable. The incidences of hematological toxicities resulting in dose reductions of bortezomib were 8.1% (n = 8) for neutropenia, 2.0% (n = 2) for thrombocytopenia, and 1.0% (n = 1) for febrile neutropenia and for leukopenia. In order to manage these hematological toxicities that are common to both MP and bortezomib, it is important to consider the condition of an individual patient and to adjust the dose of bortezomib, melphalan, and prednisolone in such a way as to balance risk and benefit.
It has also been suggested that Japanese patients have a higher risk for lung disorders when treated with bortezomib.13, 14, 15 The incidence of lung injury associated with bortezomib in Japanese patients has been reported to be 2.3%;15 in the current study, the Lung Disorders Third Party Assessment Committee confirmed 11 lung injury cases in 99 patients (11%). The high incidence of lung disorders in our study may suggest higher risk for lung disorders in Japanese patients treated with VMP; however, it could also be possible that this study detected more patients with asymptomatic lung disorders by frequent computed tomography and pulse oximeter examinations done at least once a cycle. In either case, it is highly recommended that special attention be given to this potentially life‐threatening disorder before and during VMP therapy.
The response rate of 70% in the present study is considered comparable to the response rate of 71% observed in the VISTA study,9, 10 which suggests that VMP therapy would be equally effective in Japanese patients with newly diagnosed MM as in non‐Japanese patients. However, the CR rate of 20% is lower than the 30% rate reported in the VISTA study. One plausible explanation for this difference might be that the median number of treatment cycles in the current study was five; this may not have been long enough when compared with the VISTA study's nine cycles (Table 2). Longer treatment duration may be important, as suggested by the fact that the median number of cycles to achieve CR was four while partial response was obtained after a median of one cycle in the current study. This observation is supported by the results from the VISTA study, in which 29 of the 102 patients who achieved CR did so after the start of Cycle 5,17 and the results from the Gruppo Italiano Malattie EMatologiche dell'Adulto‐03‐05 study, in which an increase in the CR rate was reported with prolonged treatment.18
In this study, the median number of treatment cycles was five, and in the first four cycles, the median dose intensity for bortezomib in the 1.3‐mg/m2 cohort was 6.86 mg/m2/cycle; in the VISTA study, these numbers for the VMP group were nine cycles and 8.32 mg/m2/cycle, respectively. When interpreting these results, it is important to remember that patients in the VISTA study were able to continue the study even if they did not receive bortezomib in one cycle. However, these results suggest that, in some Japanese patients, the 1.3‐mg/m2 dose of bortezomib might be high or the bi‐weekly schedule might need to be changed to once weekly. Recently, two large‐scale clinical studies have suggested that less intensive bortezomib therapy, including a once weekly bortezomib regimen, could reduce the risk of developing peripheral neuropathy but produce equally effective clinical outcomes.18, 19, 20 As peripheral neuropathy was the most common adverse event that resulted in discontinuation of treatment in this study, it is possible that treatment continuity may improve if bortezomib is administered once weekly. Recent recommendations for dose adjustments of bortezomib, such as those from the European Myeloma Network, which are based primarily on clinical experience with insights from clinical trials, also serve as an important reference when considering how to modify the dose and regimen based on a patient's condition and level of frailty. Subcutaneous injection of bortezomib may also be considered because it may help with managing bortezomib‐induced peripheral neuropathy.21
In conclusion, the phase I portion of the present study showed that VMP therapy was tolerable, and the phase II portion showed VMP to be effective in Japanese patients with previously untreated MM who are ineligible for HDT‐HSCT. In Japan, particular attention should be paid to the possible development of lung disorders. We acknowledge that there may be room to modify the dose and administration schedule of VMP in order to ensure that patients can continue on therapy as long as possible, as the benefit to treatment continuation has been shown in both this study and the VISTA study. However, as the dose and regimen in this study have been shown to be efficacious in this population of newly diagnosed patients that lacks effective treatment options, further refinement of the VMP regimen should be rigorously tested through carefully designed and conducted clinical studies.
Disclosure Statement
K. Suzuki received honoraria from Janssen Pharmaceutical K. K. and Celgene (Tokyo, Japan). S. Iida received honoraria from Janssen Pharmaceutical K. K. and research funding from Kyowa Hakko Kirin (Tokyo, Japan), Chugai (Tokyo, Japan), Novartis (Tokyo, Japan), Celgene, and Janssen. M. Ishii and H. Y. Mukai are employees of Janssen Pharmaceutical K. K. Y. Ogawa, A. Sakai, M. Ogura, K. Tobinai, M. Matsumoto, K. Matsue, Y. Terui, K. Ohashi, K. Ando, and T. Hotta have no conflicts of interest to declare.
Acknowledgments
We thank all of the patients who participated in this study and their families, as well as all investigators, physicians, nurses, and clinical research coordinators who helped with this study. We give special recognition to Dr H Murakami (Gunma University Faculty of Medicine, Maebashi, Japan), Dr K Shimizu (Midori Municipal Hospital, Nagoya, Japan), and Dr N Usui (The Jikei University School of Medicine, Tokyo, Japan) for their strict review of the clinical data as members of the Efficacy and Safety Evaluation Committee. This study was supported by Janssen Pharmaceutical K.K (Tokyo, Japan).
The following investigators participated in this study: Tadao Ishida, Sapporo Medical University School of Medicine (Sapporo, Japan); Takaaki Chou, Niigata Cancer Center Hospital (Niigata, Japan); Takashi Yoshida, Toyama Prefectural Central Hospital (Toyama, Japan); Shinji Nakao, Kanazawa University Hospital (Kanazawa, Japan); Atsushi Shinagawa, Hitachi General Hospital (Hitachi, Japan); Tohru Izumi, Tochigi Cancer Center (Utsunomiya, Japan); Morio Matsumoto, National Hospital Organization Nishigunma National Hospital (Shibukawa, Japan); Masahiro Kizaki, Saitama Medical University (Kawagoe, Japan); Nobuyuki Aotsuka, Japanese Red Cross Narita Hospital (Narita, Japan); Kazuteru Ohashi, Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital (Tokyo, Japan); Kensei Tobinai, National Cancer Center Hospital (Tokyo, Japan); Shuichi Taniguchi, Toranomon Hospital (Tokyo, Japan); Kenshi Suzuki, Japanese Red Cross Medical Center (Tokyo, Japan); Yasuhito Terui, Cancer Institute Hospital of the Japanese Foundation for Cancer Research (Tokyo, Japan); Kensuke Usuki, NTT Kanto Medical Center (Tokyo, Japan); Shin Fujisawa, Yokohama City University Medical Center (Yokohama, Japan); Kiyoshi Ando, Tokai University School of Medicine (Isehara, Japan); Kosei Matsue, Kameda Medical Center (Kamogawa, Japan); Kazuhito Yamamoto, Aichi Cancer Center Hospital (Nagoya, Japan); Tomohiro Kinoshita, Nagoya University Graduate School of Medicine (Nagoya, Japan); Michinori Ogura, Nagoya Daini Red Cross Hospital (Nagoya, Japan); Shinsuke Iida, Nagoya City University Graduate School of Medical Sciences (Nagoya, Japan); Isamu Sugiura, Toyohashi Municipal Hospital (Toyohashi, Japan); Masafumi Taniwaki, Kyoto Prefectural University of Medicine (Kyoto, Japan); Hirohiko Shibayama, Osaka University Hospital (Osaka, Japan); Yoichi Tatsumi, Kinki University Faculty of Medicine (Osakasayama, Japan); Takayuki Takahashi, Kobe City Medical Center General Hospital (Kobe, Japan); Kazutaka Sunami, National Hospital Organization Okayama Medical Center (Okayama, Japan); Yasunori Ueda, Kurashiki Central Hospital (Kurashiki, Japan); Akira Sakai, Hiroshima University (Hiroshima, Japan); Masahiro Abe, University of Tokushima Graduate School of Medical Sciences (Tokushima, Japan); Toshihiro Miyamoto, Kyushu University Graduate School of Medicine (Fukuoka, Japan); Naokuni Uike, National Hospital Organization Kyushu Cancer Center (Fukuoka, Japan); Takashi Okamura, Kurume University School of Medicine (Kurume, Japan); Hiroyuki Hata, Kumamoto University Hospital (Kumamoto, Japan); Tatsuro Joh, Japanese Red Cross Nagasaki Genbaku Hospital (Nagasaki, Japan).
(Cancer Sci 2013; 104: 912–919)
References
- 1. Child JA, Morgan GJ, Davies FE et al High‐dose chemotherapy with hematopoietic stem‐cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–83. [DOI] [PubMed] [Google Scholar]
- 2. Barlogie B, Jagannath S, Vesole DH et al Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood 1997; 89: 789–93. [PubMed] [Google Scholar]
- 3. Attal M, Harousseau JL, Stoppa AM et al A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med 1996; 335: 91–7. [DOI] [PubMed] [Google Scholar]
- 4. Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med 2004; 351: 1860–73. [DOI] [PubMed] [Google Scholar]
- 5. Myeloma Trialists' Collaborative Group . Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6633 patients from 27 randomized trials. Myeloma Trialists' Collaborative Group. J Clin Oncol 1998; 16: 3832–42. [DOI] [PubMed] [Google Scholar]
- 6. Brenner H, Gondos A, Pulte D. Recent major improvement in long‐term survival of younger patients with multiple myeloma. Blood 2008; 111: 2521–6. [DOI] [PubMed] [Google Scholar]
- 7. Richardson PG, Sonneveld P, Schuster M et al Extended follow‐up of a phase 3 trial in relapsed multiple myeloma: final time‐to‐event results of the APEX trial. Blood 2007; 110: 3557–60. [DOI] [PubMed] [Google Scholar]
- 8. Richardson PG, Sonneveld P, Schuster MW et al Bortezomib or high‐dose dexamethasone for relapsed multiple myeloma. N Engl J Med 2005; 352: 2487–98. [DOI] [PubMed] [Google Scholar]
- 9. San Miguel JF, Schlag R, Khuageva NK et al Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008; 359: 906–17. [DOI] [PubMed] [Google Scholar]
- 10. Mateos MV, Richardson PG, Schlag R et al Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow‐up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol 2010; 28: 2259–66. [DOI] [PubMed] [Google Scholar]
- 11. Bird JM, Owen RG, D'Sa S et al Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol 2011; 154: 32–75. [DOI] [PubMed] [Google Scholar]
- 12. Rajkumar SV. Multiple myeloma: 2011 update on diagnosis, risk‐stratification, and management. Am J Hematol 2011; 86: 57–65. [DOI] [PubMed] [Google Scholar]
- 13. Ogawa Y, Tobinai K, Ogura M et al Phase I and II pharmacokinetic and pharmacodynamic study of the proteasome inhibitor bortezomib in Japanese patients with relapsed or refractory multiple myeloma. Cancer Sci 2008; 99: 140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gotoh A, Ohyashiki K, Oshimi K et al Lung injury associated with bortezomib therapy in relapsed/refractory multiple myeloma in Japan: a questionnaire‐based report from the “lung injury by bortezomib” joint committee of the Japanese society of hematology and the Japanese society of clinical hematology. Int J Hematol 2006; 84: 406–12. [DOI] [PubMed] [Google Scholar]
- 15. Mukai H, Ohyashiki K, Katoh T et al Lung injury associated with bortezomib therapy in Japan. Rinsho Ketsueki 2011; 52: 1859–69. [PubMed] [Google Scholar]
- 16. Blade J, Samson D, Reece D et al Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high‐dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol 1998; 102: 1115–23. [DOI] [PubMed] [Google Scholar]
- 17. Harousseau JL, Palumbo A, Richardson PG et al Superior outcomes associated with complete response in newly diagnosed multiple myeloma patients treated with nonintensive therapy: analysis of the phase 3 VISTA study of bortezomib plus melphalan‐prednisone versus melphalan‐prednisone. Blood 2010; 116: 3743–50. [DOI] [PubMed] [Google Scholar]
- 18. Palumbo A, Bringhen S, Rossi D et al Bortezomib‐melphalan‐prednisone‐thalidomide followed by maintenance with bortezomib‐thalidomide compared with bortezomib‐melphalan‐prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol 2010; 28: 5101–9. [DOI] [PubMed] [Google Scholar]
- 19. Mateos MV, Oriol A, Martinez‐Lopez J et al Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol 2010; 11: 934–41. [DOI] [PubMed] [Google Scholar]
- 20. Bringhen S, Larocca A, Rossi D et al Efficacy and safety of once‐weekly bortezomib in multiple myeloma patients. Blood 2010; 116: 4745–53. [DOI] [PubMed] [Google Scholar]
- 21. Moreau P, Pylypenko H, Grosicki S et al Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non‐inferiority study. Lancet Oncol 2011; 12: 431–40. [DOI] [PubMed] [Google Scholar]


