Abstract
The powerful activating receptor NKG2D is expressed by natural killer (NK) cells and promotes cytotoxic lysis of cancer cells expressing NKG2D ligands (NKG2D‐Ls). We report the effective induction of NKG2D‐Ls, achieved with the naturally occurring polyphenol resveratrol, in a broad range of leukemia cells. In this study, resveratrol upregulated the NKG2D‐Ls MHC class I chain‐related proteins MICA and MICB, and UL16‐binding proteins ULBP1, ULBP2, and ULBP3 in most of the leukemia cells analyzed. Ligand upregulation induced by resveratrol was impaired by pharmacological and genetic disruption of ataxia–telangiectasia mutated kinase, the main regulator of NKG2D‐L expression. Leukemia cells treated with resveratrol were more susceptible to killing by NK cells than untreated cells, and the enhanced cytotoxicity of NK cells was blocked by treatment of NK cells with anti‐NKG2D mAbs. Interestingly, resveratrol consistently upregulated the NKG2D receptor expression and enhanced NKG2D‐mediated functions in resting NK cells obtained from healthy individuals. Therefore, resveratrol has attractive immunotherapeutic potential.
The potent activating receptor NKG2D is expressed on effector cells of both the innate and adaptive immune system such as natural killer (NK) cells, NK T cells, γδ T cells, and some subsets of CD8+ T cells. The NKG2D receptor plays pivotal roles in immunosurveillance of viral infections and cancer.1 NKG2D recognizes diverse and structurally different ligands, including the MHC class I chain‐related proteins (MICA and MICB), the UL16‐binding proteins (ULBP1 to 5) and retinoic acid early transcript.2 The NKG2D ligand (NKG2D‐L) transcripts are detectable in numerous normal healthy tissues; however, they are either absent or poorly expressed at the protein level.3 In response to a variety of cell stress stimuli, such as viral infections and tumorigenesis, NKG2D‐Ls are upregulated on the cell surface rendering ligands expressing cells more sensitive to destruction by NK cells through the NKG2D receptor.1, 2 Stress signals, particularly those associated with double‐strand breaks in DNA, upregulate the NKG2D ligand expression through the activation of ataxia–telangiectasia mutated (ATM) signals.4 Therefore, ATM has been postulated to be the most important regulator of NKG2D‐L expression.4
Resveratrol is a polyphenol found in grapes and other sources that possesses numerous health benefits, including anti‐inflammatory, anti‐aging, and antitumor activities.5 Resveratrol is a multitarget agent capable of modulating several proteins, including those in the nuclear factor‐κB, JAK2/signal transducer and activator of transcription‐3 (STAT3), and protein kinase B pathways.5, 6, 7, 8 Interestingly, resveratrol induces non‐mutagenic DNA damage and direct activation of ATM in tumor cells9, 10; however, it is unknown whether ATM activation induced by resveratrol is associated with the induction of NKG2D‐Ls in malignant cells.
This study showed that resveratrol not only activates ATM in leukemia cells, but also induces the expression of NKG2D‐Ls in several leukemia cells, rendering them more sensitive to NKG2D‐mediated lysis by NK cells. Given the crucial role of the NKG2D system in tumor immunosurveillance, these findings could account for the reported chemopreventive properties of this polyphenolic compound.
Materials and Methods
Cell lines
Molt4, THP1, KG1, and Jurkat cell lines were purchased from the Health Science Research Resources Bank (Ibaraki, Osaka, Japan). HL60 and Daudi cells were purchased from ATCC (Rockville, MD, USA). The chronic myeloid leukemia cell line OUN1 and the myelodysplastic syndrome cell line TF1 were provided by Dr M. Yasukawa of Ehime University (Matsuyama, Japan) and Dr S. Ogawa of the University of Tokyo (Tokyo, Japan), respectively. The TF1 cells were cultured in Iscove's modified Dulbecco's medium supplemented with 20% FBS and granulocyte/macrophage colony stimulating factors. All other cells were cultured in RPMI‐1640 medium supplemented with 10% FBS and 1% penicillin and streptomycin.
Reagents
Resveratrol was purchased from Sigma (St. Louis, MO, USA) and solubilized in DMSO. The antibodies directed against total STAT3, ERK1/2, JNK1/2, and Chk2, as well as those against phosphorylated STAT3, ERK1/2, JNK1/2, and Chk2 proteins, were purchased from Cell Signaling Technology (Tokyo, Japan). Anti‐GAPDH was purchased from Genetex (Los Angeles, CA, USA).
Natural killer cell preparation
Peripheral blood mononuclear cells were isolated using Lymphoprep (Pharmacia Biotech, Uppsala, Sweden) from heparinized blood samples of healthy volunteers collected under a protocol approved by the Institutional Review Board of Kanazawa University (Kanazawa, Japan). The NK cell fraction was purified using the untouched NK isolation kit (Invitrogen, Carlsbad, CA, USA). Flow cytometry confirmed that these cells were more than 95% CD3− CD56+ CD16+ NK cells. The cells (1 × 106 cells/mL) were resuspended in RPMI medium supplemented with 20% FBS and cultured for 24 or 48 h in the presence of several concentrations of resveratrol or vehicle (0.7% DMSO) and analyzed for NKG2D receptor expression by Western blotting and flow cytometry. A detailed description of the effect of resveratrol against activated NK cells or activated T cells is described in the supporting information (Fig. S1).
Leukemia cell culture and treatment with resveratrol
In preliminary experiments, leukemia cells lines (1 × 106 cells/mL) were cultured in the presence of several concentrations of resveratrol to determine the maximal dose of resveratrol that did not induce cell apoptosis as assessed with annexin V staining and flow cytometry analyses. As a large number of NB4, KH88, and Daudi cells underwent apoptosis after treatment with resveratrol, those cell lines were excluded from this study. The 37 μM concentration of resveratrol was selected and used throughout the study because it was found to effectively modulate NKG2D‐Ls in Molt4, THP1, KG1, Jurkat, HL60, OUN1, and TF1 cells without inducing apoptosis. Resveratrol treated and untreated cells were cultured for 48 h and their cell surface expression of NKG2D‐Ls was analyzed with flow cytometry. In some experiments, the cells were cultured for 12 or 24 h and harvested for RT‐PCR analyses.
Flow cytometry
Detection of CD3, CD56, CD16, MICA/B (BD Biosciences, San Jose, CA, USA) and NKG2D (Beckman Coulter, Brea, CA, USA) was carried out by staining the cells with appropriate fluorochrome mAbs. Detection of ULBP ligands was carried out with anti‐ULBP1, ULBP2, and ULBP3 mAbs (R&D Biosystems, Minneapolis, MN, USA) which were FITC labeled with the SureLINK FITC labeling kit (KPL, Gaithersburg, MD, USA). Data acquisition and flow cytometry analyses were carried out with a BD FACSCalibur instrument using the CellQuest software package (BD Biosciences) and analyzed using the FlowJo software package (Tri Star, Inc., Ashland, OR, USA). The NKG2D expression level on NK cells, as well as that of NKG2D‐Ls, was determined according to the mean fluorescence intensity (MFI) calculated by subtracting the MFI in the cells stained with the isotype antibody from the MFI in the corresponding cells stained with the specific antibodies. The presence of phosphorylated ATM in the leukemia cells was assessed using the FlowCellect Cell Cycle Checkpoint ATM DNA Damage Kit (Millipore, Billerica, MA, USA). Cell cycle analysis was assessed as previously described.7
Quantitative RT‐PCR
Total RNA was extracted from the cells using the Tripure RNA isolation agent following the manufacturer's instructions (Roche, Basel, Switzerland). Complementary DNA synthesis was carried out using the QuantiTect Reverse Transcription kit (Qiagen, Hilden, Germany). The NKG2D mRNA levels were measured as previously described.11 Amplification of ULBP1, ULBP2, ULBP3, and MICA cDNA was monitored using the SYBR Premix Ex Taq kit (Takara, Kyoto, Japan) on a StepOne plus instrument (Applied Biosystems, Carlsbad, CA, USA) using the primers described in a previous report.12 The amount of NKG2D‐L transcripts and that of NKG2D receptor relative to β‐actin and GAPDH mRNA, respectively, were calculated according to the comparative CT method using the relative expression function included in the StepOne version 2.2 software package (Applied Biosystems). Untreated cells were used as calibrators.
Construction of shRNA ATM lentivirus delivery system
A target sequence of the human ATM was generated by inserting the annealed oligonucleotides: 5′‐ACCGAAGAGAGACTGCTACCAAGGCGAACCTTGGTAGCAGTCTCTCTT‐3′ and 5′‐AAAAAAGAGAGACTGCTACCAAGGTTCGCCTTGGTAGCAGTCTCTCTTC‐3′ into the pENTR/U6 BLOCK‐iT plasmid (Invitrogen) to generate the pENTR/U6‐ATM‐shRNA vector. A recombination reaction using LR clonase was completed to transfer the pENTR/ATM‐shRNA or the negative control pENTR/U6‐GW/lacZshRNA cassette13 into the lentivirus delivery pLenti6/BLOCK‐iT‐DEST (Invitrogen) to construct pLenti6/Block‐iT pENTR/ATM‐shRNA (designated thereafter as shRNA‐ATM) or pLenti6/Block‐iT pENTR/U6‐GW/lacZshRNA (designated thereafter as shRNA‐NC). This lentivirus vector was used to establish ATM‐deficient OUN1 and Molt4 cells, as previously described.13 Blasticidin‐resistant clones transduced with shRNA‐ATM or shRNA‐NC from both cell lines were selected, expanded, and screened for ATM expression and sensitivity to resveratrol.
Measurement of cytokine and Granzyme B secretion
The levels of tumor necrosis factor‐α (TNF‐α), γ‐interferon, and Granzyme B secreted by primary NK cells were assessed as previously described11 with minor changes as follows. The NK cells were cultured for 24 h in the presence or absence of resveratrol. The cells were then resuspended (2 × 106 cells/mL) in RPMI medium supplemented with 100 U/mL interleukin‐2 and plated in 96‐well plates coated with human IgG or a mixture composed of 5 μg/mL each of the following recombinant human NKG2D ligands (Fc chimeras, MICA, ULBP1, and ULBP2 [R&D Systems]) and cultured for another 48 h. The levels of TNF‐α and Granzyme B in the culture supernatants were determined using specific ELISA assays (Mabtech, Nacka Strand, Sweden).
Natural killer cell cytotoxicity assay
The cytotoxic activity of NK cells was measured using a 51Cr release assay.12 Target cells were pre‐incubated with resveratrol or vehicle (0.7% DMSO) for 48 h. In the blocking experiments, the effector cells were incubated with 5 μg murine anti‐NKG2D mAbs (clone 149810) or an equal amount of isotype control antibodies (both from R&D Systems) for 30 min at 37°C before being incubated with the target cells. The effector cells were co‐incubated with the indicated effector:target ratio. The percentage of specific lysis was determined as previously described.12
Western blotting
Sample preparation and protein detection were carried out as described in a previous report.7 Where indicated, comparative blot intensities were assessed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are reported as the mean ± SE. When comparisons were made between two different groups, statistical significance was determined using Student's t‐test. The statistical significance of multiple comparisons was determined using a one‐way anova. The data were considered statistically significant at P ≤ 0.05. All statistical analyses were carried out using the Prism software package (GraphPad, San Diego, CA, USA).
Results
Resveratrol activates ATM signaling in leukemia cells
To investigate the potential activation of ATM signaling in leukemia cells by resveratrol, cells were treated with subtoxic doses of resveratrol then analyzed with flow cytometry using an antibody specific for phosphorylated ATM on Ser1981. A consistent activation of ATM was detected in the cells treated with resveratrol but not in those treated with vehicle (Fig. 1a). This effect was followed by dose‐dependent phosphorylation of the ATM substrate Chk2 on Thr68 in the resveratrol‐treated cells (Fig. 1b). Remarkably, at 37 μM, resveratrol induced no significant increases in the number of apoptotic cells (not shown). In addition, resveratrol vigorously induced G1 or S cell cycle arrest in the leukemia cells. In response to resveratrol treatment, Molt4 and THP1 cells were arrested in the G1 phase, whereas other cells, such as TF1 and KG1 cells, were arrested in the S phase of the cell cycle (Fig. 1c).
Figure 1.
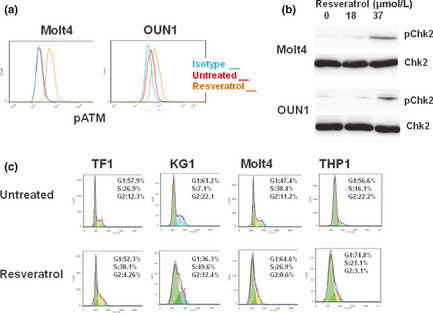
Resveratrol activates ataxia–telangiectasia mutated kinase (ATM) signaling in leukemia cells. (a) Leukemia cells were treated with resveratrol or vehicle for 6 h then stained with an antibody specific for phosphorylated ATM (at Ser188) and analyzed with flow cytometry. (b) Leukemia cells were treated for 24 h with the indicated dose of resveratrol and the expression of activated Chk2 in the cellular lysates was assessed with Western blotting. Representative results using Molt4 and OUN1 cells are shown in panels (a and b). (c) Leukemia cells were treated with resveratrol for 12 h and a cell cycle analysis was carried out using flow cytometry. Representative results of three independent experiments are shown.
Resveratrol increases expression of NKG2D‐Ls in leukemia cells
To substantiate whether the resveratrol‐induced activation of ATM signaling results in the induction of NKG2D‐Ls in leukemia cells, the expression of NKG2D‐L transcripts was assessed with an RT‐PCR analysis. By 12 h, resveratrol induced insignificant changes in NKG2D‐L transcripts (not shown); however, after 24 h of treatment, resveratrol induced a variable increase in NKG2D‐L transcripts, including MICA, ULBP1, ULBP2, and ULBP3, in the leukemia cells (Fig. S2). A flow cytometry analysis revealed that resveratrol induced heterogeneous but consistent enhancement of the expression of NKG2D‐Ls in most of the cell lines evaluated including OUN1, TF1, Molt4, and KG1 cells (Fig. 2a). Notably, PBMCs from healthy donors treated with resveratrol did not upregulate NKG2D‐Ls (Fig. 2b). Consistent with previous reports,14 ULBP1 was detectable in untreated PBMCs; however, its expression level was not modified by resveratrol.
Figure 2.
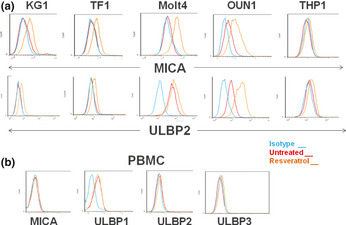
Resveratrol induces the expression of NKG2D ligands in leukemia cells. (a) Leukemia cells were treated with resveratrol or vehicle for 48 h and stained with antibodies specific for MHC class I chain‐related protein MICA and UL16‐binding proteins ULBP1, ULBP2, and ULBP3 and analyzed with flow cytometry. Representative results of cells expressing MICA and ULBP2 are shown. (b) PBMCs from three healthy donors were treated and analyzed as in panel (a). Representative results of the NKG2D ligand expression in cells obtained from a selected donor are shown.
Role of ATM signaling in resveratrol‐induced NKG2D‐L expression
A critical role for ATM kinase was suggested by the finding that resveratrol‐induced ligand upregulation was attenuated by pretreatment of leukemia cells with caffeine, a strong inhibitor of ATM signaling4, 12 (Fig. 3a). Next, ATM‐deficient leukemia cell lines were established using lentivirus delivery of a shRNA targeting the ATM gene. The effective downregulation of ATM in OUN1 and Molt4 cells was consistently achieved, as shown by Western blotting (Fig. 3b). In contrast to wild‐type cells or the shRNA‐NC transfected counterparts, the ATM knockdown cells did not upregulate NKG2D‐Ls after undergoing resveratrol treatment (Fig. 3c). These results indicate that functional ATM is required for the resveratrol‐induced expression of NKG2D‐Ls in leukemia cells. Notably, p53 was not required for NKG2D‐L induction by resveratrol, as indicated by the effective ligand upregulation in p53‐null cell line HL60 and in Jurkat cells, which harbor non‐functional p53, in response to resveratrol treatment (Fig. S3a). This was supported by the inefficacy of the p53 inhibitor pifithrin‐α to prevent the overexpression of ULBP2 and MICA in response to resveratrol treatment in the p53 active Molt4 cells (Fig. S3b). Of note, in response to resveratrol treatment, Jurkat and HL60 cells were arrested in the S phase of the cell cycle (Fig. S3c).
Figure 3.
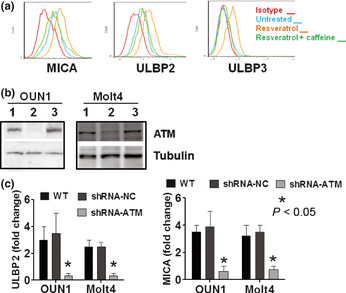
Role of ataxia–telangiectasia mutated kinase (ATM) signaling in NKG2D ligand (NKG2D‐L) expression induced by resveratrol. (a) Leukemia cells were treated with caffeine for 2 h then cultured in the presence of resveratrol for another 48 h. The NKG2D‐L expression was assessed with flow cytometry. A representative result of the NKG2D‐L expression in OUN1 cells is shown. (b) The expression of ATM in Molt4 or OUN1 cells. Whole‐cell extracts from WT or shRNA transduced Molt4 and OUN1 cells were probed for ATM expression using Western blotting. Representative figures of five independent experiments are shown: lane 1, WT cells; lane 2, shRNA‐ATM transduced cells; lane 3, cells transduced with shRNA‐NC. (c) Wild‐type or shRNA transduced Molt4 and OUN1 cells were cultured for 48 h in the presence or absence of resveratrol and their expression of NKG2D‐Ls (MHC class I chain‐related protein MICA and UL16‐binding protein ULBP2) was assessed by flow cytometry. Data are expressed as fold of increase in mean fluorescence intensity of ULBP2 and MICA in leukemia cells after treatment with resveratrol. Error bars represent SEM of three independent experiments.
Resveratrol‐induced NKG2D‐L expression enhances NK cell lysis of leukemia cells
Remarkably, the pretreatment of leukemia cells with resveratrol significantly resulted in an increase in the sensitivity of these cells to killing by NK cells. Notably, the leukemia cell sensitization to NK cell killing appears to be proportional to the levels of NKG2D‐Ls induced by resveratrol in those cells. The THP1 cells treated with resveratrol resulted in a weak induction of NKG2D‐Ls (Fig. 2a) and only a marginal increase in their sensitivity to NK cells killing (Fig. 4a, left panel). Conversely, NK cells vigorously killed Molt4 cells (Fig. 4a, right panel), which strongly upregulated NKG2D‐Ls after treatment with resveratrol (Fig. 2a). The treatment of other leukemia cells including OUN1, TF1, and KG1 cells with resveratrol resulted in a variable but significant increase in their cytotoxicity by NK cells (Fig. 4b). The increase in leukemia cell lysis was significantly reduced by pretreating NK cells with blocking anti‐NKG2D mAbs but not with isotype murine IgG (Fig. 4), supporting the involvement of an NKG2D receptor/NKG2D‐L interaction.
Figure 4.
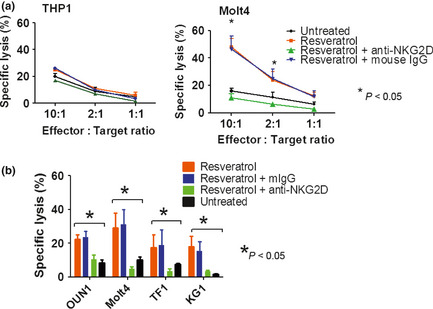
Resveratrol enhances NKG2D‐mediated killing of leukemia cells by natural killer (NK) cells. (a) THP1 and Molt4 cells were treated with resveratrol for 48 h then used as targets of resting NK cells in a Cr release assay. (b) Leukemia cells OUN1, Molt4, Jurkat, and KG1 were treated with resveratrol for 48 h then used as targets of resting NK cells in a Cr release assay at a 10:1 effector:target ratio. Data shown are average cytotoxicity of NK cells from three different donors; error bars indicate SEM.
Effects of resveratrol on primary NK cells
The effects of resveratrol on NK cells from healthy individuals were investigated. As shown in Figure 5(a,b), resveratrol induced variable but consistent enhancement of the NKG2D expression at protein level. This effect was also evident at transcriptional level (Fig. 5c). Notably, at the same dose that was effective in inducing NKG2D‐Ls in leukemia cells, resveratrol had no significant effects on the viability of in vitro activated NK cells; however, cells treated with 50 μM resveratrol proliferate less efficiently and doses higher than 100 μM resveratrol significantly impaired the viability of NK cells (Fig. S1a). These effects were also evident in activated T cells (Fig. S1b,c).
Figure 5.
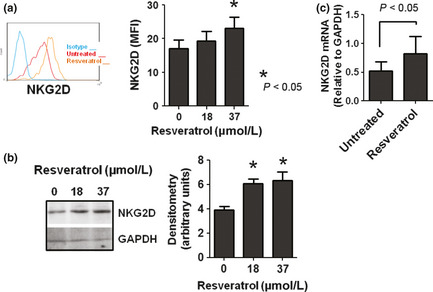
Resveratrol modulates the expression of NKG2D receptor in natural killer (NK) cells. (a) NK cells from healthy donors were cultured in the presence or absence of resveratrol for 48 h and their expression of NKG2D receptors were assessed with flow cytometry. A representative result is shown in the left panel and summarized data from four donors are shown in the right panel. (b) NK cells were treated as in panel (a) and their expression of NKG2D receptors was examined with Western blotting using anti‐NKG2D mAb (clone 149810). The same blots were stripped and reprobed with anti‐GAPDH antibodies equal protein loading. A representative result of three independent experiments is shown. Densitometric analysis of the bands obtained in three independent experiments (mean ± SEM) is shown in the right panel. (c) NK cells were treated with resveratrol for 24 h and the levels of NKG2D transcripts were assessed using RT‐PCR. Expression levels were normalized to GAPDH. Data represent mean ± SEM from four donors are shown.
The expression levels of phosphorylated protein kinases ERK1/2 and JNK1/2, as well as that of STAT3, in resveratrol‐treated NK cells were also examined. In fresh NK cells, resveratrol induced a modest increase in the phosphorylation state of ERK1/2 and robust activation of JNK1/2 (Fig. 6a), consistent with the reported effects of resveratrol in the NK92 cell line.26 Of note, resveratrol did not affect STAT3 activity in NK cells. Remarkably, resveratrol‐treated primary NK cells secreted higher levels of TNF‐α and Granzyme B (Fig. 6b,c) in response to NKG2D receptor ligation by plate‐coated NKG2D‐Ls, thus indicating that resveratrol‐induced NKG2D upregulation has functional implications.
Figure 6.
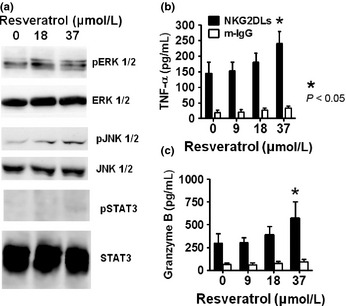
Resveratrol modulates the expression of protein kinases associated with natural killer (NK) cell activation. (a) NK cells were treated with the indicated concentrations of resveratrol for 24 h and their expressions of phosphorylated ERK1/2, JNK1/2, and signal transducer and activator of transcription‐3 (STAT3) were examined with Western blotting. Representative results of three independent experiments are shown. Natural killer cells were cultured for 24 h in the presence or absence of resveratrol. The cells were harvested and cultured for 48 h in plates coated with human IgG or a mixture of five recombinant human NKG2D ligands. The levels of tumor necrosis factor‐α (TNF‐α) (b) and Granzyme B (c) released into the culture medium were measured using ELISA. Mean ± SEM of three independent experiments is shown.
Discussion
The potent activator receptor NKG2D triggers NK cell cytotoxicity against NKG2D‐Ls expressing target cells. The specific interaction of the NKG2D receptor with its ligands plays a critical role in tumor surveillance; hence, pharmacological induction of NKG2D‐Ls in tumor cells is an attractive immunotherapeutic approach. The present study showed that resveratrol, a naturally occurring polyphenol, effectively induces the expression of NKG2D‐Ls in leukemia cells. Consequently, resveratrol‐treated cells are sensitized to NKG2D receptor‐dependent killing by NK cells. In addition, we found that resveratrol modulates the expression of NKG2D receptor in NK cells.
Our mechanistic studies indicated that ATM signaling activation is required for resveratrol‐induced NKG2D‐L upregulation in leukemia cells. This finding is consistent with previous reports suggesting that ATM is a key player in ligand overexpression in response to genotoxic stress signals such as those induced by ionizing radiation and hydroxyurea, valproic acid, and other histone deacetylase inhibitors.4, 12, 15 In addition, our data showing resveratrol induced the activation of ATM signaling in leukemia cells is consistent with previous reports showing that resveratrol is a direct activator of ATM signaling in various cellular systems.9, 16 A serine–threonine kinase, ATM phosphorylates specific targets, including p53, Chk2, BRCA1, Nbs1, and other substrates that play critical roles in the regulation of apoptosis and DNA repair as well as cell cycle arrest at G1/S, intra‐S, and G2/M checkpoints.4, 17, 18 Resveratrol has been reported to modulate key regulators of the cell cycle, thus resulting in G1/S, S, and G2/M cell cycle arrest.19 Its effects on specific cell cycle phases vary considerably among cell types and may be dependent on both the concentration of resveratrol and the characteristics of the target cells.19, 20 Notably, resveratrol‐induced ATM activation is accompanied by S phase arrest in ovarian tumor cells and malignant B cells.16, 21 This is consistent with our finding that resveratrol‐induced ATM activation is associated with G1 or S arrest in leukemia cells.
Due to its pivotal role in the regulation of cell cycle progression, DNA damage response through ATM activation constitutes an anticancer barrier in early human tumorigenesis.18 In addition, the NKG2D receptor/NKG2D‐L interaction plays a critical role in tumor immunosurveillance1 and several in vivo studies support the efficacy of resveratrol in the prevention of tumor development.5, 22 Therefore, our data are consistent with the notion that the ATM‐mediated upregulation of NKG2D‐Ls in transforming cells induced by resveratrol may account for the reported chemopreventive properties of this polyphenolic compound. The release of NKG2D‐Ls from tumor cells, either within exosomes23 or in soluble form,24 constitutes an efficient strategy by cancer cells to evade NKG2D‐mediated killing. It is unknown whether resveratrol‐induced NKG2D‐Ls in leukemia cells are accompanied by ligand shedding from tumor cells, which may impair the NK cell function, particularly in patients with advanced disease.
Several agents capable of enhancing NKG2D‐Ls in tumor cells have been reported thus far,4, 12 however, resveratrol constitutes the first agent capable of modulating NKG2D receptors in effector NK cells. In fresh NK cells, resveratrol induced an activated phenotype characterized by a higher NKG2D expression, more efficient secretion of cytokines, and activation of ERK1/2 and JNK1/2 kinases, consistent with previous observations in NK92 cells and other cellular systems treated with resveratrol.25, 26, 27
The current study showed that 37 μM resveratrol is optimal for inducing NKG2D‐Ls expression in leukemia cells. Similar concentrations of resveratrol are safe for inducing the clonal growth of normal hematopoietic progenitor cells28, 29 and are non‐toxic to human PBMCs8, 29; however, 50 μM or more of resveratrol impairs the proliferation of activated NK cells and T cells in vitro (Fig. S1) and that of highly proliferating normal cells.29 The results of phase I clinical trials show that up to 5 g/day of resveratrol is safe and well‐tolerated and does not result in plasma concentrations above 50 μM.30, 31 Accordingly, it is therefore unlikely that in vivo treatment with resveratrol is harmful for normal cells.
In summary, this study showed that resveratrol enhances the cytotoxicity of NK cells against leukemia cells through the modulation of the NKG2D receptor/NKG2D‐L system. This mechanistic finding, together with recent reports from clinical trials showing the safety of resveratrol in humans, supports the use of this polyphenol as a potential immunotherapeutic agent.
Disclosure Statement
The authors have no conflicts of interest.
Supporting information
Fig. S1. Effect of resveratrol on activated natural killer cells, T cells, and phytohaemagglutinin‐activated PBMC.
Fig. S2. Effect of resveratrol on the expression of NKG2D ligand transcripts in leukemia cells.
Fig. S3. Role of p53 signaling in NKG2D ligand expression induced by resveratrol.
Acknowledgments
We are indebted to Drs M. Yasukawa and S. Ogawa for generously providing the cell lines used in this study.
(Cancer Sci, doi: 10.1111/cas.12141, 2013)
References
- 1. Burgess S, Maasho K, Masilamani M, Narayanan S, Borrego F, Coligan J. The NKG2D receptor: immunobiology and clinical implications. Immunol Res 2008; 40: 18–34. [DOI] [PubMed] [Google Scholar]
- 2. Eagle R, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat Rev Immunol 2007; 7: 737–44. [DOI] [PubMed] [Google Scholar]
- 3. Eagle R, Jafferji I, Barrow A. Beyond stressed self: evidence for NKG2D ligand expression on healthy cells. Curr Immunol Rev 2009; 5: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005; 436: 1186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 2006; 5: 493–506. [DOI] [PubMed] [Google Scholar]
- 6. Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci 2011; 1215: 1–8. [DOI] [PubMed] [Google Scholar]
- 7. Quoc Trung L, Espinoza JL, Takami A, Nakao S. Resveratrol induces cell cycle arrest and apoptosis in malignant NK cells via JAK2/STAT3 pathway inhibition. PLoS ONE 2013; 8: e55183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Espinoza JL, Takami A, Trung LQ, Kato S, Nakao S. Resveratrol prevents EBV transformation and inhibits the outgrowth of EBV‐immortalized human B cells. PLoS ONE 2012; 7: e51306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gatz SA, Keimling M, Baumann C et al Resveratrol modulates DNA double‐strand break repair pathways in an ATM/ATR‐p53‐ and ‐Nbs1‐dependent manner. Carcinogenesis 2008; 29: 519–27. [DOI] [PubMed] [Google Scholar]
- 10. Fox JT, Sakamuru S, Huang R et al High‐throughput genotoxicity assay identifies antioxidants as inducers of DNA damage response and cell death. Proc Natl Acad Sci USA 2012; 109: 5423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Espinoza JL, Takami A, Yoshioka K et al Human microRNA‐1245 downregulates the NKG2D receptor in NK cells and impairs NKG2D‐mediated functions. Haematologica 2012; 97: 1295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu X, Ohata K, Kondo Y, Luis Espinoza J, Qi Z, Nakao S. Hydroxyurea upregulates NKG2D ligand expression in myeloid leukemia cells synergistically with valproic acid and potentially enhances susceptibility of leukemic cells to natural killer cell‐mediated cytolysis. Cancer Sci 2010; 101: 609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Espinoza J, Takamatsu H, Lu X, Qi Z, Nakao S. Anti‐moesin antibodies derived from patients with aplastic anemia stimulate monocytic cells to secrete TNF‐alpha through an ERK1/2‐dependent pathway. Int Immunol 2009; 21: 913–23. [DOI] [PubMed] [Google Scholar]
- 14. Nowbakht P, Ionescu MC, Rohner A et al Ligands for natural killer cell‐activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood 2005; 105: 3615–22. [DOI] [PubMed] [Google Scholar]
- 15. Diermayr S, Himmelreich H, Durovic B et al NKG2D ligand expression in AML increases in response to HDAC inhibitor valproic acid and contributes to allorecognition by NK‐cell lines with single KIR‐HLA class I specificities. Blood 2008; 111: 1428–36. [DOI] [PubMed] [Google Scholar]
- 16. Tyagi A, Singh RP, Agarwal C, Siriwardana S, Sclafani RA, Agarwal R. Resveratrol causes Cdc2‐tyr15 phosphorylation via ATM/ATR‐Chk1/2‐Cdc25C pathway as a central mechanism for S phase arrest in human ovarian carcinoma Ovcar‐3 cells. Carcinogenesis 2005; 26: 1978–87. [DOI] [PubMed] [Google Scholar]
- 17. Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 2001; 15: 2177–96. [DOI] [PubMed] [Google Scholar]
- 18. Bhatti S, Kozlov S, Farooqi AA, Naqi A, Lavin M, Khanna KK. ATM protein kinase: the linchpin of cellular defenses to stress. Cell Mol Life Sci 2011; 68: 2977–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: anti‐carcinogenic mechanisms. Arch Biochem Biophys 2009; 486: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann N Y Acad Sci 2002; 957: 210–29. [DOI] [PubMed] [Google Scholar]
- 21. Shimizu T, Nakazato T, Xian MJ, Sagawa M, Ikeda Y, Kizaki M. Resveratrol induces apoptosis of human malignant B cells by activation of caspase‐3 and p38 MAP kinase pathways. Biochem Pharmacol 2006; 71: 742–50. [DOI] [PubMed] [Google Scholar]
- 22. Namasivayam N. Chemoprevention in experimental animals. Ann N Y Acad Sci 2011; 1215: 60–71. [DOI] [PubMed] [Google Scholar]
- 23. Hedlund M, Nagaeva O, Kargl D, Baranov V, Mincheva‐Nilsson L. Thermal‐ and oxidative stress causes enhanced release of NKG2D ligand‐bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS ONE 2011; 6: e16899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salih H, Holdenrieder S, Steinle A. Soluble NKG2D ligands: prevalence, release, and functional impact. Front Biosci 2008; 13: 3448–56. [DOI] [PubMed] [Google Scholar]
- 25. Maher P, Dargusch R, Bodai L, Gerard PE, Purcell JM, Marsh JL. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Hum Mol Genet 2011; 20: 261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu C, Chen J. Resveratrol enhances perforin expression and NK cell cytotoxicity through NKG2D‐dependent pathways. J Cell Physiol 2010; 223: 343–51. [DOI] [PubMed] [Google Scholar]
- 27. Banerjee Mustafi S, Chakraborty PK, Raha S. Modulation of Akt and ERK1/2 pathways by resveratrol in chronic myelogenous leukemia (CML) cells results in the downregulation of Hsp70. PLoS ONE 2010; 5: e8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gautam SC, Xu YX, Dumaguin M, Janakiraman N, Chapman RA. Resveratrol selectively inhibits leukemia cells: a prospective agent for ex vivo bone marrow purging. Bone Marrow Transplant 2000; 25: 639–45. [DOI] [PubMed] [Google Scholar]
- 29. Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res 2004; 24: 2783–840. [PubMed] [Google Scholar]
- 30. Brown VA, Patel KR, Viskaduraki M et al Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin‐like growth factor axis. Cancer Res 2010; 70: 9003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kennedy DO, Wightman EL, Reay JL et al Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double‐blind, placebo‐controlled, crossover investigation. Am J Clin Nutr 2010; 91: 1590–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effect of resveratrol on activated natural killer cells, T cells, and phytohaemagglutinin‐activated PBMC.
Fig. S2. Effect of resveratrol on the expression of NKG2D ligand transcripts in leukemia cells.
Fig. S3. Role of p53 signaling in NKG2D ligand expression induced by resveratrol.


