Abstract
Human T‐cell leukemia virus type 1 (HTLV‐1) is the etiological agent of adult T‐cell leukemia (ATL). HTLV‐1 encodes the oncoprotein Tax1, which is essential for immortalization of human T‐cells and persistent HTLV‐1 infection in vivo. Tax1 has a PDZ binding motif (PBM) at its C‐terminus. This motif is crucial for the transforming activity of Tax1 to a T‐cell line and persistent HTLV‐1 infection. Tax1 through the PBM interacts with PDZ domain proteins such as Dlg1 and Scribble, but it has not been determined yet, which cellular PDZ proteins mediate the functions of Tax1 PBM. Here we demonstrate that Tax1 interacts with the PDZ domain protein MAGI‐1 in a PBM‐dependent manner, and the interaction mislocalizes MAGI‐1 from the detergent‐soluble to the detergent‐insoluble cellular fraction in 293T cells and in HTLV‐1‐infected T‐cells. In addition, Tax1‐transformation of a T‐cell line from interleukin (IL)‐2‐dependent to IL‐2‐independent growth selects cells with irreversibly reduced expression of MAGI‐1 at mRNA level. These findings imply that Tax1, like other viral oncoproteins, targets MAGI‐1 as a mechanism to suppress its anti‐tumor functions in HTLV‐1‐infected cells to contribute to the transforming activity of T‐cells and persistent HTLV‐1 infection.
Human T‐cell leukemia virus type 1 (HTLV‐1) is the etiological agent of adult T‐cell leukemia (ATL).1, 2 Among several non‐structural genes encoded by HTLV‐1, Tax1 is a crucial regulator of viral life cycle as a potent transcriptional activator for its own transcription.3, 4, 5 In addition, Tax1 is a key player involved in T‐cell immortalization, transformation, persistent infection, inflammation, and leukemogenesis.6, 7, 8, 9, 10, 11 All these pleitropic functions of Tax1 are believed to be directed by a wide spectrum of interactions with cellular factors. For instance, numerous PDZ domain containing cellular proteins have been shown to complex with Tax1 through the PDZ binding motif (PBM) located at its C‐terminus.12, 13 We have previously shown that the PBM plays a key role in Tax1 mediated activities. The motif was critically involved in Tax1‐induced transformation of a rat fibroblast cell line and a mouse T‐cell line.14, 15 Moreover, an HTLV‐1ΔPBM virus with a deletion of the Tax1 PBM in HTLV‐1, failed to establish persistent infection in rabbits as measured by lack of antibody response against HTLV‐1 and the absence of HTLV‐1 proviruses.16
Human T‐cell leukemia virus type 2 (HTLV‐2) is closely related to HTLV‐1, but it is unable to cause any malignancy.17, 18 One notable difference between the two is the lack of PBM in Tax2, and thus Tax1 PBM is proposed to be one of the major determinants of HTLV‐1 pathogenesis.14, 19 Intriguingly, PBMs have been identified in other viral oncoproteins such as the E4‐ORF1 protein of human adenovirus type 9 and the E6 proteins of high‐risk human papilloma viruses (HPV),20, 21, 22 suggesting that the oncogenic ability of these viruses may depend in part on interactions involving their PBMs with cellular proteins.
PSD‐95/Disc Large/Zona Occludens‐1 (PDZ) domain containing proteins form signaling complexes at the inner surface of cell membrane and are involved in a broad range of functions like cell signaling, cell–cell adhesion, tight junction integrity, molecular scaffolding for protein complexes, and tumor suppression.23, 24 The human genome contains hundreds of PDZ domain containing proteins,25 and some of them have been shown to be targeted by viral oncoproteins.26, 27, 28, 29 Tax1 selectively interacts with Dlg1 and Scribble.13, 30 Of the two, Dlg1 has been widely studied due to its involvement in cell growth signaling and tumor suppression.31, 32, 33 Our previous findings suggested that inactivation of Dlg1 increases the ability of Tax1 to transform a mouse T‐cell line (CTLL‐2) from interleukin (IL)‐2‐dependent to IL‐2‐independent growth, but it did not increase such transforming activity in a PBM defective Tax1 mutant.34 Hence we concluded that other yet unidentified PDZ protein(s) besides Dlg1 likely inhibit(s) transformation of CTLL‐2 by Tax1 and inactivation of these proteins by Tax1 is essential for the transformation of CTLL‐2.
MAGI‐1 (MAGUK with inverted domain structure 1) is a PDZ protein closely related to Dlg1,35 and it has also been implicated in tumor suppression in various systems. For instance, MAGI‐1 suppressed invasion of tumor cells by recruiting PTEN/β‐catenin complex to the adherent junctions and downregulation of phosphatidylinositol 3‐kinases (PI3K) signaling.36 MAGI‐1 is a known target of viral oncoproteins adenovirus‐9 E4‐ORF1 and high‐risk HPV E6.37, 38 In the present research, we provide evidence that MAGI‐1 is targeted by the HTLV‐1 oncoprotein Tax1 in two independent mechanisms. Tax1 interacts with MAGI‐1 and alters the subcellular localization. In addition, a low MAGI‐1 expression is selected during Tax1‐immortalization of human T‐cells and IL‐2‐independent transformation of CTLL‐2 cells. Interestingly, unlike Tax1, Tax2‐immortalization of human T‐cells is not associated with downregulation of MAGI‐1 expression. These findings suggest that MAGI‐1, a PDZ protein known to be associated with tumour suppression, is an important cellular target of HTLV‐1 Tax1 for T‐cell immortalization and transformation.
Materials and Methods
Cell lines and cell culture
The human embryonic kidney cell line 293T was cultured in DMEM supplemented with 10% FBS, 50 U/mL penicillin, and 50 μg/mL streptomycin. The human T‐cell lines used in the present experiments have been characterized previously.14 PBL/HTLV‐1 and PBL/HTLV‐1ΔPBM are IL‐2‐dependent HTLV‐1‐immortalized human T‐cell lines, and they were established as previously described.16 SLB‐1, MT‐4 and HUT‐102 are IL‐2‐independent HTLV‐1‐transformed human T‐cell lines. HUT78, MOLT‐4 and Jurkat are HTLV‐1‐negative human T‐cell lines. SLB‐1, HUT‐102, HUT78, MT‐4, MOLT‐4 and Jurkat cells were cultured in RPMI 1640 supplemented with 10% FBS, 4 mM glutamine, penicillin (50 U/mL), and streptomycin (50 μg/mL) (RPMI/10%FBS). PBL/HTLV‐1 and PBL/HTLV‐1ΔPBM were cultured in RPMI/20%FBS with 1 nM recombinant human IL‐2 (Takeda Chemical Industries, Osaka, Japan). CTLL‐2 is a mouse cytotoxic T‐cell line that grows in an IL‐2‐dependent manner,6, 15 and was cultured in RPMI/10%FBS containing 2‐mercaptoethanol (2‐ME) and 1 nM IL‐2. CTLL‐2 cells stably expressing hAkt1ΔmPH were described previously.39 They were cultured in RPMI/10%FBS containing 2‐ME, 1 nM IL‐2 and 0.5 mg/mL G418 (Invitrogen, Carlsbad, CA, USA). Tax1‐ and hAkt1ΔmPH‐transformed IL‐2‐independent CTLL‐2 cells were established as described previously,34, 39 and cultured in RPMI/10%FBS containing 2‐ME but without IL‐2.
Plasmids
The expression plasmids pHβPr‐neo‐Tax1 and pHβPr‐neo‐Tax1ΔC that were used for the expression of Tax1 and Tax1ΔC respectively in 293T cells together with the lentiviral vector CSII‐EF‐IG‐RfA used for the generation of recombinant lentiviruses for expression of Tax1 in T‐cells have been described previously.40, 41 Tax1ΔC is a PBM‐negative mutant, with a four amino acid deletion from the C‐terminus of Tax1.14 pcDNA3.1:FLAG‐MAGI‐1c (A gift from L. Banks, International Centre for Genetic Engineering and Biotechnology, Italy), was constructed by cloning FLAG‐MAGI‐1c into the HindIII/EcoRI site of pcDNA3.1 (Invitrogen) with a FLAG‐tag at the N‐terminus.
Western blotting analysis
Cells were lysed with the SDS‐sample buffer consisting of 62.5 mM Tris‐HCl (pH 6.8), 2% SDS, 10% glycerol. Protein concentrations of the cell lysates were measured using the DC protein assay kit (Bio‐Rad Laboratories, Hercules, CA, USA). The cell lysates (20 μg) were then treated with 50 mM DTT, 0.01% bromophenol blue, and heated at 95°C for 5 min. The resultant lysates were subjected to SDS‐PAGE separation. The proteins in the gel were transferred to a nitrocellulose membrane, which was then incubated with 5% skim milk for 1 h at room temperature to inhibit non‐specific binding, and further incubated with the primary antibody. After washing with TNN buffer (10 mM Tris–HCl [pH 7.5], 50 mM NaCl, and 0.05% NP40), the membranes were further incubated with either anti‐mouse or anti‐rabbit immunoglobulins conjugated with horseradish peroxidase (Bio‐Rad Technologies) as secondary antibodies. Proteins recognized by the antibodies in the membrane were visualized using the ECL Western blotting detection system (Amersham Biosciences, Piscataway, NJ, USA). Primary antibodies used above were mouse anti‐Tax1 mAb (TAXY‐7),42 rabbit anti‐Tax2 pAb,43 mouse anti‐FLAG M2 mAb, rabbit anti‐MAGI‐1 antibody (Sigma‐Aldrich, Tokyo, Japan), rabbit anti‐Akt mAb (Cell Signaling Technology, Beverly, MA, USA), mouse anti‐Syntrophin‐β mAb (Affinity Bioreagents, Golden, CO, USA) and mouse anti‐α‐Tubulin mAb (Oncogene Research Products, San Diego, CA, USA). The above named anti‐Tax1 and anti‐MAGI‐1 antibodies were also used for the immunoprecipitation and immunoflorescence assays.
Immunoflorescence
To analyze the subcellular localization of Tax1 and MAGI‐1 in 293T cells, the cells were cultured on glass slides in a six‐well culture plate overnight, and transiently transfected with pHβPr‐neo, pHβPr‐neo‐Tax1, or pHβPr‐neo‐Tax1ΔC plasmid using the lipofection method (FUGENE 6; Roche Diagnostics, Tokyo, Japan). At 48 h after transfection, the cells were fixed with 4% formaldehyde in PBS for 25 min at 4°C and permeabilized by 0.1% TritonX‐100. The fixed cells were then incubated with the rabbit anti‐MAGI‐1 antibody and the mouse anti‐Tax1 antibody for 30 min. After washing the slides with PBS, the cells were incubated with Alexa594‐labeled anti‐mouse IgG, Alexa488‐labeled anti‐rabbit IgG (Molecular Probes) alongside Hoechst 33258 for another 30 min. The stained cells were then examined by fluorescent microscopy (BZ‐8000; KEYENCE, Osaka, Japan) for analysis.
Co‐immunoprecipitation
The Tax1 plasmids were transiently transfected into 293T cells with or without the pcDNA3.1: FLAG‐MAGI‐1c (4 μg) by the lipofection method (FUGENE 6). At 48 h after the transfection, the cells were treated with the lysis buffer A (25 mM Tris [pH 7.2], 150 mM NaCl, 1.0 mM EDTA, 1% NP40, 2.0 mM phenylmethanesulfonyl fluoride, 20 μg/mL aprotinin, 1.0 mM Na3VO4, 1.0 mM NaF), after centrifugation of the cell lysates, the supernatants were immunoprecipitated with anti‐Tax1 antibody. The amount of Tax1 and MAGI‐1 proteins in the precipitates was analyzed by a Western blotting analysis using anti‐Tax1, and anti‐MAGI‐1 or anti‐FLAG M2 antibodies, respectively.
Cell fractionation
The 293T cells were transfected with Tax1 and its mutant plasmid by the lipofection method (FUGENE 6). At 48 h after transfection, the cells were divided into two groups. One was treated with the lysis buffer A (described above) at 4°C for 15 min. After centrifugation, the supernatant was collected and used as the soluble fraction, and the resultant pellet was further treated with the SDS‐sample buffer (125 mM Tris–HCl [pH 6.8], 2% SDS, 20% glycerol, 0.01% bromophenol blue, and 10% β‐mercaptoethanol) at 4°C for 15 min. The lysates were used as the insoluble fraction. The other group was directly treated with the SDS‐sample buffer and used as the total fraction. A similar cell fractionation assay was performed for HTLV‐1‐negative (Jurkat) and HTLV‐1‐positive (SLB‐1) T‐cells. The three sets of samples were separately size‐fractionated by SDS‐PAGE, and the amounts of proteins were measured by a Western blotting analysis.
Immortalization of PBMCs
Human peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy donors. They were stimulated with 10 μg/mL phytohemagglutinin (PHA) (Sigma Aldrich) in RPMI/20%FBS supplemented with 55 μM 2‐ME for 2 days, and then further cultured in RPMI/20%FBS with 0.5 nM IL‐2 and 2‐ME for 2 days. A portion of these cells was set aside to be used as Tax untreated PBMCs. Immortalization of PBMCs by Tax1 and Tax2 was carried out as we recently described.44
Transient transfection assays
To generate recombinant lentiviruses, 293T cells were transfected with pCAG‐HIVgp, pCMV‐VSV‐G‐RSV‐Rev (provided by Dr H. Miyoshi) and the respective lentiviral vectors encoding Tax1 or Tax1ΔC using FUGENE 6. At 72 h after the transfection, the supernatant was collected and used to infect CTLL‐2 and Jurkat cells at 4 × 105 cells/well in a 12‐well plate in a final volume of 2 mL medium. At 48 h after transfection, cell lysates were prepared from the transfected cells and used for a Western blotting analysis.
RNA extraction and quantitative real‐time RT‐PCR
All reagents used were acquired from TAKARA BIO Japan. The assay was carried out according to the manufacturer's instructions. Total RNA was extracted by the Fast Pure RNA kit and 1 μg was used for cDNA synthesis using the Prime Script RT reagent kit. Two‐step 40‐cycle quantitative real‐time RT‐PCR for MAGI‐1 expression was carried out by the Thermal Cycler Dice Real Time system and the DNA master SYBR Green using the primers 5′‐GCACTGGATGGCAAGATGGA‐3′ and 5′‐ACCAATGGGAATGGACTGGAAG‐3′ for both mouse and human MAGI‐1. Amplification of each template was conducted in triplicate. The quantity of each transcript was calculated according to the instrument's manual and normalized to the amount of GAPDH mRNA. Amplification without template was included as a control.
Results
Tax1 interacts with MAGI‐1 through the PBM
To determine any unidentified Tax1 PBM interacting proteins, a GST fusion protein of Tax1 versus that of its PBM‐deletion mutant(Tax1ΔC) were used (Fig. 1A). Proteins bound to Tax1 but not to Tax1ΔC were analyzed by mass spectrometry, and MAGI‐1 was identified as one of the Tax1 PBM interacting partners. In order to confirm the interaction of Tax1 with MAGI‐1, Tax1 or Tax1ΔC expression plasmids were transiently transfected into the 293T cells or they were co‐transfected with the FLAG‐tagged MAGI‐1 expression plasmid by the lipofection method (FUGENE 6). At 48 h after transfection, cell lysates were immunoprecipitated with anti‐Tax1 antibody. The amount of MAGI‐1 or FLAG in immunoprecipitates was analyzed by a Western blotting analysis (Fig. 1B,C). MAGI‐1 was co‐immunoprecipitated only with Tax1 but not with Tax1ΔC (Fig. 1B, IP: Tax1). As will be shown later, the amount of detergent‐soluble MAGI‐1 protein is reduced by wild type Tax1 but not Tax1ΔC (Fig. 1B). In addition to endogenous MAGI‐1, exogenously transduced MAGI‐1 was also efficiently co‐immunoprecipitated with Tax1 but not with Tax1ΔC (Fig. 1C, IP: Tax1). These results indicate that Tax1 can interact with MAGI‐1, and this interaction is dependent on the presence of the PBM in Tax1.
Figure 1.
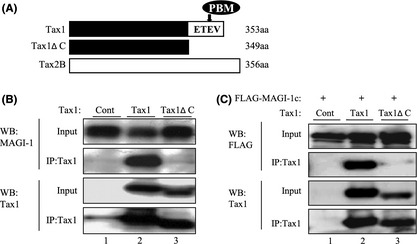
Tax1 interacts with MAGI‐1 in a PBM‐dependent manner. (A) Structures of Tax1, Tax1ΔC and Tax2 proteins used in this study. The amino acid sequence of the PBM is shown. (B, C) 293T cells were transiently transfected with pHβPr‐neo‐Tax1 (lane 2), pHβPr‐neo‐Tax1ΔC (lane 3) or pHβPr‐neo plasmid (lane 1) together with (C) or without pcDNA3.1:FLAG‐MAGI‐1c (B) by the lipofection method (FUGENE 6). At 48 h post‐transfection, the cells were treated with lysis buffer, and cell lysates immunoprecipitated with anti‐Tax1 antibody. Total cell lysates (Input) and immunoprecipitates (IP: Tax1) were characterized by a Western blotting analysis using the indicated antibodies.
Tax1 alters the subcellular localization of MAGI‐1
To further establish the association of Tax1 with MAGI‐1 in vivo, the subcellular localization of both proteins was examined. The 293T cells were grown on cover slips overnight, followed by transient transfection with Tax1 plasmids. At 48 h after the transfection, the cells were stained with anti‐Tax1 and anti‐MAGI‐1, and their subcellular localization was analyzed by fluorescent microscopy. On its own, MAGI‐1 was primarily located in the cytoplasm and on the membrane (Fig. 2, Control). In the presence of Tax1, MAGI‐1 colocalized with Tax1 as particular spots (yellow in color), mainly in the perinuclear region of the cytoplasm (Fig. 2, Tax1). In contrast, Tax1 PBM deletion mutant did not obviously show colocalization with MAGI‐1 and did not alter the subcellular localization (Fig. 2, Tax1ΔC). We and others have shown a similar behavior of Tax1 with other PDZ proteins such as MAGI‐3, Dlg1 and Scribble.14, 45, 46 Taken together, our results indicate that Tax1 mislocalizes MAGI‐1 by aberrantly sequestering it within Tax1 containing complexes.
Figure 2.
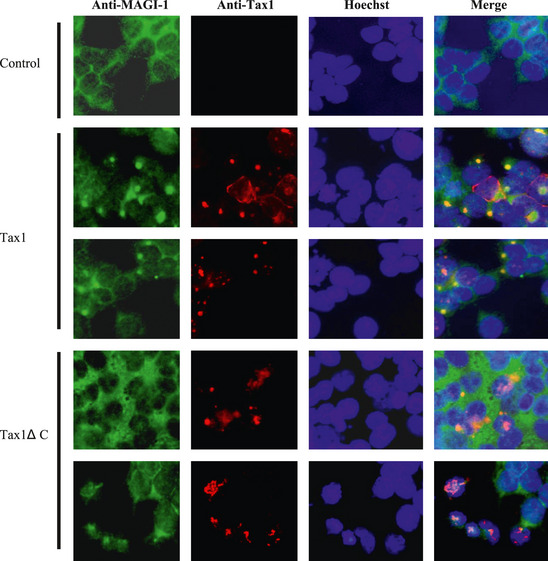
Subcellular localization of Tax1 and endogenous MAGI‐1 in 293T cells. 293T cells were transfected with either the Tax1 or Tax1ΔC plasmid as described in the methods. The cells were stained with anti‐Tax1 (red), anti‐MAGI‐1 (green), and Hoechst 33258 (blue) for nuclear staining. The stained cells were examined by fluorescent light microscopy.
Tax1 translocates MAGI‐1 from the detergent‐soluble to the detergent‐insoluble cellular fraction
Several oncoproteins of tumor viruses inactivate functions of cellular tumor suppressor proteins by altering their subcellular localizations.13, 30, 37, 46, 47 We therefore went ahead to investigate the subcellular localization of MAGI‐1 in the presence of Tax1 by cell fractionation assay. At first, 293T cells transiently transfected with Tax1 or Tax1ΔC were lysed by a mild detergent (NP40). The NP40‐insoluble fraction was further lysed by the SDS‐sample buffer, and then the NP40‐soluble fraction, the NP40‐insoluble fraction and the total fraction directly lysed by the SDS‐sample buffer were separately collected. The amounts of endogenous MAGI‐1 and transduced Tax1 protein in these three fractions were measured by a Western blotting analysis. MAGI‐1 in 293T cells transfected with Tax1ΔC was dominantly detected in the soluble fraction, with a smaller amount in the insoluble fraction, and the amounts were almost equivalent to those in cells transfected with the control plasmid (Fig. 3A, lane 1, 3). On the other hand, while MAGI‐1 in the soluble fraction of cells was drastically reduced by transfection of Tax1, MAGI‐1 in the insoluble fraction was greatly increased (Fig. 3A, lane 2). Total MAGI‐1 protein was unaffected by expression of Tax1 or Tax1ΔC (Fig. 3A, top panel). Similarly, when we examined HTLV‐1‐infected human T‐cells (SLB‐1) and uninfected human T‐cells (Jurkat), MAGI‐1 was detected in the insoluble fraction of SLB‐1 with high expression of Tax1 but not that in Jurkat (Fig. 3B). Taken together, our results show that Tax1 induces the translocation of MAGI‐1 from the detergent‐soluble to the detergent‐insoluble cellular fraction. Note worthy MAGI‐1 in the insoluble fraction of SLB‐1 was detected as a higher molecular weight band than MAGI‐1 in the soluble one. This is likely to be due to the post‐transcriptional modification of MAGI‐1 similar to the phosphorylation reported for Dlg1 in HTLV‐1‐infected T‐cells.13, 14
Figure 3.
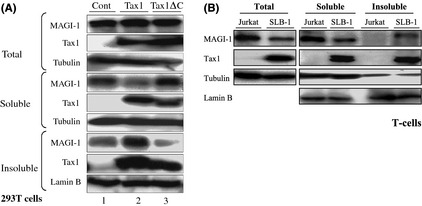
Tax1 translocates MAGI‐1 from the soluble to the insoluble cellular fraction. (A) The 293T cells transfected with Tax1 (lane 2), Tax1ΔC (lane 3) or control (lane 1) were divided into two groups. One group was treated with the NP40 lysis buffer and fractionated as described in Materials and Methods. The other group was directly treated with the sodium dodecyl sulfate (SDS)‐sample buffer and used as the total fraction. The three types of samples were size‐fractionated by SDS‐polyacrylamide gel electrophoresis (PAGE) followed by a Western blotting analysis. (B) Cell lysates from the total, soluble and insoluble fractions of Jurkat and SLB‐1 cells were prepared as describe in (A) and the amounts of MAGI‐1, Tax1, Tubulin and Lamin B proteins in the lysates were measured by a Western blotting analysis.
Downregulation of MAGI‐1 protein during Tax1‐induced transformation
We next investigated whether Tax1‐induced transformation alters MAGI‐1 expression. CTLL‐2 is a mouse T‐cell line, and it is widely used in HTLV‐1 research due to its transformation by Tax1 from IL‐2‐dependent into IL‐2‐independent growth.6, 15 Transient expression of Tax1 or Tax1ΔC in CTLL‐2 and Jurkat cells barely affected MAGI‐1 expression (Fig. 4A). In addition, stable expression of Tax1 in CTLL‐2 cells in the presence of IL‐2 little affected the expression of MAGI‐1 (Fig. 4B, left panel). On the other hand, MAGI‐1 expression in Tax1‐transformed IL‐2‐independent CTLL‐2 cells was much lower than that of their untransformed counterparts (Fig. 4B, right panel, Fig. 4C). The expression of another PDZ domain containing protein, syntrophin, with which Tax1 also interacts (Higuchi M, unpublished data, 34), was intact in Tax1‐transformed CTLL‐2 cells (Fig. 4C), indicating that MAGI‐1 reduction in Tax1‐transformed cells was a specific phenomenon. A real‐time RT‐PCR assay showed that MAGI‐1 downregulation occurred at the transcriptional level, as shown by the almost undetectable corresponding mRNA levels (Fig. 4D). We previously reported that Tax1ΔC also transforms CTLL‐2, but the activity was much lower than that of Tax1.15 Contrary to our expectations, Tax1ΔC also induced downregulation of MAGI‐1 (Fig. 4C lanes 7–9). To examine if MAGI‐1 downregulation in Tax1‐transformed CTLL‐2 cells is a consequence of IL‐2 withdrawal, we restored IL‐2 in the cultures of Tax1‐transformed cells and checked for the MAGI‐1 expression. IL‐2 restoration could not, however, rescue the expression of MAGI‐1 in two Tax1‐transformed cells (Fig. 5A). We recently reported that a constitutively active Akt1 oncoprotein (hAkt1ΔPH) transforms CTLL‐2 from IL‐2‐dependent growth into IL‐2‐independent growth.39 Like Tax1, MAGI‐1 expression in Akt1‐transformed IL‐2‐independent cells was also downregulated as compared to the untransformed CTLL‐2 cells growing in the presence of IL‐2, and restoration of IL‐2 failed to rescue MAGI‐1 expression in these transformed cells like Tax1‐transformed cells (Fig. 5B). These results suggest that Tax1‐transformation of CTLL‐2 cells is associated with an irreversibly downregulated expression of MAGI‐1.
Figure 4.
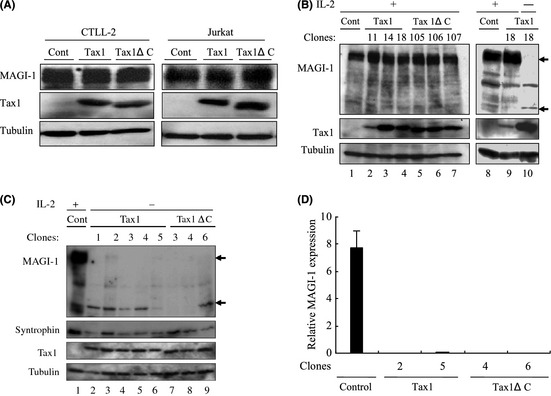
MAGI‐1 downregulation by interleukin (IL)‐2‐independent transformation of T‐cells. (A) CTLL‐2 and Jurkat cells were transfected with lentiviruses encoding Tax1. At 48 h after transfection, cell lysates were prepared and the amounts of MAGI‐1, Tax1 and Tubulin were determined by a Western blotting analysis. (B) CTLL‐2 cells stably expressing Tax1 established as previously described15 were used as IL‐2‐dependent cells (lane 2–9). All the above cells were then transferred to IL‐2 deficient medium and cultured for more than 1 month. Only one Tax1 expressing clone (Tax1–18) survived in the absence of IL‐2 (right panel, lane 10). Cell lysates were prepared from the indicated cells, and protein expression was measured by a Western blotting analysis. (C) CTLL‐2 cells transformed by Tax1 and Tax1ΔC were established as described previously.34 Cell lysates were prepared from these Tax1‐transformed (lanes 2–6), Tax1ΔC‐transformed (lanes 7–9) and parental CTLL‐2 cells (lane 1), and the expressions of MAGI‐1, Tax1, Syntrophin‐β and Tubulin proteins were measured by a Western blotting analysis. (D) A set of clones each transformed by either Tax1 or Tax1ΔC was selected from (C) above, and the relative gene expression of MAGI‐1 was evaluated by the quantitative real‐time polymerase chain reaction (PCR) method. The amounts of MAGI‐1 were normalized to those of glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) expression. The quantitative results are expressed as mean ± standard deviation (SD) of three values per sample. The experiment was independently carried out twice to confirm reproducibility.
Figure 5.
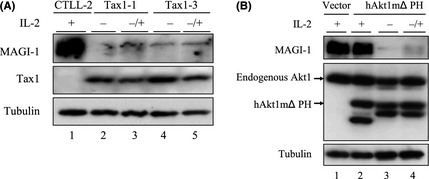
Restoration of interleukin (IL)‐2 cannot rescue MAGI‐1 expression in transformed cells. (A) Two distinct Tax1‐transformed IL‐2‐independent CTLL‐2 cells (clone 1, 3) were cultured with or without IL‐2 for 1 week, and the expression of MAGI‐1, Tax1 and Tubulin in the transformed cells with IL‐2 (lane 3, 5), the transformed cells without IL‐2 (lane 2, 4) and parental CTLL‐2 cells with IL‐2 (lane 1) were measured by a Western blotting analysis. (B) hAkt1 mΔPH‐transformed IL‐2‐independent CTLL‐2 cells were cultured with or without IL‐2 for 1 week, and the expressions of MAGI‐1, Akt1 and Tubulin proteins in the hAkt1 mΔPH‐expressing cells with IL‐2 (lane 2), the hAkt1 mΔPH‐transformed cells without IL‐2 (lane 3), the hAkt1 mΔPH‐transformed cells with IL‐2 (lane 4) as well as the vector‐transduced CTLL‐2 cells cultured in the presence of IL‐2 (lane 1) were measured by a Western blotting analysis.
Human T‐cell leukemia virus type 1 has been shown to immortalize and transform human T‐cells in an IL‐2‐dependent and IL‐2‐independent manners, respectively.9, 10, 16 These findings prompted us to examine the expression status of MAGI‐1 in human T‐cells. As shown, all five HTLV‐1‐transformed T‐cell lines including one transformed by HTLV‐1 with a Tax1 PBM deletion mutant showed lower amounts of MAGI‐1 compared to the three HTLV‐1‐negative cell lines (Fig. 6A). Similar to the observation in Tax1‐transformed CTLL‐2 cells, a real‐time RT‐PCR analysis showed that MAGI‐1 mRNA was significantly reduced in HTLV‐1‐infected cells as compared to the HTLV‐1‐uninfected ones (Fig. 6B). HTLV‐2 is a close ally of HTLV‐1, exhibiting more than 70% similarity at the nucleotide sequence level. Intriguingly, HTLV‐2 does not cause any leukemia or lymphoma in spite of its ability to immortalize human T‐cells in an IL‐2‐dependent manner in vitro as effectively as HTLV‐1.( 17 , 48 ) HTLV‐2 Tax2 protein has also been shown to play a crucial role in the immortalization ability of the virus.17, 18, 43, 49 We established peripheral T‐cells immortalized by either Tax1 or Tax2, and the expression of MAGI‐1 in these cells was determined. Our data indicated that while immortalization of human T‐cells by Tax1 abolished their expression of MAGI‐1, expression of MAGI‐1 was intact in those immortalized by Tax2 similar to HTLV‐1‐uninfected Jurkat and Tax untreated PBMCs (Fig. 6C). Taken together, these findings suggest that the low level MAGI‐1 expression phenotype in HTLV‐1‐transformed cells is as a consequence of Tax1‐mediated immortalization of human T‐cells. We observed a lower molecular weight product that was recognized by the anti‐MAGI‐1 antibody in all Tax1‐ and HTLV‐1‐transformed T‐cells (Figs 4, 6), although the identity and the function remains to be clarified.
Figure 6.
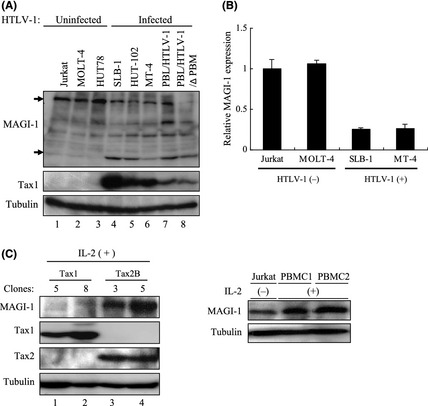
Expression of MAGI‐1 in human T‐cell lines transformed by HTLV‐1. (A) Cell lysates were prepared from five HTLV‐1‐transformed (lanes 4–8) and three human T‐cell leukemia virus type 1 (HTLV‐1)‐negative (lanes 1–3) T‐cell lines. The lysates were subjected to Western blotting analysis with the antibodies indicated. (B) Expression of MAGI‐1 mRNA in two HTLV‐1‐negative and two HTLV‐1‐positive cell lines was measured by the quantitative real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) method. The amounts of MAGI‐1 were normalized to those of glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) expression. The quantitative results are expressed as mean ± standard deviation (SD) of three values per sample. (C) Cell lysates were prepared from two Tax1 and two Tax2‐immortalized T‐cells (left panel) and HTLV‐1 uninfected Jurkat cells and Tax untreated peripheral blood mononuclear cells (PBMCs) (right panel). Western blotting analysis was performed using the corresponding antibodies.
Discussion
Recent research has focused on the PBM of HTLV‐1 Tax1 as a possible determinant of HTLV‐1 pathogenesity in reference to the non‐leukemogenic HTLV‐2 Tax2, which lacks this motif. Tax1 through the PBM has been shown to interact with cellular PDZ domain containing proteins, some of which are implicated in tumor suppression,31, 32, 33 for instance deficiency of Scribble in mice induced prostate cancer through activating MAP kinase pathway.49 Nevertheless, it remains unclear whether these PDZ proteins explain any of the Tax1 PBM functions or other cellular PDZ protein(s) play(s) a major role in Tax1 functions. The present study identifies MAGI‐1 as a novel Tax1 interacting partner and found that Tax1 aberrantly sequesters MAGI‐1 into Tax1 containing complexes and translocates it from the detergent‐soluble to the detergent‐insoluble cellular fraction (Figs 2, 3). Since MAGI‐1 is a member of the MAGUK family of proteins that function to assemble numerous cellular targets into large signaling complexes in cells,24 these results suggest that Tax1, through mislocalizing MAGI‐1, may disrupt the protein complexes and interfere with their cell signaling activities to promote aberrant cell growth of HTLV‐1‐infected cells.
Human T‐cell leukemia virus type 1‐transformed human T‐cells displayed a reduced expression of MAGI‐1 as compared to their HTLV‐1‐uninfected counterparts (Figs 6A,B). In addition, while Tax1 expression in CTLL‐2 cells in the presence of IL‐2 little affected MAGI‐1 expression (Fig. 4B), Tax1‐induced IL‐2‐independent transformation of CTLL‐2 cells downregulated MAGI‐1 expression and the downregulation was not restored by the addition of IL‐2 (Figs 4C, 5A). The downregulations of MAGI‐1 in Tax1‐transformed cells were independent of Tax1 PBM, since Tax1ΔC‐transformed cells also displayed a downregulated expression of MAGI‐1 (Fig. 4C). Moreover, IL‐2‐independent transformation of CTLL‐2 by another oncoprotein hAkt1ΔPH also downregulated MAGI‐1 expression (Fig. 5B). Collectively, these results suggest that Tax1 indirectly downregulates MAGI‐1 in CTLL‐2 cells during the IL‐2‐independent transformation process. Thus, it is tempting to speculate that cells with a low MAGI‐1 expression are selected during the transformation and immortalization of T‐cells by Tax1 or HTLV‐1.
Unlike Tax1, Tax2‐immortalized human T‐cells expressed substantial amounts of MAGI‐1 (Fig. 6C). Our recent study showed that Tax2 immortalizes human T‐cells much more efficiently than Tax1, and the difference is not mediated by the PBM,44 indicating that a difference between Tax1 and Tax2 other than the PBM controls T‐cell immortalization activities by Tax1 and Tax2. Based on these observations, we present a hypothesis to explain the distinct MAGI‐1 expression in Tax1‐immortalized cells versus Tax2‐immortalized cells; Tax2 can immortalize both MAGI‐1‐high and MAGI‐1‐low expressing cells, whereas Tax1 can immortalize only MAGI‐1‐low expressing cells. Further analysis is, however, required to elucidate the mechanism underlining the differential MAGI‐1 expression in Tax1‐ versus Tax2‐immortalized cells. It should be noted that it still remains unclear as to why HTLV‐1 is more pathogenic than HTLV‐2; however, it has been proposed that such a difference in pathogenesity is attributed to the differences in the respective Tax activities. Our current findings add to this notion that the downregulation of MAGI‐1 in Tax1‐immortalized T‐cells contributes to the pathogenic difference between HTLV‐1 and HTLV‐2.
Although the function of MAGI‐1 in T‐cells remains unknown, Tax1 can interfere with the activities of MAGI‐1 via altering the subcellular localization and downregulating the mRNA. Further research is necessary to clarify the role of MAGI‐1 in T‐cell functions and more specifically in HTLV‐1 pathogenesis. Nevertheless, MAGI‐1 has been proposed to play tumour suppressor functions in other systems. For instance, MAGI‐1 but not the related MAGI‐2/3 suppressed the transformation of baby rat kidney cells by EJ‐ras oncoprotein together with either HPV‐16 E6 or adenovirus E1A oncoprotein.32 Moreover, downregulation of MAGI‐1 was associated with poor prognosis of hepatocellular carcinoma.50 Two other viral oncoproteins, adenovirus‐9 E4‐ORF1 and high‐risk HPV E6 were also shown to sequester MAGI‐1 in the cytoplasm and target it for degradation, respectively.37 It is therefore not surprising to speculate that MAGI‐1 plays a tumour suppressor function in T‐cells.
In summary, we report herein that similar to other viral oncoproteins, Tax1 can inactivate functions of the PDZ protein MAGI‐1 by alteration of its subcellular localization and selection of cells with its downregulated mRNA expression in transformation. Since MAGI‐1 has been implicated in tumour suppression in other systems, this can be as a mechanism to suppress its potential anti‐tumor functions in HTLV‐1‐infected cells.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
We appreciate Dr H. Miyoshi, Dr L. Banks, Dr R. Mahieux, Dr W. Hall and Takeda Pharmaceutical Company for providing us with the respective reagents. We also express our gratitude to Misako Tobimatsu for her technical assistance. This research was supported in part by the Ministry of Education, Science, Sports and Culture of Japan and a Grant from the Niigata University Kyowakai Society.
(Cancer Sci, doi: 10.1111/cas.12087, 2013)
References
- 1. Yao J, Wigdahl B. Human T cell lymphotropic virus type I genomic expression and impact on intracellular signaling pathways during neurodegenerative disease and leukemia. Front Biosci 2000; 5: D138–68. [DOI] [PubMed] [Google Scholar]
- 2. Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T‐cell leukemia and its implication in the disease. Proc Natl Acad Sci USA 1982; 79: 2031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsuoka M, Jeang KT. Human T‐cell leukaemia virus type 1 (HTLV‐1) infectivity and cellular transformation. Nat Rev Cancer 2007; 7: 270–80. [DOI] [PubMed] [Google Scholar]
- 4. Grassmann R, Aboud M, Jeang KT. Molecular mechanisms of cellular transformation by HTLV‐1 Tax. Oncogene 2005; 24: 5976–85. [DOI] [PubMed] [Google Scholar]
- 5. Nerenberg M, Hinrichs SH, Reynolds RK, Khoury G, Jay G. The tat gene of human T‐lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 1987; 237: 1324–9. [DOI] [PubMed] [Google Scholar]
- 6. Iwanaga Y, Tsukahara T, Ohashi T et al Human T‐cell leukemia virus type 1 tax protein abrogates interleukin‐2 dependence in a mouse T‐cell line. J Virol 1999; 73: 1271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasegawa H, Sawa H, Lewis MJ et al Thymus‐derived leukemia‐lymphoma in mice transgenic for the Tax gene of human T‐lymphotropic virus type I. Nat Med 2006; 12: 466–72. [DOI] [PubMed] [Google Scholar]
- 8. Ohsugi T, Kumasaka T, Okada S, Urano T. The Tax protein of HTLV‐1 promotes oncogenesis in not only immature T cells but also mature T cells. Nat Med 2007; 13: 527–8. [DOI] [PubMed] [Google Scholar]
- 9. Grassmann R, Berchtold S, Radant I et al Role of human T‐cell leukemia virus type 1× region proteins in immortalization of primary human lymphocytes in culture. J Virol 1992; 66: 4570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akagi T, Shimotohno K. Proliferative response of Tax1‐transduced primary human T cells to anti‐CD3 antibody stimulation by an interleukin‐2‐independent pathway. J Virol 1993; 67: 1211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robek MD, Ratner L. Immortalization of CD4(+) and CD8(+) T lymphocytes by human T‐cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J Virol 1999; 73: 4856–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rousset R, Fabre S, Desbois C, Bantignies F, Jalinot P. The C‐terminus of the HTLV‐1 Tax oncoprotein mediates interaction with the PDZ domain of cellular proteins. Oncogene 1998; 16: 643–54. [DOI] [PubMed] [Google Scholar]
- 13. Suzuki T, Ohsugi Y, Uchida‐Toita M, Akiyama T, Yoshida M. Tax oncoprotein of HTLV‐1 binds to the human homologue of Drosophila discs large tumor suppressor protein, hDLG, and perturbs its function in cell growth control. Oncogene 1999; 18: 5967–72. [DOI] [PubMed] [Google Scholar]
- 14. Hirata A, Higuchi M, Niinuma A et al PDZ domain‐binding motif of human T‐cell leukemia virus type 1 Tax oncoprotein augments the transforming activity in a rat fibroblast cell line. Virology 2004; 318: 327–36. [DOI] [PubMed] [Google Scholar]
- 15. Tsubata C, Higuchi M, Takahashi M et al PDZ domain‐binding motif of human T‐cell leukemia virus type 1 Tax oncoprotein is essential for the interleukin 2 independent growth induction of a T‐cell line. Retrovirology 2005; 2: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xie L, Yamamoto B, Haoudi A, Semmes OJ, Green PL. PDZ binding motif of HTLV‐1 Tax promotes virus‐mediated T‐cell proliferation in vitro and persistence in vivo . Blood 2006; 107: 1980–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feuer G, Green PL. Comparative biology of human T‐cell lymphotropic virus type 1 (HTLV‐1) and HTLV‐2. Oncogene 2005; 24: 5996–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hall WW, Fujii M. Deregulation of cell‐signaling pathways in HTLV‐1 infection. Oncogene 2005; 24: 5965–75. [DOI] [PubMed] [Google Scholar]
- 19. Higuchi M, Fujii M. Distinct functions of HTLV‐1 Tax1 from HTLV‐2 Tax2 contribute key roles to viral pathogenesis. Retrovirology 2009; 6: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee SS, Weiss RS, Javier RT. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA 1997; 94: 6670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tomaić V, Gardiol D, Massimi P, Ozbun M, Myers M, Banks L. Human and primate tumour viruses use PDZ binding as an evolutionarily conserved mechanism of targeting cell polarity regulators. Oncogene 2009; 28: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high‐risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA 1997; 94: 11612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Craven SE, Bredt DS. PDZ proteins organize synaptic signaling pathways. Cell 1998; 93: 495–8. [DOI] [PubMed] [Google Scholar]
- 24. Fanning AS, Anderson JM. PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest 1999; 103: 767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giallourakis C, Cao Z, Green T et al A molecular‐properties‐based approach to understanding PDZ domain proteins and PDZ ligands. Genome Res 2006; 16: 1056–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas M, Laura R, Hepner K et al Oncogenic human papillomavirus E6 proteins target the MAGI‐2 and MAGI‐3 proteins for degradation. Oncogene 2002; 21: 5088–96. [DOI] [PubMed] [Google Scholar]
- 27. Gardiol D, Kühne C, Glaunsinger B, Lee SS, Javier R, Banks L. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome‐mediated degradation. Oncogene 1999; 18: 5487–96. [DOI] [PubMed] [Google Scholar]
- 28. Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin‐mediated degradation by the high‐risk papillomavirus E6 proteins and the E6AP ubiquitin‐protein ligase. Mol Cell Biol 2000; 20: 8244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Javier RT. Cell polarity proteins: common targets for tumorigenic human viruses. Oncogene 2008; 27: 7031–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okajima M, Takahashi M, Higuchi M et al Human T‐cell leukemia virus type 1 Tax induces an aberrant clustering of the tumor suppressor Scribble through the PDZ domain‐binding motif dependent and independent interaction. Virus Genes 2008; 37: 231–40. [DOI] [PubMed] [Google Scholar]
- 31. Senda T, Shimomura A, Iizuka‐Kogo A. Adenomatous polyposis coli (Apc) tumor suppressor gene as a multifunctional gene. Anat Sci Int 2005; 80: 121–31. [DOI] [PubMed] [Google Scholar]
- 32. Massimi P, Gammoh N, Thomas M, Banks L. HPV E6 specifically targets different cellular pools of its PDZ domain‐containing tumour suppressor substrates for proteasome‐mediated degradation. Oncogene 2004; 23: 8033–9. [DOI] [PubMed] [Google Scholar]
- 33. Ishidate T, Matsumine A, Toyoshima K, Akiyama T. The APC‐hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene 2000; 19: 365–72. [DOI] [PubMed] [Google Scholar]
- 34. Ishioka K, Higuchi M, Takahashi M et al Inactivation of tumor suppressor Dlg1 augments transformation of a T‐cell line induced by human T‐cell leukemia virus type 1 Tax protein. Retrovirology 2006; 3: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dobrosotskaya I, Guy RK, James GL. MAGI‐1, a membrane‐associated guanylate kinase with a unique arrangement of protein‐protein interaction domains. J Biol Chem 1997; 272: 31589–97. [DOI] [PubMed] [Google Scholar]
- 36. Kotelevets L, van Hengel J, Bruyneel E, Mareel M, van Roy F, Chastre E. Implication of the MAGI‐1b/PTEN signalosome in stabilization of adherens junctions and suppression of invasiveness. FASEB J 2005; 19: 115–7. [DOI] [PubMed] [Google Scholar]
- 37. Glaunsinger BA, Lee SS, Thomas M, Banks L, Javier R. Interactions of the PDZ‐protein MAGI‐1 with adenovirus E4‐ORF1 and high‐risk papillomavirus E6 oncoproteins. Oncogene 2000; 19: 5270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kranjec C, Banks L. A systematic analysis of human papillomavirus (HPV) E6 PDZ substrates identifies MAGI‐1 as a major target of HPV type 16 (HPV‐16) and HPV‐18 whose loss accompanies disruption of tight junctions. J Virol 2011; 85: 1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoshita M, Higuchi M, Takahashi M, Oie M, Tanaka Y, Fujii M. Activation of mTOR by human T‐cell leukemia virus type 1 Tax is important for the transformation of mouse T cells to interleukin‐2‐independent growth. Cancer Sci 2012; 2: 369–74. [DOI] [PubMed] [Google Scholar]
- 40. Endo K, Hirata A, Iwai K et al Human T‐cell leukemia virus type 2 (HTLV‐2) Tax protein transforms a rat fibroblast cell line but less efficiently than HTLV‐1 Tax. J Virol 2002; 76: 2648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Higuchi M, Tsubata C, Kondo R et al Cooperation of NF‐kappaB2/p100 activation and the PDZ domain binding motif signal in human T‐cell leukemia virus type 1 (HTLV‐1) Tax1 but not HTLV‐2 Tax2 is crucial for interleukin‐2‐independent growth transformation of a T‐cell line. J Virol 2007; 81: 11900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanaka Y, Yoshida A, Tozawa H, Shida H, Nyunoya H, Shimotohno K. Production of a recombinant human T‐cell leukemia virus type‐I trans‐activator (tax1) antigen and its utilization for generation of monoclonal antibodies against various epitopes on the tax1 antigen. Int J Cancer 1991; 48: 623–30. [DOI] [PubMed] [Google Scholar]
- 43. Meertens L, Chevalier S, Weil R, Gessain A, Mahieux R. A 10‐amino acid domain within human T‐cell leukemia virus type 1 and type 2 tax protein sequences is responsible for their divergent subcellular distribution. J Biol Chem 2004; 279: 43307–20. [DOI] [PubMed] [Google Scholar]
- 44. Imai M, Higuchi M, Kawamura H et al Human T cell leukemia virus type 2 (HTLV‐2) Tax2 has a dominant activity over HTLV‐1 Tax1 to immortalize human CD4(+) T cells. Virus Genes 2012, doi: 10.1007/s11262-012-0831-9. [DOI] [PubMed] [Google Scholar]
- 45. Ohashi M, Sakurai M, Higuchi M et al Human T‐cell leukemia virus type 1 Tax oncoprotein induces and interacts with a multi‐PDZ domain protein, MAGI‐3. Virology 2004; 320: 52–62. [DOI] [PubMed] [Google Scholar]
- 46. Arpin‐Andre C, Mesnard JM. The PDZ domain‐binding motif of the human T cell leukemia virus type 1 tax protein induces mislocalization of the tumor suppressor hScrib in T cells. J Biol Chem 2007; 282: 33132–41. [DOI] [PubMed] [Google Scholar]
- 47. Aoyagi T, Takahashi M, Higuchi M et al The PDZ domain binding motif (PBM) of human T‐cell leukemia virus type 1 Tax can be substituted by heterologous PBMs from viral oncoproteins during T‐cell transformation. Virus Genes 2010; 40: 193–9. [DOI] [PubMed] [Google Scholar]
- 48. Ross TM, Pettiford SM, Green PL. The tax gene of human T‐cell leukemia virus type 2 is essential for transformation of human T lymphocytes. J Virol 1996; 70: 5194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pearson HB, Perez‐Mancera PA, Dow LE et al SCRIB expression is deregulated in human prostate cancer, and its deficiency in mice promotes prostate neoplasia. J Clin Invest 2011; 121: 4257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang G, Liu T, Wang Z. Downregulation of MAGI1 associates with poor prognosis of hepatocellular carcinoma. J Invest Surg 2012; 25: 93–9. [DOI] [PubMed] [Google Scholar]


