Abstract
Tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL) has been recognized as a promising target for cancer therapy because it can induce apoptotic cell death in tumor cells but not normal cells. Although TRAIL shows specific tumoricidal activity, resistance to TRAIL‐induced apoptosis in some tumor cells has been considered a clinical obstacle of its application. It has been shown that TRAIL provides inflammatory signals that may contribute to the TRAIL‐resistance of cancer cells; however, it is not known whether TRAIL itself is involved in malignant cancer cell behavior. In the present study, we examined the functional role of TRAIL in B16F10 mouse melanoma cells, which are totally insensitive to TRAIL‐induced apoptosis. By establishing B16F10 cells stably expressing the nuclear factor‐κB (NFκB)‐luciferase reporter gene, we found that TRAIL can activate NFκB through its death receptor DR5 in B16F10 cells. Furthermore, TRAIL–DR5 interaction not only promoted malignant behaviors of B16F10 cells, such as cell proliferation and MMP‐9 production, but also induced lung metastasis of B16F10 cells in vivo. These findings may imply a contrary role for the TRAIL–DR5 pathway in the inflammatory tumor microenvironment, in its ability to induce the metastatic potential of B16F10 melanoma cells instead of inducing apoptosis.
Tumor necrosis factor (TNF)‐related apoptosis‐inducing ligand (TRAIL), also known as Apo2 ligand, is a type II transmembrane protein belonging to the TNF family1, 2 of cytokines that play important roles in inflammation and immunity.3, 4, 5, 6 It has been recognized as a promising target for cancer therapy, because TRAIL can induce apoptotic cell death in a variety of tumor cells but not in most normal cells.7, 8, 9 Some studies have shown that this ligand has the potential to suppress the metastatic ability of cancer cells.10, 11 So far, two cell death‐inducing receptors (TRAIL‐R1/DR4, TRAIL‐R2/DR5) and two non‐cell death‐inducing receptors (TRAIL‐R3/DcR1, TRAIL‐R4/DcR2) have been identified for TRAIL in humans; the latter two of these may act as decoys.5, 6, 7, 12, 13 In mice, only one death‐inducing receptor homologous to human DR5 (mTRAIL‐R2/mDR5), and two potential decoy receptors have been identified.4, 14 These death receptors signal apoptosis through a Fas‐associated death domain and the caspase‐8‐dependent pathway.4, 6, 7, 13, 15, 16 Moreover, the cytoplasmic regions of DR5 and mDR5 contain potential TNF receptor‐associated factor (TRAF)‐binding motifs, which may be responsible for NFκB and MAPK activation by this receptor.13, 15, 16, 17, 18
Although TRAIL has shown specific tumoricidal activity, some cancer cells are totally insensitive to TRAIL‐induced apoptosis and such resistance may account for a clinical obstacle. Some studies have shown that the resistance to TRAIL‐induced apoptosis is caused by lower expression levels of functional TRAIL receptors.19, 20, 21, 22 The B16F10 murine melanoma cell line is known to show resistance in spite of the high expression of mDR5 on the cell surface.23, 24 Therefore, the effects of TRAIL on B16F10 cells have not been comprehensively explored.
As a critical transcription factor for inflammation, NFκB regulates the expression of pro‐inflammatory genes associated with invasion, angiogenesis, and metastasis.25, 26 Some reports indicated that the activation of NFκB maintains resistance to TRAIL‐induced apoptosis.19, 27, 28, 29 However, it is not known whether the TRAIL pathway is involved in cancer cell behavior by providing inflammatory signals.
In the present study, we investigated the role of NFκB‐mediated inflammatory signals in cancer progression, particularly through the TRAIL–DR5 receptor pathway in B16F10 melanoma cells. We found that TRAIL activated the NFκB pathway through DR5 in B16F10 cells and induced a tumor‐promoting effect with MMP‐9 production, proliferation ability in vitro, and also induced lung metastasis potential in vivo, instead of inducing apoptosis.
Materials and Methods
Reagents
Tumor necrosis factor‐α was purchased from Peprotech (Rocky Hill, NJ, USA). The pGL4.32 (luc2P/NF‐kappaB‐RE/Hygro) vector and d‐luciferin were obtained from Promega (Madison, WI, USA). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA, USA). Hygromycin B was obtained from Nacalai Tesque (Kyoto, Japan). Anti‐TRAIL (N2B2) and anti‐DR5 (MD5‐1) antibodies were purchased from Biolegend (San Diego, CA, USA).
Cells
Mouse melanoma B16F10 cells were maintained in DMEM and Ham's F12 medium containing 10% bovine serum (Nissui, Tokyo, Japan). Mouse B lymphoma 2PK3 cells and 2PK3 expressing mouse TRAIL (TRAIL‐2PK3) cells were cultured in RPMI‐1640 (Nissui) containing 0.03% l‐glutamine, 0.01 M HEPES, 0.2% NaHCO3, and 10% bovine serum. To establish NFκB‐mediated luciferase gene expressing B16F10 cells (B16F10 NFκB), B16F10 cells (5 × 105/well) were seeded in a 6‐well plate and pGL4.32 vector was transfected using Lipofectamine 2000. The cells were selected with Hygromycin B (200 μg/mL) and cloned by limiting dilution. To evaluate the response of NFκB in vitro, B16F10 NFκB transfectants or B16F10 CMV control cells (1 × 105/well) were cultured in a 96‐well plate and treated with TNF‐α (0.1–100 ng/mL). After incubation for 6 h, luciferase activity was measured with a multiplate reader (2030 ARVO X; Perkin Elmer Life Sciences, Boston, MA, USA).
Analysis of NFκB activation with TRAIL
The B16F10 NFκB cells (1.25 × 105/well) were cocultured with either TRAIL‐expressing 2PK3 (TRAIL‐2PK3) cells or control 2PK3 cells (1.25 × 105, 6.25 × 104, 1.25 × 104/well) in a 96‐well plate. After 6 h incubation, luciferase activity was measured with a multiplate reader. To evaluate the specificity of TRAIL on NFκB activation in B16F10 cells, TRAIL‐2PK3 (1 × 105/well) cells were pretreated with anti‐TRAIL mAb (clone N2B2, 10 μg/mL) at 37°C for 1 h then cocultured with B16F10 NFκB cells at 37°C for 6 h. After the incubation, luciferase activity was measured with a multiplate reader.
Cell proliferation assay
The B16F10 CMV cells (5 × 103/well) were cocultured with TRAIL‐2PK3 or control 2PK3 cells (1 × 104, 5 × 103, 2.5 × 103/well) in a 96‐well plate for 48 h at 37°C. After the incubation, luciferase activity was measured with a multiplate reader.
Gelatin zymography
The B16F10 NFκB cells (5 × 105/well) were cultured with serum‐free medium in a 24‐well plate then treated with TNF‐α (50 ng/mL) or cocultured with TRAIL‐2PK3 (5 × 105/well) for another 48 h. After the incubation, cell‐free supernatants were collected and mixed with sample buffer containing 2% SDS (without 2‐mercaptoethanol) and incubated at 37°C for 20 min. Comparative gelatin zymography was carried out on 10% SDS‐PAGE with 0.1% gelatin. Samples were electrophoresed at 10 mA for 4–5 h at 4°C. Gels were washed with buffer containing 2.5% Triton X‐100 and 0.01 M Tris‐HCl for 2 h at 4°C and washed with 0.01 M Tris‐HCl for 40 min at room temperature. Gels were incubated in the buffer containing 0.05 M Tris‐HCl, 0.5 mM CaCl2, and 1 μM ZnCl2 for 48 h at 37°C. After the incubation, gels were stained with Coomassie Brilliant Blue for 6 h and destained with 5% acetic acid and 10% methanol. The bands were quantified using ImageQuant LAS 4010 (GE Healthcare Japan, Tokyo, Japan).
Experimental lung metastasis model
Inbred wild‐type C57BL/6 mice were purchased from Japan SLC (Tokyo, Japan). All experiments were carried out according to the guidelines of the Care and Use of Laboratory Animals of the University of Toyama (Toyama, Japan). The B16F10 CMV cells were inoculated i.v. (3 × 105) with or without pretreatment with anti‐DR5 mAb (30 min, 4°C). Mice were injected with d‐luciferin (150 mg/kg i.p.; Promega) 4 days after the tumor inoculation, then the lungs were removed 20 min after the d‐luciferin injection to measure luminescence using an in vivo imaging system (IVIS Lumina II; Caliper Life Sciences, Hopkinton, MA, USA). The data was presented as the mean luminescence ± SEM.
Statistical analysis
Data were analyzed for statistical significance using Student's t‐test. P‐values <0.05 were considered significant.
Results
Establishment of NFκB‐mediated luciferase gene stably expressing B16F10 cells
In order to determine whether TRAIL–DR5 interaction may have biological roles in B16F10 metastatic melanoma cells through NFκB‐mediated inflammatory signals, we established luciferase gene‐expressing B16F10 cells under an NFκB reporter (B16F10 NFκB cells). We first characterized the association between cell numbers and luciferase activity of B16F10 NFκB cells or control B16F10 CMV cells under stable cell culture conditions. There was a strong correlation between luciferase activity and cell number not only in B16F10 CMV cells (Fig. S1A,B) but also in B16F10 NFκB cells (Fig. S1C,D). These results clearly indicated that the luminescence represents cell number or viability without any stimulation in those reporter cells.
We further examined the response of B16F10 NFκB cells to TNF‐α, known to be a typical inflammatory cytokine to activate the NFκB pathway. As shown in Figure 1, TNF‐α induced luciferase activity in a dose‐dependent manner (Fig. 1a) and appeared to have a peak response at 6–8 h after the TNF‐α stimulation (Fig. 1b). Such induction of luciferase activity in response to TNF‐α was specific for B16F10 cells expressing NFκB reporter, because B16F10 CMV cells did not show any response in its luciferase activity after TNF‐α stimulation (Fig. 1c). These results indicate that B16F10 NFκB cells but not B16F10 CMV cells induce their luminescence in response to inflammatory stimulation through the NFκB pathway.
Figure 1.
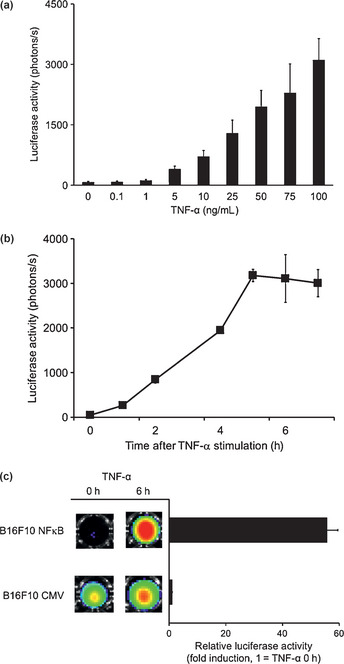
Nuclear factor‐κB (NFκB) activation after tumor necrosis factor‐α (TNF‐α) treatment in B16F10 mouse melanoma cells. (a) B16F10 NFκB cells were incubated with indicated concentrations of TNF‐α for 6 h and the luminescence was measured. (b) B16F10 NFκB cells were treated with TNF‐α (100 ng/mL) and the luminescence was measured at the indicated time after the TNF‐α stimulation. (c) B16F10 NFκB and B16F10 CMV cells were stimulated TNF‐α (100 ng/mL) for 6 h. The luminescence was measured at 0 h and 6 h after TNF‐α stimulation. Error bars represent SEM.
Interaction between TRAIL and DR5 activates NFκB in B16F10 cells
It is known that highly metastatic B16F10 melanoma cells are resistant to TRAIL‐induced apoptosis despite their expression of DR5 receptor (Fig. S1E,F). To investigate whether TRAIL–DR5 interaction activates the inflammatory signaling pathway in B16F10 cells, we tested B16F10 NFκB cells to monitor NFκB activation in response to TRAIL stimulation. After coculture with TRAIL‐2PK3 transfectants, B16F10 NFκB cells showed increased luminescence, but not with control 2PK3 cells (Fig. 2a). The reporter activity was associated with the amount of TRAIL availability within the coculture (Fig. 2a). Importantly, such induction of reporter activity was diminished in the presence of anti‐TRAIL mAb (Fig. 2b). Furthermore, the activation of TRAIL receptor by agonistic anti‐DR5 mAb also activated NFκB reporter in a dose‐dependent manner (Fig. S2). Collectively, these results indicate that TRAIL–DR5 interaction activates the NFκB pathway in B16F10 cells.
Figure 2.
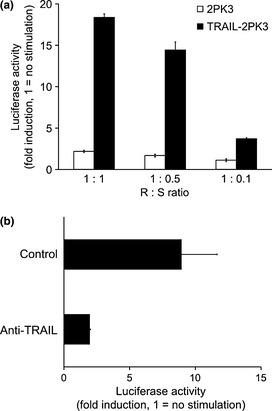
Nuclear factor‐κB (NFκB) activation through tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL)–DR5 interaction in B16F10 mouse melanoma cells. (a) B16F10 NFκB cells were cocultured with TRAIL‐2PK3 or 2PK3 at indicated responder (R):stimulator (S) ratios (B16F10 NFκB : TRAIL‐2PK3 or 2PK3). After 6 h incubation, the luminescence was measured. (b) B16F10 NFκB cells were cocultured with TRAIL‐2PK3 (at R:S 1:1) and N2B2 (10 μg/mL). After 6 h incubation, the luminescence was measured. Error bars represent SEM.
Interaction between TRAIL and DR5 functionally activates B16F10 cells
We next examined whether TRAIL shows any functional roles in B16F10 cells in association with NFκB activation. In concert with NFκB activation, the proliferation rate of B16F10 cells was increased after 48 h of coculture with TRAIL‐2PK3 cells but not with control 2PK3 cells (Fig. 3a). In addition to its activity in promoting proliferation, TRAIL–DR5 interaction also increased the production of MMP‐9 from B16F10 cells, which is known to be a typical target molecule for NFκB activation. As shown in Figure 3(B), the activity of MMP‐9 in the cell culture supernatant of TRAIL‐stimulated B16F10 cells was higher than that of the control, and the induction of MMP‐9 by TRAIL stimulation was comparable to TNF‐α. These results clearly indicate that TRAIL–DR5 functionally activates B16F10 cells to facilitate their proliferation and MMP‐9 production.
Figure 3.
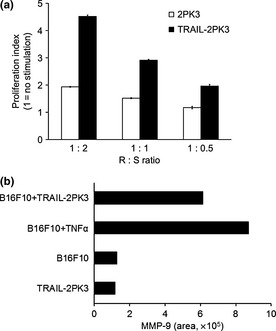
Functional roles of the tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL)–DR5 pathway in B16F10 mouse melanoma cells. (a) B16F10 CMV cells were cocultured with TRAIL‐2PK3 or control 2PK3 cells at responder (R):stimulator (S) ratio of 1:1 for 48 h and luminescence was measured. Error bars represent SEM. (b) B16F10 cells were stimulated with tumor necrosis factor‐α (TNF‐α; 50 ng/mL) or cocultured with TRAIL‐2PK3 (at R:S 1:1) for 48 h, and the cell‐free supernatant was collected. Gelatin zymography was used to determine MMP‐9 production and the band intensity was measured.
Finally, we examined the physiological significance of TRAIL–DR5 interactions in cancer metastasis. In an experimental lung metastasis model of B16F10 melanoma cells, we found that the activation of TRAIL receptor by pretreatment with agonistic anti‐DR5 mAb enhanced the metastatic colonization of B16F10 cells (Fig. 4). Together with the functional role of TRAIL engagement in B16F10 cells in vitro, these results strongly imply that TRAIL–DR5 interactions have a physiological potential to enhance metastasis of B16F10 melanoma cells rather than to induce apoptosis of the cells expressing these receptors.
Figure 4.
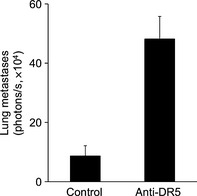
Stimulation of tumor necrosis factor‐related apoptosis‐inducing ligand receptor enhances experimental metastasis of B16F10 mouse melanoma cells. B16F10 CMV cells were inoculated i.v. with or without pretreatment with anti‐DR5 mAb. Lungs were removed 4 days after tumor inoculation to measure luminescence for determining lung metastasis. Data presented as the mean luminescence ± SEM.
Discussion
Tumor necrosis factor‐related apoptosis‐inducing ligand is associated with one of the important effector pathways in the tumor immune surveillance, and the TRAIL signal has been known to induce the suppression of tumor metastasis by inducing apoptosis of malignantly transformed cells.4, 10, 12, 30 In contrast, it is also suggested that TRAIL may be involved in cancer cell activation by providing inflammatory signals similar to other TNF superfamily members.3, 13, 15, 16, 17, 18 Previous studies have indicated that NFκB activation can be critical for acquiring resistance to TRAIL‐induced apoptosis in some tumor cells.19, 27, 28 In the present study, we showed the contribution of TRAIL–DR5 interaction to the activation of the NFκB pathway in B16F10 mouse melanoma cells, which is resistant to the TRAIL‐induced apoptosis pathway. The TRAIL–DR5 interaction also plays a functional role in B16F10 cells by inducing their proliferation, MMP‐9 production, and acquisition of metastatic potential in vivo.
It has been shown that cancer cells can evade TRAIL‐induced apoptosis or acquire TRAIL resistance through several different mechanisms.6, 7, 12, 15 One particular mechanism can be the lower expression of death receptors for TRAIL, such as DR4 and DR5.19, 20, 21, 22 Furthermore, the intrinsic activation of anti‐apoptotic machinery was also shown to be involved in acquiring TRAIL resistance in cancer cells.12, 31, 32, 33 It is also suggested that NFκB can be a key regulator for the expression of pro‐inflammatory genes, including those for cancer cell proliferation and survival.25, 26 Despite the significant expression of DR5 on their cell surface,24 murine B16F10 melanoma cells were known to be resistant to TRAIL inducing apoptosis.23 Our present results clearly show that TRAIL stimulation in B16F10 cells activates NFκB and further promotes their cellular functions, including MMP‐9 production and proliferation, which might contribute to cancer progression and metastasis. We did not find significant differences in B16F10 proliferation after a relatively short time (24 h) of coculture with TRAIL‐2PK3 cells (data not shown), therefore, TRAIL–DR5 interaction may require more persistent interaction with its receptor in promoting B16F10 cell proliferation compared to its induction of apoptosis, which is generally seen 8–16 h after TRAIL ligation. Considering the TRAIL receptor ligation activates the NFκB pathway through interaction with the TNF receptor 1‐associated death domain adaptor protein to recruit receptor‐interacting protein kinase and TRAF2,5, 13, 15, 17 similar mechanisms might underlie the TRAIL‐induced activation of NFκB in B16F10 cells to induce such cellular functions. Further study will be required to determine which signaling pathway is involved in the functional activation of B16F10 cells in response to TRAIL. Known to specifically express on host immune cells such as natural killer cells, dendritic cells, and activated T cells, TRAIL plays an important role in antitumor immune responses.23, 34, 35, 36, 37 In contrast, we have shown that B16F10 melanoma cells may use TRAIL–DR5 interaction to promote their metastatic potential. Consistent with our current finding, it has been reported that TRAIL enhanced survival and/or proliferation in TRAIL‐resistant primary leukemia cells in an NFκB‐dependent manner.38 Interestingly, it has also been reported that the NFκB pathway plays a role in the induction and maintenance of epithelial–mesenchymal transition,26, 39, 40 considered to be an important process of tumor invasion and metastasis spread. Furthermore, the metastasis of TRAIL‐resistant human pancreatic ductal carcinoma was promoted by TRAIL in a xenograft model.41 Additional study is clearly required to determine whether endogenous TRAIL could be involved in the malignant progression of B16 melanoma cells; however, our current findings support a contrary role of the TRAIL–DR5 pathway in the inflammatory tumor microenvironment, in inducing the metastatic potential of cancer cells rather than inducing apoptosis in B16 melanoma cells. Considering several clinical trials of agonistic human TRAIL receptor antibodies have been undertaken to test their efficacy in cancer patients,42, 43, 44, 45, 46 it would be very important to characterize patients' cancer cell types in their response to TRAIL‐induced apoptosis. Collectively, our present findings propose a pro‐tumor role of TRAIL–DR5 interaction in murine B16F10 melanoma cells by enhancing metastatic potential. Thus, a careful approach is required in the clinical application of the TRAIL pathway in cancer treatment, especially in TRAIL apoptosis‐resistant cancer cell types.
Supporting information
Fig. S1. Establishment of B16F10 CMV and B16F10 nuclear factor‐κB (NFκB) mouse melanoma cells.
Fig. S2. Nuclear factor‐κB (NFκB) activation by DR5 stimulation in B16F10 mouse melanoma cells.
Acknowledgments
We are grateful to Kaori Denda‐Nagai and Nobuaki Higashi for their discussion, and Satomi Yoshinaga for her technical assistance. This work was supported by the Suzuken Memorial Foundation.
Disclosure Statement
The authors have no conflict of interest.
(Cancer Sci 2013; 104: 558–562)
References
- 1. Wiley SR, Schooley K, Smolak PJ et al Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995; 3: 673–82. [DOI] [PubMed] [Google Scholar]
- 2. Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo‐2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 1996; 271: 12687–90. [DOI] [PubMed] [Google Scholar]
- 3. Ashkenazi A. Targeting death and decoy receptors of the tumour‐necrosis factor superfamily. Nat Rev Cancer 2002; 2: 420–30. [DOI] [PubMed] [Google Scholar]
- 4. Yagita H, Takeda K, Hayakawa Y, Smyth MJ, Okumura K. TRAIL and its receptors as targets for cancer therapy. Cancer Sci 2004; 95: 777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene 2010; 29: 4752–65. [DOI] [PubMed] [Google Scholar]
- 6. Wang S, El‐Deiry WS. TRAIL and apoptosis induction by TNF‐family death receptors. Oncogene 2003; 22: 8628–33. [DOI] [PubMed] [Google Scholar]
- 7. Holoch PA, Griffith TS. TNF‐related apoptosis‐inducing ligand (TRAIL): a new path to anti‐cancer therapies. Eur J Pharmacol 2009; 625: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashkenazi A, Pai RC, Fong S et al Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999; 104: 155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ichikawa K, Liu W, Zhao L et al Tumoricidal activity of a novel anti‐human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med 2001; 7: 954–60. [DOI] [PubMed] [Google Scholar]
- 10. Seki N, Hayakawa Y, Brooks AD et al Tumor necrosis factor‐related apoptosis‐inducing ligand‐mediated apoptosis is an important endogenous mechanism for resistance to liver metastases in murine renal cancer. Cancer Res 2003; 63: 207–13. [PubMed] [Google Scholar]
- 11. Takeda K, Hayakawa Y, Smyth MJ et al Involvement of tumor necrosis factor‐related apoptosis‐inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med 2001; 7: 94–100. [DOI] [PubMed] [Google Scholar]
- 12. Dimberg LY, Anderson CK, Camidge R, Behbakht K, Thorburn A, Ford HL. On the TRAIL to successful cancer therapy? Predicting and counteracting resistance against TRAIL‐based therapeutics. Oncogene 2013; 32: 1341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Falschlehner C, Emmerich CH, Gerlach B, Walczak H. TRAIL signalling: decisions between life and death. Int J Biochem Cell Biol 2007; 39: 1462–75. [DOI] [PubMed] [Google Scholar]
- 14. Schneider P, Olson D, Tardivel A et al Identification of a new murine tumor necrosis factor receptor locus that contains two novel murine receptors for tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL). J Biol Chem 2003; 278: 5444–54. [DOI] [PubMed] [Google Scholar]
- 15. Hersey P, Zhang XD. How melanoma cells evade trail‐induced apoptosis. Nat Rev Cancer 2001; 1: 142–50. [DOI] [PubMed] [Google Scholar]
- 16. Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD‐dependent apoptosis and activate the NF‐kappaB pathway. Immunity 1997; 7: 821–30. [DOI] [PubMed] [Google Scholar]
- 17. Hu WH, Johnson H, Shu HB. Tumor necrosis factor‐related apoptosis‐inducing ligand receptors signal NF‐kappaB and JNK activation and apoptosis through distinct pathways. J Biol Chem 1999; 274: 30603–10. [DOI] [PubMed] [Google Scholar]
- 18. Schneider P, Thome M, Burns K et al TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD‐dependent apoptosis and activate NF‐kappaB. Immunity 1997; 7: 831–6. [DOI] [PubMed] [Google Scholar]
- 19. Khanbolooki S, Nawrocki ST, Arumugam T et al Nuclear factor‐kappaB maintains TRAIL resistance in human pancreatic cancer cells. Mol Cancer Ther 2006; 5: 2251–60. [DOI] [PubMed] [Google Scholar]
- 20. Büneker C, Mohr A, Zwacka RM. The TRAIL‐receptor‐1: TRAIL‐receptor‐3 and ‐4 ratio is a predictor for TRAIL sensitivity of cancer cells. Oncol Rep 2009; 21: 1289–95. [DOI] [PubMed] [Google Scholar]
- 21. Nguyen T, Zhang XD, Hersey P. Relative resistance of fresh isolates of melanoma to tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL)‐induced apoptosis. Clin Cancer Res 2001; 7: 966s–73s. [PubMed] [Google Scholar]
- 22. Zhang XD, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF‐related apoptosis‐inducing ligand (TRAIL) receptor and FLICE‐inhibitory protein expression to TRAIL‐induced apoptosis of melanoma. Cancer Res 1999; 59: 2747–53. [PubMed] [Google Scholar]
- 23. Kayagaki N, Yamaguchi N, Nakayama M et al Expression and function of TNF‐related apoptosis‐inducing ligand on murine activated NK cells. J Immunol. 1999; 163: 1906–13. [PubMed] [Google Scholar]
- 24. Drobits B, Holcmann M, Amberg N et al Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor‐killing effector cells. J Clin Invest. 2012; 122: 575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karin M, Greten FR. NF‐kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005; 5: 749–59. [DOI] [PubMed] [Google Scholar]
- 26. Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol 2009; 9: 351–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Franco AV, Zhang XD, Van Berkel E et al The role of NF‐kappa B in TNF‐related apoptosis‐inducing ligand (TRAIL)‐induced apoptosis of melanoma cells. J Immunol. 2001; 166: 5337–45. [DOI] [PubMed] [Google Scholar]
- 28. Deeb D, Jiang H, Gao X et al Curcumin sensitizes prostate cancer cells to tumor necrosis factor‐related apoptosis‐inducing ligand/Apo2L by inhibiting nuclear factor‐kappaB through suppression of IkappaBalpha phosphorylation. Mol Cancer Ther 2004; 3: 803–12. [PubMed] [Google Scholar]
- 29. Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS. NF‐kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c‐IAP1 and c‐IAP2 to suppress caspase‐8 activation. Science 1998; 281: 1680–3. [DOI] [PubMed] [Google Scholar]
- 30. Wajant H, Pfizenmaier K, Scheurich P. TNF‐related apoptosis inducing ligand (TRAIL) and its receptors in tumor surveillance and cancer therapy. Apoptosis 2002; 7: 449–59. [DOI] [PubMed] [Google Scholar]
- 31. Hinz S, Trauzold A, Boenicke L et al Bcl‐XL protects pancreatic adenocarcinoma cells against CD95‐ and TRAIL‐receptor‐mediated apoptosis. Oncogene 2000; 19: 5477–86. [DOI] [PubMed] [Google Scholar]
- 32. Schimmer AD, Welsh K, Pinilla C et al Small‐molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell 2004; 5: 25–35. [DOI] [PubMed] [Google Scholar]
- 33. Sinicrope FA, Penington RC, Tang XM. Tumor necrosis factor‐related apoptosis‐inducing ligand‐induced apoptosis is inhibited by Bcl‐2 but restored by the small molecule Bcl‐2 inhibitor, HA 14–1, in human colon cancer cells. Clin Cancer Res 2004; 10: 8284–92. [DOI] [PubMed] [Google Scholar]
- 34. Takeda K, Smyth MJ, Cretney E et al Involvement of tumor necrosis factor‐related apoptosis‐inducing ligand in NK cell‐mediated and IFN‐gamma‐dependent suppression of subcutaneous tumor growth. Cell Immunol 2001; 214: 194–200. [DOI] [PubMed] [Google Scholar]
- 35. Smyth MJ, Takeda K, Hayakawa Y, Peschon JJ, van den Brink MR, Yagita H. Nature's TRAIL–on a path to cancer immunotherapy. Immunity 2003; 18: 1–6. [DOI] [PubMed] [Google Scholar]
- 36. Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte‐mediated tumoricidal activity via the tumor necrosis factor‐related cytokine. TRAIL. J Exp Med. 1999; 189: 1343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fanger NA, Maliszewski CR, Schooley K, Griffith TS. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL). J Exp Med 1999; 190: 1155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ehrhardt H, Fulda S, Schmid I, Hiscott J, Debatin KM, Jeremias I. TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL‐induced apoptosis mediated by NF‐kappaB. Oncogene 2003; 22: 3842–52. [DOI] [PubMed] [Google Scholar]
- 39. Wu Y, Zhou BP. TNF‐alpha/NF‐kappaB/Snail pathway in cancer cell migration and invasion. Br J Cancer 2010; 102: 639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Min C, Eddy SF, Sherr DH, Sonenshein GE. NF‐kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem 2008; 104: 733–44. [DOI] [PubMed] [Google Scholar]
- 41. Trauzold A, Siegmund D, Schniewind B et al TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene 2006; 25: 7434–9. [DOI] [PubMed] [Google Scholar]
- 42. Herbst RS, Kurzrock R, Hong DS et al A first‐in‐human study of conatumumab in adult patients with advanced solid tumors. Clin Cancer Res 2010; 16: 5883–91. [DOI] [PubMed] [Google Scholar]
- 43. Wakelee HA, Patnaik A, Sikic BI et al Phase I and pharmacokinetic study of lexatumumab (HGS‐ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol 2010; 21: 376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Camidge DR. Apomab: an agonist monoclonal antibody directed against Death Receptor 5/TRAIL‐Receptor 2 for use in the treatment of solid tumors. Expert Opin Biol Ther. 2008; 8: 1167–76. [DOI] [PubMed] [Google Scholar]
- 45. Tolcher AW, Mita M, Meropol NJ et al Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor‐related apoptosis‐inducing ligand receptor‐1. J Clin Oncol 2007; 25: 1390–5. [DOI] [PubMed] [Google Scholar]
- 46. Hotte SJ, Hirte HW, Chen EX et al A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL‐R1) in patients with advanced solid malignancies. Clin Cancer Res 2008; 14: 3450–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Establishment of B16F10 CMV and B16F10 nuclear factor‐κB (NFκB) mouse melanoma cells.
Fig. S2. Nuclear factor‐κB (NFκB) activation by DR5 stimulation in B16F10 mouse melanoma cells.


