Abstract
By definition, ductal carcinoma in situ (DCIS) – pre‐invasive breast cancer – does not metastasize to the lymph nodes. However, since the introduction of molecular whole‐node analysis using the one‐step nucleic acid amplification assay for sentinel node (SN) biopsies, the number of patients with DCIS and SN metastasis has increased. The pathogenesis and clinical management of DCIS with SN metastasis remain controversial. In this case–control study, in order to elucidate the pathogenesis of SN metastasis in DCIS, we compared occult invasions between the SN‐positive and SN‐negative DCIS and investigated predictive factors of occult invasion. The subjects were 24 patients selected from 285 patients with a routine postoperative diagnosis of DCIS who had undergone SN biopsy using the one‐step nucleic acid amplification whole‐node assay between 2009 and 2011. Of these 24 patients, 12 were SN‐positive, and 12 were SN‐negative. The 12 SN‐negative patients make up the control group and were selected from the 273 SN‐negative patients based on patient characteristics. All paraffin blocks of the primary tumor from each patient were step‐sectioned with 500‐μm intervals until the block was exhausted and histopathologically examined. We analyzed 1830 step‐sectioned slides and found occult invasions were more frequent in the SN‐positive group (7/12, 58.3%) than in the SN‐negative group (3/12, 25.0%). All occult invasions were <5 mm. There was no correlation between occult invasion and SN tumor burden, non‐SN metastasis, or patient characteristics. Our results suggest true metastasis from occult invasion may be a potent pathogenesis indicating nodal metastasis in postoperatively diagnosed DCIS. Patient follow‐up is required to elucidate the prognostic impact of nodal metastasis and occult invasion.
The standard procedure for axillary staging of clinically node‐negative breast cancer is SN biopsy.1 However, conventional histopathological examinations may lead to underestimation of nodal staging because they only partially evaluate specimens, limiting the accuracy of metastases detection, particularly micrometastases.
The OSNA assay (Sysmex Corporation, Kobe, Japan) was developed to overcome this limitation of histopathological methods in examining lymph nodes. It is accepted and routinely used in more than 200 institutions in countries such as Spain, Japan, Italy, the UK, and France.2 This assay can assess whole lymph nodes, and its detection and amplification of CK19 mRNA can yield semi‐quantitative results for detecting clinically relevant nodal metastases (size, >0.2 mm).3 Calibration and validation studies have offered reasonable evidence that the CK19 mRNA copy numbers detected by the OSNA assay can provide good estimates of macrometastasis, micrometastasis, and isolated tumor cells, as defined by the Cancer Staging Manual of the American Joint Committee on Cancer.3, 4, 5 Previously, we have shown that when the OSNA whole‐node assay is applied to invasive breast cancers, it detects a greater number of metastases, particularly micrometastases, than routine histopathological examinations.6, 7
The most common type of pre‐invasive breast cancer, DCIS is characterized by the clonal proliferation of cells that appear malignant and accumulate within the lumen of mammary ducts.8 By definition, DCIS does not metastasize to the lymph nodes because the tumor is limited to the epithelial layer and does not reach lymphatic vessels. However, since the OSNA whole‐node assay was introduced for SN biopsy, the number of patients with a postoperative DCIS diagnosis and SN metastasis has significantly increased.9 Most of these metastases are reported to be micrometastases, and none of the characteristics of high‐risk DCIS (i.e. mass formation, size, grade, and comedo type) or preoperative breast biopsy (i.e. methods or time to surgery) has been significantly correlated with OSNA assay‐positive DCIS.9
The clinical management for patients with DCIS and SN metastasis remains controversial. As discussed in our previous publication,9 according to the current staging system,5 these tumors can be treated as one of two different tumor stages: stage IB (pT1mi/1a‐pN1mi‐M0), invasive cancer with true nodal metastasis, or stage 0 (pTis‐pN0‐M0), true DCIS with iatrogenic dissemination of benign epithelial or tumor cells into the lymph nodes as a result of the preoperative breast biopsy. For pT1mi/1a‐pN1mi‐M0 tumors, the current guideline recommends adjuvant endocrine therapy with or without chemotherapy for hormone receptor‐positive tumors. For hormone receptor‐negative pT1mi/1a‐pN1mi‐M0 tumors, chemotherapy should be considered.10 For pTis‐pN0‐M0 tumors, tamoxifen should be considered for reducing the risk of ipsilateral breast events.10
In the present case–control study, we analyzed multiple sections obtained through exhaustive step‐sectioning of primary tumors. Our objective was to investigate and compare the incidence of occult invasion in SN‐positive and SN‐negative DCIS and to elucidate predictive factors of occult invasion. We aimed to reveal the pathogenesis of nodal metastases in postoperatively diagnosed DCIS and to improve the clinical management of these patients.
Materials and Methods
Patients and tumors
Between April 2009 and March 2011, 285 patients were postoperatively diagnosed with DCIS and underwent SN biopsy analyzed with the OSNA whole‐node assay at the Cancer Institute Hospital (Tokyo, Japan). This is the same population as the OSNA cohort of our previous study.9 Of the 285 patients, 12 (4.2%) had SN metastasis and 273 (95.8%) did not. The subjects of the present study were 12 SN‐positive patients and 12 SN‐negative patients. The SN‐negative patients were selected from the 273 patients based on patient characteristics; they represent the control group. The study protocol was reviewed and approved by the Institutional Review Board of the Cancer Institute Hospital.
SN Biopsy with the OSNA assay
All patients underwent SN mapping and identification with a radioisotope tracer, sometimes with a vital dye. Whole SN from each patient were evaluated with the OSNA assay without histopathological examination. Since September 2009, we have also used the OSNA assay for non‐SN in patients who underwent axillary dissection following metastatic SN biopsy.
The OSNA assay procedure has been previously described in detail.3 Briefly, whole lymph nodes were homogenized with 4‐mL lysis buffer solution (Lynorhag; Sysmex Corporation) and centrifuged at 10 000 g at room temperature. A total of 2‐μL supernatant was analyzed with the RD‐100i System (Sysmex Corporation), an automated molecular detection system that uses a reverse transcription loop‐mediated isothermal amplification method, and the LynoampBC Kit (Sysmex Corporation).11 The degree of amplification was detected on the basis of a reaction by‐product, pyrophosphate.12 The resultant change in turbidity from precipitation of magnesium pyrophosphate was then correlated with the CK19 mRNA copy number per microliter of the original lysate; the correlation was performed with a standard curve using three calibrators containing different CK19 mRNA copy numbers. The number of CK19 mRNA copies per microliter was extrapolated from the standard curve for both the measurement sample and a 1:10 diluted sample. Positive results and macrometastasis were confirmed at 250 and 5000 copies/μL, respectively.3 In situations where the reaction was inhibited in the measurement sample, the copy numbers in the diluted sample were employed.
Routine postoperative diagnosis of DCIS
After formalin fixation, partial and total mastectomy materials were sectioned continuously from the nipple side to the periphery at 5‐mm and 5–7‐mm intervals, respectively. All sections from partial mastectomy materials and most sections covering the macroscopic and/or radiologic tumor spread from total mastectomy materials were histologically examined with H&E staining. In addition, immunohistochemical myoepithelial markers were used to resolve any ambiguity in determining the presence of invasive cancer nests.
Step‐sectioning of the primary tumor
All paraffin blocks containing the primary tumor were step‐sectioned with 500‐μm (0.5‐mm) intervals until the breast tissue was exhausted. At each level, six microscopic slides were made: one was used for H&E staining and five were stored unstained. All H&E‐stained slides were microscopically examined. The unstained slide was used for additional immunohistochemical examinations as described above.
Definition of occult invasion and iatrogenic tumor displacement
Occult invasion was defined as a naturally occurring invasive lesion between the specimen surfaces of the surgical materials (Fig. 1). Tumor displacement resulting from preoperative breast biopsy was excluded from the occult invasion. This iatrogenic tumor displacement was histologically defined as the presence of cancer cells entirely within the scar or granulation tissue at the biopsy site (Fig. 2).
Figure 1.
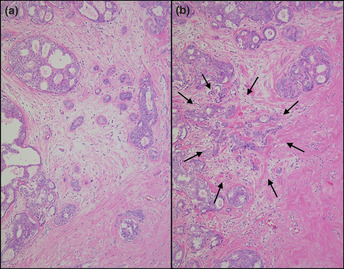
Specimens obtained by exhaustive step‐sectioning of the primary tumor showing an occult invasive lesion. (a) No invasive lesion is observed in the original specimen with H&E staining. (b) An invasive lesion (arrow) is seen in the step‐sectioned specimen with H&E staining.
Figure 2.
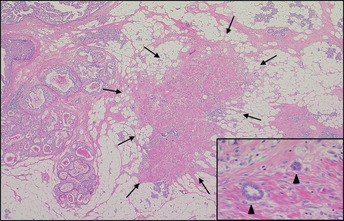
A preoperative breast biopsy indicating iatrogenic tumor displacement. Displaced cancer cells (arrow head) are observed within the scar at the biopsy site (with H&E staining).
Statistical analyses
To compare patient characteristics between SN‐positive and SN‐negative groups, the Mann–Whitney U‐test (for the age, time from breast biopsy to surgery, pathological size, and number of step‐section specimens) and the Fisher's exact test (for other characteristics) were performed. In order to reveal the predictive factors of occult invasion, the Mann–Whitney U‐test and the Fisher's exact test were performed for the above mentioned characteristics of patients with and without occult invasion. P < 0.05 was considered statistically significant. All statistical analyses were performed with the statistical software R version 2.10.1 (http://www.r-project.org/).
Results
Patient characteristics
Patient characteristics between both SN‐positive and SN‐negative groups were well matched (Table 1). All patients were Japanese women. In total, 1830 H&E‐stained step‐section specimens from the 24 patients (SN‐positive group, 984; SN‐negative group, 846) were examined.
Table 1.
Patient characteristics of the sentinel node‐positive and sentinel node‐negative groups
| Characteristics | Sentinel node positive | Sentinel node negative | P‐value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Number of patients | 12 | 100.0 | 12 | 100.0 | |
| Age (years) | |||||
| Median (range) | 53.5 (37–75) | 49.5 (40–75) | 0.91 | ||
| Palpability | |||||
| Palpable | 4 | 33.3 | 3 | 25.0 | 1.00 |
| Non‐palpable | 8 | 66.7 | 9 | 75.0 | |
| Mammographic findings | |||||
| Mass | 2 | 16.7 | 4 | 33.3 | 0.85 |
| Calcification | 7 | 58.3 | 6 | 50.0 | |
| Others | 2 | 16.7 | 1 | 8.3 | |
| None | 1 | 8.3 | 1 | 8.3 | |
| Breast biopsy method | |||||
| Needle with vacuum assist | 7 | 58.3 | 8 | 66.7 | 1.00 |
| Needle without vacuum assist | 3 | 25.0 | 3 | 25.0 | |
| Ductoscopic | 2 | 16.7 | 1 | 8.3 | |
| Period from breast biopsy to surgery (days) | |||||
| Median (range) | 82 (13–281) | 77.5 (19–154) | 1.00 | ||
| Breast surgery | |||||
| Partial mastectomy | 7 | 58.3 | 7 | 58.3 | 1.00 |
| Total mastectomy | 5 | 41.7 | 5 | 41.7 | |
| Pathological size (cm) | |||||
| Median (range) | 4.7 (1.7–6.8) | 5.05 (2–6.5) | 0.79 | ||
| Subtype | |||||
| Comedo | 3 | 25.0 | 3 | 25.0 | 1.00 |
| Non‐comedo | 9 | 75.0 | 9 | 75.0 | |
| Nuclear grade | |||||
| 1 | 6 | 50.0 | 8 | 66.7 | 0.85 |
| 2 | 4 | 33.3 | 2 | 16.7 | |
| 3 | 2 | 16.7 | 2 | 16.7 | |
| Estrogen receptor | |||||
| + | 11 | 91.7 | 11 | 91.7 | 1.00 |
| − | 1 | 8.3 | 1 | 8.3 | |
| Progesterone receptor | |||||
| + | 9 | 75.0 | 10 | 83.3 | 1.00 |
| − | 3 | 25.0 | 2 | 16.7 | |
| Number of step‐section specimen (slides) | |||||
| Median (range) | 53.5 (17–344) | 59 (27–168) | 0.73 | ||
Detection of occult invasion and iatrogenic tumor displacement
Of the 12 SN‐positive patients, seven (58.3%) were found to have occult invasion in the primary tumor and five (41.7%) did not demonstrate an invasive phenotype (Fig. 3). Of the seven occult invasions, four were <0.5 mm, one occult invasion was 1 mm, and two were 2–3 mm in size. In contrast, of the 12 SN‐negative patients, three (25.0%) had occult invasion, three (25.0%) had iatrogenic tumor displacement, and six (50.0%) had neither. Of the three occult invasions, one was 0.5 mm, and two were 2–3 mm in size.
Figure 3.
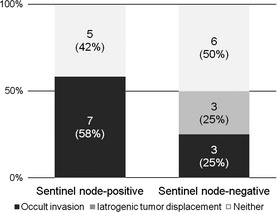
A graph showing occult invasions in the sentinel node‐positive and sentinel node‐negative groups that were obtained by exhaustive step‐sectioning of the primary tumor.
Occult invasion and SN/non‐SN status
There was one SN metastasis in each of the 12 patients in the SN‐positive group (Table 2). In patients with occult invasion (cases 1–7), the CK19 mRNA copy numbers in the SN were 280, 366, and 250–1300 copies/μL, respectively, for median, mean and range values. In patients without occult invasion (cases 8–12), CK19 mRNA copy numbers in the SN were calculated as 780, 888, and 400–1400 copies/μL, respectively, for median, mean and range values. Regarding the non‐SN status, two of seven patients with occult invasion (cases 6 and 7, 28.6%) and one of five patients without occult invasion (case 12, 20.0%) were positive for non‐SN metastasis.
Table 2.
Occult invasions and sentinel/non‐sentinel node status in the sentinel node‐positive group
| Case no. | Occult invasion | Sentinel node status | Non‐sentinel node status | |||
|---|---|---|---|---|---|---|
| Number of positive/removed nodes | CK19 mRNA (copy/μL) | Method | Number of positive/removed nodes | CK19 mRNA (copy/μL) | ||
| 1 | (+) | 1/1 | 260 | Histology† | 0/12 | NA |
| 2 | (+) | 1/4 | 270 | OSNA | 0/14 | 0 |
| 3 | (+) | 1/2 | 280 | Histology† | 0/11 | NA |
| 4 | (+) | 1/6 | 400 | OSNA | 0/14 | 0 |
| 5 | (+) | 1/3 | 620 | Histology† | 0/15 | NA |
| 6 | (+) | 1/2 | 250‡ | OSNA | 1/6 | 330‡ |
| 7 | (+) | 1/3 | 1300‡ | OSNA | 2/22 | 7800 |
| 8 | (−) | 1/5 | 400 | OSNA | 0/26 | 240 |
| 9 | (−) | 1/3 | 460 | OSNA | 0/9 | 2 |
| 10 | (−) | 1/4 | 780 | OSNA | 0/13 | 150 |
| 11 | (−) | 1/1 | 1400 | Histology† | 0/20 | NA |
| 12 | (−) | 1/1 | 1400 | OSNA | 1/15 | 780 |
CK19, cytokeratin 19; OSNA, one‐step nucleic acid amplification assay; NA, not available.
†Permanent histology using a single‐sectioned lymph node. ‡CK19 mRNA copy numbers in the diluted samples.
Predictive factor of occult invasion
Patients found to have occult invasion did not display significantly different characteristics compared to patients without occult invasion (Table 3).
Table 3.
Characteristics of patients with and without occult invasion
| Characteristics | Invasion detected | Invasion undetected | P‐value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Number of patients | 10 | 100.0 | 14 | 100.0 | |
| Age (years) | |||||
| Median (range) | 52.5 (37–75) | 50.5 (40–75) | 0.86 | ||
| Palpability | |||||
| Palpable | 3 | 30.0 | 4 | 28.6 | 1.00 |
| Non‐palpable | 7 | 70.0 | 10 | 71.4 | |
| Mammographic findings | |||||
| Mass | 2 | 20.0 | 4 | 28.6 | 0.84 |
| Calcification | 5 | 50.0 | 8 | 57.1 | |
| Others | 2 | 20.0 | 1 | 7.1 | |
| None | 1 | 10.0 | 1 | 7.1 | |
| Breast biopsy method | |||||
| Needle with vacuum assist | 6 | 60.0 | 9 | 64.3 | 1.00 |
| Needle without vacuum assist | 3 | 30.0 | 3 | 21.4 | |
| Ductoscopic | 1 | 10.0 | 2 | 14.3 | |
| Period from breast biopsy to surgery (days) | |||||
| Median (range) | 74 (47–98) | 81.5 (13–281) | 0.40 | ||
| Breast surgery | |||||
| Partial mastectomy | 5 | 50.0 | 9 | 64.3 | 0.68 |
| Total mastectomy | 5 | 50.0 | 5 | 35.7 | |
| Pathological size (cm) | |||||
| Median (range) | 3.95 (1.7–6.6) | 5.05 (2.6–6.8) | 0.27 | ||
| Subtype | |||||
| Comedo | 2 | 20.0 | 4 | 28.6 | 1.00 |
| Non‐comedo | 8 | 80.0 | 10 | 71.4 | |
| Nuclear grade | |||||
| 1 | 6 | 60.0 | 8 | 57.1 | 1.00 |
| 2 | 2 | 20.0 | 4 | 28.6 | |
| 3 | 2 | 20.0 | 2 | 14.3 | |
| Estrogen receptor | |||||
| + | 8 | 80.0 | 14 | 100.0 | 0.16 |
| − | 2 | 20.0 | 0 | 0.0 | |
| Progesterone receptor | |||||
| + | 8 | 80.0 | 11 | 78.6 | 1.00 |
| − | 2 | 20.0 | 3 | 21.4 | |
| Number of step‐section specimen (slides) | |||||
| Median (range) | 56 (17–344) | 57.5 (29–168) | 0.52 | ||
Discussion
To the best of our knowledge, this is the first report where postoperatively diagnosed DCIS samples were exhaustively examined to identify occult invasive lesions. The diagnostic accuracy of both DCIS and lymph node metastases is dependent on the rigor of the examination, as small invasions or metastases may not be identified when fewer portions of the primary tumor or lymph node are examined. Thus, the present study represents an exhaustive examination of both primary tumors and lymph nodes.
As a result of our examination, approximately 60% of patients with DCIS and SN metastasis were found to have invasive disease, which is more than twice the rate of DCIS patients without SN metastasis. This result suggests that true metastasis from occult invasive lesions of primary tumors is a potent pathogenesis indicating nodal metastasis in postoperatively diagnosed DCIS. All occult invasions were <5 mm in size. Although our routine histological procedure for postoperative DCIS diagnosis is detailed, small invasive foci may be present between the surfaces of surgical materials that are 5–7 mm thick.
In contrast, occult invasion could not be detected in 40% of patients with DCIS and SN metastasis. This cannot completely exclude the presence of occult invasive foci. The present study has three limitations that may have affected their detection. First, the formalin‐fixed breast tissue slices had been shaved so that they could be put in the cassettes before paraffin embedding. Second, the paraffin blocks that did not contain tumor cells in the original H&E specimens were not step‐sectioned. Third, invasions <0.5 mm in size that potentially present between the cut surfaces may have been missed in the step‐section histological specimens. In fact, 40% (4/10) of the occult invasions detected in the present study were <0.5 mm in size. Therefore, there is a possibility that some of the occult invasive lesions were lost or not examined.
Another possible pathogenesis of nodal metastases in DCIS, in which occult invasion was not found, may be an iatrogenic dissemination of epithelial cells. A previous invasive diagnostic biopsy can iatrogenically displace epithelial or tumor cells into the lymphatic system; these dislocated cells can be passively transported to the SN.13, 14, 15, 16 The results of a large cohort study show that surgical excision biopsy increases the presence of isolated tumor cells and micrometastases in SN.16 However, there is no clear definition to make a histopathological diagnosis of the iatrogenic dissemination. In addition, the present study is limited by the lack of histological information from the nodal metastases because a molecular whole‐node assay was used to determine SN metastasis. Thus, follow‐up of patients with DCIS and SN metastasis is needed to elucidate the prognostic effect of nodal metastases because iatrogenic dissemination does not have any prognostic value.
The adjuvant systemic therapy for patients with DCIS and SN metastasis remains controversial.17 According to the present study, more than half of SN‐positive DCIS may have benefited from treatment recommended for pT1mi/1a‐pN1mi‐M0 tumors. However, it is difficult to identify patients with DCIS with underlying the occult invasive lesions. No predictive factor of the occult invasion was found when SN tumor burden, presence of non‐SN metastasis, or characteristics of high‐risk DCIS (i.e. size, grade, comedo type, and palpable/mammographic mass) were analyzed.17 Moreover, the exhaustive step‐sectioning for the detection of occult invasion imposes a heavy workload on technicians and pathologists, making it impractical in daily practice.
In addition, the prognostic impact of nodal metastasis and occult invasion is unclear. Regarding nodal metastasis, immunohistochemically detected tumor deposits in the node have no clinical significance in DCIS.18 However, the OSNA assay detects metastases >0.2 mm in size and diagnoses low‐volume metastases ≤0.2 mm as negative.3 Regarding occult invasion, the natural history of DCIS with microinvasion ≤1 mm closely resembles that of DCIS, with a low incidence of local‐regional and distant failures.19 However, we found occult invasions >1 mm in size. The 15‐year cumulative incidence of breast cancer deaths was 2–5% among patients with DCIS,19 and these DCIS patients may have been positive for occult invasive disease and/or nodal metastasis.
In conclusion, through this exhaustive step‐sectioning of primary tumors, approximately 60% of patients with DCIS and SN metastasis were found to have invasive foci, which is more than twice the rate of patients with DCIS but without SN metastasis. Therefore, true metastasis from occult invasive lesions of primary tumor is a potent pathogenesis indicating nodal metastasis in DCIS. Patient follow‐up is needed to elucidate the prognostic impact of the nodal micrometastasis and occult invasion.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- CK19
cytokeratin 19
- DCIS
ductal carcinoma in situ
- OSNA
one‐step nucleic acid amplification
- SN
sentinel lymph node
Acknowledgments
This work was supported in part by a Grant‐in‐Aid for Young Scientists (B) (No. 21791264) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (Tokyo, Japan) and a research grant from the Foundation for the Promotion of Cancer Research (Tokyo, Japan). The authors wish to thank Motoyoshi Iwakoshi, Tomoyo Kakita, and Mayumi Ogawa for their technical assistance and Tadashi Kiniwa, Kazuki Kishi, Kenji Iwakabe, and Reiko Watanabe (Sysmex Corporation) for their helpful advice.
(Cancer Sci 2013; 104: 453–457)
References
- 1. Lyman GH, Giuliano AE, Somerfield MR et al American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early‐stage breast cancer. J Clin Oncol 2005; 23: 7703–20. [DOI] [PubMed] [Google Scholar]
- 2. Sysmex Corporation . [Cited 28 October 2012] Available from URL: http://lifescience.sysmex.co.jp/ls/products/osna/index.html. (In Japanese.)
- 3. Tsujimoto M, Nakabayashi K, Yoshidome K et al One‐step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res 2007; 13: 4807–16. [DOI] [PubMed] [Google Scholar]
- 4. Tamaki Y, Akiyama F, Iwase T et al Molecular detection of lymph node metastases in breast cancer patients: results of a multicenter trial using the one‐step nucleic acid amplification assay. Clin Cancer Res 2009; 15: 2879–84. [DOI] [PubMed] [Google Scholar]
- 5. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer Cancer Staging Manual, 7th edn New York, NY: Springer, 2010. [Google Scholar]
- 6. Osako T, Iwase T, Kimura K et al Intraoperative molecular assay for sentinel lymph node metastases in early stage breast cancer: a comparative analysis between one‐step nucleic acid amplification whole node assay and routine frozen section histology. Cancer 2011; 117: 4365–74. [DOI] [PubMed] [Google Scholar]
- 7. Osako T, Iwase T, Kimura K, Yamashita K, Horii R, Akiyama F. Accurate staging of axillary lymph nodes from breast cancer patients using a novel molecular method. Br J Cancer 2011; 105: 1197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med 2004; 350: 1430–41. [DOI] [PubMed] [Google Scholar]
- 9. Osako T, Iwase T, Kimura K, Masumura K, Horii R, Akiyama F. Incidence and possible pathogenesis of sentinel node micrometastases in ductal carcinoma in situ of the breast detected using molecular whole lymph node assay. Br J Cancer 2012; 106: 1675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Comprehensive Cancer Network . Clinical practice guidelines in oncology. Breast cancer ver. 3, 2012. [Cited 24 Sepember 2012] Available from URL: http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
- 11. Notomi T, Okayama H, Masubuchi H et al Loop‐mediated isothermal amplification of DNA. Nucleic Acids Res 2000; 28: E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop‐mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun 2001; 289: 150–4. [DOI] [PubMed] [Google Scholar]
- 13. Bleiweiss IJ, Nagi CS, Jaffer S. Axillary sentinel lymph nodes can be falsely positive due to iatrogenic displacement and transport of benign epithelial cells in patients with breast carcinoma. J Clin Oncol 2006; 24: 2013–8. [DOI] [PubMed] [Google Scholar]
- 14. Moore KH, Thaler HT, Tan LK, Borgen PI, Cody HS 3rd. Immunohistochemically detected tumor cells in the sentinel lymph nodes of patients with breast carcinoma: biologic metastasis or procedural artifact? Cancer 2004; 100: 929–34. [DOI] [PubMed] [Google Scholar]
- 15. King TA, Ganaraj A, Fey JV et al Cytokeratin‐positive cells in sentinel lymph nodes in breast cancer are not random events: experience in patients undergoing prophylactic mastectomy. Cancer 2004; 101: 926–33. [DOI] [PubMed] [Google Scholar]
- 16. Tvedskov TF, Jensen MB, Kroman N, Balslev E. Iatrogenic displacement of tumor cells to the sentinel node after surgical excision in primary breast cancer. Breast Cancer Res Treat 2012; 131: 223–9. [DOI] [PubMed] [Google Scholar]
- 17. Ansari B, Ogston SA, Purdie CA, Adamson DJ, Brown DC, Thompson AM. Meta‐analysis of sentinel node biopsy in ductal carcinoma in situ of the breast. Br J Surg 2008; 95: 547–54. [DOI] [PubMed] [Google Scholar]
- 18. Lara JF, Young SM, Velilla RE, Santoro EJ, Templeton SF. The relevance of occult axillary micrometastasis in ductal carcinoma in situ: a clinicopathologic study with long‐term follow‐up. Cancer 2003; 98: 2105–13. [DOI] [PubMed] [Google Scholar]
- 19. Wapnir IL, Dignam JJ, Fisher B et al Long‐term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B‐17 and B‐24 randomized clinical trials for DCIS. J Natl Cancer Inst 2011; 103: 478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]


