Abstract
Concurrent chemoradiotherapy is the standard treatment for unresectable stage III non‐small cell lung cancer (NSCLC). The long‐term feasibility and efficacy of vinorelbine and cisplatin with concurrent thoracic radiotherapy were investigated. Eighteen patients received cisplatin (80 mg/m2) on day 1 and vinorelbine (20 mg/m2 in level 1, and 25 mg/m2 in level 2) on days 1 and 8 every 4 weeks for four cycles in a phase I trial. Ninety‐three patients received the same chemotherapy regimen except for the fixed vinorelbine (20 mg/m2) dosage and consolidation therapy with docetaxel (60 mg/m2, every 3 weeks). The thoracic radiotherapy consisted of a single dose of 2 Gy once daily to a total dose of 60 Gy. A total of 111 patients were analyzed in the present study: male/female, 91/20; median age, 60 years; stage IIIA/IIIB, 50/61; and squamous/non‐squamous histology, 26/85. The 3‐, 5‐, and 7‐year overall survival rates (95% CI) were 43.2% (33.9–52.2), 25.2% (17.6–33.5), and 23.2% (15.8–31.4), respectively. The median progression‐free survival and median survival time (95% CI) were 13.5 (10.1–16.7) months and 30.0 (24.3–38.8) months, respectively. Four patients (4%) experienced Grade 5 pulmonary toxicities from 4.4 to 9.4 months after the start of treatment. In conclusion, approximately 15% of patients with unresectable stage III NSCLC could be cured with chemoradiotherapy without severe late toxicities after 10 months of follow‐up. Although based on the data from highly selected population participated in phase I and phase II trial, this analysis would strengthen and confirm the previous reports concerning concurrent chemoradiotherapy with third generation cytotoxic agents. (Cancer Sci 2013; 104: 93–97)
Stage III locally advanced non‐small cell lung cancer (NSCLC) accounts for 25–30% of all lung cancer cases.1, 2 Because of the equal frequency of local and distant recurrences, the combination of systemic chemotherapy and thoracic radiotherapy has been established as a standard of care for patients with stage III NSCLC.3 Concurrent chemoradiotherapy is superior to a sequential approach, as shown by phase III trials in stage III NSCLC.4, 5
Ohe et al.6 reported the long‐term follow‐up analysis of concurrent chemoradiotherapy with former generation chemotherapy agents (median survival time 16.1 months, and 7‐year overall survival rate 12.0%). Few researchers, however, have reported follow‐up data of longer than 5 years after concurrent chemoradiotherapy with third‐generation chemotherapy. The long‐term safety and efficacy of vinorelbine and cisplatin with concurrent thoracic radiotherapy were investigated.
Materials and Methods
Study selection
Two previous studies were included in this analysis. One was a phase I study of concurrent thoracic radiotherapy with cisplatin plus vinorelbine, and the other evaluated docetaxel consolidation therapy following concurrent chemoradiotherapy.7, 8 These studies were approved by the institutional review board at each institution. Written, informed consent was obtained from all participating patients.
Patient selection
The two studies had similar eligibility criteria. They were: histologically or cytologically proven NSCLC; unresectable stage IIIA or IIIB disease; no previous treatment; measurable disease; tumor within an estimated irradiation field no larger than half the hemithorax; age between 20 years and 74 years; Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1; and adequate organ function, including bone marrow, liver, kidney, and lung. Patients were diagnosed to have unresectable disease based on a consensus of thoracic oncologists including surgeons in each institution. The exclusion criteria were reported in previous papers.7, 8
Treatment schedule
In the phase I study, treatment consisted of chemotherapy with four cycles of cisplatin and vinorelbine (20 mg/m2 in level 1, and 25 mg/m2 in level 2) and concurrent thoracic radiotherapy (see below). In the other study, treatment consisted of a chemoradiotherapy portion with three cycles of cisplatin and vinorelbine followed by a consolidation portion with three cycles of docetaxel. Cisplatin (80 mg/m2) was administered every 4 weeks by intravenous infusion for 60 min with 2500–3000 mL of fluid for hydration. Vinorelbine 20 mg/m2 diluted in 50 mL of normal saline was administered intravenously on days 1 and 8 every 4 weeks. All patients received prophylactic antiemetic therapy consisting of a 5HT3‐antagonist and a steroid. In the docetaxel (60 mg/m2, every 3 weeks) consolidation trial, consolidation therapy was started sequentially in patients whose general condition was acceptable. Follow‐up computed tomographies after chemoradiotherapy were scheduled as follows; every 2–4 months during the 1 year, every 6 months in the 2 and 3 years, and every 1 year thereafter.
Thoracic radiotherapy was delivered with megavoltage equipment (≥6 MV) using anterior/posterior opposed fields up to 40 Gy in 20 fractions, including the primary tumor, the metastatic lymph nodes, and the regional nodes. A booster dose of 20 Gy in 10 fractions was given to the primary tumor and the metastatic lymph nodes for a total dose of 60 Gy using bilateral oblique fields. Computed tomography (CT) scan‐based treatment planning was used in all patients. The clinical target volume (CTV) for the primary tumor was defined as the gross tumor volume (GTV) plus 1 cm taking into account subclinical extension. CTV and GTV for the metastatic nodes (>1 cm in the shortest dimension) were the same. Regional nodes, excluding the contralateral hilar and supraclavicular nodes, were included in the CTV, but the lower mediastinal nodes were included only if the primary tumor was located in the lower lobe of the lung. The planning target volumes for the primary tumor, the metastatic lymph nodes, and regional nodes were determined as CTVs plus 0.5–1.0‐cm margins laterally and 1.0–2.0‐cm margins craniocaudally, taking into account setup variations and internal organ motion. Lung heterogeneity corrections were not used.
Toxicity assessment
Toxicities were graded according to the National Cancer Institute (NCI) Common Toxicity Criteria version 2.0 issued in 1998, and late toxicities associated with thoracic radiotherapy were graded according to the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer late radiation morbidity scoring scheme.9 Late toxicities were defined as those that occurred or persisted 90 days after completion of radiotherapy. The detailed methods of treatment modification due to toxicity were reported in previous papers.7, 8
Response evaluation
In the phase I trial, the objective tumor response was evaluated according to the World Health Organization (WHO) criteria issued in 1979.10 The Response Evaluation Criteria in Solid Tumors were used to evaluate objective tumor response in the docetaxel consolidation trial.11 Local recurrences were defined as tumor progression in the primary site and in the hilar, mediastinal, and supraclavicular lymph nodes after a partial or complete response; regional recurrence was defined as the development of malignant pleural and pericardial effusions; and distant recurrence was defined as the appearance of distant metastases.
Statistical analyses
Progression‐free and overall survival times were estimated by the Kaplan–Meier method, and confidence intervals (CIs) were based on Greenwood's formula.12 Progression‐free survival time was measured from the date of registration to the date of disease progression, death (from any cause), or the last follow‐up. Overall survival time was measured from the date of registration to the date of death (from any cause) or to the last follow‐up. Patients who were lost to follow‐up without an event were censored at the date of their last known follow‐up. A CI for response rate (RR) was calculated using methods for exact binomial CIs. To investigate the association between survival and factors related to patient characteristics, the Cox regression model was used. Potential factors investigated were as follows: age (in 10‐year increments), sex, body weight loss (≤5.0% vs ≥5.1%), histology (squamous cell carcinoma versus non‐squamous cell carcinoma), T factor (T1/2 vs T3/4), N factor (N0‐2 vs N 3), and stage (IIIA vs IIIB). The STATA 10 for Windows software package (StataCorp LP, College Station, TX, USA) was used for statistical analyses.
Results
Characteristics of the patients
From October 1999 to June 2003, 13 patients were registered at dose level 1 and five at dose level 2 of the phase I study, and 93 patients were enrolled in the docetaxel consolidation trial. Thus, a total of 111 patients were analyzed in the present study. The participants' characteristics were as follows (Table 1): male/female 91/20; median age (range) 60 (31–74) years; body weight loss ≤5.0%/≥5.1% 96/14; stage IIIA/IIIB 50/61; and squamous/non‐squamous histology 26/85.
Table 1.
Patients' characteristics
| Clinical trial | |||
|---|---|---|---|
| Phase I trial† | DTX consolidation‡ | Total | |
| Number of patients | 18 | 93 | 111 |
| Age (years) | |||
| Median | 58.5 | 60 | 60 |
| Range | 48–69 | 31–74 | 31–74 |
| Sex | |||
| Male | 15 | 76 | 91 |
| Female | 3 | 17 | 20 |
| Performance status | |||
| 0 | 4 | 32 | 36 |
| 1 | 14 | 51 | 65 |
| Unknown | 0 | 10 | 10 |
| Body weight loss (minus, %) | |||
| 0 | 11 | 72 | 83 |
| 0.1‐5.0 | 4 | 9 | 13 |
| 5.1– | 3 | 11 | 14 |
| Unknown | 0 | 1 | 1 |
| Clinical stage | |||
| IIIA | 9 | 41 | 50 |
| IIIB | 9 | 52 | 61 |
| N factor | |||
| N0 | 0 | 6 | 6 |
| N1 | 0 | 3 | 3 |
| N2 | 11 | 58 | 69 |
| N3 | 7 | 26 | 33 |
| T factor | |||
| T1 | 1 | 18 | 19 |
| T2 | 6 | 31 | 37 |
| T3 | 7 | 13 | 20 |
| T4 | 4 | 30 | 34 |
| Unknown | 0 | 1 | 1 |
| Histology | |||
| Adenocarcinoma | 14 | 57 | 71 |
| Squamous cell carcinoma | 3 | 23 | 26 |
| Adenosquamous | 1 | 0 | 1 |
| Large cell carcinoma | 0 | 6 | 6 |
| NOS§ | 0 | 6 | 6 |
| Others | 0 | 1 | 1 |
†The phase I study of concurrent thoracic radiotherapy with cisplatin plus vinorelbine.‡The docetaxel consolidation therapy following concurrent chemoradiotherapy study.§Non‐small cell lung cancer not otherwise specified.
Treatment delivery
Full cycles (four in the phase I trial, three in the docetaxel consolidation trial) of cisplatin and vinorelbine and the full dose (60 Gy) of thoracic radiotherapy were administered in 94 (85%) and 102 (92%) patients, respectively. The delay in radiotherapy was less than 5 days in 74 (67%) patients. In the docetaxel consolidation trial, 59 (63%) patients could enter the consolidation phase, and only 34 (37%) patients completed three cycles of docetaxel chemotherapy, mainly because of toxicities. Of 91 patients with relapses, 27 (30%) received gefitinib as salvage treatments.
Objective tumor response and survival
The objective response rate was 82.0% (95% CI, 74.5–89.1). The 3‐, 5‐, and 7‐year progression‐free and overall survival rates (95% CI) were 21.0% (13.9–29.1), 15.7% (9.5–23.4), 14.4% (8.4–22.0), and 43.2% (33.9–52.2), 25.2% (17.6–33.5), and 23.1% (15.7–31.4), respectively (Fig. 1). The median progression‐free survival and median survival time (95% CI) were 13.4 (9.8–16.4) months and 30.0 (24.5–38.8) months, respectively (Fig. 2). There was no significant difference in survival results between subgroups; patients with or without docetaxel consolidation and patients with or without gefitinib.
Figure 1.
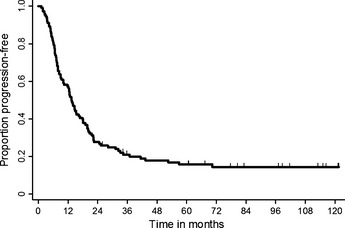
Progression‐free survival (n = 111). The median progression‐free survival is 13.5 months (95% confidence interval [CI] 10.1–16.7).
Figure 2.
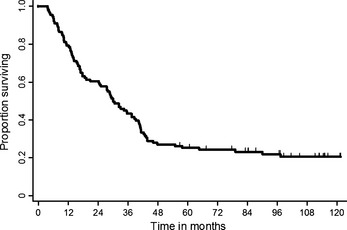
Overall survival (n = 111). The median overall survival is 30.0 months (95% confidence interval [CI] 24.3–38.6).
Pattern of relapse
Relapses were noted in 91 (82%) of 111 patients. Initial relapse sites were local alone in 39 (42%) patients, regional alone in 5 (5%), and distant alone in 38 (41%), including 17 (18%) patients with brain metastases as a sole recurrence site. Brain metastases were detected in 19 (21%) patients and were the most frequent sites of distant metastases. Brain metastases were detected within 3 years of initial treatment, and the last brain relapse was observed after 33 months of follow‐up (Table 2). Three (3%) patients experienced adrenal metastases as a first relapse site.
Table 2.
Sites of initial relapse
| Site of recurrences | Number of relapses | |||
|---|---|---|---|---|
| <1 year | 1–3 years | >3 years | Total (%) | |
| Local | 16 | 21 | 2 | 39 (42) |
| Distant | 23 | 12 | 3 | 38 (41) |
| Distant without brain | 12 | 4 | 3 | 19 (21) |
| Distant including brain | 1 | 1 | 0 | 2 (2) |
| Brain only | 10 | 7 | 0 | 17 (18) |
| Regional | 3 | 2 | 0 | 5 (5) |
| Others (L/D/R)a | 3 | 5 | 1 | 9 (10) |
| Unknown | – | – | – | 2 (2) |
Others includes 2 Local+Regional relapses, 6 Local+Distant relapses, and 1 Local+Regional+Distant relapse.
Late toxicities
Grade 1, 2, 3, and 5 late pulmonary toxicities were observed in 18 (16%), 15 (13%), 3 (3%), and 4 (4%) patients, respectively. Seventy‐two (64%) patients did not experience late pulmonary toxicities (Table 3). Four cases of grade 5 pulmonary toxicity developed at 4.4, 5.9, 9.4, and 9.6 months, respectively, after the treatment started. Late esophageal toxicities were observed in three patients (one grade 1 and two grade 3).
Table 3.
Late pulmonary toxicities§
| Toxicity grades | Clinical trial | Total (%) | |
|---|---|---|---|
| Phase I tria† | DTX consolidation‡ | ||
| Without late toxicity | 10 | 62 | 72 (64) |
| Grade 1 | 4 | 14 | 18 (16) |
| Grade 2 | 3 | 12 | 15 (13) |
| Grade 3 | 1 | 2 | 3 (3) |
| Grade 4 | 0 | 0 | 0 |
| Grade 5¶ | 0 | 4 | 4 (4) |
†The phase I study of concurrent thoracic radiotherapy with cisplatin plus vinorelbine. ‡The docetaxel consolidation therapy following concurrent chemoradiotherapy study. §Late toxicities were defined as those that occurred or persisted 90 days after completion of radiotherapy. ¶The Grade 5 pulmonary toxicities developed at 4.4, 5.9, 9.4, and 9.6 months after the treatment started.
Causes of death in long‐term survivors
There were 67 (60%) patients that survived 24 months or more from the initial treatment. Among them, five patients died because of reasons other than lung cancer. One patient was diagnosed as having pharyngeal cancer at the point of 35 months and died 4 months later. Other than malignancies, community‐acquired pneumonia (one patient at 43 months), sudden death due to unknown etiology (two patients at 41 and 42 months) and suicide (one patient at 29 months) were reported, respectively.
Predictive factors for survival
The associations between overall survival and patients' characteristics (age [in 10‐year increments], sex, body weight loss [≤5.0% vs ≥5.1%], histology [squamous cell carcinoma versus non‐squamous cell carcinoma], T factor [T1/2 vs T3/4], N factor [N0‐2 vs N 3], and stage [IIIA vs IIIB]) were also examined using Cox regression analysis. Age was significantly associated with survival (hazard ratio [HR] 1.34, 95% CI 1.02–1.75, Table 4).
Table 4.
Cox proportional hazard model for assessment of overall survival
| Factors | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Age | |||
| 10‐year increment | 1 | ||
| 1.34 | 1.02–1.75 | 0.03 | |
| Sex | |||
| Female | 1 | ||
| Male | 1.23 | 0.69–2.31 | 0.46 |
| Body Weight Loss | |||
| <5.0% | 1 | ||
| >5.1% | 1.19 | 0.69–2.11 | 0.51 |
| Histology | |||
| Non‐squamous | 1 | ||
| Squamous | 1.31 | 0.80–2.19 | 0.28 |
| T factor | |||
| T1/2 | 1 | ||
| T3/4 | 0.91 | 0.53–1.61 | 0.77 |
| N factor | |||
| N 0–2 | 1 | ||
| N 3 | 1.05 | 0.55–2.08 | 0.85 |
| Stage | |||
| IIIA | 1 | ||
| IIIB | 0.97 | 0.52–1.83 | 0.93 |
Discussion
Concurrent chemoradiotherapy has been established as a standard treatment for patients with unresectable locally advanced NSCLC. The long‐term feasibility and efficacy of vinorelbine and cisplatin chemotherapy with concurrent thoracic radiotherapy were investigated. The 3‐, 5‐, and 7‐year overall survival rates (95% CI) were 43.2% (33.9–52.2), 25.2% (17.6–33.5), and 23.1% (15.7–31.4), respectively. Older age was associated with poor survival on multivariate analysis (HR 1.34, 95% CI 1.02–1.75).
Two phase III trial examined the efficacy and safety of newer generation cytotoxic agents in concurrent chemoradiotherapy for patients with locally advanced NSCLC.13, 14 The 5‐year survival rates (around 20%) were comparable to current analysis. To date, the present report (median survival time 30 months and 7‐year overall survival rate 23.1%) is one of the longest observation periods after concurrent chemoradiotherapy using third‐generation agents for locally advanced NSCLC. Recently, Tokuda et al.15 reported a favorable long‐term survival data (median survival time 2.1 years and 5‐year survival rate 31%) of concurrent thoracic radiotherapy with docetaxel and cisplatin in a phase II trial conducted by Okayama Lung Cancer Study Group (OLCSG). It seems that the result of these analyses were about twice better than that of the previous long‐term report of chemoradiotherapy with former generation agents by Ohe et al.6 (median survival time 16.1 months and 7‐year overall survival rate 12.0%) and others.16
Of the 91 patients with relapses, 85 (93%) experienced recurrence within 3 years after initial treatment. Local relapses (37 patients, 41%) and distant relapses (35 patients, 38%) were equally frequent. After 3 years of follow‐up, two local, three distant (without brain), and one mixed‐site recurrence was observed. Considering the proportion of local recurrence was similar to the OLCSG 0007 trial, a better strategy to control local relapse is a key to improving survival in locally advanced NSCLC.13 To gain a better local control, the radiation therapy oncology group (RTOG) conducted a phase III trial (RTOG 0617) to examine a higher dose (74 Gy) of radiotherapy with concurrent chemotherapy. However, the experimental arms of higher radiotherapy were terminated early because of survival futility.17 We recently reported early termination of a multicenter phase II trial of high‐dose thoracic radiotherapy (72 Gy) because of slow accrual and pulmonary toxicities.18 Based on these results, development of another strategy such as surgery followed by induction therapy might offer a better local control in selected patients.19 On the other hand, 11 of 20 brain relapses as a first recurrence were found within a year of initial treatment. Several authors reported that brain metastases were frequent early in the course after the initial treatment of stage III NSCLC.20, 21 According to our findings and previous reports, intensive brain surveys might be indicated for such patients no longer than 3 years from initial chemoradiotherapy.
The frequency and control of late toxicities, especially lung injury, have been emphasized along with the improvement of survival by concurrent chemoradiotherapy in stage III NSCLC. In the present analysis, four patients (4%) in the docetaxel consolidation trial experienced grade 5 pulmonary toxicities 4.4–9.6 months from initial treatments. On the other hand, life‐threatening pulmonary toxicities were not reported in phase I trial. (Table 3) This difference in the frequency of severe pulmonary toxicities might be related to consolidation docetaxel because the dose of cisplatin (80 mg/m2), vinorelbine (20 mg/m2) and thoracic radiotherapy (60 Gy) were the same in these two trials except for five patients who received 25 mg/m2 of vinorelbine in the phase I trial.7, 8 A relatively higher frequency of pulmonary complications was also reported in the experimental arm of the previous phase III trial that examined docetaxel as a consolidation therapy after concurrent chemoradiotherapy.22, 23 Although a note of caution might be indicated with docetaxel, the present result suggests that severe pulmonary toxicities were rare after 10 months from concurrent chemoradiotherapy.
According to recent trials, about half of Japanese patients with locally advanced lung cancer survive more than 2 years after concurrent chemoradiotherapy.13, 14 In those who survived more than 2 years, mortalities due to second primary malignancies and etiologies other than lung cancer were reported by several authors.15, 24 Five patients (4.5%) died without recurrence of lung cancer and whose causes of death were as follows: second primary malignancy (pharyngeal cancer, one patient), community‐acquired pneumonia (one patient), sudden death due to unknown etiology (two patients) and suicide (one patient), respectively. With an even greater proportion of patients cured by modern therapies including combined modality treatments, it would be increasingly important to consider and evaluate an appropriate care and monitoring for survivors.
In the present analysis, older age was significantly associated with poor survival (HR 1.34, 95% CI 1.02–1.75) after adjusting for sex, degree of weight loss, histology, T factor, N factor, and stage. In the previous literature on concurrent chemoradiotherapy with cisplatin and vinorelbine, age (≥70 years) was marginally associated with poor survival (HR 1.79, 95% CI 0.94–3.39).25 Several investigators reported higher incidences of adverse events in elderly patients with locally advanced NSCLC, even though they had a similar survival benefit.26, 27, 28 Furthermore, better clinical outcomes were reported in elderly patients (>70 years) by thoracic radiotherapy rather than chemoradiotherapy with a similar regimen for younger patients.29, 30 Based on these reports, it is necessary to develop an optimal treatment strategy, especially to find the best chemotherapy regimen combined with thoracic radiotherapy, for elderly patients with stage III NSCLC.
This study had several limitations. First, the proportion of patients with stage IIIA disease was relatively high compared to previous phase III trials, which might have a favorable effect on overall survival.13, 14 Second, the population included in this analysis was relatively younger than those reported by Segawa et al.13 and had better prognosis than real world patients. As discussed in this article, younger age might be a better prognostic factor in concurrent chemoradiotherapy (Table 3). The third limitation is potential selection bias in a highly selected population suitable for early phase clinical trials. To enable to follow clinical and prognostic information with the least missing data, however, we selected the patients that participated in the current phase I and feasibility trial of docetaxel consolidation.
In conclusion, approximately 15% of patients with unresectable stage III NSCLC could be cured with chemoradiotherapy without severe late toxicities after 10 months of follow‐up. Although based on the data from a highly selected population participated in phase I and phase II trial, this analysis would strengthen and confirm the previous reports concerning concurrent chemoradiotherapy with third generation cytotoxic agents.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgement
We thank Ms Mika Nagai for her assistance in the preparation of this manuscript.
(Cancer Sci, doi: 10.1111/cas.12028, 2012)
References
- 1. Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997; 111: 1710–7. [DOI] [PubMed] [Google Scholar]
- 2. Meerbeeck V. Staging of non‐small cell lung cancer: consensus, controversies and challenges. Lung Cancer 2001; 34: S95–107. [DOI] [PubMed] [Google Scholar]
- 3. Jett JR, Scott WJ, Rivera MP, Sause WT. Guidelines on treatment of stage IIIB non‐small cell lung cancer. Chest 2003; 123: 221S–5S. [DOI] [PubMed] [Google Scholar]
- 4. Furuse K, Fukuoka M, Kawahara M et al Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non‐small‐cell lung cancer. J Clin Oncol 1999; 17: 2692–9. [DOI] [PubMed] [Google Scholar]
- 5. Fournel P, Robinet G, Thomas P et al Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non‐small‐cell lung cancer: Groupe Lyon‐Saint‐Etienne d'Oncologie Thoracique‐Groupe Francais de Pneumo‐Cancerologie NPC 95‐01 Study. J Clin Oncol 2005; 23: 5910–7. [DOI] [PubMed] [Google Scholar]
- 6. Ohe Y, Ishizuka N, Tamura T, Sekine I, Nishiwaki Y, Saijo N. Long‐term follow‐up of patients with unresectable locally advanced non‐small cell lung cancer treated with chemoradiotherapy: a retrospective analysis of the data from the Japan Clinical Oncology Group trials (JCOG0003A). Cancer Sci 2003; 94: 729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sekine I, Noda K, Oshita F et al Phase I study of cisplatin, vinorelbine, and concurrent thoracic radiotherapy for unresectable stage III non‐small cell lung cancer. Cancer Sci 2004; 95: 691–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sekine I, Nokihara H, Sumi M et al Docetaxel consolidation therapy following cisplatin, vinorelbine, and concurrent thoracic radiotherapy in patients with unresectable stage III non‐small cell lung cancer. J Thorac Oncol 2006; 1: 810–5. [PubMed] [Google Scholar]
- 9. National Institutes of Health . National Cancer Institute Common Toxicity Criteria, Version 2.0. 1998. [Cited 30 Apr 1999.] Available from URL: http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf. [Google Scholar]
- 10. WHO . Handbook for Reporting Results of Cancer Treatment. Geneva: WHO Offset Publication, No. 48; 1979. [Google Scholar]
- 11. Therasse P, Arbuck SG, Eisenhauer EA et al New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 12. Armitage P, Berry G, Matthews J. Survival analysis In: Armitage P, Berry G, Matthews J, eds. Statistical Methods in Medical Research, 4th edn Oxford: Blackwell Science Ltd, 2002; 568–90. [Google Scholar]
- 13. Segawa Y, Kiura K, Takigawa N et al Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non‐small‐cell lung cancer: OLCSG 0007. J Clin Oncol 2010; 28: 3299–306. [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto N, Nakagawa K, Nishimura Y et al Phase III study comparing second‐ and third‐generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non‐small‐cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol 2010; 28: 3739–45. [DOI] [PubMed] [Google Scholar]
- 15. Tokuda Y, Takigawa N, Kozuki T et al Long‐term follow‐up of phase II trial of docetaxel and cisplatin with concurrent thoracic radiation therapy for locally advanced non‐small cell lung cancer. Acta Oncol 2012; 51: 537–40. [DOI] [PubMed] [Google Scholar]
- 16. Blackstock AW, Govindan R. Definitive chemoradiation for the treatment of locally advanced non small‐cell lung cancer. J Clin Oncol 2007; 25: 4146–52. [DOI] [PubMed] [Google Scholar]
- 17. Bradley RP, Komaki R, Masters G et al A Randomized Phase III Comparison of Standard‐Dose (60 Gy) Versus High‐dose (74 Gy) Conformal Chemoradiotherapy ± Cetuximab for Stage IIIA/IIIB Non‐Small Cell Lung Cancer: Preliminary Findings on Radiation Dose in RTOG 0617. 53rd Annual Meeting of the American Society of Radiation Oncology 2011.
- 18. Horinouchi H, Sumi M, Satouchi M et al Multicenter phase II study of concurrent high‐dose (72Gy) three‐dimensional conformal radiotherapy (3D‐CRT) without elective nodal irradiation with chemotherapy using cisplatin and vinorelbine for unresectable stage III non‐small cell lung cancer (NSCLC). ASCO annual conference. J Clin Oncol 2012; 30: 7070. [DOI] [PubMed] [Google Scholar]
- 19. Toyooka S, Kiura K, Takemoto M et al Long‐term outcome of induction chemoradiotherapy with docetaxel and cisplatin followed by surgery for non‐small‐cell lung cancer with mediastinal lymph node metastasis. Interact Cardiovasc Thorac Surg 2012; 14: 565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gaspar LE, Chansky K, Albain KS et al Time from treatment to subsequent diagnosis of brain metastases in stage III non‐small‐cell lung cancer: a retrospective review by the Southwest Oncology Group. J Clin Oncol 2005; 23: 2955–61. [DOI] [PubMed] [Google Scholar]
- 21. Horinouchi H, Sekine I, Sumi M et al Brain metastases after definitive concurrent chemoradiotherapy in patients with stage III lung adenocarcinoma: carcinoembryonic antigen as a potential predictive factor. Cancer Sci 2012; 103: 756–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gandara DR, Chansky K, Albain KS et al Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non‐small‐cell lung cancer: Phase II Southwest Oncology Group Study S9504. J Clin Oncol 2003; 21: 2004–10. [DOI] [PubMed] [Google Scholar]
- 23. Hanna N, Neubauer M, Yiannoutsos C et al Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non‐small‐cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 2008; 26: 5755–60. [DOI] [PubMed] [Google Scholar]
- 24. Takigawa N, Kiura K, Segawa Y et al Second primary cancer in survivors following concurrent chemoradiation for locally advanced non‐small‐cell lung cancer. Br J Cancer 2006; 95: 1142–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Naito Y, Kubota K, Nihei K et al Concurrent chemoradiotherapy with cisplatin and vinorelbine for stage III non‐small cell lung cancer. J Thorac Oncol 2008; 3: 617–22. [DOI] [PubMed] [Google Scholar]
- 26. Schild SE, Stella PJ, Geyer SM et al The outcome of combined‐modality therapy for stage III non‐small‐cell lung cancer in the elderly. J Clin Oncol 2003; 21: 3201–6. [DOI] [PubMed] [Google Scholar]
- 27. Schild SE, Mandrekar SJ, Jatoi A et al The value of combined‐modality therapy in elderly patients with stage III nonsmall cell lung cancer. Cancer 2007; 110: 363–8. [DOI] [PubMed] [Google Scholar]
- 28. Yuen AR, Zou G, Turrisi AT et al Similar outcome of elderly patients in intergroup trial 0096: Cisplatin, etoposide, and thoracic radiotherapy administered once or twice daily in limited stage small cell lung carcinoma. Cancer 2000; 89: 1953–60. [DOI] [PubMed] [Google Scholar]
- 29. Movsas B, Scott C, Sause W et al The benefit of treatment intensification is age and histology‐dependent in patients with locally advanced non‐small cell lung cancer (NSCLC): a quality‐adjusted survival analysis of radiation therapy oncology group (RTOG) chemoradiation studies. Int J Radiat Oncol Biol Phys 1999; 45: 1143–9. [DOI] [PubMed] [Google Scholar]
- 30. Sause W, Kolesar P, Taylor SI et al Final results of phase III trial in regionally advanced unresectable non‐small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000; 117: 358–64. [DOI] [PubMed] [Google Scholar]


