Abstract
Expression of MDM2 protein appears to be increased in malignancy and correlated to prognosis of tumors, but its role in gastric cancer remains controversial. Our recent investigations indicated that JWA was a novel candidate biomarker for gastric cancer. To evaluate the impact of MDM2 protein expression alone, and in combination with JWA, on the prognostic and predictive of patients with resectable gastric cancer, expression of MDM2 and JWA were examined by immunohistochemistry in three large cohorts (total n = 1131) of patient with gastric cancer. We found that MDM2 protein levels were significantly upregulated in gastric cancer (70.4%, 57 of 81) compared with adjacent non‐cancerous tissues. High tumoral MDM2 expression significantly correlated with clinicopathologic characteristics, as well as with shorter overall survival (OS; P < 0.001 for all cohorts) in patients without adjuvant treatment. The effect of adjuvant fluorouracil–leucovorin–oxaliplatin (FLO) in improving OS compared with surgery alone was evident only in the high MDM2 group (hazard ratio = 0.57; 95% confidence interval, 0.37–0.89; P = 0.013). Furthermore, knockdown of MDM2 and overexpression of JWA had a synergistic effect on suppression of gastric cancer cell proliferation and migration. Patients with low MDM2 and high JWA expression had a better outcome of survival compared with the other groups (P < 0.001 for all cohorts). For the first time, our data suggest that MDM2 is a potent prognostic and predictive factor for benefit from adjuvant fluorouracil–leucovorin–oxaliplatin chemotherapy in resectable gastric cancer. The combination of MDM2 expression and JWA could serve as a more effective candidate prognostic biomarker for gastric cancer.
Gastric cancer is one of the most common malignancies and remains the second most frequent cause of cancer‐related death worldwide. Despite recent advances in surgical techniques and medical treatment, the 5‐year survival rate for gastric cancer patients is <30%.1 Investigation of biomarkers for screening at an early curative stage would have great clinical value. Moreover, a large percentage of cancer patients are treated unnecessarily.2 Biomarkers may be especially useful in the group being more likely to benefit from chemotherapeutics.
The MDM2 protein is a negative regulator of the p53 tumor suppressor. The p53 protein functions as a tumor suppressor by inducing the expression of genes that inhibit cell growth and upregulate apoptosis,3 and its deletion or loss of function is associated with a large fraction of human cancers.4, 5 It has been shown that MDM2 can inhibit p53 bioactivity by blocking the transcriptional activity of p53 and promoting p53 protein degradation.6 However, p53 can also regulate the synthesis of MDM2.7 The imbalance of the functions of MDM2 and p53 has been related with several cancers.8 Molecular epidemiological studies have shown the association between single nucleotide polymorphisms of MDM2 and the risk of various cancers, including gastric cancer.9, 10, 11, 12, 13 Several studies have shown that MDM2 overexpression is associated with poor survival and is a useful predictive factor for poor prognosis in humans with hepatocellular carcinoma and breast carcinomas.6, 14, 15 However, the predictive value of MDM2 for gastric cancer patients with adjuvant chemotherapy remains controversial.
The JWA gene, also known as ADP‐ribosylation‐like factor 6 interacting protein 5 (ARL6ip5), is a structurally novel microtubule‐binding protein, which regulates cancer cell migration through MAPK cascades16 and inhibits cell adhesion, invasion, and the metastasis of melanoma cells by suppressing integrin αVβ3 signaling.17 We also showed that polymorphisms in the JWA gene are associated with gastric cancer in a Chinese population.18 Furthermore, our recent data have indicated that JWA protein expression in tumor is a novel candidate prognostic marker and predictive factor for benefit from adjuvant platinum‐based chemotherapy in resectable gastric cancer.19 Considering the versatile roles of MDM2 and JWA in oncopathology, we are interested in whether JWA would work as a cooperator with MDM2 to improve predictive potency in gastric cancer.
In the present study, to examine the possible prognostic and predictive value of MDM2 and JWA, we evaluated staining results in three large independent cohorts of gastric cancer biopsies using TMA technology.
Materials and Methods
Patients and specimens
Three independent retrospective patient cohorts were studied.19 The training cohort included 103 patients who underwent radical gastrectomy at Nantong Cancer Hospital (Nantong, China) from May 1, 1990 to June 1, 1995. A TMA including the gastric cancer samples and matched non‐cancerous gastric mucosa more than 5 cm from the tumoral margins was constructed. Two more independent tumor TMAs were constructed to validate training cohort data. The first, the testing cohort, consisted of all 640 surgical cases from the Nantong Cancer Hospital from December 1, 2000 to April 1, 2005, and the second, the validation cohort, included all 1022 surgical cases in Yixing People's Hospital (Yixing, China) from January 1, 1999 to December 31, 2006. These patients were treated only with radical gastrectomy or with postoperative adjuvant chemotherapy (for details, see Data S1 and Fig. S1). For the patients with resectable gastric cancer treated with chemotherapy in the validation cohort, the distributions of demographic characteristics and the selected clinicopathologic variables of patients between the FLO group and the FLP group were similar (all P > 0.05), except histological type (P < 0.001; Table S1). Written informed consent was obtained from each patient prior to tissue acquisition. Institutional approval was acquired from the Ethical Review Board of Nanjing Medical University (Nanjing, China) prior to this study.
Detailed clinicopathologic information was obtained. The histological types of gastric cancer were classified according to Lauren20 and staged according to the TNM guidelines.21 Only confirmed intestinal, diffuse, and mixed types were included.
Tissue microarray construction and immunohistochemistry
This procedure is described in detail in Data S1.
Evaluation of immunohistochemistry
By applying a semiquantitative IRS in the training cohort, staining of MDM2 and JWA in the tissue was scored independently by two pathologists blinded to the clinical data, as reported elsewhere.22 Category A documented the intensity of immunostaining as 0–3 (0, negative; 1, weak; 2, moderate; 3, strong; Fig. S2). Category B documented the percentage of immunoreactive cells as one (0–25%), two (26–50%), three (51–75%), and four (76–100%). Multiplication of category A and B resulted in an IRS ranging from 0 to 12 for each tumor or non‐tumor.
The optimum cut‐off value of IRS was obtained by ROC analysis, the AUCs at different cut‐off values of MDM2 IRS for 1, 3, and 5 years of OS time were calculated. The optimum value of cut‐off points of the MDM2 IRS in the Nantong district cohort (combined training cohort and testing cohort) was 3, as the predictive value of this cut‐off point for death was the best (Fig. S3). Under these conditions, samples with IRS 0–3 and IRS 4–12 were classified as low and high expression of MDM2 in tumors. The expression of MDM2 in the validation cohort was scored by the same pathologists with exactly the same procedure.
Cell culture
Details regarding the source of cells and culture conditions are presented in Data S1.
Short interfering RNA, plasmids, and cell growth assay
Control siRNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or MDM2 siRNA (Santa Cruz Biotechnology) was transfected by Lipofectamine 2000 (Invitrogen, Life Technologies, Grand Island, NY, USA) according to the manufacturer's instructions. The FLAG‐vector and FLAG‐JWA plasmids were described previously.23
For measurement of cell growth, a colorimetric water‐soluble tetrazolium salt assay (Cell Counting Kit‐8; Dojindo Laboratories, Kumamoto, Japan) was used to access the number of viable cells at various time points after transfection. The inhibition rate (I%) after treatment with oxaliplatin for 48 h was calculated using the background corrected absorbance by the following equation: I% = 100 × (A untreated control well − A experimental well) / A untreated control well.
Apoptosis assay
This procedure is described in Data S1.
Western blot analysis
This procedure is described in Data S1.
Transwell migration assay
This procedure is described in Data S1.
Scratch migration assay
This procedure is described in Data S1.
Statistical analysis
This procedure is described in detail in Data S1.
Results
Increased MDM2 expression in gastric cancer versus non‐cancer tissues
To test MDM2 protein expression by Western blot, we selected seven pairs of human primary gastric cancer tissues and matched normal gastric mucosa. Elevated expression of MDM2 occurred in six of seven (85.7%) gastric cancer tissues compared with the paired normal mucosa (Fig. 1a). Furthermore, immunohistochemical staining of gastric TMA was used to further confirm MDM2 expression in 81 gastric cancer patients of the training cohort. Staining of MDM2 was mainly localized in the nuclei (Fig. 1b). Regarding the distribution of the differences of IRS, MDM2 expression was significantly increased in 57 of 81 (70.4%) of gastric cancer tissues compared with the matched normal tissues (P < 0.001, Wilcoxon test; Fig. 1c). The abovementioned results show the MDM2 protein elevation in gastric cancer versus non‐cancer tissues.
Figure 1.
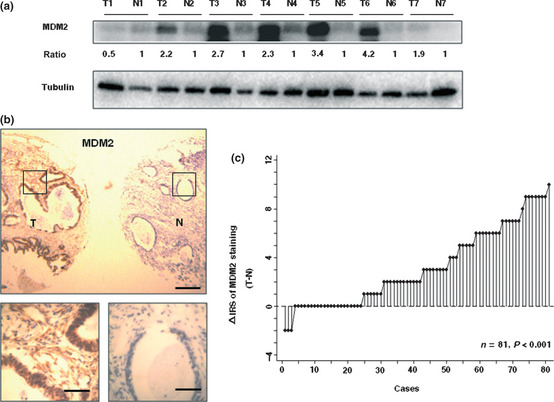
Comparative expression of MDM2 in primary tumors and corresponding non‐tumors in gastric cancer patients. (a) MDM2 protein levels in seven cancer tissues (T) and paired non‐cancerous normal tissues (N) of gastric cancer patients were analyzed by Western blotting. The level of each protein was normalized against tubulin, and the protein levels in cancer tissues indicated as a ratio paired to non‐cancerous normal tissues. (b) Immunohistochemical staining for MDM2 in tissue microarray. Scale lines: top panel, 250 μm; bottom panel, 50 μm. (c) Distribution of the differences in MDM2 staining expressed as immunoreactivity score (IRS; Δ IRS = IRS T − IRS N). P‐values were calculated using the Wilcoxon test.
Association of MDM2 expression with clinicopathologic features in patients treated only with surgery
Immunohistochemical staining of MDM2 levels was analyzed to determine their relationship with clinicopathologic features of the three cohorts. As shown in Table 1, the protein expression of MDM2 was significantly associated with clinicopathologic features, such as lymph node metastasis (N category), TNM stage, and tumor diameter in the cancerous tissues of the training and testing cohorts (P < 0.05). Moreover, the association in the validation cohort had the same tendency, although not statistically significant (Table 1). Expression of MDM2 was positively correlated with distant metastasis (M category) in the training and validation cohorts (P < 0.05). In addition, more cases with depth of T3/T4 invasion (T category) were seen in the group with high MDM2 expression in the testing and validation cohorts (P < 0.05). These observations suggest that elevated functional MDM2 expression is associated with clinical gastric cancer progression.
Table 1.
Relationship between expression levels of MDM2 and clinicopathologic features of gastric cancer patients treated with surgery alone, split in three cohorts
| Variables | Training cohort (n = 81 cases) | Testing cohort (n = 368 cases) | Validation cohort (n = 357 cases) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (%) | High (%) | P * | Low (%) | High (%) | P * | Low (%) | High (%) | P * | |
| All patients | 21 (25.9) | 60 (74.1) | 197 (53.5) | 171 (46.5) | 114 (31.9) | 243 (68.1) | |||
| Age, years | |||||||||
| ≤65 | 15 (71.5) | 49 (81.7) | 0.358 | 122 (61.9) | 113 (66.1) | 0.447 | 45 (39.5) | 110 (45.3) | 0.360 |
| >65 | 6 (28.5) | 11 (18.3) | 75 (38.1) | 58 (33.9) | 69 (60.5) | 133 (54.7) | |||
| Gender | |||||||||
| Males | 16 (76.2) | 45 (75.0) | 1.000 | 138 (70.1) | 118 (69.0) | 0.910 | 90 (78.9) | 187 (76.9) | 0.786 |
| Females | 5 (23.8) | 15 (25.0) | 59 (29.9) | 53 (31.0) | 24 (21.1) | 56 (23.1) | |||
| Depth of invasion | |||||||||
| T1/T2 | 3 (14.3) | 2 (3.3) | 0.107 | 50 (25.4) | 22 (12.9) | 0.003 | 51 (44.7) | 73 (30.0) | 0.009 |
| T3/T4 | 18 (85.7) | 58 (96.7) | 147 (74.6) | 149 (87.1) | 63 (55.3) | 170 (70.0) | |||
| Lymph node metastasis | |||||||||
| N0 | 14 (66.7) | 6 (10.0) | <0.001 | 77 (39.1) | 27 (15.8) | <0.001 | 50 (43.9) | 86 (35.4) | 0.130 |
| N1/N2/N3 | 7 (33.3) | 54 (90.0) | 120 (60.9) | 144 (84.2) | 64 (56.1) | 157 (64.6) | |||
| Distant metastasis | |||||||||
| M0 | 21 (100.0) | 45 (75.0) | 0.009 | 187 (94.9) | 164 (95.9) | 0.805 | 113 (99.1) | 230 (94.7) | 0.044 |
| M1 | 0 (0.0) | 15 (25.0) | 10 (5.1) | 7 (4.1) | 1 (0.9) | 13 (5.3) | |||
| TNM stage | |||||||||
| I | 6 (28.6) | 3 (5.0) | <0.001 | 34 (17.3) | 6 (3.5) | <0.001 | 35 (30.7) | 55 (22.6) | 0.103 |
| II | 8 (38.1) | 10 (16.7) | 50 (25.4) | 31 (18.1) | 30 (26.3) | 50 (20.6) | |||
| III | 7 (33.3) | 27 (45.0) | 90 (45.7) | 104 (60.8) | 46 (40.4) | 131 (53.9) | |||
| IV | 0 (0.0) | 20 (33.3) | 23 (11.6) | 30 (17.5) | 3 (2.6) | 7 (2.9) | |||
| Tumor diameter | |||||||||
| ≤5 cm | 15 (71.4) | 26 (43.3) | 0.041 | 101 (51.3) | 48 (28.1) | <0.001 | 68 (59.7) | 135 (55.6) | 0.493 |
| >5 cm | 6 (28.6) | 34 (56.7) | 96 (48.7) | 123 (71.9) | 46 (40.3) | 108 (44.4) | |||
| Histological type† | |||||||||
| Intestinal | 11 (52.4) | 31 (51.7) | 1.000 | 119 (60.4) | 90 (52.6) | 0.141 | 52 (46.4) | 96 (39.7) | 0.248 |
| Diffuse | 10 (47.6) | 29 (48.3) | 78 (39.6) | 81 (47.4) | 60 (53.6) | 146 (60.3) | |||
| JWA expression | |||||||||
| Low | 5 (23.8) | 45 (75.0) | <0.001 | 22 (11.2) | 139 (81.3) | <0.001 | 30 (26.3) | 151 (62.1) | <0.001 |
| High | 16 (76.2) | 15 (25.0) | 175 (88.8) | 32 (18.7) | 84 (73.7) | 92 (37.9) | |||
*Two‐sided Fisher's exact tests. †Excluded three patients with mixed intestinal and diffuse types in validation cohort.
Expression of MDM2 an independent prognostic predictor for survival of gastric cancer patients treated only with surgery
Kaplan–Meier survival curves showed that MDM2 expression was significantly correlated with overall 5‐year survival in patients treated only with surgery in the three independent cohorts (P < 0.001, Fig. 2). Moreover, univariate analysis indicated that significant negative predictors for survival in the three independent cohorts were lymph node metastasis (P < 0.01 for all), TNM stage (P < 0.001 for all), and MDM2 expression (P < 0.01 for all; Table S2). The multivariate Cox regression analysis indicated that low MDM2 expression was an independent positive prognostic factor for gastric cancer (P < 0.001 for the two larger cohorts; Table 2), whereas non‐tumoral MDM2 expression was not correlated with OS (Fig. S4). Overall, MDM2 expression is an independent prognostic predictor for patient survival of gastric cancer.
Figure 2.
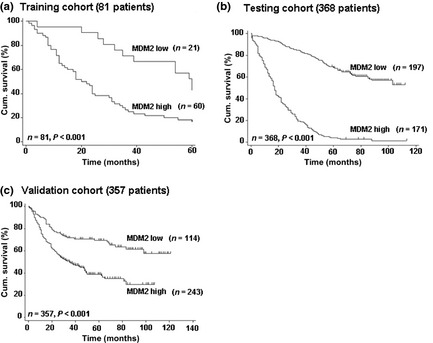
Kaplan–Meier curves depicting overall survival according to expression pattern of MDM2 in three cohorts of gastric cancer patients. P‐values were calculated using the log–rank test. Cum., cumulative.
Table 2.
Multivariate Cox regression analysis of MDM2 or MDM2/JWA expression and clinicopathologic variables predicting survival in three cohorts of patients with gastric cancer treated with surgery alone
| Variables | Training cohort (n = 81 cases) | Testing cohort (n = 368 cases) | Validation cohort (n = 357 cases) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P a | HR (95% CI) | P a | HR (95% CI) | P a | |
| MDM2 | ||||||
| Age, years (≤65 vs >65) | 1.60 (0.78–3.28) | 0.195 | 1.12 (0.86–1.46) | 0.400 | 0.84 (0.62–1.12) | 0.230 |
| Gender (male vs female) | 1.49 (0.84–2.65) | 0.169 | 1.05 (0.80–1.38) | 0.716 | 1.01 (0.71–1.44) | 0.939 |
| Histological type (diffuse vs intestinal) | 0.90 (0.52–1.57) | 0.723 | 1.23 (0.95–1.59) | 0.118 | 1.56 (1.12–2.17) | 0.009 |
| Tumor diameter (≤5 cm vs >5 cm) | 1.01 (0.60–1.70) | 0.985 | 1.34 (0.99–1.80) | 0.057 | 1.48 (1.08–2.01) | 0.014 |
| TNM stage (I/II vs III/IV) | 2.85 (1.35–6.03) | 0.006 | 1.62 (1.17–2.24) | 0.004 | 4.32 (2.91–6.40) | <0.001 |
| MDM2 expression (low vs high) | 1.91 (0.96–3.81) | 0.065 | 6.91 (5.10–9.36) | <0.001 | 1.88 (1.31–2.70) | 0.001 |
| MDM2/JWA | ||||||
| Age, years (≤65 vs >65) | 1.47 (0.72–2.99) | 0.293 | 1.18 (0.90–1.53) | 0.227 | 0.82 (0.67–1.10) | 0.185 |
| Gender (male vs female) | 1.65 (0.92–2.94) | 0.090 | 0.97 (0.74–1.28) | 0.843 | 1.04 (0.73–1.48) | 0.819 |
| Histological type (diffuse vs intestinal) | 0.91 (0.52–1.57) | 0.728 | 1.02 (0.79–1.32) | 0.885 | 1.45 (1.04–2.03) | 0.030 |
| Tumor diameter (≤5 cm vs >5 cm) | 1.05 (0.62–1.76) | 0.857 | 1.42 (1.05–1.92) | 0.023 | 1.50 (1.10–2.05) | 0.010 |
| TNM stage (I/II vs III/IV) | 1.37 (0.57–3.27) | 0.479 | 1.38 (0.98–1.93) | 0.062 | 4.14 (2.79–6.16) | <0.001 |
| MDM2/JWA expression | ||||||
| MDM2 high JWA low vs both low/high | 0.36 (0.17–0.77) | 0.008 | 0.42 (0.29–0.59) | <0.001 | 0.67 (0.48–0.94) | 0.020 |
| MDM2 high JWA low vs MDM2 low JWA high | 0.18 (0.06–0.51) | 0.001 | 0.09 (0.06–0.13) | <0.001 | 0.45 (0.29–0.69) | <0.001 |
Multivariate Cox regression analysis including age, gender, TNM stage, tumor diameter, histological type, JWA or MDM2 or combined protein expression status. CI, confidence interval; HR, hazard ratio.
Protein MDM2 is a potent prognostic and predictive factor for benefit from adjuvant FLO chemotherapy in resectable gastric cancer
To further determine the prognostic value of MDM2 in patients with adjuvant chemotherapy, in the validation cohort, we analyzed OS between the patients who received adjuvant chemotherapy versus those who did not. Significant difference was seen in OS between the surgery only group and the group receiving adjuvant FLO (n = 85, log–rank test, P = 0.033, Fig. 3a). A statistically significant benefit for gastric cancer patients from FLO chemotherapy compared with surgery alone was only found in high MDM2 expression patients (HR = 0.57, 95% confidence interval, 0.37–0.89, Table S3; log–rank test, P = 0.013, Fig. 3b). Patients with low MDM2 expression in tumors had no additional survival benefit from adjuvant FLO chemotherapy (Table S3, Fig. 3c).
Figure 3.
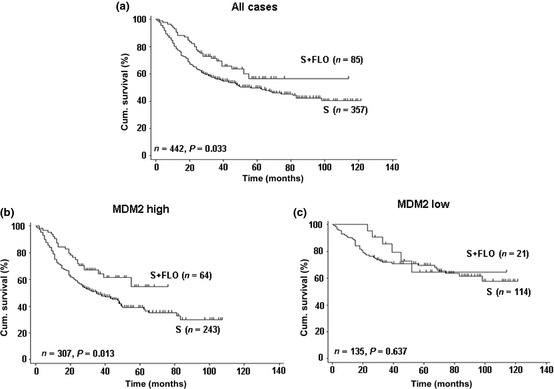
Kaplan–Meier curves depicting overall survival according to MDM2 expression patterns in gastric cancer patients in the validation cohort treated with or without fluorouracil–leucovorin–oxaliplatin (FLO). P‐values were calculated using the log–rank test. Cum., cumulative; S, surgery alone.
Overall survival was also analyzed for patients treated with surgery alone and with FLP chemotherapy. The results did not present a significant survival difference (log–rank test, P = 0.076; Fig. S5A). Regardless of high or low MDM2 expression, data showed no significant survival discrepancy between the FLP treatment and surgery alone (P = 0.462 for MDM2 high expression, P = 0.119 for MDM2 low expression; Fig. S5B,C). Further multivariate analysis elucidated that patients receiving the FLP treatment had no significant benefit in OS compared with surgery alone (HR = 0.98, P = 0.901 for MDM2 high expression, HR = 0.92, P = 0.828 for MDM2 low expression; Table S4). Taken together, MDM2 is a potent prognostic and predictive factor for benefit from adjuvant FLO chemotherapy in resectable gastric cancer.
Effect of MDM2 knockdown on chemosensitivity to oxaliplatin and expression of p53 in SGC‐7901 cells
These results showed that patients with high MDM2 expression received survival benefit from adjuvant FLO therapy. We then investigated the effect of MDM2 knockdown on chemosensitivity to oxaliplatin in SGC‐7901 gastric cancer cells by cell growth assay and apoptosis assay. Cell growth assay showed that oxaliplatin increased the cytotoxicity of SGC‐7901 cells in a dose‐dependent pattern. Reduced inhibition rate and increased p53 expression were seen in MDM2 knockdown cells (Fig. 4a,b). Furthermore, we found that the percentage of apoptotic cells produced by oxaliplatin was significantly decreased by the presence of siRNA‐MDM2 treatment (Fig. 4c,d).
Figure 4.
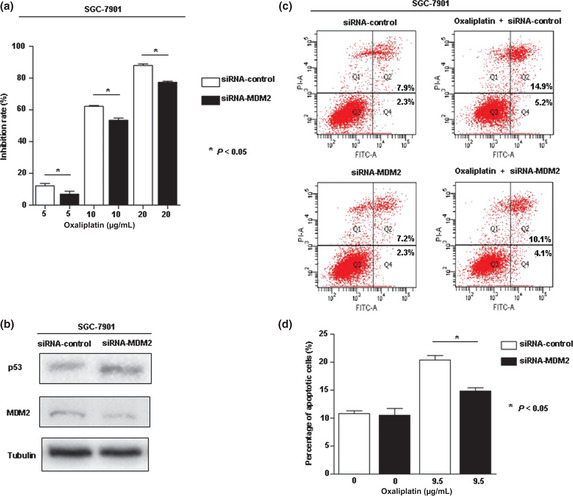
(a) Dose–response curve of siRNA‐MDM2 and oxaliplatin alone or in combination in SGC‐7901 gastric cancer cells. Data point, means of at least three independent experiments; bars indicate standard deviation. (b) Protein expression of p53 shown in SGC‐7901 cells by Western blot. (c,d) Effect of MDM2 knockdown on oxaliplatin‐induced apoptosis in SGC‐7901 cells. The dose of oxaliplatin chosen was close to IC 50.
Synergistic effect of MDM2 with JWA expression on suppression of gastric cancer cell proliferation and migration
Our previous report showed that JWA regulates cancer cell migration through MAPK cascades,16 and JWA was a novel candidate biomarker for gastric cancer.19 To study the potential synergistic role of MDM2 and JWA in gastric carcinogenesis, we first investigated the involvement of MDM2 and JWA in gastric cancer cell proliferation using siRNA‐MDM2 and FLAG‐JWA. In the SGC‐7901 gastric cancer cell line, expression of the MDM2 protein was efficiently knocked down after the transient introduction of the MDM2‐specific siRNA (siRNA‐MDM2) than with the control siRNA (siRNA‐control; Fig. 5a). The JWA protein was efficiently overexpressed after the transfection of FLAG‐JWA plasmid compared with the control plasmid (FLAG‐vector; Fig. 5b). We found that the ability of cell proliferation was drastically decreased after MDM2 knockdown and the transfection of FLAG‐JWA further reduced the cell numbers in the SGC‐7901 cell line (Fig. 5c). The same effect was seen in the protein expression of proliferating cell nuclear antigen (Fig. 5d). A Transwell migration assay revealed that the numbers of SGC‐7901 cells that migrated through the membrane into the lower chamber were significantly lower in siRNA‐MDM2 transfected cells than control; moreover, the FLAG‐JWA transfection further reduced the cell numbers (Fig. 5e,f). Similarly, MDM2 deficient and JWA overexpression cells showed a marked delay in wound closure by scratch migration assay (Fig. 5g,h), indicating that MDM2 and JWA have a synergistic effect on suppression of gastric cancer cell proliferation and migration.
Figure 5.
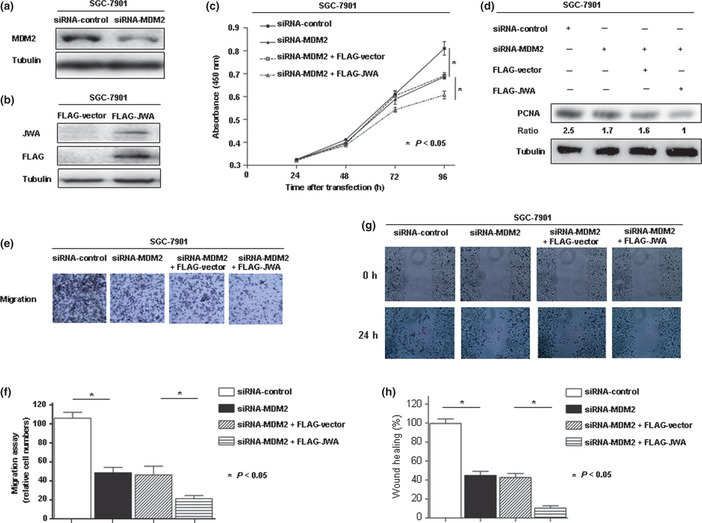
(a) Loss‐of‐function screening was done using siRNAs targeting MDM2 in SGC‐7901 gastric cancer cells. The knockdown of a target gene was confirmed by Western blot. (b) Overexpression of JWA protein using FLAG‐JWA in SGC‐7901 cells was confirmed by Western blot analysis. (c) Number of viable cells at various time points after transfection was assessed by colorimetric water‐soluble tetrazolium salt assay. (d) Protein expression of proliferating cell nuclear antigen (PCNA) shown in SGC‐7901 cells by Western blot. (e,f) Transwell migration assay. (g,h) Scratch migration assay.
Synergistic effect of MDM2 with JWA expression on OS in patients treated only with surgery
In our recent study, statistically significant positive correlations between JWA and overall 5‐year survival in the three independent cohorts have been elucidated.19 Moreover, the expression of MDM2 was negatively correlated with JWA in the cancerous tissues of all three independent cohorts of patients treated only with surgery only (P < 0.001 for all; Table 1, Fig. S6). Next, we stratified the patients into three distinct groups depending on staining for MDM2 and JWA: MDM2 low and JWA high; both low/high; MDM2 high and JWA low. It was shown that patients with MDM2 low and JWA high had significantly better OS than other groups (P < 0.001, log–rank test; Fig. 6).
Figure 6.
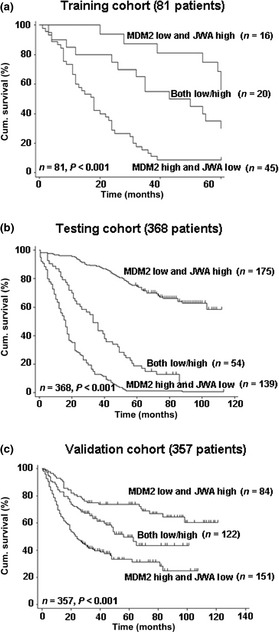
Kaplan–Meier curves depicting overall survival according to expression patterns of MDM2/JWA in three cohorts of gastric cancer patients. P‐values were calculated using the log–rank test. Cum., cumulative.
Furthermore, the multivariate and univariate Cox regression analysis indicated that low MDM2 combined with high JWA expression was an independent positive prognostic factor for gastric cancer in all three cohorts (Tables 2, S2).
To further evaluate the prognostic value of MDM2 and JWA expression, a time‐dependent ROC analysis was carried out, which indicated that the combination of the clinical risk score (TNM stage, histologic type, and tumor diameter) and MDM2 or MDM2 plus JWA contributed much more than either one alone in both the training and testing cohorts (Fig. 7). However, this effect was not apparent in the validation cohort due to the relatively higher AUC of clinical predictors (Fig. S7). These findings show us the synergistic effect of MDM2 with JWA expression on OS in patients treated only with surgery.
Figure 7.
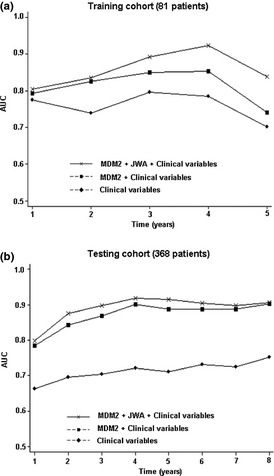
Time‐dependent receiver operating characteristic analyses for the clinical risk score (TNM stage, histological type, and tumor diameter), the combined MDM2 or MDM2 plus JWA, and clinical risk score in patients with surgery alone in the training cohort (a) and testing cohort (b). AUC, area under the curve.
Discussion
Prognostic studies of tumor biomarkers are valuable as they assist in the improvement of diagnosis, treatment, and prevention of malignancies.24 However, even more important are those biomarkers determining which patients can benefit from adjuvant therapy.25 Adjuvant chemotherapy without radiation for gastric cancer has recently become the standard of care in some countries,26 whereas unnecessary treatments are used in many cancer patients. Even among gastric cancers that share identical clinicopathological features, there are significant differences among individuals in terms of sensitivity to the same regimen.2 Thus, the discovery of potential biomarkers could play an important role in personalized cancer therapies. In this study, patients with high MDM2 expression in tumors had significant survival benefit from adjuvant FLO chemotherapy. Moreover, we showed the synergistic effect of MDM2 with JWA expression in predicting OS in gastric cancer patients.
An interesting question that attracted out attention is how could high expression of MDM2 play precisely opposite roles on prognostic and predictive effect? This phenomenon in cancer biology and treatment resistance has been documented: MDM2 plays an important role in the development, invasion, and metastasis of malignancies.27, 28 This conclusion was confirmed and extended by our study, in which we found an association between high MDM2 expression with clinicopathological features and a negative prognosis in resectable gastric cancer patients. Conversely, the positive predictive effect of high MDM2 on survival in FLO treated patients was highly significant. This may point to a role of MDM2 in chemoresistance, probably related to oxaliplatin, and it was confirmed in this study. Dysregulation of several DNA repair mechanisms are important modulators of platinum effect.29 Oxaliplatin‐induced DNA lesions, including interstrand DNA cross‐links and DNA protein cross‐links are likely to contribute to the drug's biological properties.30 Thus, high MDM2 expression might play an important role in the susceptibility to DNA‐damaging agents though the direct role of MDM2 in FLO chemotherapy treatment. This point needs to be studied further. It must be noted that the patients received more survival benefit from FLO than from FLP, which may be partially due to the fact that FLO reduces toxicity as compared with FLP, as explained previously.19
Despite the large numbers of studies that attempt to identify molecular predictors of response and toxicity to treatment, none of the tests and molecular markers thus far have proven to be reliable in prospective clinical trials.31 An important conclusion we would like to emphasize here is that, despite cohorts with different types of patients, from different hospitals in different districts and different time periods, MDM2 expression maintained its strong independent prognostic value. Moreover, the synergistic of MDM2 with JWA presented a more effective and convincing candidate prognostic biomarker for gastric cancer.
Nantong Cancer Hospital is a specialized cancer hospital whereas Yixing People's Hospital is a comprehensive one, therefore, patients in the former are often at more advanced stages of disease compared to the latter. However, the distributions of these variables of patients between the training cohort and testing cohort in Nantong district were mostly matched.19 All the cases of the validation cohort in this retrospective study were collected during 1999– 2006. The final decision regarding which chemotherapy regimen should be used mostly depended on the patients in many cases, such as regional and personal economic status or the rules of medical insurance in China. In our study, more patients received platinum‐based first‐line chemotherapy regimens, so we focused our attention on these patients. The database built upon this information is thus as complete as possible. Nevertheless, despite highly significant results in such a large patient group, these markers should be validated in different ethnic populations and prospective clinical studies are warranted.
In conclusion, we found that MDM2 was expressed at higher levels in gastric cancer tissues than in non‐cancerous gastric mucosa, and MDM2 expression was associated with clinicopathologic features in patients treated only with surgery. Moreover, knockdown of MDM2 and overexpression of JWA has the synergistic effect on suppression of gastric cancer cell proliferation and migration. Expression of MDM2 combined with JWA could serve as a more effective candidate prognostic biomarker for gastric cancer. To our knowledge, this is the first report of MDM2 as a potential predictor for adjuvant chemotherapy with platinum‐based regimen FLO in resectable gastric cancer patients.
Supporting information
Data S1. Details of: patients and specimens; tissue microarray construction and immunohistochemistry; cell culture; apoptosis assay; Western blot analysis; Transwell migration assay; scratch migration assay; and statistical analysis.
Fig. S1. Schematic representation of this study.
Fig. S2. Representative images of MDM2 immunohistochemical staining in gastric cancer. (A) Negative staining; (B) weak positive staining; (C) moderate positive staining; (D) strong positive staining. Scale line = 25 μm.
Fig. S3. Receiver operating characteristic curves were obtained to show the relation between areas under the curves (AUC) at different cut‐off values of MDM2 immunoreactivity scores (IRS) for 1, 3, and 5 years of overall survival time.
Fig. S4. Kaplan–Meier curves depicting overall survival according to MDM2 expression patterns in non‐tumors in the training cohort of gastric cancer patients. P‐values were calculated using the log–rank test. Cum., cumulative.
Fig. S5. Kaplan–Meier curves depicting overall survival according to MDM2 expression patterns in gastric cancer patients in the validation cohort treated with or without fluorouracil–leucovorin–platinol (FLP). P‐values were calculated using the log–rank test. S, surgery alone.
Fig. S6. Immunoreactivity score (IRS) comparison of the absolute value of ΔIRS (tumor tissue [T] – non‐cancerous gastric tissue [N]) of MDM2 staining in different absolute values of ΔIRS of JWA staining subgroups.
Fig. S7. Time‐dependent receiver operating characteristic analyses for the clinical risk score (TNM stage, histological type, and tumor diameter), the combined MDM2 or MDM2 plus JWA, and clinical risk score in the validation cohort of gastric cancer patients. AUC, area under the curve.
Table S1. Distributions of demographic and clinicopathologic characteristics of gastric cancer patients treated with or without chemotherapy.
Table S2. Univariate Cox regression analysis of MDM2 or MDM2/JWA expression and clinicopathologic variables predicting survival in three cohorts of patients with gastric cancer treated with surgery alone.
Table S3. Multivariate Cox regression analysis assessing the predictive significance of MDM2 expression in radical gastrectomy patients treated with or without fluorouracil–leucovorin–oxaliplatin (FLO).
Table S4. Multivariate Cox regression analysis assessing the predictive significance of MDM2 expression in radical gastrectomy patients treated with or without fluorouracil–leucovorin–platinol (FLP).
Acknowledgments
This work was supported by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions, the National Natural Science Foundation of China (Grant nos. 30930080, 81161120537/H16), and the Innovative Research Project for Graduate Students in Jiangsu Province (for Y. Ye).
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- AUC
area under the curve
- FLO
fluorouracil–leucovorin–oxaliplatin
- FLP
fluorouracil–leucovorin–platinol
- HR
hazard ratio
- IRS
immunoreactivity score
- MDM2
protein encoded by murine double minute 2 gene
- OS
overall survival
- ROC
receiver operating characteristic
- TMA
tissue microarray
(Cancer Sci 2013; 104: 590–598)
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62: 10–29. [DOI] [PubMed] [Google Scholar]
- 2. Nabeya Y, Suzuki T, Furuya A et al Calpain regulates thymidylate synthase‐5‐fluoro‐dUMP complex levels associated with response to 5‐fluorouracil in gastric cancer cells. Cancer Sci 2011; 102: 1509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vousden KH. p53: death star. Cell 2000; 103: 691–4. [DOI] [PubMed] [Google Scholar]
- 4. Jin S, Levine AJ. The p53 functional circuit. J Cell Sci 2001; 114: 4139–40. [DOI] [PubMed] [Google Scholar]
- 5. Michael D, Oren M. The p53‐Mdm2 module and the ubiquitin system. Semin Cancer Biol 2003; 13: 49–58. [DOI] [PubMed] [Google Scholar]
- 6. Schoniger‐Hekele M, Hanel S, Wrba F, Muller C. Hepatocellular carcinoma–survival and clinical characteristics in relation to various histologic molecular markers in Western patients. Liver Int 2005; 25: 62–9. [DOI] [PubMed] [Google Scholar]
- 7. Bond GL, Hu W, Bond EE et al A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell 2004; 119: 591–602. [DOI] [PubMed] [Google Scholar]
- 8. Chen H, Ma X, Li Z et al Functionalization of single‐walled carbon nanotubes enables efficient intracellular delivery of siRNA targeting MDM2 to inhibit breast cancer cells growth. Biomed Pharmacother 2012; 66: 334–8. [DOI] [PubMed] [Google Scholar]
- 9. Fang F, Yu XJ, Yu L, Yao L. MDM2 309 T/G polymorphism is associated with colorectal cancer risk especially in Asians: a meta‐analysis. Med Oncol 2011; 28: 981–5. [DOI] [PubMed] [Google Scholar]
- 10. Li G, Zhai X, Zhang Z, Chamberlain RM, Spitz MR, Wei Q. MDM2 gene promoter polymorphisms and risk of lung cancer: a case‐control analysis. Carcinogenesis 2006; 27: 2028–33. [DOI] [PubMed] [Google Scholar]
- 11. Ohmiya N, Taguchi A, Mabuchi N et al MDM2 promoter polymorphism is associated with both an increased susceptibility to gastric carcinoma and poor prognosis. J Clin Oncol 2006; 24: 4434–40. [DOI] [PubMed] [Google Scholar]
- 12. Chrisanthar R, Knappskog S, Lokkevik E et al Predictive and prognostic impact of TP53 mutations and MDM2 promoter genotype in primary breast cancer patients treated with epirubicin or paclitaxel. PLoS ONE 2011; 6: e19249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Terry K, McGrath M, Lee IM, Buring J, De Vivo I. MDM2 SNP309 is associated with endometrial cancer risk. Cancer Epidemiol Biomarkers Prev 2008; 17: 983–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Endo K, Ueda T, Ohta T, Terada T. Protein expression of MDM2 and its clinicopathological relationships in human hepatocellular carcinoma. Liver 2000; 20: 209–15. [DOI] [PubMed] [Google Scholar]
- 15. Iwakuma T, Lozano G. MDM2, an introduction. Mol Cancer Res 2003; 1: 993–1000. [PubMed] [Google Scholar]
- 16. Chen H, Bai J, Ye J et al JWA as a functional molecule to regulate cancer cells migration via MAPK cascades and F‐actin cytoskeleton. Cell Signal 2007; 19: 1315–27. [DOI] [PubMed] [Google Scholar]
- 17. Bai J, Zhang J, Wu J et al JWA regulates melanoma metastasis by integrin alphaVbeta3 signaling. Oncogene 2010; 29: 1227–37. [DOI] [PubMed] [Google Scholar]
- 18. Tang WY, Wang L, Li C et al Identification and functional characterization of JWA polymorphisms and their association with risk of gastric cancer and esophageal squamous cell carcinoma in a Chinese population. J Toxicol Environ Health A 2007; 70: 885–94. [DOI] [PubMed] [Google Scholar]
- 19. Wang S, Wu X, Chen Y et al Prognostic and predictive role of JWA and XRCC1 expressions in gastric cancer. Clin Cancer Res 2012; 18: 2987–96. [DOI] [PubMed] [Google Scholar]
- 20. Lauren P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. An attempt at a histo‐clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 21. Aiko T, Sasako M. The new Japanese Classification of Gastric Carcinoma: points to be revised. Gastric Cancer 1998; 1: 25–30. [DOI] [PubMed] [Google Scholar]
- 22. Weichert W, Roske A, Gekeler V et al Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: a retrospective analysis. Lancet Oncol 2008; 9: 139–48. [DOI] [PubMed] [Google Scholar]
- 23. Shen L, Xu W, Li A, Ye J, Zhou J. JWA enhances As(2)O(3)‐induced tubulin polymerization and apoptosis via p38 in HeLa and MCF‐7 cells. Apoptosis 2011; 16: 1177–93. [DOI] [PubMed] [Google Scholar]
- 24. Yang X, Takano Y, Zheng HC. The pathobiological features of gastrointestinal cancers (Review). Oncol Lett 2012; 3: 961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gould Rothberg BE, Bracken MB, Rimm DL. Tissue biomarkers for prognosis in cutaneous melanoma: a systematic review and meta‐analysis. J Natl Cancer Inst 2009; 101: 452–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paoletti X, Oba K, Burzykowski T et al Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta‐analysis. JAMA 2010; 303: 1729–37. [DOI] [PubMed] [Google Scholar]
- 27. Zietz C, Rossle M, Haas C et al MDM‐2 oncoprotein overexpression, p53 gene mutation, and VEGF up‐regulation in angiosarcomas. Am J Pathol 1998; 153: 1425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang JY, Zong CS, Xia W et al MDM2 promotes cell motility and invasiveness by regulating E‐cadherin degradation. Mol Cell Biol 2006; 26: 7269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 2007; 33: 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woynarowski JM, Faivre S, Herzig MC et al Oxaliplatin‐induced damage of cellular DNA. Mol Pharmacol 2000; 58: 920–7. [DOI] [PubMed] [Google Scholar]
- 31. Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni‐Ehrig A. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol 2012; 3: 251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Details of: patients and specimens; tissue microarray construction and immunohistochemistry; cell culture; apoptosis assay; Western blot analysis; Transwell migration assay; scratch migration assay; and statistical analysis.
Fig. S1. Schematic representation of this study.
Fig. S2. Representative images of MDM2 immunohistochemical staining in gastric cancer. (A) Negative staining; (B) weak positive staining; (C) moderate positive staining; (D) strong positive staining. Scale line = 25 μm.
Fig. S3. Receiver operating characteristic curves were obtained to show the relation between areas under the curves (AUC) at different cut‐off values of MDM2 immunoreactivity scores (IRS) for 1, 3, and 5 years of overall survival time.
Fig. S4. Kaplan–Meier curves depicting overall survival according to MDM2 expression patterns in non‐tumors in the training cohort of gastric cancer patients. P‐values were calculated using the log–rank test. Cum., cumulative.
Fig. S5. Kaplan–Meier curves depicting overall survival according to MDM2 expression patterns in gastric cancer patients in the validation cohort treated with or without fluorouracil–leucovorin–platinol (FLP). P‐values were calculated using the log–rank test. S, surgery alone.
Fig. S6. Immunoreactivity score (IRS) comparison of the absolute value of ΔIRS (tumor tissue [T] – non‐cancerous gastric tissue [N]) of MDM2 staining in different absolute values of ΔIRS of JWA staining subgroups.
Fig. S7. Time‐dependent receiver operating characteristic analyses for the clinical risk score (TNM stage, histological type, and tumor diameter), the combined MDM2 or MDM2 plus JWA, and clinical risk score in the validation cohort of gastric cancer patients. AUC, area under the curve.
Table S1. Distributions of demographic and clinicopathologic characteristics of gastric cancer patients treated with or without chemotherapy.
Table S2. Univariate Cox regression analysis of MDM2 or MDM2/JWA expression and clinicopathologic variables predicting survival in three cohorts of patients with gastric cancer treated with surgery alone.
Table S3. Multivariate Cox regression analysis assessing the predictive significance of MDM2 expression in radical gastrectomy patients treated with or without fluorouracil–leucovorin–oxaliplatin (FLO).
Table S4. Multivariate Cox regression analysis assessing the predictive significance of MDM2 expression in radical gastrectomy patients treated with or without fluorouracil–leucovorin–platinol (FLP).


