Abstract
The purpose of this study was to clarify the appropriate combination of targeting antibody and conjugate‐design of anti‐tumor immunoconjugate depending on a quantity of tumor stroma. Most human solid tumors including pancreatic cancer (PC) forming hypovascular and stroma‐rich tumor hinders the penetration of monoclonal antibodies (mAbs) into the cells, and that leads to failure of the conventional cell‐targeting immunoconjugate strategy. To overcome this drawback, SN‐38 as topoisomerase 1 inhibitor was conjugated to a mAb to collagen 4, a plentiful component of the tumor stroma via ester‐bond. The immunoconjugate, which was able to release SN‐38 in physiological condition outside the cells, was effective to stroma‐rich PC‐tumor. On the other hand, anti‐CD 20 mAb‐PEG‐SN‐38 via carbamate‐bond as conventional immunoconjugate, enabled SN‐38 to be released by a carboxylesterase inside of the tumor cell following the internalization, showed strong anti‐tumor activity against malignant lymphoma as hypervascular and stroma‐poor tumor. The conjugate‐design, in parallel with the choice of targeting antibodies, should be selected to maximize the therapeutic effect in each individual tumor having a distinct stromal structure.
Monoclonal antibody (mAb), which can target the tumor cell actively by the specific binding ability against corresponding antigen, easily extravasates from leaky tumor vessels but not from normal vessels, is long retained in the tumor by using active targeting and passive targeting based on the enhanced permeability and retention (EPR) effect.1, 2, 3, 4 Therefore, numerous mAbs have been developed and conjugated with anticancer agents (ACAs) or toxins to create an “immunoconjugate strategy”.5, 6, 7, 8 Recent examples of the conjugates include anti‐CD33 immunoconjugate‐calicheamicin and anti‐CD20 radiolabeled immunoconjugate, were effective to hematological malignancy such as malignant lymphoma and leukemia.5 Heterogeneity of the tumor cells, however, prevents development of the immunoconjugate chemotherapy based on cell‐specific antigen.9, 10, 11, 12 Moreover, conventional immunoconjugates depend on cleavage of conjugation site with intracellular biochemical (enzymatic) process after the cell‐uptake of the conjugate.13, 14, 15, 16 In addition to such annoying characteristics of cancer cells themselves, most human solid tumors such as pancreatic cancer and gastric cancer, possess abundant stroma that hinders the distribution of mAbs (Fig. 1a).17, 18, 19, 20 To overcome these drawbacks, we developed a unique strategy whereby the cancer‐stromal targeting (CAST) therapy by cytotoxic immunoconjugate bound to the collagen 4 or fibrin network in the tumor stroma, from which the payload released gradually and distributed throughout the tumor, resulting in the arrest of tumor growth due to induced damage to tumor cells and tumor vessels.21, 22 Besides, there have been a few reports describing tumor stromal targetingimmunoconjugates, a mAb against a cell surface antigen FAP as fibroblast targeting therapy, or a mAb against fibronectin for the targeting of tumor vascular endothelial cell in photodynamic therapy.23, 24 However, the merits and drawbacks of anti‐stromal targeting immunoconjugate therapy in relation to the conjugate‐design and the amount of tumor stroma have not yet been fully elucidated.
Figure 1.
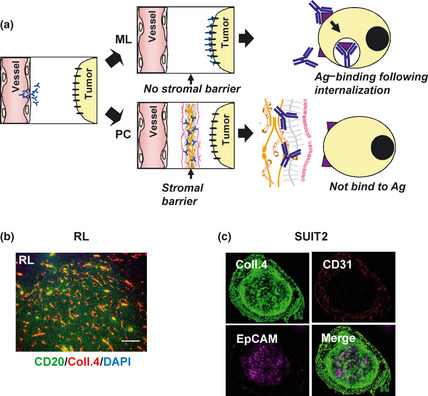
The difference of tumor tissue stromal component as stromal barrier between malignant lymphoma and pancreatic cancer. (a) The schema of antibody delivery into the tumor cells. In the tumor having no stromal barrier like malignant lymphoma (ML), antibodies were delivered into the cancer cells, and can be internalized after antigen‐binding. However, many human solid tumors including pancreatic cancer (PC) possess stromal barrier hindering the distribution of the immuno‐conjugates into cancer cells such that antigen‐binding following antibody‐intrernalization never occur. Ag, Antigen. (b) RL‐tumor (ML) was stained with anti‐CD20 (green), anti‐collagen 4 (red) mAb and 4′6′‐diamidino‐2‐phenylindole dihydrochloride (DAPI) (blue). Scale bar: 100 μm. (c) SUIT2‐tumor (PC) was stained with anti‐EpCAM (purple), anti‐collagen 4 (green) and anti‐CD31 (red) mAb. Co‐existence of collagen 4 and CD 31 (yellow in Merge). Coll., collagen.
The purpose of this study was to clarify the appropriate combination of targeting antibody and conjugate‐design of anti‐tumor immunoconjugate depending on the quantity of tumor stroma. Hence, we selected two types of conjugate linker: ester‐bond and carbamate‐bond. We hypothesized that a combination of anti‐stromal targeting mAb and a linker composed of ester‐bond to release ACA outside the cells would be effective against the stroma‐rich cancer. Conversely, anti‐cancer cell targeting via carbamate‐bond to release ACA inside the cells would be effective against stroma‐poor cancer. It seemed that the outcome of immunoconjugate therapy against each individual tumor having distinct stromal structure was dependent on the selection of conjugation‐design, as well as targeting mAb.
Materials and Methods
Antibodies and cells
Anti‐EpCAM (B8‐4) and Anti‐collagen 4 antibody (35‐4) were prepared as previously reported.21 Anti‐human CD20 antibody (rituximab) was purchased from Daiichi‐Sankyo (Tokyo, Japan). Human malignant lymphoma cell line RL was purchased from the American Type Culture Collection (Rockville, MD, USA). Human PC cell line SUIT2 was purchased from the Health Science Research Resources Bank (Osaka, Japan).
In vivo imaging and immunohistochemistry
Immunohistochemistry was conducted using anti CD31 antibody (R&D Systems, Minneapolis, MN, USA), anti‐collagen 4 antibody and anti‐CD20 (rituximab), or anti‐EpCAM antibody as first antibodies, Alexa 488‐, 555‐ or 647‐labeled anti‐human, mouse, rat or goat IgG (Invitrogen, Carlsbad, CA, USA) as second antibodies.
For mouse‐systemic in vivo imaging or tracking of antibody in the tissue, IRDye 800 (Li‐Cor Biosciences, Lincoln, NE, USA) alexa‐647 (Invitrogen) or Qdot 625 (Invitrogen) labeled antibodies were injected into the mice tail vein at 100 μg/body. Fluorescence images were obtained using OV110 (Olympus, Tokyo, Japan), BZ‐9000 (Keyence, Osaka, Japan), LSM 710 (Carl Zeiss, LinkedIn Germany).
Immunoconjugate
The detailed process of chemical synthesis is shown in the Data S1. The final structure was composed of one maleimide for attachment of mAb, one PEG12 (MW 865) spacer and one PEG27 (MW 1422) ester‐bond or carbamate‐bond for attachment of one SN‐38 molecule. Inter‐chain disulfides of the antibodies were reduced with 10 mM DTT (Sigma).14 The numbers of free thiols were quantified with dinitrothiocyanobenzene (DNTB, Wako, Osaka, Japan). Reduced antibodies were reacted with maleimide‐linker‐SN‐38 prodrugs in PBS containing 5 mM EDTA (pH 6) at room temperature for 1 h, then at 4°C overnight. The concentration of antibody‐prodrug conjugates was determined using the Bradford method (Bio‐Rad Protein Assay, 500‐0006JA, Bio‐Rad, Hercules, CA, USA). The numbers of residual thiols were quantified with dNTP. Each drug (SN‐38)/antibody ratio was determined by comparing the numbers of free and residual thiols. In the characterization of the conjugates, statistical analysis was performed using Student's t‐test.
Animal model and anti‐tumor effects
Female BALB/c nude mice (5 weeks old) were purchased from SLC Japan (Shizuoka, Japan). Mice were inoculated subcutaneously in the flank with 5 × 106 cells of RL, or 2 × 106 of SUIT2. The length (L) and width (W) of tumor masses and body weight were measured every 4 days, and tumor volume was calculated using (L × W2)/2. All animal procedures were performed in compliance with the Guidelines for the Care and Use of Experimental Animals established by the Committee for Animal Experimental of the National Cancer Center. These guidelines meet the ethical standards required by law and also comply with the guidelines for the use of experimental animals in Japan. When the mean tumor volume reached approximately 140 mm3 (RL) and approximately 70 mm3 (SUIT2), mice were randomly divided into groups consisting of five mice. Immunoconjugates were administered on day 0 by the mice tail vein injection. The injection doses of antibody‐SN‐38 prodrug equal to an SN‐38 dose of 3 mg/kg were determined by calculations based on drug (SN‐38)/antibody ratio (range from 2629.08 to 3296.16 mg SN‐38 per 1 mM antibody) for each drug. Statistical analysis was performed using anova.
Biochemistry and hematological examination
Blood samples were taken from the healthy mice at 7 days after i.v. administration of immunoconjugates (at an equivalent SN‐38 dose of 3 mg/kg). Hemograms were measured by using an auto‐analyzer Celltacα MEK6358 (Nihon Kohden, Tokyo, Japan), and blood chemistry examinations were carried out by Nagahama LSL (Shiga, Japan).
Anti‐collagen antibody induced arthritis
Female DBA/1J mice (5 weeks old) were purchased from SLC Japan. Anti‐collagen 2 antibody (Chondrex, Redmond, WA, USA) or anti‐collagen 4 antibody (clone 35‐4, the same mAb in the immunoconjugate) were intraperitoneally administered on day 0 at 2 mg. Fifty micrograms of LPS (Chondrex) was intraperitoneally injected on day 3.
Results
Difference of tumor stromal component between malignant lymphoma and pancreatic cancer
We first examined the difference of the stromal component influencing the drug delivery between malignant lymphoma RL and pancreatic cancer SUIT2. Anti‐CD20‐ or anti‐EpCAM‐mAb, which is specific to lymphoma or epithelial carcinoma, respectively, was used as cancer cell‐specific mAb.5, 25 Anti‐collagen 4 mAb was prepared to evaluate the stromal component. RL tumor consisted of CD20‐positive tumor cells and collagen‐4‐positive blood vessels, which was stained fine‐linearly but not interspersed‐fibrously like the intercellular‐stroma (Fig. 1b). On the other hand, SUIT2‐tumor reported as the histopathology relatively resembling original human pancreatic cancer,21, 26 consisted of EpCAM‐positive cancer‐cells and collagen‐4‐positive extracellular component, the latter was composed of both CD31‐positive blood vessel wall (yellow in Merge, Fig. 1c) and high amount of CD31‐negative stroma (green in Merge, Fig. 1c).
Internalization and biodistribution of mAbs against malignant lymphoma or pancreatic cancer
Cell‐uptake of fluorescent anti‐CD20‐ or anti‐EpCAM‐mAb against RL cells or SUIT2 cells was evaluated, respectively. Anti‐CD20 mAb was internalized and colocalized with intracellular lysosome in RL cells at 12 h after the incubation. On the other hand, anti‐EpCAM mAbs, the majority of which was still retained on cell‐surface membrane of SUIT2 cells, was poorly internalized at the same period (Fig. 2a). We next investigated the kinetics of entry of three mAbs into the tumors using an in vivo imaging system with near infrared fluorescence, which can provide deep tissue imaging with high fidelity.27, 28 Until day 3, all fluorescent‐mAbs were delivered and retained both in RL‐tumor, and SUIT2‐tumor, indicating passive targeting (Fig. 2b). On Day 7, anti‐EpCAM mAb in RL‐tumor and anti‐CD20 mAb in SUIT‐2‐tumor as a negative control, were almost eliminated, but anti‐CD20 mAb in RL‐tumor, anti‐EpCAM mAb in PC‐tumor and anti‐collagen 4 mAb in both RL‐tumor and SUIT‐2‐tumor were still retained in each tumor, indicating active targeting, that is, binding to their respective antigens within the lesion. There were also clear differences in the degree of intratumor accumulation among three mAbs. Anti‐CD20 mAb accumulation was higher in RL‐tumors than anti‐EpCAM mAb in SUIT2‐tumors (Fig. 2b). On the other hand anti‐collagen 4 mAb accumulation was higher in SUIT2‐tumors than in RL‐tumors (Fig. 2b). We then examined the histological distribution of mAbs in each tumor. In RL tumor, fluorescent anti‐CD20 mAb was distributed in the whole tumor area and bound to the cancer cells (Fig. 2c). Qdot‐labeling system detecting lower fluorescent signals in SUIT‐2 tumor was conducted to evaluate the biodistribution of each mAb. The quantity of anti‐CD20 mAb observed in SUIT2 tumor was small (Fig. 2d). Anti‐EpCAM mAb was observed mainly around the tumor cell‐abundant area, in which collagen 4 was negative (Fig. 2d). In contrast, anti‐collagen 4 mAb was mainly observed in collagen 4‐positive stroma, and rarely in the tumor cell‐abundant area (Fig. 2d). Thus, we succeeded in preparing three mAbs: anti‐CD 20 mAb for cell‐targeting against RL tumor, anti‐EpCAM mAb for cell‐targeting against SUIT2‐tumor, and anti‐collagen 4 mAb for stroma‐targeting against both tumors. These three mAbs can selectively exit the vascular system through the leaky tumor vessels and distribute within each tumor according to the nature of tissue component.
Figure 2.
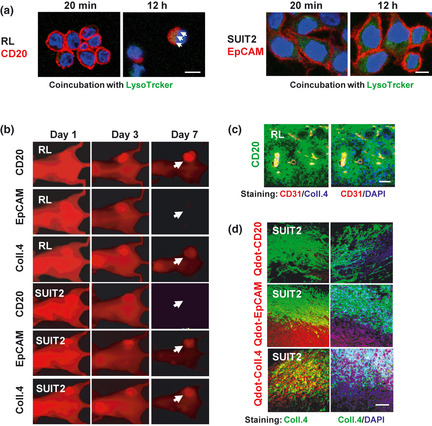
Internalization and biodistribution of anti‐CD20, anti‐EpCAM monoclonal antibody (mAb) and anti‐collagen 4 mAb. (a) Internalization of Alexafluor‐633‐anti‐CD20 or anti‐EpCAM mAb (Red) on RL or SUIT2 cells was examined at 20 min or 12 h after the incubation. Arrow shows merged yellow as co‐localization with lysostracker (Green). Scale bar: 10μm. (b) In vivo imaging analysis of RL‐, SUIT2‐tumor was conducted using near‐infrared labeled anti‐CD20, EpCAM and collagen 4 mAbs on days 1, 3 and 7 after injection. Arrows indicate each tumor position. (c) The intra‐RL‐tumor distribution of Alexa 647 (green)‐labeled anti‐CD20 mAb was examined by confocal laser scanning microscopy at 24 h after injection. CD31 (red), Collagen 4 (blue, left panel) and nuclei (blue, right panel) were stained by immunohistochemistry or DAPI. Scale bar: 100 μm. (d) The intra‐SUIT2‐tumor distribution of each Qdot (red)‐labeled antibody was examined by confocal laser scanning microscopy on day 7 after injection. Collagen 4 (green) or nuclei (blue) were stained by immunohisochemisty or DAPI. Scale bar: 100 μm.
Preparation and characterization of cell‐targeting or stroma‐targeting Immunoconjugate‐PEG‐SN‐38 via a carbamate‐bond or ester‐bond
To specify the appropriate immunoconjugatge therapy against malignant lymphoma or pancreatic cancer, we prepared two types of the conjugates, one being mAb‐PEG‐SN‐38 via a carbamate‐bond29 (Fig. 3a) and another being mAb‐PEG‐SN‐38 via an ester‐bond21, 22 (Fig. 3b). Consequently, six types of immunoconjugates, anti‐CD20, anti‐EpCAM or anti‐collagen 4 mAb‐ SN‐38 via a carbamate‐bond or an ester‐bond, were obtained. The average number of conjugated SN‐38 per one mAb (drugs/mAb), ranging from 7.0 to 8.5, was shown in Figure 3c. There was no clear loss of antigen‐binding activity of each mAb after the conjugation (Fig. 3D). An in vitro release experiment, both bonds can be cut by a carboxylesterase localized in cytoplasm to release SN‐38 inside various cells (Fig. 3e). However, in physiological conditions (non‐enzymatic hydrolysis), the immunoconjugate prepared via an ester‐bond can release SN‐38 gradually and effectively. In contrast, the immunoconjugate via a carbamate‐bond cannot release SN‐38 effectively in the conditions outside the cells (Fig. 3e). We then evaluated the release profiles of SN‐38 from both types of immunoconjugate in mouse blood, which contained high amounts of carboxylesterase.30 In vivo analysis of mouse plasma, the concentration of unbound SN‐38 or bound and unbound of SN‐38 from the immunoconjugate via an ester‐bond or a carbamate‐bond at 72 h after the mice tail vein injection were shown. Most of the immunoconjugates in the mouse blood were protected from the enzymatic cleavage (Fig. 3f). Next, we examined the difference between carbamate‐bond and ester‐bond in combination with cell‐targeting or stromal‐targeting antibody by the cytotoxicity assay. In RL cells, anti‐CD 20 immunoconjugate via carbamate‐bond showed strong cytotoxicity compared with anti‐CD 20 immunoconjugate via ester‐bond significantly. In SUIT2 cells, although there was no significant difference, anti‐EpCAM immunoconjugate via carbamate‐bond had a lower tendency in the cytotoxic effect compared to anti‐EpCAM immunoconjugate via ester‐bond. Anti‐collagen 4 immunoconjugate via ester‐bond showed higher cytotoxic activity than anti‐collagen 4 immunoconjugate via carbamate‐bond in both cells significantly (Table 1). These results indicated that a carbamate‐bond was useful for the immunoconjugate linker to work inside of the cells and an ester‐bond to work outside the cells.
Figure 3.
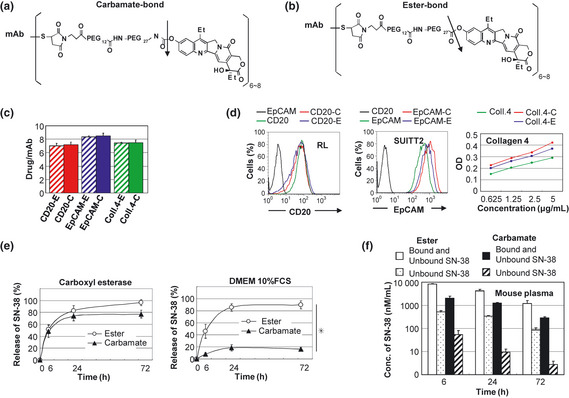
Preparation and characterization of two types of immunoconjugqates‐PEG‐SN‐38 via carbamate‐bond and eser‐bond. (a,b) Drug design of two types of immunoconjugates; monoclonal antibody (mAb) ‐PEG‐SN‐38 via carbamate‐bond (a) and mAb‐PEG‐SN‐38 via ester‐bond (b). One antibody bears six to eight molecules of SN‐38. The arrow indicates the cleavage site for releasing free active SN‐38. (c)The average number of conjugated SN‐38 per one mAb was shown (n = 3). Bar = standard deviation (SD). (d) Antigen‐binding activity of the mAb before and after the conjugation was shown. Anti‐CD 20 and EpCAM mAb were examined by fluorescence‐activated cell sorting (FACS) analysis using RL cells and SUIT2 cells, respectively. Anti‐collagen 4 mAb was examined by enzyme linked immunosorbent assay (ELISA) using purified protein. (e) In vitro release of SN‐38 from two types of immunoconjugates in carboxylesterase‐contained solution (left) and Dulbecco's modified eagle medium (DMEM) 10% fetal calf serum (FCS) (right) (n = 3). Bar standard deviation (SD), *P < 0.05. (f) Concentration of bound and unbound form of SN‐38, and unbound form of SN‐38 from two types of immunoconjugates in the mouse plasma at 6, 24, 72 h after the mice tail vein injection, were shown (n = 3). Concentrations of SN‐38 were determined by high performance liquid chromatography (HPLC). Bar = standard deviation (SD).
Table 1.
IC50 of free SN‐38 and SN‐38 conjugated to monoclonal antibody (mAb) (immunoconjugate) for malignant lymphoma and pancreatic cancer cell lines (WST‐8 assay)
| Malignant lymphoma cell lines | Free SN‐38 | SN‐38 conjugated to mAb | |
| CD20 | Collagen 4 | ||
| Ester vs carbamate | Ester vs carbamate | ||
| RL | >4.6 ± 3.7 | >8.7 ± 2.9 vs 2.1 ± 1.0* | 34 ± 17 vs 90 ± 30* |
| Pancreatic cancer cell lines | Free SN‐38 | SN‐38 conjugated to mAb | |
| EpCAM | Collagen 4 | ||
| Ester vs carbamate | Ester vs carbamate | ||
| SUIT2 | 7.8 ± 3.6 | 24 ± 13 vs 15 ± 9 | 29 ± 15 vs 75 ± 22* |
IC50 (50% cell survival) (nM), Mean ± standard deviation (n = 3), *P < 0.05.
Cell‐targeting or stroma‐targeting immunoconjugate‐PEG‐SN‐38 via carbamate‐bond or ester‐bond differs drastically in their anti‐tumor effects depending on tumor stromal component in mice
Three mAbs conjugated with SN‐38 via carbamate‐bond or ester‐bond (administered once, at an equivalent SN‐38 dose of 3 mg/kg) were evaluated in order to know their anti‐tumor effects in RL (CD20‐positive stroma‐poor human malignant lymphoma), SUIT2 (EpCAM‐positive stroma‐rich human pancreatic tumor). In RL lymphomas, cell‐targeting anti‐CD20 mAb‐ SN‐38 via carbamate‐bond showed superior anti‐tumor activity compared to anti‐CD20 mAb‐ SN‐38 via ester‐bond after the treatment (Fig. 4a). Stroma‐targeting anti‐collagen 4 mAb‐ SN‐38 via ester‐bond showed significant superior anti‐tumor activity as compared to saline as control, but inferior to anti‐CD20 mAb‐ SN‐38 via carbamate‐bond (Fig. 4a). On the contrary to RL tumor, in SUIT2 tumor, the most potent anti‐tumor activity was obtained by the stroma‐targeting anti‐collagen 4 mAb‐ SN‐38 via ester‐bond (Fig. 4b). However, there was no significant difference in anti‐tumor activity between anti‐EpCAM mAb‐ SN‐38 via carbamate‐bond and via ester‐bond, whereas the anti‐tumor activity of anti‐collagen 4 mAb‐ SN‐38 via carbamate‐bond was inferior to that of anti‐collagen 4 mAb‐ SN‐38 via ester‐bond (Fig. 4b). These results clearly indicated that, in stroma‐poor solid tumors like malignant lymphoma, cytotoxic immunoconjugate should target to the tumor cell surface and ACA should be conjugated to mAb through carbamate‐bond, which can be specifically cut by a carboxylesterase inside the tumor cell after the internalization. On the other hand, in stroma‐rich tumors, the immunoconjugate should target to the stroma within tumor tissue and ACA should be attached to the mAb via ester‐bond, which can be cut gradually outside the tumor cell following the accumulation of the cytotoxic immunoconjugate in the tumor stroma. It is remarkable that the feature of tumor stromal component influence the outcome of the two types of immunoconjugation drugs, cell‐targeting mAb‐PEG‐SN‐38 via carbamate‐bond, or stroma‐targeting mAb‐PEG‐SN‐38 via ester‐bond.
Figure 4.
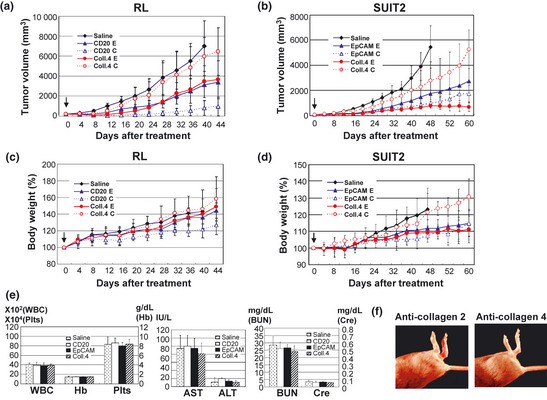
Antitumor effects and toxicities of immunoconjugates‐PEG‐SN‐38 in the combinations of anti‐cell or anti‐stroma targeting, carbonate‐bond or ester‐bond. (a,b) Anti‐tumor activities and (c,d) percent changes of body weight were examined. In animal models of RL (a,c) and SUIT2 (b,d), the six types of immunoconjugates (combined anti‐CD20 monoclonal antibody (mAb) =CD20, anti‐EpCAM mAb=EpCAM or anti‐collagen 4 mAb=Coll.4 and ester‐bond=E or carbamate‐bond=C), or saline as control, were administered once at an equivalent SN‐38 dose of 3 mg/kg to separate groups of mice (n = 5) by intravenous bolus injection to the mice on day 0. Arrows indicate day of administration and the curves illustrate the effect of treatment on tumor size. P < 0.0001 (saline versus CD20‐E or CD20‐C, CD20‐C versus CD20‐E, Coll.4‐E or Coll.4‐C in RL tumor; saline versus EpCAM‐E, EpCAM‐C or Coll.4‐E, Coll.4‐E versus EpCAM‐C or Coll.4‐C, EpCAM‐C versus Coll.4‐C in SUIT2 tumor), P < 0.001 (Saline versus Coll.4‐E in RL tumor; saline versus Coll.4‐C, Coll.4‐E versus EpCAM‐E in SUIT2 tumor). Bar = standard deviation (SD). (e) Hematological (WBC, Hb and Plts) and biochemical (aspartate aminotransferase [AST], alanine aminotransferase [ALT], blood urea nitrogen [BUN] and creatinine [Cre]) examination were conducted at 7 days after i.v. administration of immunoconjugates via ester‐bond or saline as control. Bar = standard deviation (SD). (f) Anti‐collagen antibody induced arthritis in DBA/1J mice. The arthritis was admitted on day 7 only after the administration of anti‐collagen 2 (left) but not anti‐collagen 4 (right) antibodies.
Regarding normal tissue distribution and elimination of antibodies and SN‐38, there was no difference among immunoconjugates on day 7 after the administration. The dose in this study did not cause significant toxicity as shown by the change of mouse body weight (Fig. 4c,d). Moreover, there was no hepatotoxicity, nephrotoxicity, or bone marrow toxicity in mice treated with all three immunoconjugates as compared to controls (Fig. 4e). In addition, no autoimmune disease‐like adverse effects such as arthritis and nephritis were observed in the administration of anti‐collagen 4 mAb, whereas anti‐collagen 2 mAb combined with lipopolysaccharide caused severe arthritis (Fig. 4f).
Discussion
Conventional immunoconjugate is composed of cell‐targeting mAb, ACA as payload and linker for the conjugation. The linker technology is an important part of the immunoconjugate strategy, and various linkers have been exploited to date. Among them, acid labile hydrazine linkage, thiol reduction of disulfide linkers, and lysosomal peptidase proteolysis of peptide linkers were favorably applied to ensure stability in blood.6, 7, 8 For these types of linkers, cell‐mediated endocytosis of antibody (antibody‐internalization) and intracellular biochemical (enzymatic) processing of the immunoconjugate were indispensable to make the active ACA work. Our carbamate‐bond based linker, which is used in a clinically approved anticancer prodrug CPT‐11 to release an active component SN‐38 within the tumor cell but not in blood circulation29, 31, 32 can be classified into the conventional type mentioned above. Anti‐CD 20 Immunoconjugate‐PEG‐SN‐38 via carbamate‐bond showed strong anti‐tumor activity against malignant lymphoma, in which the distribution within the tumor tissue and antibody‐internalization into tumor cells occur effectively. Although there were negative reports concerning the internalization of anti‐CD20 mAbs, several authors, recently demonstrated internalization of anti‐CD20 mAbs including rituximab in malignant lymphoma and leukemia cells.33, 34, 35, 36 These conflicting results might reflect the differences of cell types, mAbs or methodologies used.35, 36 In contrast to malignant lymphoma, most human solid tumors possess abundant stroma that hinders the tissue‐distribution of antibodies.17, 18, 19, 20, 21, 22 Cell–cell interaction between malignant epithelial cells also inhibits the penetration of mAb besides tumor‐stroma.18, 37 Moreover, heterogeneity of the cells in the tumor prevents development of immunoconjugate therapy based on cancer cell‐specific antigen.9, 10, 11, 12 This led us to design an anti‐stromal targeting immunoconjugate strategy using the tumor stroma both as a scaffold for binding and assembling immunoconjugates and as a relay base for a second attack by payload‐ACA persistently released from the scaffold.21, 22 In this drug design we selected a specially selected linker using ester‐bond, which can release SN‐38 in physiological condition (non‐enzymatic hydrolysis) outside the cells. Both ester‐bond and carbamate‐bond were concerned to be cleaved by plasma carboxyl‐esterase in the circulation after the injection. Cleavages of our conjugates were very low in mouse plasma, which has much higher levels of carboxyl‐esterase activity than in human.30 Recently, we conducted clinical trials of NK012, a SN‐38 incorporating polymeric micelle. In this formulation, SN‐38 was conjugated to poly‐Glu‐chain via ester‐bond. From these trials, we learned that human blood also contains high amounts of carboxylesterase. Nevertheless, NK012 proved good stability in human blood circulation.38, 39
Anti‐collagen 4 immunoconjugate exiting from vessel can bind to the outer vessel wall and cells surrounding the stroma. Anti‐collagen 4 immunoconjugate via carbamate‐bond was not useful because it can scarcely release SN‐38 outside the cells. On the other hand, anti‐collagen 4 immunoconjugate via ester‐bond can release SN‐38 on the stroma. Low molecular weight agent SN‐38 can penetrate through stroma into the cells. SN‐38 released from the scaffold of adjacent collagen‐4‐positive vascular wall also attack tumor endothelium.21 Anti‐collagen 4 mAb‐SN‐38 via ester‐bond exerted more potent antitumor activity compared to anti‐EpCAM mAb‐SN‐38 via carbamate‐bond or ester‐bond. It is too complicated to explain these data. However, we speculate that in addition to the insufficient attainability of anti‐EpCAM mAb to tumor cells by stromal barrier and its low internalization into the cells, the retention of anti‐EpCAM mAb within the tumor cell lesion is lower than that of anti‐collagen 4 mAb within the tumor stroma. Consequently, the amount of SN‐38 released inside of the cells from anti‐EpCAM mAb‐SN‐38 via carbamate‐bond or outside of the cells from anti‐EpCAM mAb‐SN‐38 via ester‐bond, may be less than that of SN‐38 released outside of the cells from anti‐collagen 4 mAb‐SN‐38 via ester‐bond.
Although there had been a concern about the influence of anti‐collagen 4 immunoconjugate on normal tissues having high level of collagen 4, we observed the safety of the immunoconjugate in several mouse models. We think that cancer stromal targeting (CAST) therapy is dependent on the fundamental concept that antibodies or immunoconjugates are generally too large to pass through the normal vessel walls, whereas they can extravasate from leaky tumor vessels to achieve tumor selective targeting by using EPR effect and bind to collagen 4, a plentiful component of the tumor stroma.1, 2, 3, 4, 40 We also speculate that such a passive targeting effect is one of the reasons why recent anti‐EGFR antibody therapies show no serious adverse effects in spite of high level EGFR expression in normal tissues including intestinal mucosa, dermis and others.9, 10, 41
In general, human cancer is classified into three types according to the tissue component. One is hypervascular stroma‐poor tumor such as malignant lymphoma, the second is hypovascular stroma‐rich tumor such as pancreatic cancer and stomach cancer, and the third is intermediated tumor between the two types such as breast cancer and colorectal cancer. We thus propose the new therapeutic strategy of immunoconjugates to the feature of individual tumor as tissue stromal component: (i) cell‐targeting mAb conjugated with ACAs via carbamate‐bond for hypervascular and stroma‐poor tumor; (ii) stroma‐targeting mAb conjugated with ACAs via ester‐bond for hypovascular and stroma‐rich tumor; (iii) both cell‐targeting immunoconjugate via carbamate‐bond and stroma‐tgargeting via ester‐bond for intermediated type of tumor (Fig. 5).
Figure 5.
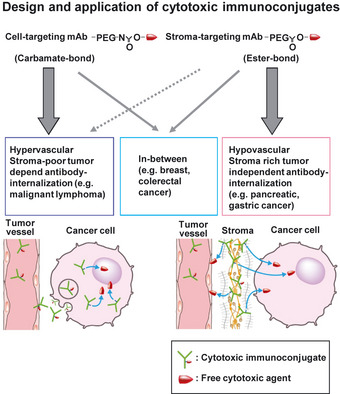
Diagram of Immunoconjugate strategy to tumor tissue component and characteristic of cancer‐cells. Design and application of cytotoxic immunoconjugates. SN‐38 conjugated cell‐targeting monoclonal antibody (mAb) via carbamate‐bond is suitable for hypervascular, stroma‐poor tumor dependent antibody‐internalization. SN‐38 conjugated stroma‐targeting mAb via ester‐bond is suitable for hypovascular, stroma‐rich tumor independent antibody‐internalization.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Data S1. Detailed methods including linker‐SN‐38 derivative synthesis.
Acknowledgments
This work was supported by the Funding Program for World‐Leading Innovative R&D on Science and Technology (FIRST Program) (YM), Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labour and Welfare of Japan (YM), a Grant‐in‐Aid for Scientific Research or Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology, the Princess Takamatsu Cancer Research Fund (YM), Japanese Foundation for Multidisciplinary Treatment of Cancer (YM), Japanese Foundation for Promotion of Cancer Research (MY), National Cancer Center Research and Development Fund (MY), the Grant‐in‐Aid for Scientific Research from Japan Society for the Promotion of Science (MY) and Kobayashi Foundation for Cancer Research (MY). We thank Dr T. Sugino for his helpful discussion. We also thank Mrs H. Koike, Mrs M. Araake‐MIzoguchi for their technical assistance and Mrs K. Shiina for her secretarial support.
(Cancer Sci, doi: 10.1111/cas.12062, 2012)
References
- 1. Imai K, Takaoka A. Comparing antibody and small‐molecule therapies for cancer. Nat Rev Cancer 2006; 6: 714–27. [DOI] [PubMed] [Google Scholar]
- 2. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986; 46: 6387–92. [PubMed] [Google Scholar]
- 3. Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov 2003; 2: 347–60. [DOI] [PubMed] [Google Scholar]
- 4. Matsumura Y, Kataoka K. Preclinical and clinical studies of anticancer agent‐incorporating polymer micelles. Cancer Sci 2009; 100: 572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ricart AD, Tolcher AW. Technology insight: cytotoxic drug immunoconjugates for cancer therapy. Nat Clin Pract Oncol 2007; 4: 245–55. [DOI] [PubMed] [Google Scholar]
- 6. Doronina SO, Toki BE, Torgov MY et al Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 2003; 21: 778–84. [DOI] [PubMed] [Google Scholar]
- 7. Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol 2005; 23: 1137–46. [DOI] [PubMed] [Google Scholar]
- 8. Doronina SO, Bovee TD, Meyer DW et al Novel peptide linkers for highly potent antibody‐auristatin conjugate. Bioconjug Chem 2008; 19: 1960–3. [DOI] [PubMed] [Google Scholar]
- 9. Koenders PG, Peters WH, Wobbes T, Beex LV, Nagengast FM, Benraad TJ. Epidermal growth factor receptor levels are lower in carcinomatous than in normal colorectal tissue. Br J Cancer 1992; 65: 189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Messersmith W, Oppenheimer D, Peralba J et al Assessment of epidermal growth factor receptor (EGFR) signaling in paired colorectal cancer and normal colon tissue samples using computer‐aided immunohistochemical analysis. Cancer Biol Ther 2005; 4: 1381–6. [DOI] [PubMed] [Google Scholar]
- 11. Hayden E. Cancer complexity slows quest for cure. Nature 2006; 455: 148. [DOI] [PubMed] [Google Scholar]
- 12. Heng HH, Bremer SW, Stevens JB, Ye KJ, Liu G, Ye CJ. Genetic and epigenetic heterogeneity in cancer: a genome‐centric perspective. J Cell Physiol 2009; 220: 538–47. [DOI] [PubMed] [Google Scholar]
- 13. Collins BE, Blixt O, Han S et al High‐affinity ligand probes of CD22 overcome the threshold set by cis ligands to allow for binding, endocytosis, and killing of B cells. J Immunol 2006; 177: 2994–3003. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt MM, Thurber GM, Wittrup KD. Kinetics of anti‐carcinoembryonic antigen antibody‐internalization: effects of affinity, bivalency, and stability. Cancer Immunol Immunother 2008; 57: 1879–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burke PJ, Senter PD, Meyer DW et al Design, synthesis, and biological evaluation of antibody‐drug conjugates comprised of potent camptothecin analogues. Bioconjug Chem 2009; 20: 1242–50. [DOI] [PubMed] [Google Scholar]
- 16. Coyne CP, Jones T, Pharr T. Synthesis of a covalent gemcitabine‐(carbamate)‐[anti‐HER2/neu] immunochemotherapeutic and its cytotoxic anti‐neoplastic activity against chemotherapeutic‐resistant SKBr‐3 mammary carcinoma. Bioorg Med Chem 2011; 19: 67–76. [DOI] [PubMed] [Google Scholar]
- 17. Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315: 1650–9. [DOI] [PubMed] [Google Scholar]
- 18. Minchinton AI, Tannock IF. Drug penetration in solid tumors. Nat Rev Cancer 2006; 6: 583–92. [DOI] [PubMed] [Google Scholar]
- 19. Trédan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 2007; 99: 1441–54. [DOI] [PubMed] [Google Scholar]
- 20. Ghajar CM, Bissell MJ. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochem Cell Biol 2008; 130: 1105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yasunaga M, Manabe S, Tarin D, Matsumura Y. Cancer‐stroma targeting therapy by cytotoxic immunoconjugate bound to the collagen 4 network in the tumor tissue. Bioconjug Chem 2011; 22: 1776–83. [DOI] [PubMed] [Google Scholar]
- 22. Yasunaga M, Manabe S, Matsumura Y. New concept of cytotoxic immunoconjugate therapy targeting cancer‐induced fibrin clots. Cancer Sci 2011; 102: 1396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ostermann E, Garin‐Chesa P, Heider KH et al Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin Cancer Res 2008; 14: 4584–92. [DOI] [PubMed] [Google Scholar]
- 24. Palumbo A, Hauler F, Dziunycz P et al A chemically modified antibody mediates complete eradication of tumors by selective disruption of tumor blood vessels. Br J Cancer 2011; 104: 1106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Armstrong A, Eck SL. EpCAM: a new therapeutic target for an old cancer antigen. Cancer Biol Ther 2003; 2: 320–6. [DOI] [PubMed] [Google Scholar]
- 26. Iwamura T, Katsuki T, Ide K. Establishment and characterization of a human pancreatic cancer cell line (SUIT‐2) producing carcinoembryonic antigen and carbohydrate antigen 19–9. Jpn J Cancer Res 1987; 78: 54–62. [PubMed] [Google Scholar]
- 27. Folli S, Westermann P, Braichotte D et al Antibody‐indocyanin conjugates for immunophotodetection of human squamous cell carcinoma in nude mice. Cancer Res 1994; 54: 2643–9. [PubMed] [Google Scholar]
- 28. Mariani G, Lasku A, Balza E et al Tumor targeting potential of the monoclonal antibody BC‐1 against oncofetal fibronectin in nude mice bearing human tumor implants. Cancer 1997; 80: 2378–84. [DOI] [PubMed] [Google Scholar]
- 29. Senter PD, Beam KS, Mixan B, Wahl AF. Identification and activities of human carboxylesterases for the activation of CPT‐11, a clinically approved anticancer drug. Bioconjug Chem 2001; 12: 1074–80. [DOI] [PubMed] [Google Scholar]
- 30. Li B, Sedlacek M, Manoharan I et al Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem Pharmacol 2005; 70: 1673–84. [DOI] [PubMed] [Google Scholar]
- 31. Pommier Y, Topoisomerase I. inhibitors: camptothecins and beyond. Nat Rev Cancer 2006; 6: 789–802. [DOI] [PubMed] [Google Scholar]
- 32. Zhao H, Rubio B, Sapra P et al Novel prodrugs of SN38 using multiarm poly(ethylene glycol) linkers. Bioconjug Chem 2008; 19: 849–59. [DOI] [PubMed] [Google Scholar]
- 33. Michel RB, Mattes MJ. Intracellular accumulation of the anti‐CD20 antibody 1F5 in B‐lymphoma cells. Clin Cancer Res 2002; 8: 2701–13. [PubMed] [Google Scholar]
- 34. Luqman M, Klabunde S, Lin K et al The antileukemia activity of a human anti‐CD40 antagonist antibody, HCD122, on human chronic lymphocytic leukemia cells. Blood 2008; 112: 711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lim SH, Vaughan AT, Ashton‐Key M et al Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 2011; 118: 2530–40. [DOI] [PubMed] [Google Scholar]
- 36. Beers SA, French RR, Chan HTC et al Antigenic modulation limits the efficacy of anti‐CD20 antibodies: implications for antibody selection. Blood 2010; 115: 5191–201. [DOI] [PubMed] [Google Scholar]
- 37. Sutherland R, Buchegger F, Schreyer M, Vacca A, Mach J‐P. Penetration and binding of radiolabeled anti‐carcinoembryonic antigen monoclonal antibodies and their antigen binding fragments in human colon multicellular tumor spheroids. Cancer Res 1987; 47: 1627–33. [PubMed] [Google Scholar]
- 38. Hamaguchi T, Doi T, Eguchi‐Nakajima T et al Phase I study of NK012, a novel SN‐38‐incorporating micellar nanoparticle, in adult patients with solid tumors. Clin Cancer Res 2010; 16: 5058–66. [DOI] [PubMed] [Google Scholar]
- 39. Matsumura Y. Preclinical and clinical studies of NK012, an SN‐38‐incorpo‐rating polymeric micelles, which is designed based on EPR effect. Adv Drug Deliv Rev 2011; 63: 184–92. [DOI] [PubMed] [Google Scholar]
- 40. Matsumura Y. Cancer stromal targeting (CAST) therapy. Adv Drug Deliv Rev 2012; 64: 710–9. [DOI] [PubMed] [Google Scholar]
- 41. Cunningham D, Humblet Y, Siena S et al Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan‐refractory metastatic colorectal cancer. N Engl J Med 2004; 351: 337–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Detailed methods including linker‐SN‐38 derivative synthesis.


