Abstract
Many clinical trials of peptide vaccines have been carried out since the first clinical trial of a melanoma antigen gene‐1‐derived peptide‐based vaccine was reported in 1995. The earlier generations of peptide vaccines were composed of one to several human leukocyte antigen class I‐restricted CTL‐epitope peptides of a single human leukocyte antigen type. Currently, various types of next‐generation peptide vaccines are under development. In this review, we focus on the clinical trials of the following categories of peptide vaccines mainly published from 2008 to 2012: (i) multivalent long peptide vaccines; (ii) multi‐peptide vaccines consisting of CTL‐ and helper‐epitopes; (iii) peptide cocktail vaccines; (iv) hybrid peptide vaccines; (v) personalized peptide vaccines; and (vi) peptide‐pulsed dendritic cell vaccines. (Cancer Sci 2013; 104: 15–21)
A cDNA‐expression cloning technique to identify genes and peptides of tumor‐associated antigens was first reported by van der Bruggen et al. in 1991.1 Subsequently, a technique using autologous antibodies was introduced for identification of genes and peptides recognized by the host immune system.2 These advanced techniques have provided a large number of antigens and peptides applicable as cancer vaccines. Many clinical trials of peptide vaccines have been carried out since the first clinical trial of a melanoma antigen gene‐1 (MAGE‐1)‐derived peptide‐based vaccine was reported in 1996 by Hu et al.3 The earlier generations of peptide vaccines were composed of one to several human leukocyte antigen (HLA)‐class I‐restricted peptides of a single HLA‐type. The peptides were emulsified with Montanide ISA51, a clinical grade of Freund's incomplete adjuvant, or pulsed on antigen‐presenting cells and used for vaccination. Various types of new generation peptide vaccines have since been developed (Figs 1, 2). In this review, we discuss the recent clinical trials of the latest generation of peptide‐based cancer vaccines mainly published from 2008 to 2012.
Figure 1.
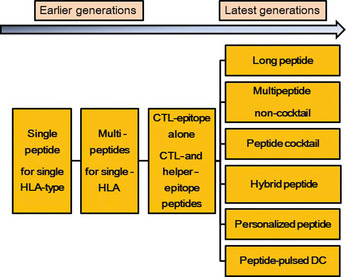
Transition of peptide vaccine development for advanced cancer. DC, dendritic cells.
Figure 2.
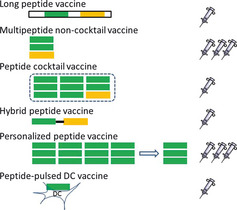
Various types of latest generation peptide vaccines. The number of syringes indicates that of the final preparation for injection. Green, CTL‐epitopes; orange, helper‐epitopes. DC, dendritic cells.
Multivalent long peptide vaccines
The classical types of peptide vaccines only contain one to several epitope peptides, which are recognized by CTLs or helper T cells. In contrast, the mother proteins of the peptide vaccines usually contain several HLA‐type restricted epitopes recognized by both CTLs and helper T cells. Although the importance of helper T cells in the induction of CTLs has been established and protein vaccines are able to induce both CTLs and helper T cells, the protein vaccines have several demerits in terms of manufacturing and safety controls. To avoid these drawbacks, synthetic long peptide vaccines have been developed. Synthetic long peptide vaccines are predominantly taken up by antigen presenting cells (APCs), where they are processed for presentation by both MHC class I and II molecules.
Several clinical studies using mixes of synthetic long peptides have been reported, as mixes of synthetic long peptide are likely to contain multiple HLA class I and II T‐cell epitopes, which allows the use of this type of peptide vaccine in all patients irrespective of the type of HLA of each patient. Kenter et al.4 carried out a phase I study of high‐risk type human papilloma virus (HPV) 16 E6 and E7 overlapping long peptides in end‐stage cervical cancer patients. Cocktails of nine E6 peptides and/or four E7 peptides, each 25–35‐mer, covering the entire sequences of E6 and E7 proteins, were given s.c. with Montanide ISA51 four times at 3‐week intervals. Co‐injection of E6 and E7 long peptides induced a strong and broad T‐cell response dominated by immunity against E6. Subsequently, they carried out a phase II study of this vaccine in patients with HPV‐positive grade 3 vulvar intraepithelial neoplasia.5 Vulvar intraepithelial neoplasia is a chronic disorder caused by HPV 16. At 3 months after the last vaccination, 12 of 20 patients (60%) had clinical responses and reported relief of symptoms. Five women had complete regression of the lesions. At 12 months of follow‐up, 15 of 19 patients (79%) had clinical responses with a complete response in 9 of 19 patients (47%).
A synthetic long peptide vaccine targeted for p53 was reported by Speetjens et al.6 The p53 synthetic long peptide vaccine consisted of 10 synthetic 25–30‐mer long overlapping peptides, spanning amino acids 70–248 of the wild type p53 protein. Ten patients with metastatic colorectal cancer were vaccinated with this vaccine. The p53‐specific T cell responses were induced in 9 of 10 patients as measured by γ‐interferon (IFN‐γ). Subsequently, a phase II study of a p53 synthetic long overlapping peptide vaccine in patients with ovarian cancer was carried out by the same group.7 Twenty patients with recurrent elevation of CA‐125 were immunized with the vaccine. Stable disease, as determined by CA‐125 levels and computed tomography scans, was observed in 2/20 (10%) patients as the best clinical response, but no relationship was found with vaccine‐induced immunity. Interferon‐γ‐producing p53‐specific T‐cell responses were induced in all patients who received all four immunizations. Interestingly, the IFN‐γ secreted cells were CD4 T‐cells and no CD8 T‐cell/CTL responses were detected. The absence of CD8 T‐cell/CTL responses may be attributable to the dominant production of Th2 cytokines, whose inhibitory effects on CTL induction are well known, although the vaccine immunization resulted in the expansion of p53‐specific Th1 and Th2 CD4 T‐cell responses.
Kakimi et al.8 carried out a phase I trial of an NY‐ESO‐1 synthetic long peptide vaccine. A 20‐mer NY‐ESO‐1f peptide, which includes multiple epitopes recognized by antibodies, and CD4 and CD8 cells, was given along with OK‐432 and Montanide ISA51 to patients with advanced cancers. Both CD4 and CD8 T cell responses, as well as NY‐ESO‐1 antibody, were increased or induced in 9 of 10 patients.
Multipeptide vaccines consisting of CTL‐ and helper‐epitopes
As mentioned above, helper T cells play crucial roles in the induction of CTLs. Some of the latest generation of peptide vaccines consist of HLA class‐II restricted helper epitope peptides recognized by CD4 T cells in addition to class‐I restricted CTL‐epitope peptides to induce both CTLs and helper T cells. Numerous helper epitopes had been identified from the same target molecules of CTL‐epitope vaccines and co‐used as cancer vaccines.9, 10, 11, 12, 13, 14, 15, 16, 17 A helper epitope peptide capable of binding pan HLA‐DR (pan‐DR epitope [PADRE]) has been reported,18 and a clinical trial of a peptide vaccine using this helper epitope was reported. Kuball et al.15 carried out a phase I study of CTL‐epitope peptides of Wilms' tumor gene, proteinase 3, and mucin 1, and PADRE or mucin 1‐helper epitope peptide with Montanide ISA51 and CpG oligonucleotide. Each peptide was formulated independently of the others and injected at a separate site. An increase in PADRE‐specific CD4 T cells was observed after vaccination but these appeared unable to produce interleukin 2 (IL2), and the regulatory T cells were increased. This study indicates that helper epitope peptides have the potential to induce both helper T cells and regulatory T cells.
Peptide cocktail vaccines
Different peptides have different binding affinities to the corresponding HLA molecules. Therefore, if different CTL‐epitope peptides with different binding affinities are loaded to APCs, there may be competition among the individual peptides to bind HLA molecules on the APCs. To prevent this, individual peptides of multipeptide vaccines were formulated independently of each other and injected at separate sites in most of the former clinical trials. In our case, a maximum of four peptides were individually mixed with Montanide ISA51 and injected s.c. at different sites on the same day. The maximum number of four peptides was similar to the maximum acceptable number of doses for patients on the same day, and no more than five peptides were used for vaccination. One of the strategies for overcoming the limitation of peptide number is the use of multipeptide cocktail vaccines. The multipeptide cocktail vaccines have no limitation of peptide number, as one preparation can contain more than 10 peptides. However, the issue of competition between the individual peptides of a cocktail vaccine for the binding of HLA molecules on the APCs still remains.
Different types of multipeptide cocktail vaccines have been developed, that is, vaccines consisting of CTL‐epitope peptides alone,19, 20, 21 or CTL‐epitope and helper‐epitope peptides.9, 10, 11, 12, 13, 16, 17 The number of component peptides in the cocktail vaccines varies from around four to more than 10. Barve et al.9 carried out a phase I/II study of a cocktail vaccine IDM‐2101 consisting of nine CTL‐epitope peptides and the PADRE helper‐epitope peptide with Montanide ISA51 in patients with metastatic non‐small‐cell lung cancer. No significant adverse events were noted except for low‐grade erythema and pain at the injection site. One‐year survival in the treated patients was 60%, and median overall survival was 17.3 months. One complete response case was observed in the total of 63 patients. Feyerabend and colleagues reported cocktail vaccines for patients with prostate cancer.12 The cocktail vaccine consisted of 13 synthetic peptides, 11 HLA‐A*0201 restricted CTL epitopes and two helper epitopes derived from prostate tumor antigens. A phase I/II trial of the vaccine was carried out in HLA‐A2‐positive patients with hormone‐sensitive prostate cancer with biochemical recurrence after primary surgical treatment. The same group also developed another cocktail vaccine for renal cell cancer.17 The vaccine, IMA901, consisted of nine HLA‐A*0201 restricted CTL‐epitopes and one helper epitope from renal cell cancer antigens with hepatitis B virus epitope as a marker peptide. A randomized phase II trial with a single dose of cyclophosphamide reduced the number of regulatory T cells and confirmed that immune responses to the vaccine component peptides were associated with longer overall survival.
Hybrid peptide vaccines
Peptide sequences of most of the single epitope vaccines as well as multi‐epitope long peptide vaccines are native sequences with or without modification of anchor amino acids. Some of the latest generation of peptide vaccines are of hybrid‐type, that is, a peptide fused with two epitopes. The Ii‐Key/HER‐2/neu hybrid peptide vaccine is a fusion peptide made up of the Ii‐Key 4‐mer peptide and human epidermal growth factor receptor‐2 (HER‐2)/neu (776–790) helper epitope peptide.22, 23 The Ii protein catalyzes direct charging of MHC class II epitopes to the peptide‐binding groove, circumventing the need for intracellular epitope processing, and the shortest active sequence of the Ii protein is the Ii/Key peptide.24 Holmes et al.22 and Perez et al. 23 reported the results of phase I studies of the Ii‐Key/HER ‐2/neu hybrid peptide vaccine in patients with prostate cancer. Significant decreases in circulating regulatory T cell frequencies, plasma HER‐2/neu, and serum transforming growth factor‐β levels were observed when compared with the native HER‐2/neu (776–790) peptide vaccination.
Takahashi and colleagues developed a hybrid peptide of a helper‐epitope and CTL‐epitope of MAGE‐A4.25 The phase I study of the vaccine was carried out in patients with advanced cancers who were vaccinated with MAGE‐A4‐H/K‐HELP combined with OK432 and Montanide ISA51. In a case report, there were no severe side‐effects except for a skin reaction at the injection site. The vaccine induced MAGE‐A4‐specific Th1 and Tc1 immune responses and the production of MAGE‐A4‐specific complement‐fixing IgG antibodies. Tumor growth and the carcinoembryonic antigen tumor marker were significantly decreased in the final diagnosis.
Personalized peptide vaccines
Virtually all prevaccination patients already have a weak immunity to cancer cells. However, the characteristics of cancer cells and of the immunological status against cancers differ widely among patients, even among those with the same histological types of cancer and identical HLA types. One of the reasons for the low clinical efficacies of the earlier generations of peptide vaccines might be a mismatch between the vaccine peptides and pre‐existing immunity to the cancer cells. We therefore attempted to optimize the vaccine peptides so that they were appropriately matched to the pre‐existing immunity of each patient (Fig. 3). There are two ways to detect pre‐existing immunity, detection of CTL‐precursors and detection of IgG in the peripheral blood. The PBMCs were cultured with vaccine peptide panels and the CTL responses to each peptide were measured. The second method is to detect IgG antibodies to the vaccine peptide panels. It is well known that the production of the IgG class of antibodies requires T‐cell help. Therefore, the presence of a specific IgG indicates the presence of helper T cells. We carried out a series of clinical trials using personalized peptide vaccines (PPVs) for advanced cancer patients.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 In this PPV formulation, appropriate peptide antigens for vaccination are screened and selected from a panel of vaccine candidates in each patient, based on pre‐existing host immunity as mentioned above. Currently, we use 31 HLA class I‐restricted peptide candidates, which were identified from a variety of tumor‐associated antigens mainly through the cDNA expression cloning method with tumor‐infiltrating T‐lymphocyte lines, 12 peptides for HLA‐A2, 14 peptides for HLA‐A24, 9 peptides for HLA‐A3 supertype (A3, A11, A31, or A33), and 4 peptides for HLA‐A26. The safety and potential immunological effects of these vaccine candidates have been shown in previous clinical studies.26, 27 A maximum of four peptides, which were selected based on the results of HLA typing and the pre‐existing immune responses specific to each of the 31 different vaccine candidates, were injected s.c. with Montanide ISA51 weekly or bi‐weekly.
Figure 3.
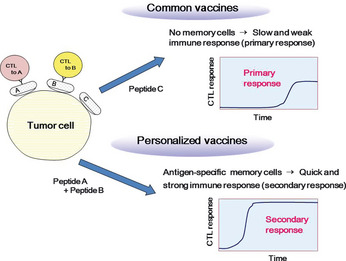
Personalized peptide vaccine. In the classical type of vaccine, peptides derived from tumor‐specific or overexpressed antigens are used as vaccine peptides and often mismatched to the pre‐existing immunity of patients. In personalized peptide vaccines, appropriate peptides for vaccination are screened and selected from a panel of vaccine candidates in each patient, based on pre‐existing host immunity and HLA types.
Currently, we evaluate the pre‐existing immune responses to vaccine candidates by B cell responses, but not by T cell responses, as the performance characteristics, such as the sensitivity and reproducibility, of the current T cell assays are far from satisfactory. In contrast to these drawbacks inherent to T cell assays, B cell assays have more potential for screening and/or monitoring antigen‐specific immune responses even to HLA class I‐restricted peptides. For example, we have recently published several papers describing the clear correlations between clinical benefits and antigen‐specific B cell responses measured by IgG antibody production in patient plasma after vaccination. Notably, the multiplex bead‐based Luminex technology that we have developed for monitoring B cell responses allow simple, quick, and highly reproducible high‐throughput screening of IgG responses specific to large numbers of peptide antigens with a tiny amount of plasma.
In the clinical trials of PPV carried out during the past decade, we have shown promising results in various types of cancers.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 Table 1 shows the summary of the immunological and clinical responses in 460 advanced cancer patients who received PPV. The best clinical responses assessed in the 436 evaluable patients were a partial response in 43 patients (10%), stable disease in 144 patients (33%), and progressive disease in 249 patients (57%), with a median overall survival of 9.9 months. Of note, a recent phase II randomized clinical trial of PPV for 57 castration‐resistant prostate cancer patients showed that patients receiving PPV in combination with low‐dose estramustine phosphate (EMP) showed a significantly longer progression‐free (median survival time, 8.5 months vs 2.8 months; hazard ratio, 0.28 [95% confidence interval, 0.14–0.61]; P = 0.0012) and overall survival (median survival time, undefined vs 16.1 months; hazard ratio, 0.30 [95% confidence interval, 0.1–0.91]; P = 0.0328) than those receiving standard‐dose EMP alone, suggesting the feasibility of this combination therapy (Fig. 4).44 In addition, PPV was also used in an early phase clinical trial of patients with recurrent or progressive glioblastoma multiforme, one of the most aggressive brain tumors, with a median overall survival of 10.6 months.47 Based on these promising results, randomized phase III trials are currently underway in glioblastoma. To prove the clinical benefits of PPV for accelerating cancer vaccine development, further randomized phase III trials would also be recommended in other types of cancers.
Table 1.
Immunological and clinical responses to personalized peptide vaccines for advanced cancer
| Disease status | Phase | HLA restriction | Total no. of patients | Humoral response (%) | Cellular response (%) | Clinical response (%) | MST (months) | Grade 3/4 toxicities | Ref. no. |
|---|---|---|---|---|---|---|---|---|---|
| Advanced CRPC | PI | A24 | 10 | 60 | 40 | SD 50 | Not ref. | 0 | 31 |
| Advanced CRPC | PI | A24 | 13 | 91 | 55 | PR 63 | 24 | G3, 5% | 32 |
| Advanced CRPC | PI | A2 | 10 | 70 | 40 | SD 30 | 22 | 0 | 33 |
| Advanced CRPC | PI/II | A24 | 16 | 50 | 71 | PR 43 | 17 | 0 | 37 |
| Advanced CRPC | PI/II | A2/A24 | 58 | 88 | 78 | PR 24 | 17 | G3, 7% | 38 |
| Localized PC | PII | A24 | 10 | 80 | 80 | PR 20 | Not ref. | 0 | 39 |
| Advanced CRPC | PI, extension | A24 | 15 | 47 | 67 | PR 13 | 24 | 0 | 46 |
| Advanced CRPC | PII, randomized | A2/A24 | 57 | 64 | 50 | PFS 8.5 (vaccine) vs 2.8M (control) | 22.4 (vaccine) vs 16.1M (control) | 0 | 44 |
| Advanced CRPC | PII |
A2/A24/ A3sup/A26 |
42 | 44 | 34 | PR 12 | 17.8 | 0 | 49 |
| Advanced malignant glioma | PI | A2/A24 | 21 | 40–64 | 50–82 | PR 24, SD 38 | Not reached | 0 | 36 |
| Advanced glioblastoma multiforme | PI, extension | A24 | 12 | 17 | 75 | PR 17, SD 42 | 10.6 | 0 | 47 |
| Advanced corolectal cancer | PI | A24 | 10 | 70 | 50 | PR 10 | Not ref. | 0 | 34 |
| Advanced corolectal cancer | PI/II | A2/A24 | 7 | 71 | 57 | SD 14 | Not ref. | G3, 20% | 40 |
| Advanced pancreatic cancer | PI | A2/A24 | 13 | 69 | 69 | PR 15, SD 54 | 7.6 | 0 | 41 |
| Non‐resectable pancreatic cancer | PII | A2/A24 | 21 | 72 | 78 | PR 33, SD 43 | 9 | 0 | 45 |
| Advanced gastric cancer | PI | A2/A24 | 13 | 80 | 50 | SD 45 | Not ref. | 0 | 30 |
| Advanced lung cancer | PI | A24 | 10 | 40 | 40 | SD 80 | 15.2 | 0 | 29 |
| Refractory SCLC | PII |
A2/A24/ A3sup/A26 |
10 | 83 | 83 | SD 20 | 6.2 | G3, 4% | 50 |
| Refractory NSCLC | PII |
A2/A24/ A3sup/A26 |
41 | 49 | 34 | SD 56 | 10.1 | G3, 7% | 42 |
| Metastatic RCC | PI | A2/A24 | 10 | 80 | 5 | SD 60 | 23 | 0 | 43 |
| Malignant melanoma | PI | A2/A24 | 7 | 57 | 86 | SD 43 | Not ref. | 0 | 28 |
| Recurrent gynecologic cancer | PI | A2/A24 | 14 | 86 | 85 | SD 36 | Not ref. | G3, 8% | 35 |
| Advanced urotherial cancer | PI | A2/A24 | 10 | 80 | 80 | CR 10, PR 10 | 24 | 0 | 48 |
A3sup, A3 super type; CR, complete response; CRPC, castration‐resistant prostate cancer; G3, grade 3; HLA, human leukocyte antigen; M, months; MST, median survival time; Not ref., not referred; NSCLC, non‐small‐cell lung cancer; PI, phase I clinical trial; PII, phase II clinical trial; PC, prostate cancer; PD, progressive disease; PFS, progression‐free survival; PR, partial response; RCC, renal cell carcinoma; Ref., reference; SCLC, small‐cell lung cancer; SD, stable disease.
Figure 4.
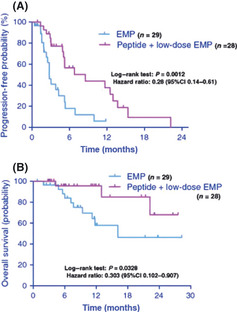
Randomized phase II trial of personalized peptide vaccine (PPV) plus low‐dose estramustine phosphate (EMP) versus standard‐dose EMP in patients with castration‐resistant prostate cancer. Patients were randomized into groups receiving either PPV plus low‐dose EMP (280 mg/day) or standard‐dose EMP (560 mg/day). (A) Duration of progression‐free survival in the first treatment. (B) Overall survival of patients treated with PPV plus low‐dose EMP and standard‐dose EMP. CI, confidence interval.
Peptide‐pulsed dendritic cell vaccines
Many clinical trials of dendritic cell (DC)‐based vaccinations using autologous DC and tumor‐associated antigen peptides have been carried out to assess the ability of these vaccines to induce clinical responses in cancer patients.51, 52, 53, 54 Rahma et al.54 carried out a comparative study of DC‐based vaccine versus non‐DC‐based authentic peptide vaccine. Twenty‐one advanced ovarian cancer patients were divided two groups: arm A received a p53 CTL‐epitope peptide with Montanide with IL2; arm B received the same peptide‐pulsed DCs with IL2. The median progression‐free survival and overall survival were 4.2 (arm A) i 8.7 (arm B) months and 40.8 (arm A) versus 29.6 (arm B) months, respectively. This study suggests that the simple peptide vaccination and labor‐consuming DC‐based vaccination therapy are similarly effective.
Conclusion
Many investigators have attempted to develop more effective cancer vaccines, and in this review we discussed the resulting progress in the latest generation of peptide vaccines. The pros and cons of each type of vaccine are shown in Table 2. Each study used different adjuvants, cytokines, and/or other combination therapies with different doses. Moreover, the individual peptides themselves had different immunological and clinical potency as well as different amino acid sequences. Therefore, it is very hard to conclude that one type of vaccine was more efficient than another. The role of immune checkpoint molecules, such as CTLA‐4 and programmed cell death‐1, on antitumor immunity was clarified, and promising results have been reported in the clinical trials using combination therapies with peptide vaccines and immune checkpoint blockades.55, 56, 57 Further randomized phase III trials would be essential to prove the clinical benefits of these vaccine therapies, including immune checkpoint blockade combination therapies.
Table 2.
Pros and cons of the latest generation of peptide vaccines
| Vaccine type | Pros | Cons | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Induction of CTL | Induction of Th | Applicable for multi‐HLA type | Activation of memory T‐cells | High efficiency of antigen presentation | Synthetic chemicals | No induction of Th | Possible induction of Treg | Not applicable for multi‐HLA type | Multi formula | Induction of primary response | Biologics | |
| Long peptide vaccine | Yes | Yes | Yes | No | No | Yes | No | Yes | No | No | Yes | No |
| Multipeptide non‐cocktail vaccine | Yes | Yes | Yes | No | No | Yes | No | Yes | No | Yes | Yes | No |
| Peptide cocktail vaccine | Yes | Yes | Yes | No | No | Yes | No | Yes | No | No | Yes | No |
| Hybrid peptide vaccine | Yes | Yes | No | No | Yes | No | Yes | Yes | No | Yes | No | |
| Personalized peptide vaccine | Yes | No | Yes | Yes | No | Yes | Yes | No | No | Yes | No | No |
| Peptide‐pulsed DC vaccine | Yes | No | No | No | Yes | No | Yes/No | No | Yes | No | Yes | Yes |
DC, dendritic cell; HLA, human leukocyte antigen; Th, helper T‐cells; Treg, regulatory T‐cells.
Disclosure Statement
The author Akira Yamada is an Executive Officer for Green Peptide Company, Ltd.
(Cancer Sci, doi: 10.1111/cas.12050, 2012)
References
- 1. van der Bruggen P, Traversari C, Chomez P et al A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991; 254: 1643–7. [DOI] [PubMed] [Google Scholar]
- 2. Chen YT, Scanlan MJ, Sahin U et al A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A 1997; 94: 1914–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu X, Chakraborty NG, Sporn JR, Kurtzman SH, Ergin MT, Mukherji B. Enhancement of cytolytic T lymphocyte precursor frequency in melanoma patients following immunization with the MAGE‐1 peptide loaded antigen presenting cell‐based vaccine. Cancer Res 1996; 56: 2479–83. [PubMed] [Google Scholar]
- 4. Kenter GG, Welters MJ, Valentijn AR et al Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high‐risk human papillomavirus 16 in end‐stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res 2008. (Jan 1); 14 (1): 169–77. [DOI] [PubMed] [Google Scholar]
- 5. Kenter GG, Welters MJ, Valentijn AR et al Vaccination against HPV‐16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009; 361: 1838–47. [DOI] [PubMed] [Google Scholar]
- 6. Speetjens FM, Kuppen PJ, Welters MJ et al Induction of p53‐specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer. Clin Cancer Res 2009; 15: 1086–95. [DOI] [PubMed] [Google Scholar]
- 7. Leffers N, Vermeij R, Hoogeboom BN et al Long‐term clinical and immunological effects of p53‐SLP® vaccine in patients with ovarian cancer. Int J Cancer 2012. (Jan 1); 130 (1): 105–12. doi: 10.1002/ijc.25980. [DOI] [PubMed] [Google Scholar]
- 8. Kakimi K, Isobe M, Uenaka A et al A phase I study of vaccination with NY‐ESO‐1f peptide mixed with Picibanil OK‐432 and Montanide ISA‐51 in patients with cancers expressing the NY‐ESO‐1 antigen. Int J Cancer 2011; 129: 2836–46. [DOI] [PubMed] [Google Scholar]
- 9. Barve M, Bender J, Senzer N et al Induction of immune responses and clinical efficacy in a phase II trial of IDM‐2101, a 10‐epitope cytotoxic T‐lymphocyte vaccine, in metastatic non‐small‐cell lung cancer. J Clin Oncol 2008; 26: 4418–25. [DOI] [PubMed] [Google Scholar]
- 10. Chianese‐Bullock KA, Irvin WP Jr, Petroni GR et al A multipeptide vaccine is safe and elicits T‐cell responses in participants with advanced stage ovarian cancer. J Immunother 2008; 31: 420–30. [DOI] [PubMed] [Google Scholar]
- 11. Slingluff CL Jr, Petroni GR, Olson WC et al Effect of granulocyte/macrophage colony‐stimulating factor on circulating CD8+ and CD4+ T‐cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res 2009; 15: 7036–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feyerabend S, Stevanovic S, Gouttefangeas C et al Novel multi‐peptide vaccination in Hla‐A2+ hormone sensitive patients with biochemical relapse of prostate cancer. Prostate 2009; 69: 917–27. [DOI] [PubMed] [Google Scholar]
- 13. Maslak PG, Dao T, Krug LM et al Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T‐cell responses in patients with complete remission from acute myeloid leukemia. Blood 2010; 116: 171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krug LM, Dao T, Brown AB et al WT1 peptide vaccinations induce CD4 and CD8 T cell immune responses in patients with mesothelioma and non‐small cell lung cancer. Cancer Immunol Immunother 2010; 59: 1467–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuball J, de Boer K, Wagner E et al Pitfalls of vaccinations with WT1‐, Proteinase3‐ and MUC1‐derived peptides in combination with MontanideISA51 and CpG7909. Cancer Immunol Immunother 2011; 60: 161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slingluff CL Jr, Petroni GR, Chianese‐Bullock KA et al Randomized multicenter trial of the effects of melanoma‐associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol 2011; 29: 2924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walter S, Weinschenk T, Stenzl A et al Multipeptide immune response to cancer vaccine IMA901 after single‐dose cyclophosphamide associates with longer patient survival. Nat Med 2012. (Jul 29). doi: 10.1038/nm.2883. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18. Alexander J, Sidney J, Southwood S et al Development of high potency universal DR‐restricted helper epitopes by modification of high affinity DR‐blocking peptides. Immunity 1994; 1 : 751–61. [DOI] [PubMed] [Google Scholar]
- 19. Slingluff CL Jr, Petroni GR, Chianese‐Bullock KA et al Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res 2007; 13 : 6386–95. [DOI] [PubMed] [Google Scholar]
- 20. Meyer RG, Korn S, Micke P et al An open‐label, prospective phase I/II study evaluating the immunogenicity and safety of a ras peptide vaccine plus GM‐CSF in patients with non‐small cell lung cancer. Lung Cancer 2007; 58(1): 88–94. [DOI] [PubMed] [Google Scholar]
- 21. Morse MA, Secord AA, Blackwell K et al MHC class I‐presented tumor antigens identified in ovarian cancer by immunoproteomic analysis are targets for T‐cell responses against breast and ovarian cancer. Clin Cancer Res 2011; 17: 3408–19. [DOI] [PubMed] [Google Scholar]
- 22. Holmes JP, Benavides LC, Gates JD et al Results of the first phase I clinical trial of the novel II‐key hybrid preventive HER‐2/neu peptide (AE37) vaccine. J Clin Oncol 2008; 26: 3426–33. [DOI] [PubMed] [Google Scholar]
- 23. Perez SA, Kallinteris NL, Bisias S et al Results from a phase I clinical study of the novel Ii‐Key/HER‐2/neu(776–790) hybrid peptide vaccine in patients with prostate cancer. Clin Cancer Res 2010; 16: 3495–506. [DOI] [PubMed] [Google Scholar]
- 24. Kallinteris NL, Lu X, Blackwell CE, von Hofe E, Humphreys RE, Xu M. Ii‐Key/MHC class II epitope hybrids: a strategy that enhances MHC class II epitope loading to create more potent peptide vaccines. Expert Opin Biol Ther 2006; 6: 1311–21. [DOI] [PubMed] [Google Scholar]
- 25. Takahashi N, Ohkuri T, Homma S et al First clinical trial of cancer vaccine therapy with artificially synthesized helper/killer‐hybrid epitope long peptide of MAGE‐A4 cancer antigen. Cancer Sci 2012. (Jan); 103 (1): 150–3. doi: 10.1111/j.1349-7006.2011.02106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Itoh K, Yamada A. Personalized peptide vaccines: a new therapeutic modality for cancer. Cancer Sci 2006; 97: 970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Itoh K, Yamada A, Mine T, Noguchi M. Recent advances in cancer vaccines: an overview. Jpn J Clin Oncol 2009; 39: 73–80. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka S, Harada M, Mine T et al Peptide vaccination for patients with melanoma and other types of cancer based on pre‐existing peptide‐specific cytotoxic T‐lymphocyte precursors in the periphery. J Immunother 2003; 26: 357–366. [DOI] [PubMed] [Google Scholar]
- 29. Mine T, Gouhara R, Hida N et al Immunological evaluation of CTL precusor‐oriented vaccines for advanced lung cancer patients. Cancer Sci 2003; 94: 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sato Y, Shomura H, Maeda Y et al Immunological evaluation of peptide vaccination for patients with gastric cancer based on pre‐existing cellular response to peptide. Cancer Sci 2003; 94: 802–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Noguchi M, Kobayashi K, Suetsugu N et al Induction of cellular and humoral immune responses to tumor cells and peptides in HLA‐A24 positive hormone‐refractory prostate cancer patients by peptide vaccination. Prostate 2003; 57: 80–92. [DOI] [PubMed] [Google Scholar]
- 32. Noguchi M, Itoh K, Suekane S et al Immunological monitoring during combination of patient‐oriented peptide vaccination and estramustine phosphate in patients with metastatic hormone refractory prostate cancer. Prostate 2004; 60: 32–45. [DOI] [PubMed] [Google Scholar]
- 33. Noguchi M, Itoh K, Suekane S et al Phase I trial of patient‐oriented vaccination in HLA‐A2 positive patients with metastatic hormone refractory prostate cancer. Cancer Sci 2004; 95: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sato Y, Maeda Y, Shomura H et al A phase I trial of cytotoxic T‐lymphocyte precursor‐oriented peptide vaccines for colorectal carcinoma patients. Br J Cancer 2004; 90: 13334–13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsuda N, Mochizuki K, Harada M et al Vaccination with pre‐designated or evidence‐based peptides for patients with recurrent gynecologic cancers. J Immunother 2004; 27: 60–67. [DOI] [PubMed] [Google Scholar]
- 36. Yajima N, Yamanaka R, Mine T et al Immunologic evaluation of personalized peptide vaccination for patients with advanced malignant glioma. Clin Cancer Res 2005; 11: 5900–5911. [DOI] [PubMed] [Google Scholar]
- 37. Noguchi M, Itoh K, Yao A et al Immunological evaluation of individualized peptide vaccination with a low‐dose of estramustine for HLA‐A24+ HRPC patients. Prostate 2005; 63: 1–12. [DOI] [PubMed] [Google Scholar]
- 38. Noguchi M, Mine T, Yamada A et al Combination therapy of personalized peptide vaccination and low‐dose estramustine phosphate for metastatic hormone refractory prostate cancer patients: an analysis of prognostic factors in the treatment. Oncol Res 2007; 16: 341–349. [DOI] [PubMed] [Google Scholar]
- 39. Noguchi M, Yao A, Harada M et al Immunological evaluation of neoadjuvant peptide vaccination before radical prostatectomy for patients with localized prostate cancer. Prostate 2007; 67: 933–942. [DOI] [PubMed] [Google Scholar]
- 40. Sato Y, Fujiwara T, Mine T et al Immunological evaluation of personalized peptide vaccination in combination with a 5‐fluorouracil derivative (TS‐1) for advanced gastric or colorectal carcinoma patients. Cancer Sci 2007; 98: 1113–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yanagimoto H, Mine T, Yamamoto K et al Immunological evaluation of personalized peptide vaccination with gemcitabine for pancreatic cancer. Cancer Sci 2007; 98: 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoshiyama K, Terazaki Y, Matsueda S et al Personalized peptide vaccination in patients with refractory non‐small cell lung cancer. Int J Oncol 2012; 40: 1492–500. [DOI] [PubMed] [Google Scholar]
- 43. Suekane S, Nishitani M, Noguchi M et al Phase I trial of personalized peptide vaccination for cytokine‐refractory metastatic renal cell carcinoma patients. Cancer 2007; 98: 1965–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Noguchi M, Kakuma T, Uemura H et al A randomized phase II trial of personalized peptide vaccine plus low dose estramustine phosphate (EMP) versus standard dose EMP in patients with castration resistant prostate cancer. Cancer Immunol Immunother 2010; 59: 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yanagimoto H, Shiomi H, Satoi S et al A phase II study of personalized peptide vaccination combined with gemcitabine for non‐resectable pancreatic cancer patients. Oncol Rep 2010; 24: 795–801. [DOI] [PubMed] [Google Scholar]
- 46. Noguchi M, Uemura H, Naito S, Akaza H, Yamada A, Itoh K. A phase I study of personalized peptide vaccination using 14 kinds of vaccine in combination with low‐dose estramustine in HLA‐A24‐positive patients with castration‐resistant prostate cancer. Prostate 2011; 71: 470–479. [DOI] [PubMed] [Google Scholar]
- 47. Terasaki M, Shibui S, Narita Y et al Phase I trial of a personalized peptide vaccine for patients positive for human leukocyte antigen–A24 with recurrent or progressive glioblastoma multiforme. J Clin Oncol 2011; 29: 337–344. [DOI] [PubMed] [Google Scholar]
- 48. Matsumoto K, Noguchi M, Satoh T et al A phase I study of personalized peptide vaccination for advanced urothelial carcinoma patients who failed treatment with methotrexate, vinblastine, adriamycin and cisplatin. BJU Int 2011; 108: 831–8. [DOI] [PubMed] [Google Scholar]
- 49. Noguchi M, Moriya F, Suekane S et al Phase II study of personalized peptide vaccination for castration‐resistant prostate cancer patients who failed in docetaxel‐based chemotherapy. Prostate 2012; 72: 834–45. [DOI] [PubMed] [Google Scholar]
- 50. Terazaki Y, Yoshiyama K, Matsueda S et al Immunological evaluation of personalized peptide vaccination in refractory small cell lung cancer. Cancer Sci 2012; 103: 638–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Svane IM, Pedersen AE, Johansen JS et al Vaccination with p53 peptide‐pulsed dendritic cells is associated with disease stabilization in patients with p53 expressing advanced breast cancer; monitoring of serum YKL‐40 and IL‐6 as response biomarkers. Cancer Immunol Immunother 2007; 56: 1485–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kavanagh B, Ko A, Venook A et al Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides. J Immunother 2007; 30: 762–72. [DOI] [PubMed] [Google Scholar]
- 53. Okada H, Kalinski P, Ueda R et al Induction of CD8+ T‐cell responses against novel glioma‐associated antigen peptides and clinical activity by vaccinations with {alpha}‐type 1 polarized dendritic cells and polyinosinic‐polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol 2011; 29: 330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rahma OE, Ashtar E, Czystowska M et al A gynecologic oncology group phase II trial of two p53 peptide vaccine approaches: subcutaneous injection and intravenous pulsed dendritic cells in high recurrence risk ovarian cancer patients. Cancer Immunol Immunother 2012; 61: 373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hodi FS, O'Day SJ, McDermott DF et al Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–23. Epub 2010 Jun 5. Erratum in: N Engl J Med. 2010;363(13):1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yuan J, Ginsberg B, Page D et al CTLA‐4 blockade increases antigen‐specific CD8(+) T cells in prevaccinated patients with melanoma: three cases. Cancer Immunol Immunother 2011; 60: 1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sarnaik AA, Yu B, Yu D et al Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high‐risk stage IIIc/IV melanoma. Clin Cancer Res 2011; 17: 896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]


