Abstract
While COVID-19, the disease driven by SARS-CoV-2 has ignited interest in the host immune response to this infection, it has also highlighted the lack of treatment options for the damaging inflammatory responses driven by pathogens that precipitate the acute respiratory distress syndrome (ARDS). With the global prevalence of SARS-CoV-2 and the likelihood of a second winter spike alongside seasonal flu, the need for effective and targeted anti-inflammatory agents is even more pressing. Here we discuss the aetiology of COVID-19 and the common signalling pathways driven by SARS-CoV-2, namely p38 MAP kinase. We highlight that p38 MAP kinase becomes elevated with increasing age, thereby driving many of the inflammatory pathways that precipitate death in old people with the added drawback of impairing vaccine efficacy in this susceptible age group. Finally, we review drugs available to inhibit p38 MAP kinase, their risks-versus-benefits as well as suggested dosing regimen to combat over-exuberant innate immune responses and potentially reverse vaccine inefficacy in older patients.
Keywords: ARDS, COVID-19, Inflammation, p38 MAP kinase, SARS-CoV-2
Abbreviations: ARDS, Acute respiratory distress syndrome; Ang, Angiotensin; ACE, Angiotensin converting enzyme; COPD, Chronic obstructive pulmonary disorder; COVID-19, Coronavirus disease 2019; IFN, Interferon; IL, Interleukin; LPS, Lipopolysaccharide; MAP kinase, Mitogen-activated protein kinase; NETs, Neutrophil extracellular traps; PHE, Public Health England; SASP, Senescence-associated secretory phenotype; SARS-CoV, Severe acute respiratory syndrome coronavirus; TNF, Tumour necrosis factor
1. Background
In the United Kingdom, between 10,000-25,000 people die/per year from seasonal flu infection. While the flu season spans about six months of the year, SARS-CoV-2 has resulted in 50,427 excess deaths in England, contributing between 10.4% and 100% of excess mortality depending on place of death (Public Health England, PHE). At the time of writing, the worldwide death toll has surpassed a million and continues unabated. Certainly, vaccines and anti-viral agents combat flu and remdesivir is showing some efficacy against SARS-CoV-2 (Beigel et al., 2020). However, a significant clinical challenge arises from the exuberant and non-resolving inflammatory response triggered by these respiratory infections. This has become particularly apparent with SARS-CoV-2 and the unique pathologic sequalae that this infection triggers. In essence, despite the prevalence and pervasiveness of ARDS triggered by infectious stimuli, no therapeutic intervention exists to counterbalance the associated detrimental immune response. The United Kingdom NIHR-funded RECOVERY (Randomised Evaluation of COVid-19 thERapY) trial has found that dexamethasone reduced the risk of dying by one-third in ventilated patients and by 20% in patients receiving oxygen only (The RECOVERY Collaborative Group et al., 2020). There was no benefit among those who did not need respiratory intervention. With dexamethasone being a potent anti-inflammatory agent, these findings underline that the pathological sequalae triggered by SARS-CoV-2 infection are largely immune mediated. While the effects of dexamethasone on COVID-19 patients are heartening, there is scope to build upon this clinically meaningful success to improve outcomes for a broader infected population with urgent unmet clinical needs.
Arising from our growing understanding of the inflammatory response seen in COVID-19, we suggest that p38 MAP kinase is a tractable target for inhibiting the underlying pathology driven by SARS-CoV-2 and indeed other respiratory viral infections. By associating the pathogenesis of COVID-19 with elevated p38 MAP kinase activity we will draw strong similarities with immune senescence (Nikolich-Žugich, 2018). The term immune senescence refers to a state of immune dysfunction seen in older people. Two key features of immune senescence are the increased attraction of monocytes to inflamed tissues and their acquisition of a dysregulated pro-resolution phenotype as well as the senescence-associated secretory phenotype (SASP), a phenomenon where the immune system of older people generates more cytokines and clotting factors. We will argue that this may explain why older individuals with COVID-19 and other respiratory viral infections have significantly higher mortality rates than younger individuals. Finally, we will discuss the potential limitations of developing a vaccine to SARS-CoV-2 and flu, particularly in older people and suggest how a specific p38 MAP kinase inhibitor, at the point of vaccination, may enhance vaccine efficacy in COVID-19 infected patients.
2. The pathogenesis of COVID-19
In principle, there are several approaches we can use to protect ourselves from SARS-CoV-2. These include (1) develop a vaccine to re-programme our immune system against the virus, (2) develop an antiviral agent including antibodies/human convalescent serum and (3) deal with the deadly overexuberant immune response and pulmonary inflammation to the virus.
Currently there are numerous clinical trials ongoing to develop a treatment for patients infected with SARS-CoV-2. In no particular order, these include antiviral drugs, immune boosters as well as anti-inflammatory agents including anti-IL-1, anti-IL-6, mucolytics, anticoagulants, vasodilators, anti-angiogenesis as well as an antioxidant (α-lipoic acid), an antibiotic (azithromycin), and an anti-parasitic drug (Levamisole). As of April 2020, a novel therapeutic approach in the form of a Bruton Tyrosine Kinase inhibitor, acalabrutinib has been initiated to target the hyper-inflammatory response and resulting cytokine storm associated with COVID-19 infection in severely ill patients [NCT04346199] (Roschewski et al., 2020).
While the above largely target single pathways, from our perspective, the therapeutic ploy in preventing COVID-19 and flu-related deaths is to look at the pathobiology of the host response more holistically. So far, we have learned that a cytokine storm, including TNF, IL-6 and IL-2Rα generation; clotting disorder/micro emboli; pronounced monocyte influx and oedema leading to failed inflammatory resolution are cardinal signs of severe COVID-19 pathogenesis (Giamarellos-Bourboulis et al., 2020; Meftahi, Jangravi, Sahraei, & Bahari, 2020; Moore & June, 2020).
Airway neutrophilia is increased during respiratory viral infections, and in combating the pathogenic insult, neutrophils generate neutrophil extracellular traps (NETs) that can precipitate NETopathic lung inflammation (Uddin, Watz, Malmgren, & Pedersen, 2019). Elevated NETs and serum levels of NET-associated products, including MPO–DNA complexes and citrullinated histone H3 in hospitalised patients with COVID-19 has been reported (Barnes et al., 2020; Zuo et al., 2020). Moreover, the extent of neutrophilia is a strong predictor of outcome in patients with COVID-19 (D. Wang et al., 2020; J. Wang et al., 2020). Thus, dysregulation of neutrophil effector functions provoked by SARS-CoV-2 infection provides an important contribution to the pathobiology of COVID-19.
In addition, it is clear that the older one is, the greater the likelihood that one will succumb to infection. In England, 90% of all COVID-19 related deaths occur in those over the age of 60 (36,961 out of 39,829 deaths as of July 7th, source: PHE). Indeed, the mortality rate in the over 60s is 16 times higher than in those under 60 (35% in ≥60 compared to 2% <60, source: PHE). Furthermore, a recent UK study on more than 17 million patients has identified in-hospital mortality from SARS-CoV-2 infections was strongly associated with increasing age (The OpenSAFELY Collaborative et al., 2020). Older patients were disproportionally affected by COVID-19 with the ≥80 years age group having major prognostic implications. In certain older patients, this may be attributable to an exaggerated immune response (cytokine storm) leading to the development of ARDS requiring assisted ventilation with subsequent multi-organ failure and ultimately death. It should be noted that pre-existing age-related co-morbidities such as hypertension, diabetes, obesity and cardiovascular disease play a role in the severity of COVID-19 in the elderly (Casucci, Acanfora, & Incalzi, 2020). As investigative immunopathologists, we suggest that one common molecular master switch that links each of these disease pathways to old people, is p38 mitogen-activated protein kinase (MAP) kinase. The p38 MAP kinases are highly conserved proline-directed protein kinases composed of α, β, γ and δ, isoforms which are encoded by distinct genes. The p38α and p38β isoforms are expressed ubiquitously and have been best studied to date (Cuenda & Rousseau, 2007).
3. p38 MAP kinase and immunosenescence
Immunosenescence refers to the age-acquired changes to the innate and adaptive immune system affecting our ability to efficiently respond to infections as well as develop long term immune memory, especially vaccinations (Nichol, Margolis, Wuorenma, & Sternberg, 1994; Pawelec & McElhaney, 2020). Naïve B cell and T cell numbers, antigen presentation by dendritic cells and cellular proliferation are all negatively affected (Nikolich-Žugich, 2018). In addition to arrested growth and altered function, senescent cells, including not only haematopoietic but also epithelial and endothelial cells as well as adipose tissue show widespread changes in gene expression. These changes include the secretion of numerous proinflammatory cytokines, chemokines, growth factors, clotting factors and proteases, a feature termed the senescence-associated secretory phenotype (SASP) (Coppé, Desprez, Krtolica, & Campisi, 2010). The SASP has powerful endocrine activities, the evolutionary benefit of which has been suggested to prevent the development of cancer and to promote tissue repair or regeneration following injury. Importantly, p38 MAP kinase activity is both necessary and sufficient for development of SASP (Alimbetov et al., 2015; Freund, Patil, & Campisi, 2011). Finally, the SASP is mainly driven by the alpha isoform of p38 MAP kinase, as blocking this particular isoform resulted in SASP inhibition (Freund et al., 2011).
IFNs are not typically associated with the SASP and in fact are found to decline with age (Pillai et al., 2016; Prakash, Agrawal, Cao, Gupta, & Agrawal, 2012; Qian et al., 2011). Delayed type 1 IFN responses in murine SARS-CoV infection models exhibit an accumulation of inflammatory monocytes and macrophages in the lung, resulting in vascular leakage, increased lung inflammation and impaired virus-specific T cell immunity. Depletion of these inflammatory myeloid cells protects mice from infection and prevents the lethal pneumonia that is otherwise observed (Channappanavar et al., 2016). Interestingly, decreased IFNγ expression in CD4+ T cells are associated with severe COVID-19 (Channappanavar et al., 2016). We do not know why p38 MAP kinase becomes elevated with age driving SASP or whether it directly or indirectly dampens IFNs.
At the downstream level, p38 MAP kinase activation is associated with induction of transcription factors, such as c-Fos which coordinate the induction of pro-inflammatory mediator expression (Frödin & Gammeltoft, 1999). The disease relevance of this signalling pathway in regulating immune responses in the context of COVID-19 disease was reported in a recent study. The blood expression profile of c-Fos was markedly upregulated in established COVID-19 infected patients but found to be downregulated in individuals undergoing disease recovery (L. Huang et al., 2020). These findings suggest that regulation of the p38 MAP kinase pathway could serve as an auto-regulatory mechanism, therefore limiting ensuing cytokine storm and thereby prompting resolution in COVID-19 infected patients and a return to homeostasis. It is unclear to what extent this p38 MAP kinase regulation of immune responses in COVID-19 patients is influenced by age.
Consequently, we hypothesise that the underlying pathologies driven by SARS-CoV-2 and that manifest more profoundly in older (>60 years) COVID-19 patients are a consequence of p38 MAP kinase overexpression or enzymatic activity. For instance, p38 MAP kinase inhibitors dampen the synthesis of pro-inflammatory cytokines and would therefore block the lethal COVID-19-induced cytokine storm (Panigrahy et al., 2020). p38 MAP kinase inhibitors can also block the degree of sputum neutrophilia and levels of neutrophil-promoting factors in the airways, such as TNF-α that is important in regulating persistent neutrophilic lung inflammation (Patel et al., 2018). p38 MAP kinase inhibitors improve vasodilation/vascular dysfunction, prevent clotting, and inhibiting viral replication (Banerjee, Narayanan, Mizutani, & Makino, 2002; Cheriyan et al., 2011; Klemm et al., 2017; Koch et al., 2012; Sakurai et al., 2004; P. Shi et al., 2017). Indeed, multiple viruses including SARS-CoV and influenza activate p38 MAP kinase as part of their life cycle suggesting that targeting p38 MAP kinase will not only influence the host response but might in fact block viral action itself (Börgeling et al., 2014; Kopecky-Bromberg, Martinez-Sobrido, & Palese, 2005). In addition, from our recent work we found that the immune system of old people takes longer to resolve compared to younger counterparts (De Maeyer et al., 2020). We reported that older people have high levels of monocyte p38 MAP kinase phosphorylation, recruit more monocytes to sites of inflammation with these cells losing their ability to efferocytose immune debris, a key determinant of inflammatory resolution. As a result of dysfunctional efferocytosis, inflammatory monocytes do not acquire a resolution phenotype, but are more M1-like thereby generating Th-1 like cytokines (De Maeyer et al., 2020).
SARS-CoV-2 infects lung cells by interacting with angiotensin (Ang) converting enzyme (ACE)2 essentially downregulating this marker in lung epithelium (Glowacka et al., 2009; Kuba et al., 2005; Zhang, Penninger, Li, Zhong, & Slutsky, 2020). Work has shown that ACE2 and its enzymatic products Ang(1-7) protect the lung from injury and subsequent inflammation, whereas increased ACE1 can skew AngII signaling towards adverse effects and excessive inflammatory damage (Meng et al., 2014; Sriram & Insel, 2020; L. Wang, Wang, Yang, Guo, & Sun, 2015). Bleomycin-induced lung damage models in rats have shown this is in part due to blockade of MAP kinases by ACE2 activity (Meng et al., 2014). Conversely, AngII can downregulate ACE2 via activation of p38 MAP kinase resulting in ACE1/ACE2 imbalance (Koka et al., 2008), whereas p38 MAP kinase blockade using SB203580 results in overexpression of ACE2 in lung fibroblasts in vitro (Meng et al., 2014). It is possible, therefore, that targeting p38 MAP kinase could help to restore ACE1/ACE2 balance in the lung, counteracting fibrosis and inflammation, and ultimately improving outcome.
Another mechanism of interest is the upregulation of pyroptosis, a process of inflammatory cells death associated with old age, which has been shown to occur in cultured macrophages challenged with SARS-CoV proteins (Chen, Moriyama, Chang, & Ichinohe, 2019; C.-S. Shi, Nabar, Huang, & Kehrl, 2019). This occurred via activation of the inflammasome, a process that has been shown to be amenable to p38 MAP kinase regulation in murine models of acute lung injury (D. Li, Ren, Jiang, & Zhu, 2018). It is becoming apparent that blood lactate dehydrogenase levels, a key pyroptosis-related protein early in COVID-19 disease correlates with severe disease progression (Y. Huang et al., 2020; Wynants et al., 2020; Xiang et al., 2020; Zhao et al., 2020). Interestingly, we found in experimental skin blister that LDH is increased in those of our older volunteers in a manner that was reversed following p38 MAP kinase inhibition (De Maeyer et al., 2020).
Taken together, with earlier reports demonstrating that p38 MAP kinase is activated during lung aging with a corresponding increase in pro-inflammatory cytokines (Z. Li et al., 2011; Ren et al., 2014), we argue that p38 MAP kinase is a master regulator of many of the negative hallmarks of COVID-19. Indeed, infection of Vero E6 cells by SARS-CoV induced phosphorylation of p38 MAP kinase as well as its downstream targets including MAPKAPK-2, HSP-27, CREB, and eIF4E; events that were reversed using the p38 MAP kinase inhibitor, SB203580 (Mizutani, Fukushi, Saijo, Kurane, & Morikawa, 2004). More recently, phospho-proteomic analysis revealed that p38 MAP kinase is activated in both Vero E6 and ACE2-A549 human lung carcinoma cells infected with SARS-CoV-2 resulting in cytokine production and cell cycle arrest. p38 inhibition, using SB203580, decreased both viral replication and inflammatory cytokine production in a dose-dependent manner (Bouhaddou et al., 2020). The effects of p38 MAP kinase inhibition on cell cycle arrest was not evaluated, but a link between the two exists and could play a role in SARS-CoV-2 infection (Bouhaddou et al., 2020; Lee et al., 2002; Yee et al., 2004).
Consequently, its inhibition will have more profound effects on the disease severity and duration than targeting single pathways. More importantly, as p38 MAP kinase becomes elevated with age and as the pathogenesis of COVID-19 reflects an overactive p38 MAP kinase, we suggest that acute inhibition of p38 MAP kinase would be more effective in old people, the high-risk cohort that are most likely to die if infected with SARS-CoV-2.
4. Will a vaccine benefit the most vulnerable – old people?
There is the expectation that a vaccine against SARS-CoV-2 will be developed. The problem is that vaccines lose their efficacy in older people. For instance, the flu vaccine is 80% effective in 25-50 years old but is only 40% effective in people over 75 (Goodwin, Viboud, & Simonsen, 2006; Nichol et al., 1994; Pawelec & McElhaney, 2020); the very patient group that is predominantly dying in response to SARS-CoV-2 infections. The therapeutic challenge, therefore, is to boost vaccine efficacy in older patients. It has been postulated by our group that too much, or inappropriate inflammation, generated by the SASP, may impair the development of optimal host immunity as we age. Indeed, p38 MAP kinase inhibition was shown to restore antigen-specific, T cell mediated immunity in older subjects in vivo (Vukmanovic-Stejic et al., 2017). Therefore, short-term inhibition of p38 MAP kinase will enhance rather than inhibit immunity in vivo. In essence, we have always contended that inflammation arising from senescent cell and monocyte interactions is associated with immune decline during ageing and can be reversed. Importantly, this reversal can be achieved by concomitant administration of a p38 MAP kinase inhibitor alongside vaccines, which will arguably result in greater efficacy in old people.
5. Side effects of p38 MAP kinase inhibition
In addition to immune responses, there is experimental evidence across several organ systems suggesting that p38 MAP kinase also mediates developmental, differentiation and proliferation processes (Martínez-Limón, Joaquin, Caballero, Posas, & Nadal, 2020). Therefore, as a consequence of the wide-ranging regulatory role of p38 MAP kinase in diverse cellular processes, the possibility of adverse events resulting from undesired pharmacological activity is a concern for the p38 MAP kinase inhibitor drug class. However, p38 MAP kinase inhibitors have been administered to patients with rheumatoid arthritis, Crohn’s disease and chronic obstructive pulmonary disorder (COPD) for as long as 12 weeks (Fisk et al., 2018; Genovese et al., 2011; Schreiber et al., 2006). Whilst treatment-emergent adverse have been reported that include dizziness and skin rash, these drugs are generally well tolerated.
Certainly, clinical development of orally active p38 MAP kinase inhibitors have flagged safety signals, probably due to “off target” effects on other enzymatic pathways resulting in dose-limiting toxicity (Dominguez, Powers, & Tamayo, 2005; Fisk, Gajendragadkar, Mäki-Petäjä, Wilkinson, & Cheriyan, 2014; Genovese et al., 2011). Avoidance of off-target effects have been the primary safety concerns, hence spurring the development of inhalable p38 MAP kinase inhibitors to minimise side effects by reducing systemic exposure. In a recent phase I study, inhaled administration of the specific inhibitor of p38α MAP kinase (AZD7624) reduced the raised neutrophil numbers in induced sputum in response to LPS challenge in normal volunteers. The incidence of adverse events reported was similar across both treatment groups, and the most commonly reported adverse event was upper respiratory tract infection (Patel et al., 2018; Pehrson et al., 2018).
In the phase II stage of the trial, inhaled AZD7624 failed to show any clinical benefit in patients with COPD. Although AZD7624 was well tolerated, the reported incidence for the majority of adverse events was higher for participants in the active treatment group compared with placebo (Patel et al., 2018). The most commonly reported adverse events in this trial was indeed COPD, followed by dyspnoea and cough. There was no evidence of AZD7624-related hepatoxicity or rash, unlike with orally active p38 MAP kinase inhibitors (Dominguez et al., 2005; Fisk et al., 2014; Genovese et al., 2011).
More specific p38 MAP kinase inhibitors were well-tolerated in a studies of COPD patients displaying an adequate safety profile but resulting in increased drug-related adverse events compared to placebo (Pascoe et al., 2017; Watz, Barnacle, Hartley, & Chan, 2014). In a phase II trial of the oral p38α MAP kinase inhibitor, PH-797804 given to patients with COPD was shown to demonstrate improvements in lung function parameters and dyspnoea relative to placebo. However, rash was the most frequently reported treatment-related adverse event in that study (MacNee, Allan, Jones, Salvo, & Tan, 2013). A larger clinical trial of another oral p38α inhibitor, losmapimod (GW856553), failed to improve any clinical outcomes of exercise tolerance or lung function in patients with COPD. Although losmapimod was well tolerated and showed no evidence of hepatotoxicity, the most common treatment-emergent adverse event observed was dose-related dermatological reactions (pruritus) (Watz et al., 2014). Indeed, in another study systemic dilmapimod was well tolerated in severely injured subjects at risk of ARDS. At the dose levels tested in this study, there was no evidence of hepatotoxicity in trauma patients (Christie et al., 2015); this is an important finding given that development of other orally active p38 MAP kinase inhibitors has been discontinued because they have caused an increase in liver transaminases.
6. Treatment strategy of COVID-19 with p38 MAP kinase inhibitors
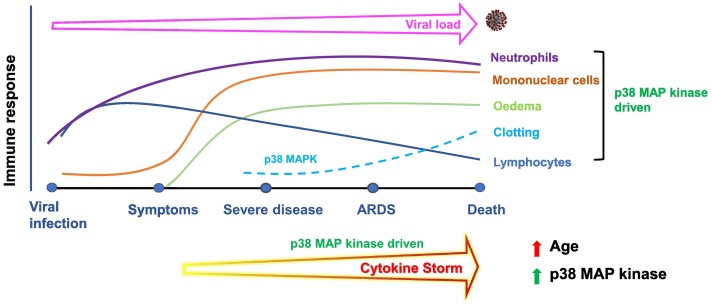
The recent success of the RECOVERY trial highlights the tractability of targeting overexuberant immune responses in critically ill COVID-19 patients. However, such a blunt approach might work in some but not others and the multifactorial nature of this infectious disease needs to be accounted for. Recently, it has been suggested that we should aim to achieve the optimal “Goldilocks level of immune activity” in patients, where hyperimmune responses are inhibited, but not to the point that viral spread becomes an issue. Indeed, given how p38 MAP kinase is involved in regulating these processes, we believe that p38 MAP kinase blockade might be able to effectively target the damaging virally driven inflammation observed. The advantage of p38 MAP kinase is that in addition to oral inhibitors that have been developed over the years, there are also inhaled p38 MAP kinase inhibitors for local delivery into the lung (Pehrson et al., 2018). Given that the respiratory mucosa is one of the main portals of entry for SARS-CoV-2 and consequently, the pathology of COVID-19 initiates in the lung, then local delivery represents a logical and potentially very efficacious approach. Indeed, this could be accompanied with administration of an oral or parenteral p38 MAP kinase inhibitor to counter-regulate any cytokine storm spill over, thereby tackling the disease both locally and systemically. Examining the temporal profile of pathological events driven by SARS-CoV-2 infection, it is clear that most phases of the disease will benefit from acute p38 MAP kinase inhibition (Fig. 1 ). Therefore, administering a p38 MAP kinase inhibitor, especially in combination with an anti-viral agent at onset of symptoms (usually with seven days of infection) will immediately tackle viral load. If symptoms are ignored by the infected individual or rapidly progress as in old people or people with multi-morbidities, then the ensuing cytokine storm, and viral load, will be dampened by p38 MAP kinase inhibition. Indeed, coagulation and pulmonary oedema will also be inhibited by continued p38 MAP kinase inhibition. Thus, the relatively well-defined disease trajectory has suggested that most phases of COVID-19 will benefit from the acute inhibition of p38 MAP kinase; however, the sooner it is administered, the better.
Fig. 1.
Time course of immune activation following SARS-CoV-2 viral infection in the context of p38 MAP kinase activation. In essence, many of the pathological events underpinning mortality following SARS-CoV-2 infection including neutrophilic and monocytic infiltrates, cytokine storm, oedema formation and clotting as well as risk factors are driven by elevated p38 MAP kinase activity.
7. Conclusion
We propose the acute inhibition of p38α MAP kinase for adjunct treatment of COVID-19 as many of the pathogenic processes that have been thus far associated with death, including cytokine storm, clotting, oedema and pulmonary inflammation are driven by this signalling pathway. Of benefit, is the combination of a local as well as systemic p38α MAP kinase inhibitor delivery that over the course of an acute treatment regimen (days rather than weeks) would be expected to carry a low/no burden of side effects. If, however, inhibiting p38 MAP kinase compromised host defence during infection-driven acute respiratory distress syndromes, such prior knowledge, in light of an effective treatment, at least pre-warns of appropriate prophylactic antibiotic/antifungal treatment.
Declaration of Competing Interest
DWG and MT have equity ownership in Senex Therapeutics Inc. MU is an employee of AstraZeneca and holds share in the company. The other authors declare no competing interests.
References
- Alimbetov D., Davis T., Brook A.J.C., Cox L.S., Faragher R.G.A., Nurgozhin T.…Kipling D. Suppression of the senescence-associated secretory phenotype (SASP) in human fibroblasts using small molecule inhibitors of p38 MAP kinase and MK2. Biogerontology. 2015;17(2):305–315. doi: 10.1007/s10522-015-9610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S., Narayanan K., Mizutani T., Makino S. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. Journal of Virology. 2002;76(12):5937–5948. doi: 10.1128/jvi.76.12.5937-5948.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M.…Egeblad M. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. Journal of Experimental Medicine. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C.…Members, A.-1 S. G Remdesivir for the treatment of Covid-19 — preliminary report. New England Journal of Medicine. 2020 doi: 10.1056/nejmoa2007764. [DOI] [PubMed] [Google Scholar]
- Börgeling Y., Schmolke M., Viemann D., Nordhoff C., Roth J., Ludwig S. Inhibition of p38 mitogen-activated protein kinase impairs influenza virus-induced primary and secondary host gene responses and protects mice from lethal H5N1 infection. Journal of Biological Chemistry. 2014;289(1):13–27. doi: 10.1074/jbc.m113.469239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhaddou M., Memon D., Meyer B., White K.M., Rezelj V.V., Marrero M.C.…Krogan N.J. The global phosphorylation landscape of SARS-CoV-2 Infection. Cell. 2020 doi: 10.1016/j.cell.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casucci G., Acanfora D., Incalzi R.A. The cross-talk between age, hypertension and inflammation in COVID-19 patients: Therapeutic targets. Drugs & Aging. 2020:1–7. doi: 10.1007/s40266-020-00808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host & Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I.-Y., Moriyama M., Chang M.-F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Frontiers in Microbiology. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyan J., Webb A.J., Sarov-Blat L., Elkhawad M., Wallace S.M.L., Mäki-Petäjä K.M.…Wilkinson I.B. Inhibition of p38 mitogen-activated protein kinase improves nitric oxide-mediated vasodilatation and reduces inflammation in hypercholesterolemia. Circulation. 2011;123(5):515–523. doi: 10.1161/circulationaha.110.971986. [DOI] [PubMed] [Google Scholar]
- Christie J.D., Vaslef S., Chang P.K., May A.K., Gunn S.R., Yang S.…Lazaar A.L. A randomized dose-escalation study of the safety and anti-inflammatory activity of the p38 mitogen-activated protein kinase inhibitor dilmapimod in severe trauma subjects at risk for acute respiratory distress syndrome. Critical Care Medicine. 2015;43(9):1859–1869. doi: 10.1097/ccm.0000000000001132. [DOI] [PubMed] [Google Scholar]
- Coppé J.-P., Desprez P.-Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annual Review of Pathology. 2010;5(1):99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A., Rousseau S. p38 MAP-Kinases pathway regulation, function and role in human diseases. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2007;1773(8):1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- De Maeyer R.P.H., Merwe van de R.C., Louie R., Bracken O.V., Devine O.P., Goldstein D.R.…Gilroy D.W. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nature Immunology. 2020;21(6):615–625. doi: 10.1038/s41590-020-0646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez C., Powers D.A., Tamayo N. p38 MAP kinase inhibitors: many are made, but few are chosen. Current Opinion in Drug Discovery & Development. 2005;8(4):421–430. [PubMed] [Google Scholar]
- Fisk M., Cheriyan J., Mohan D., Forman J., Mäki-Petäjä K.M., McEniery C.M.…Wilkinson I.B. The p38 mitogen activated protein kinase inhibitor losmapimod in chronic obstructive pulmonary disease patients with systemic inflammation, stratified by fibrinogen: A randomised double-blind placebo-controlled trial. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0194197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk M., Gajendragadkar P.R., Mäki-Petäjä K.M., Wilkinson I.B., Cheriyan J. Therapeutic potential of p38 MAP kinase inhibition in the management of cardiovascular disease. American Journal of Cardiovascular Drugs. 2014;14(3):155–165. doi: 10.1007/s40256-014-0063-6. [DOI] [PubMed] [Google Scholar]
- Freund A., Patil C.K., Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype: p38 regulates the senescence secretory phenotype. The EMBO Journal. 2011;30(8):1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frödin M., Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Molecular and Cellular Endocrinology. 1999;151(1–2):65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Genovese M.C., Cohen S.B., Wofsy D., Weinblatt M.E., Firestein G.S., Brahn E.…Tong S.E. A 24-Week, randomized, double-blind, placebo-controlled, parallel group study of the efficacy of oral SCIO-469, a p38 mitogen-activated protein kinase inhibitor, in patients with active rheumatoid arthritis. The Journal of Rheumatology. 2011;38(5):846–854. doi: 10.3899/jrheum.100602. [DOI] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N.…Koutsoukou A. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host & Microbe. 2020;27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O.…Pöhlmann S. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63 ▿. Journal of Virology. 2009;84(2):1198–1205. doi: 10.1128/jvi.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin K., Viboud C., Simonsen L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine. 2006;24(8):1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- Huang L., Shi Y., Gong B., Jiang L., Liu X., Yang J.…Yang Z. Blood single cell immune profiling reveals the interferon-MAPK pathway mediated adaptive immune response for COVID-19. MedRxiv. 2020 doi: 10.1101/2020.03.15.20033472. 2020.03.15.20033472. [DOI] [Google Scholar]
- Huang Y., Zhou H., Yang R., Xu Y., Feng X., Gong P. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. MedRxiv. 2020 doi: 10.1101/2020.02.27.20029009. 2020. 02.27.20029009. [DOI] [Google Scholar]
- Klemm C., Bruchhagen C., Krüchten A., Niemann S., Löffler B., Peters G.…Ehrhardt C. Mitogen-activated protein kinases (MAPKs) regulate IL-6 over-production during concomitant influenza virus and Staphylococcus aureus infection. Scientific Reports. 2017;7(1):42473. doi: 10.1038/srep42473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch L., Hofer S., Weigand M.A., Frommhold D., Poeschl J., Ruef P. Inhibition of LPS-induced activation of coagulation by p38 MAPK inhibitor. ISRN Hematology. 2012;2012:1–5. doi: 10.5402/2012/762614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka V., Huang X.R., Chung A.C.K., Wang W., Truong L.D., Lan H.Y. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. The American Journal of Pathology. 2008;172(5):1174–1183. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky-Bromberg S.A., Martinez-Sobrido L., Palese P. 7a protein of severe acute respiratory syndrome coronavirus inhibits cellular protein synthesis and activates p38 mitogen-activated protein kinase. Journal of Virology. 2005;80(2):785–793. doi: 10.1128/jvi.80.2.785-793.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B.…Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nature Medicine. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Hong F., Kwon S., Kim S.S., Kim D.O., Kang H.S.…Kim S.S. Activation of p38 MAPK induces cell cycle arrest via inhibition of Raf/ERK pathway during muscle differentiation. Biochemical and Biophysical Research Communications. 2002;298(5):765–771. doi: 10.1016/s0006-291x(02)02562-7. [DOI] [PubMed] [Google Scholar]
- Li D., Ren W., Jiang Z., Zhu L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Molecular Medicine Reports. 2018;18(5):4399–4409. doi: 10.3892/mmr.2018.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Li J., Bu X., Liu X., Tankersley C.G., Wang C., Huang K. Age-induced augmentation of p38 MAPK phosphorylation in mouse lung. Experimental Gerontology. 2011;46(8):694–702. doi: 10.1016/j.exger.2011.04.005. [DOI] [PubMed] [Google Scholar]
- MacNee W., Allan R., Jones I., Salvo M., Tan L. Efficacy and safety of the oral p38 inhibitor PH-797804 in chronic obstructive pulmonary disease: A randomised clinical trial. Thorax. 2013;68(8):738–745. doi: 10.1136/thoraxjnl-2012-202744. [DOI] [PubMed] [Google Scholar]
- Martínez-Limón A., Joaquin M., Caballero M., Posas F., Nadal E.d. The p38 pathway: From biology to cancer therapy. International Journal of Molecular Sciences. 2020;21(6):1913. doi: 10.3390/ijms21061913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meftahi G., Jangravi Z., Sahraei H., Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of “inflame-aging”. Inflammation Research: Official Journal of the European Histamine Research Society. 2020:1–15. doi: 10.1007/s00011-020-01372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y., Yu C.-H., Li W., Li T., Luo W., Huang S.…Li X. Angiotensin-converting enzyme 2/Angiotensin-(1-7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF-κB pathway. American Journal of Respiratory Cell and Molecular Biology. 2014;50(4):723–736. doi: 10.1165/rcmb.2012-0451oc. [DOI] [PubMed] [Google Scholar]
- Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S. Phosphorylation of p38 MAPK and its downstream targets in SARS coronavirus-infected cells. Biochemical and Biophysical Research Communications. 2004;319(4):1228–1234. doi: 10.1016/j.bbrc.2004.05.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- Nichol K., Margolis K., Wuorenma J., Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. The New England Journal of Medicine. 1994;331(12):778–784. doi: 10.1056/nejm199409223311206. [DOI] [PubMed] [Google Scholar]
- Nikolich-Žugich J. The twilight of immunity: Emerging concepts in aging of the immune system. Nature Immunology. 2018;19(1):10–19. doi: 10.1038/s41590-017-0006-x. [DOI] [PubMed] [Google Scholar]
- Panigrahy D., Gilligan M.M., Huang S., Gartung A., Cortés-Puch I., Sime P.J.…Hammock B.D. Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Reviews. 2020:1–4. doi: 10.1007/s10555-020-09889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoe S., Costa M., Marks-Konczalik J., McKie E., Yang S., Scherbovsky P. Biological effects of p38 MAPK inhibitor losmapimod does not translate to clinical benefits in COPD. Respiratory Medicine. 2017;130:20–26. doi: 10.1016/j.rmed.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Patel N.R., Cunoosamy D.M., Fagerås M., Taib Z., Asimus S., Hegelund-Myrbäck T.…Jansson P. The development of AZD7624 for prevention of exacerbations in COPD: a randomized controlled trial. International Journal of Chronic Obstructive Pulmonary Disease. 2018;13:1009–1019. doi: 10.2147/copd.s150576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G., McElhaney J. Recent advances in influenza vaccines. F1000Research. 2020;9:305. doi: 10.12688/f1000research.22611.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson R., Hegelund-Myrbäck T., Cunoosamy D., Asimus S., Jansson P., Patel N.…Lundin S. AZD7624, an inhaled p38 inhibitor, demonstrates local lung inhibition of LPS-induced TNF α with minimal systemic exposure. Journal of Pharmacology and Experimental Therapeutics. 2018;365(3):567–572. doi: 10.1124/jpet.117.246132. [DOI] [PubMed] [Google Scholar]
- Pillai P.S., Molony R.D., Martinod K., Dong H., Pang I.K., Tal M.C.…Iwasaki A. Mx1 reveals innate pathways to antiviral resistance and lethal influenza disease. Science. 2016;352(6284):463–466. doi: 10.1126/science.aaf3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Agrawal S., Cao J., Gupta S., Agrawal A. Impaired secretion of interferons by dendritic cells from aged subjects to influenza: Role of histone modifications. Age (Dordrecht, Netherlands) 2012;35(5):1785–1797. doi: 10.1007/s11357-012-9477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F., Wang X., Zhang L., Lin A., Zhao H., Fikrig E., Montgomery R.R. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. Journal of Infectious Diseases. 2011;203(10):1415–1424. doi: 10.1093/infdis/jir048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Du H., Li Y., Yao X., Huang J., Li Z.…Huang K. Age-related activation of MKK/p38/NF-κB signaling pathway in lung: From mouse to human. Experimental Gerontology. 2014;57:29–40. doi: 10.1016/j.exger.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Roschewski M., Lionakis M., Sharman J., Roswarski J., Goy A., Monticelli M.…Wilson W. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Science Immunology. 2020;5(48):eabd0110. doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K., Matsuo Y., Sudo T., Takuwa Y., Kimura S., Kasuya Y. Role of p38 mitogen-activated protein kinase in thrombus formation. Journal of Receptors and Signal Transduction. 2004;24(4):283–296. doi: 10.1081/rrs-200040324. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Feagan B., D’Haens G., Colombel J., Geboes K., Yurcov M.…Group, B. 796 S Oral p38 mitogen-activated protein kinase inhibition with BIRB 796 for active Crohn’s disease: A randomized, double-blind, placebo-controlled trial. Clinical Gastroenterology and Hepatology. 2006;4(3):325–334. doi: 10.1016/j.cgh.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Shi C.-S., Nabar N.R., Huang N.-N., Kehrl J.H. SARS-coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discovery. 2019;5(1):101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P., Zhang L., Zhang M., Yang W., Wang K., Zhang J.…Liu J. Platelet-specific p38α deficiency improved cardiac function after myocardial infarction in mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;37(12):e185–e196. doi: 10.1161/atvbaha.117.309856. [DOI] [PubMed] [Google Scholar]
- Sriram K., Insel P.A. A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance. British Journal of Pharmacology. 2020;177(21):4825–4844. doi: 10.1111/bph.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E., Walker A.J., Bhaskaran K.J., Bacon S., Bates C.…Goldacre B., The OpenSAFELY Collaborative OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. MedRxiv. 2020 doi: 10.1101/2020.05.06.20092999. 2020.05.06.20092999. [DOI] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L.…Landray M.J., The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19 — Preliminary report. The New England Journal of Medicine. 2020 doi: 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M., Watz H., Malmgren A., Pedersen F. NETopathic inflammation in chronic obstructive pulmonary disease and severe asthma. Frontiers in Immunology. 2019;10:47. doi: 10.3389/fimmu.2019.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M., Chambers E.S., Farinas M., Sandhu D., Fuentes-Duculan J., Patel N.…Akbar A.N. Enhancement of cutaneous immunity during ageing by blocking p38 MAPkinase induced inflammation. Journal of Allergy and Clinical Immunology. 2017 doi: 10.1016/j.jaci.2017.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Yin Y., Hu C., Liu X., Zhang X., Zhou S.…Peng Z. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Critical Care. 2020;24(1):188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li Q., Yin Y., Zhang Y., Cao Y., Lin X.…Qiu Y. Excessive neutrophils and neutrophil extracellular traps in COVID-19. Frontiers in Immunology. 2020;11:2063. doi: 10.3389/fimmu.2020.02063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang Y., Yang T., Guo Y., Sun T. Angiotensin-converting enzyme 2 attenuates bleomycin-induced lung fibrosis in mice. Cellular Physiology and Biochemistry. 2015;36(2):697–711. doi: 10.1159/000430131. [DOI] [PubMed] [Google Scholar]
- Watz H., Barnacle H., Hartley B.F., Chan R. Efficacy and safety of the p38 MAPK inhibitor losmapimod for patients with chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. The Lancet Respiratory Medicine. 2014;2(1):63–72. doi: 10.1016/s2213-2600(13)70200-5. [DOI] [PubMed] [Google Scholar]
- Wynants L., Calster B.V., Collins G.S., Riley R.D., Heinze G., Schuit E.…van M. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang J., Wen J., Yuan X., Xiong S., Zhou X., Liu C., Min X. Potential biochemical markers to identify severe cases among COVID-19 patients. MedRxiv. 2020 doi: 10.1101/2020.03.19.20034447. 2020.03.19.20034447. [DOI] [Google Scholar]
- Yee A.S., Paulson E.K., McDevitt M.A., Rieger-Christ K., Summerhayes I., Berasi S.P.…Zhang X. The HBP1 transcriptional repressor and the p38 MAP kinase: unlikely partners in G1 regulation and tumor suppression. Gene. 2004;336(1):1–13. doi: 10.1016/j.gene.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Medicine. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Zhang B., Li P., Ma C., Gu J., Hou P.…Bai Y. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: A systematic review and meta-analysis. MedRxiv. 2020 doi: 10.1101/2020.03.17.20037572. 2020.03.17.20037572. [DOI] [Google Scholar]
- Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A.…Knight J.S. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5(11) doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]