Abstract
Objective:
Despite advances made in the treatment of ischemic stroke, it still remains one of the leading causes of mortality and disability worldwide. The main objective of this study was to identify from a panel of 10 inflammatory markers and chemokines those biomarkers that have a potential predictive role in the evolution of disability and functional dependence in daily activities after an ischemic stroke.
Methods:
The study included 116 patients with ischemic stroke and 40 healthy volunteers matched for gender and age. Stroke severity was assessed by the National Institute of Health Stroke Scale (NIHSS) on admission and during hospitalization and functional mobility in daily activities by Barthel index (BI). Multiplex panel with 10 biomarkers [brain-derived neurotrophic factor (BDNF), platelet-derived growth factor (PDGF)-AA, PDGF-AB/BB, neural cell adhesion molecule (NCAM), cathepsin D, soluble vascular cell adhesion molecule (sVCAM), soluble intercellular cell adhesion molecule (sICAM), myeloperoxidase (MPO), regulated on activation, normal T cell expressed and secreted (RANTES), plasminogen activator inhibitor (PAI)-1] was analyzed on days 1 and 5 after admission using the xMAP technology.
Results:
Plasma concentrations of RANTES and NCAM were significantly lower in patients with ischemic stroke compared with healthy controls, while MPO and sICAM were significantly higher in patients versus controls. Plasma concentrations of sICAM, sVCAM, and RANTES significantly decreased during the analyzed period. For the first-day measurement, the bivariate analysis revealed the association of NIHSS on admission with sVCAM, and on discharge negative association with PDGF-AA, PDGR-AB/BB, BDNF, and RANTES. Plasma levels of PDGF-AA, PDGF-AB/BB, BDNF, and RANTES were found to be significantly lower in patients with BI ≤ 80, on day 5 after disease onset. PDGF-AA, PDGF-AB/BB, and BDNF were univariate and multivariate predictors for functional dependence in daily life activity (BI ≤ 80), having a protective effect (odds ratio < 1).
Conclusion:
Plasma levels of BDNF, PDGF-AA, and PDGF-AB/BB are independent predictors for functional dependency in daily life activities and may be useful prognostic markers in the evaluation of ischemic stroke patients.
Keywords: Barthel index, BDNF, blood biomarker, PAI-1, PDGF, RANTES, stroke, neurotrophins
INTRODUCTION
Among noncommunicable diseases, stroke has become the second leading cause of death and disability worldwide (http://www.who.int/news-room/fact-sheets/detail). Although there is a promising treatment for stroke, not all patients are in the eligible time window. Thus, many of them face long-term suffering. These facts suggest that there is an immediate need to find both new stroke therapies and peripheral biomarkers for patient assessment regarding the evolution of disability and functional dependence in daily activities. Results from experimental and observational data suggest that some inflammatory chemokines, such as regulated on activation, normal T cell expressed and secreted (RANTES), exert both deleterious and neuroprotective effects after an ischemic stroke.[1,2,3] Also, experimental data suggest a strong relation between RANTES and some neurotrophic factors.[3] Data regarding the evolution of the peripheral concentration of RANTES in relation to stroke outcome, or the relation between neurotrophic factors such as brain-derived neurotrophic factor (BDNF), platelet-derived growth factors (PDGF), and RANTES, are scarce.
The objectives of the present study were to asses (i) differences in the profiles of biomarkers in stroke patients versus the control group, (ii) the temporal changes in a panel of 10 inflammatory markers and chemokines with possible relevance on the pathophysiological processes during the acute phase of cerebral ischemia, and (iii) the impact of these 10 inflammatory markers and chemokines on functional dependence in daily life activities. The panel included inflammatory markers RANTES and myeloperoxidase (MPO); markers of vascular activation, such as soluble vascular cell adhesion molecule (sVCAM) and soluble intercellular cell adhesion molecule (sICAM); and several neurotrophins: BDNF and PDGF isoforms AA and AB/BB. Also, an apoptosis marker cathepsin D, neural cell adhesion molecule (NCAM), and plasminogen activator inhibitor 1 (PAI-1) were evaluated in relation to stroke severity and the degree of the daily disability.
MATERIALS AND METHODS
In the study, a convenience sample of 124 patients with a diagnosis of ischemic stroke from the Neurological Department of the Emergency Clinical County Hospital of Tîrgu Mureş was prospectively enrolled, between October 2014 and July 2016. Of these, 116 fulfilled the inclusion criteria: stroke onset <72 h, age above 18, and without a history of traumatic brain injury or stroke in the last 3 months. For comparison, 40 healthy volunteers with a similar distribution of gender and age, who attended the laboratory outpatient service for a regular checkup, were included. Other exclusion criteria were hemorrhagic stroke, thrombolyzed patients, multiple sclerosis, other neurological conditions, or obvious inflammatory disorders. The study protocol was approved by the Ethical Committee of the University of Medicine and Pharmacy of Tîrgu Mureş.
Patients and clinical evaluation
Ischemic stroke was defined as a neurological deficit with sudden onset in patients with a CT scan without acute heterodense lesions within the first 12 h or with established ischemic lesions, resulting from a sudden loss of blood circulation to an area of the brain. After admission and during hospitalization, stroke severity was assessed by National Institute of Health Stroke Scale (NIHSS); patients were classified into two groups: mild stroke (NIHSS ≤ 7) and moderate/severe stroke (NIHSS > 7).[4] Barthel index (BI) was used in order to assess the functional mobility in daily life activities, with BI > 80 representing independence with minor assistance.[5] Stroke subtype was classified into large artery atherosclerosis (LAA), cardioembolic (CE), small vessels occlusion (SVO), and other causes according to TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria.[6] Considering that in a large number of patients the cerebral CT scan was performed within the first 12 h of onset and was not repeated subsequently, we do not have exact data on the percentage of patients with and without cortical involvement.
Sample collection and measurement
The dynamic evaluation of the peripheral biomarkers was performed on days 1 and 5 after admission. Blood was collected between 7 and 9 a.m., in EDTA blood collection tubes, and immediately centrifuged at 1500g for 15 min at 4°C; plasma aliquots were stored at −80°C for subsequent analysis.
Multiplex panel with 10 biomarkers was analyzed using the xMAP technology and human neurodegeneration magnetic beads kit (EMD, Millipore, USA), on Flexmap 3D analyzer (Luminex Corp, Austin USA). The xMAP technology allows evaluating multiple biomarkers in a small sample volume. The final antigen–antibody complex at the end of the reaction was formed on magnetic beads.
The analytical performances of the studied analytes were as follows: detection limits of 0.47 pg/ml for BDNF, 0.51 pg/ml for PDGF-AA, 6.87 pg/ml for PDGF-AB/BB, 13.48 pg/ml for NCAM, 23.91 pg/ml for cathepsin D, 12.24 pg/ml for sVCAM, 16.13 pg/ml for sICAM, 550 pg/ml for MPO, 1.72 pg/ml for RANTES, and 0.92 pg/ml for PAI-1. The overall coefficients of variations were <6% for intra-assay and <13% for inter-assay measurements.
Statistical analysis
Statistical analysis was performed with the advanced environment for statistical computing, R (v. 3.4.0, Vienna, Austria). The variables with a Gaussian distribution were expressed as mean ± SD, while the nonparametric variables were expressed as median and interquartile range (25th and 75th percentile). The comparisons of the distributions of biomarkers across studied groups were released using the Student t-test or Mann–Whitney test.
The associations between qualitative variables were analyzed using contingency tables and the Chi-square or exact Fisher's test while the correlations between quantitative studied parameters were tested and quantified by Spearman's correlation coefficient.
The possible impact of plasma markers on stroke severity, the unfavorable functional outcome at 3 months, and functional dependence in daily life activities (measured by BI) were tested by binomial logistic regression, the effect size being estimated by crude and adjusted odds ratio (OR) and 95% confidence interval (CI) associated.
Thereby, each biomarker was studied both individually and in relation to the known risk factors of the poor outcome of the ischemic event. In the receiver operating characteristic ( ROC) analysis, the addition of each biomarker to the basal clinical model (model included known risk factors as age, sex, TOAST classification, stroke history, and NIHSS on admission) was compared with DeLong test.
Statistical significance was established at an estimated significance level (P < 0.05).
RESULTS
Comparative analysis between ischemic stroke patients and controls
Demographic characteristics of patients and healthy controls are presented in Table 1, without noticeable differences between subgroups, with the hospitalization duration [median interquartile range (IQR): 10 (7–13)] days. There was a difference in several peripheral biomarkers between ischemic stroke patients measured on day 1 after admission and healthy controls [Table 1]. Plasma concentrations of RANTES and NCAM were significantly lower in ischemic stroke patients compared with healthy controls, while MPO and sICAM were significantly higher in stroke patients versus controls.
Table 1.
Comparative analysis of peripheral biomarkers between stroke patients measured on day 1 after admission and healthy controls
| Characteristics | Stroke patients (n=114) | Controls (n=40) | P |
|---|---|---|---|
| Age (years) | 71.7±10.2 | 67.9±13.9 | 0.121 |
| Gender (MF) | 65 (57.0)/49 (43.0) | 23 (57.5)/17 (42.5) | 0.958 |
| Parameters (pg/ml) at baseline | |||
| PDGF-AA | 1.08 (0.71, 2.14) | 0.76 (0.24, 1.70) | 0.097 |
| PDGF-AB/BB | 9.83 (5.11, 20.26) | 10.29 (4.16, 23.01) | 0.730 |
| PAI-1 | 150.08 (102.96, 185.90) | 145.78 (84.91, 187.36) | 0.548 |
| BDNF | 4.1 (2.73, 9.24) | 5.59 (2.57, 8.38) | 0.943 |
| RANTES | 17.01 (10.08, 31.28) | 38.28 (16.99, 53.21) | 0.004 |
| sICAM | 136.65 (110.26, 167.23) | 118.07 (55.88, 160.18) | 0.024 |
| sVCAM | 1537.89 (1280.62, 1856.16) | 1416.52 (1261.35, 1788.73) | 0.312 |
| NCAM | 370.00 (317.79, 430.80) | 416.72 (346.95, 477.54) | 0.019 |
| MPO | 539.93 (286.80, 1065.61) | 418.72 (311.81, 535.89) | 0.025 |
| Cathepsin D | 252.28 (210.79, 361.58) | 242.58 (180.85, 416.64) | 0.689 |
Data are expressed as mean±standard deviation, median and IQR (25th-75th percentile), or absolute (relative) frequencies; the estimated significance level obtained from the Mann-Whitney test. PDGF=Platelet-derived growth factor, PAI=plasminogen activator inhibitor, BDNF=brain-derived neurotrophic factor, RANTES=Regulated on Activation, Normal T Cell Expressed and Secreted, sICAM=soluble intercellular cell adhesion molecule, sVCAM=soluble vascular cell adhesion molecule, NCAM=neural cell adhesion molecule, MPO=myeloperoxidase; values in bold typeface denote statistical significance
Comparison of demographic and baseline paraclinical characteristics between stroke patients and controls
There was a significantly positive strong correlation between inflammatory markers RANTES and neurotrophins: BDNF (Spearman coefficient: ρ = 0.85, P < 0.001) and PDGF isoforms AA (ρ = 0.94, P < 0.001) and AB/BB (ρ = 0.95, P < 0.001). The RANTES inflammatory marker was positively correlated with the PAI-1 (ρ = 0.47, P < 0.001) and cathepsin D (ρ = 0.29, P = 0.002). The same type of correlations between RANTES and neurotrophins were found in the control group, but the magnitude of correlations was lower than for stroke patients (RANTES and BDNF: ρ = 0.65, P < 0.001, RANTES and PDGF-AA: ρ =0.63, P < 0.001, RANTES and PDGF-AB/BB: ρ = 0.68, P < 0.001). In addition, RANTES was positively correlated with MPO (ρ = 0.39, P = 0.017), but there was no significant correlation with the other markers in the control group.
The relation of peripheral biomarkers and TOAST subtype of stroke
According to TOAST classification, 58.8% (n = 67) were LAA, 37.1% (n = 43) were CE subtype, and only 3.4% (n = 4) were included in SVO stroke subtype. Lower levels of MPO were observed in LAA and CE [median (IQR): 539.9 (223.7, 1052.5) pg/ml and 580.8 (403.9, 1066.1) pg/ml, respectively, compared with SVO subtype 256.7 (172.5, 323.8) pg/ml, P = 0.028), but the SVO group was too small. sVCAM was higher in CE and SVO [median (IQR): 1732.1 (1374.6, 2095.7) pg/ml and 1534.9 (1294.6, 1944.3) pg/ml versus LAA 1425.1 (1238.4, 1711.1) pg/ml, P = 0.012]. For other biomarkers, there was no significant difference among stroke subtypes.
Temporal profile of peripheral biomarkers in stroke patients
The dynamic evolution of the analyzed biomarkers was evaluated on days 1 and 5 after admission; plasma concentrations of sICAM, sVCAM, and RANTES significantly decreased during the analyzed period [Table 2].
Table 2.
Dynamic evolution of peripheral biomarkers, between days 1 and 5 after admission in ischemic stroke patients
| Parameters (pg/ml) | Day 1 after admission | Day 5 after admission | P |
|---|---|---|---|
| PDGF-AA | 1.04 (0.62, 2.21) | 1.10 (0.41, 3.13) | 0.288 |
| PDGF-AB/BB | 8.60 (4.71, 20.58) | 11.77 (4.76, 24.15) | 0.162 |
| PAI-1 | 151.23 (105.30, 185.96) | 152.32 (102.35, 222.39) | 0.154 |
| BDNF | 4.56 (2.47, 9.31) | 4.83 (2.44, 14.92) | 0.173 |
| RANTES | 16.43 (9.56, 35.99) | 14.38 (8.90, 19.57) | 0.016 |
| sICAM | 136.65 (108.69, 165.86) | 109.15 (66.12, 172.97) | 0.013 |
| sVCAM | 1578.38 (1294.69, 886.04) | 1470.26 (1220.17, 1686.31) | 0.001 |
| NCAM | 370.51 (317.63, 422.07) | 352.30 (328.47, 432.20) | 0.898 |
| MPO | 564.65 (299.85, 1160.86) | 780.08 (437.81, 1160.70) | 0.352 |
| Cathepsin D | 270.80 (212.08, 270.80) | 239.09 (199.05, 357.04) | 0.551 |
Data are expressed as median and IQR (25th and 75th percentile); the estimated significance level obtained from the Mann-Whitney test; values in bold typeface denote statistical significance. PDGF=Platelet-derived growth factor, PAI=plasminogen activator inhibitor, BDNF=brain-derived neurotrophic factor, RANTES=Regulated on Activation, Normal T Cell Expressed and Secreted, sICAM=soluble intercellular cell adhesion molecule, sVCAM=soluble vascular cell adhesion molecule, NCAM=neural cell adhesion molecule, MPO=myeloperoxidase; values in bold typeface denote statistical significance
The association between biomarkers and stroke severity (measured on admission, at day 5, and at discharge)
For the first-day measurement, the bivariate analysis revealed the association only of sVCAM with stroke severity assessed by NIHSS score on admission >7 points (Mann–Whitney test, P = 0.018). However, NIHSS assessed on discharge was in negative association with PDGF-AA (Mann–Whitney test, P = 0.013), PDGR-AB/BB (P = 0.008), BDNF (P = 0.014), and RANTES (P = 0.012); patients with NIHSS score at discharge >7 had lower plasma levels than patients with NIHSS score ≤7 points. Univariate and multivariate logistic regression indicated basal NCAM as a significant predictor for NIHSS >7 points 5 days after the onset of the disease.
The association between the studied biomarkers and functional dependency in daily life activity assessed with Barthel index
Plasma levels of PDGF-AA, PDGF-AB/BB, BDNF, and RANTES were found to be significantly lower in patients with higher degree of dependency in daily life activities and BI ≤ 80, on day 5 after the onset of the disease [Table 3].
Table 3.
The bivariate association between plasma biomarkers on day 1 and BI on day 5 after admission
| Parameters (pg/ml) | Stroke patients with BI >80, day 5 (n1=46) | Stroke patients with BI ≤80, day 5 (n2=68) | P |
|---|---|---|---|
| PDGF-AA | 1.13 (0.82, 2.50) | 0.90 (0.59, 1.50) | 0.010 |
| PDGF-AB/BB | 10.66 (6.80, 27.32) | 7.70 (3.14, 16.73) | 0.013 |
| PAI-1 | 153.51 (103.38, 182.52) | 131.05 (97.46, 190.96) | 0.677 |
| BDNF | 5.36 (3.50, 12.30) | 4.09 (1.92, 7.79) | 0.012 |
| RANTES | 19.41 (12.41, 42.33) | 12.84 (8.10, 22.58) | 0.010 |
| sICAM | 137.72 (118.74, 69.89) | 135.39 (102.68, 165.34) | 0.291 |
| sVCAM | 1517.43 (1249.57, 1730.93) | 1669.76 (1318.71, 2086.03) | 0.142 |
| NCAM | 361.14 (315.33, 412.78) | 396.32 (329.68, 451.07) | 0.134 |
| MPO | 505.73 (291.38, 938.11) | 581.23 (262.11, 1234.99) | 0.365 |
| Cathepsin D | 258.49 (215.58, 342.12) | 250.79 (205.34, 368.61) | 0.994 |
Data are expressed as median and IQR (25th and 75th percentile); the estimated significance level obtained from the Mann-Whitney test; values in bold typeface denote statistical significance. PDGF=Platelet-derived growth factor, PAI=plasminogen activator inhibitor, BDNF=brain-derived neurotrophic factor, RANTES=Regulated on Activation, Normal T Cell Expressed and Secreted, sICAM=soluble intercellular cell adhesion molecule, sVCAM=soluble vascular cell adhesion molecule, NCAM=neural cell adhesion molecule, MPO=myeloperoxidase; values in bold typeface denote statistical significance
PDGF-AA (per 1 unit increase), PDGF-AB/BB (per 10 units increase), and BDNF (per 10 units increase) were univariate and multivariate predictors for functional dependence in daily life activity (BI ≤ 80), having a protective effect (OR < 1) [Table 4 and Figure 1].
Table 4.
Univariate and multivariate association of the studied parameters with functional dependence evaluated with BI on day 5
| Parameters (pg/ml) | Estimated value of COR (95% CI) | P | Estimated value of AORa (95% CI) | P |
|---|---|---|---|---|
| PDGF-AAb | 0.56 (0.39, 0.89) | 0.012 | 0.55 (0.32, 0.93) | 0.027 |
| PDGF-ABc | 0.70 (0.51, 0.97) | 0.034 | 0.64 (0.43, 0.96) | 0.030 |
| PAId | 0.87 (0.50, 1.52) | 0.620 | 0.79 (0.40, 1.57) | 0.503 |
| BDNFc | 0.50 (0.26, 0.97) | 0.039 | 0.42 (0.18, 0.97) | 0.042 |
| RANTESa | 0.86 (0.74, 1.01) | 0.058 | 0.85 (0.71, 1.02) | 0.072 |
| sICAMd | 0.81 (0.50, 1.32) | 0.402 | 0.84 (0.49, 1.44) | 0.532 |
| sVCAMd | 1.08 (0.99, 1.16) | 0.069 | 1.04 (0.93, 1.15) | 0.518 |
| NCAMd | 1.51 (0.95, 2.39) | 0.080 | 1.68 (0.90, 3.17) | 0.106 |
| MPOe | 1.17 (0.78, 1.76) | 0.456 | 1.17 (0.72, 1.91) | 0.532 |
| Cathepsind | 1.11 (0.88, 1.42) | 0.379 | 1.01 (0.74, 1.37) | 0.969 |
The estimated significance level obtained from univariate and multivariate logistic regression; aadjusting for age >70, gender, stroke history, TOAST classification, leucoaraiosis, NIHSS on admission; bper 1 unit increase of biomarker; cper 10 units increase of biomarker; dper 100 units increase of biomarker; eper 1000 units increase. 95% CI=Confidence interval (lower limit; upper limit); COR=crude odds ratio; AOR=adjusted odds ratio; PDGF=platelet-derived growth factor, PAI=plasminogen activator inhibitor, BDNF=brain-derived neurotrophic factor, RANTES=Regulated on Activation, Normal T Cell Expressed and Secreted, sICAM=soluble intercellular cell adhesion molecule, sVCAM=soluble vascular cell adhesion molecule, NCAM=neural cell adhesion molecule, MPO=myeloperoxidase; values in bold typeface denote statistical significance
Figure 1.
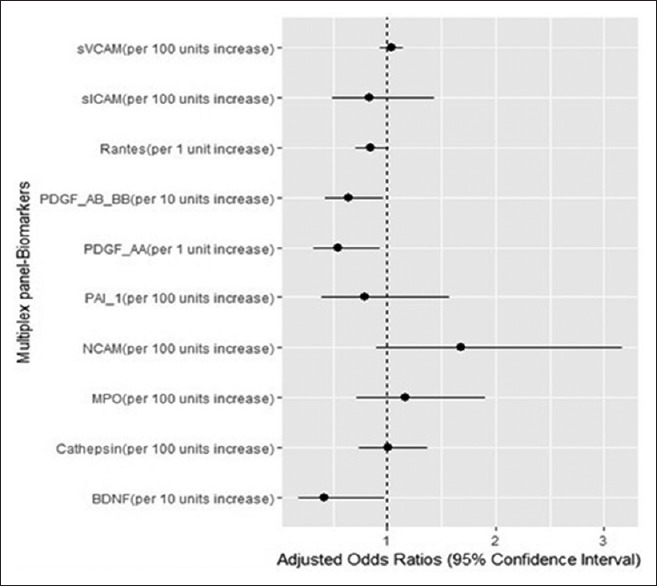
The impact of the studied biomarkers on functional dependency in daily life activities in patients with ischemic stroke. PDGF-AA, PDGF-AB/BB, and BDNF were independent predictors for lower degree of functional dependence
For each individual significant biomarker, the optimal cutoff value for poor functional dependency in daily life activity at day 5 was estimated. For BDNF, the following results were obtained: cutoff value = 2.33 pg/ml as the optimal value, sensitivity (Se) = 91.1% (95% CI: 81.8–96.7%), specificity (Sp) =31.1% (95% CI: 18.2–46.7%), PPV = 66.7% (95% CI: 49.6–84.9%), and NPV = 70% (95% CI: 50.3–81.9%). For PDGF-AB/BB, the following results were obtained: cutoff = 4.90 pg/ml, Se = 89.7% (95% CI: 79.9–95.8%), Sp = 37.8% (95% CI: 23.7–53.5%), PPV = 68.5% (95% CI: 52.8–82.1%), and NPV = 70.8% (95% CI: 52.6–82.1%). For PDGF-AA parameter, the following results were obtained: cutoff = 0.80 pg/ml, Se = 82.3% (95% CI: 71.2–90.5%), Sp = 44.4% (95% CI: 29.6–60.0%), PPV = 69.1% (95% CI: 54.1–82.1%), and NPV = 62.5% (95% CI: 46.9–75.7%).
Although we did not find a significant difference in the discriminatory capacity of models improved with significant predictors for functional dependence, markers with significant effect in the prediction of functional dependence had a clinically relevant prognostic value, the area under the ROC curves related to each biomarker being greater than the threshold value of 0.80 [Table 5].
Table 5.
Assessment of discriminatory capacity of basal clinical model improved with biomarkers found significant in regression analysis, for functional dependence
| Models tested | AUC (95% CI) | P* |
|---|---|---|
| Clinical model# | 0.843 (0.766, 0.920) | - |
| +PDGF-AA | 0.864 (0.798, 0.929) | 0.290 |
| +PDGF-AB | 0.863 (0.797, 0.929) | 0.285 |
| +BDNF | 0.861 (0.795, 0.928) | 0.288 |
| +RANTES | 0.853 (0.782, 0.924) | 0.459 |
#Model with known clinical predictors for mRS: age, gender, stroke history, leucoaraiosis, NIHSS on admission, and TOAST subtype; +clinical model after addition of individual biomarkers; *the estimated significance level obtained from the DeLong test for comparison between ROC curves associated with clinical basal model and the model obtained by adding biomarkers with significant predictor effect. AUC=Area under the ROC curve, 95% CI=95% confidence interval for AUC, PDGF=platelet-derived growth factor, BDNF=brain-derived neurotrophic factor, RANTES=Regulated on Activation, Normal T Cell Expressed and Secreted
DISCUSSION
In an attempt to find possible biomarkers for outcome prediction in stroke patients, the present study aimed to evaluate several neurotrophic factors and chemokines. Using the xMAP technology, the present study aimed to assess the relationship between a panel of chemokines and neurotrophic factors and stroke severity, functional outcome, and degree of daily life activity. Also, a temporal profile of peripheral biomarkers and comparison with a healthy group were done, due to scarce information about the reference range in a healthy population.
MPO is an enzyme secreted in the first phase as an apopro-MPO, which is activated after the enzymatic conversion in an active MPO, a 140-kDa hemeprotein, mainly found in azurophilic granules of polymorphonuclear neutrophils (PMNs) and to a lesser extent in lysosomes of monocytes and macrophages,[7] influencing the NO availability and modulating the vascular response,[8] thus having a harmful effect during acute inflammation, contributing to tissue damage after ischemia/reperfusion injury.[9] Although ICAM-1 is a key molecule after ischemic stroke, enhancing the neutrophile accumulation,[10] a recent experimental study revealed that PMN accumulation is not exclusively dependent of ICAM, and the absence of ICAM neither inhibits neutrophil accumulation during cerebral ischemia nor enhances protection against stroke.[11] In our study, MPO and sICAM basal levels were higher in ischemic stroke patients compared with controls. Similar results regarding MPO were revealed by Tay et al., where MPO levels were higher on day 1 after stroke onset compared with controls; after 5 days, the values returned to normal.[12] Additionally, MPO levels were positively associated with stroke severity and mortality in the ischemic group.[12] In our study, there was a continuous increment of MPO levels during the first 5 days, but without correlation and with stroke severity and outcome. In a Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial, MPO was found to be an independent predictor for risk of fatal stroke, while markers of endothelial dysfunction such as sVCAM and sICAM were without significance.[13] sICAM and sVCAM were higher in ischemic stroke patients compared with controls, similar to other data in the literature,[14,15] with statistical significance only for sICAM (P = 0.024). The continuously increasing levels of soluble adhesion molecules are in line with other reports. Manolescu et al. also found an increasing trend for sICAM during hospitalization, without influence on BI.[16]
Another marker with a projection on unfavorable outcome prediction was PAI-1, the main endogenous inhibitor of plasminogen activator, important in fibrinolysis regulation.[17] Secreted by platelets, smooth muscular and endothelial cells and by hepatic and splenic cells, it also has, besides its procoagulant effect, a proinflammatory effect, influencing the inflammatory cell migration at the injury site.[17,18] An experimental study revealed that PAI-1 originated from astrocytes also exerts neuroprotective effects, either by modulation of Bax/Bcl-2 gene expression or by reducing N-methyl-D-aspartate (NMDA) excitotoxicity.[19] The neuroprotective effects of PAI-1 originated from astrocytes, pericytes, and endothelial cells of the blood–brain barrier (BBB) reduce BBB destruction, consequently protecting the neurovascular unit, apart from tissue plasminogen activator (t-PA) inhibition.[20] In our study, the plasma level of PAI-1 was found to be slightly higher in stroke patients compared with controls, without statistical significance, but in relation to stroke severity assessed with NIHSS at discharge, PAI-1 levels were lower in patients with severe stroke (NIHSS > 7), with a tendency toward statistical significance. The multivariate regression analysis revealed that PAI-1 is an independent predictor for unfavorable outcome, lower levels being associated with unfavorable outcome (data not shown), suggesting that in the long-term evolution of ischemic stroke patients, PAI-1 might have neuroprotective effects. However, further studies on larger cohorts need to be analyzed in order to clarify these aspects, as data regarding the protective role of PAI-1 are from experimental studies.
RANTES (CCL5) is a proinflammatory chemokine produced by several cell types, such as T lymphocytes, endothelial cells, platelets, or smooth muscle cells from vascular endothelium, involved in inflammatory cell migration and infiltration at the site of injury; after an ischemic episode, the CCL5 expression in brain tissue is enhanced.[21] Experimental studies showed a reduced BBB permeability after an ischemic episode in RANTES−/− animals[1] and involvement of this chemokine in vascular dysfunction through a perivascular inflammation interferon gamma-induced mechanism.[22] In our study groups, the basal plasma level of RANTES was significantly lower in stroke patients compared with controls and decreased during the first 5 days after admission, divergent with results of Tokami et al., where levels were found greater in patients compared with controls.[3] Similar to the data from the literature, we found a significant positive correlation of RANTES not only with BDNF (r = 0.846, P ≤ 0.001)[3] but also with other neurotrophins such as PDGF-AA (r = 0.825, P ≤ 0.001) and PDGF-AB/BB (r = 0.842, P ≤ 0.001), suggesting the beneficial effects on neural tissue. Exogen administration of RANTES in rats undergoing MCAO revealed a reduced BBB extravasation.[2] Dual effects of RANTES could be explained by the different types of receptors involved in signal modulation, while proinflammatory effects are exerted through CCR1 receptors and the neuroprotective effects are exerted through CCR3 and CCR5 receptors.[3] In our study, RANTES was found to be significantly higher in patients with favorable outcome 3 months after discharge, and in patients with BI > 80 with a lower degree of dependency in daily life activities, thus suggesting the dual role of this chemokine.
NCAM belongs to the superfamily of immunoglobulins, involved in neural cell migration, synaptic plasticity, and cognitive processes.[23] There are only few data regarding the difference in NCAM concentration between healthy persons and patients with different conditions such as schizophrenia or multiple sclerosis,[24,25] where levels were found to be lower in patients compared with control groups. Our results are in line with these data, the peripheral levels of NCAM being higher in the healthy group compared with stroke patients. There was no relationship between NCAM and BI or modified Rankin Scale (mRS) at discharge, but surprisingly, the plasma NCAM in day 1 after admission was higher and was a univariate predictor for the long-term unfavorable outcome (data not shown). This might be explained by a higher degree of proteolytic cleavage of transmembrane NCAM by MMP-9, increasing the concentration of proteolytic fragments (NCAM – 65 kDa).[26] After adjustment for age (>70 years), gender, history of stroke, TOAST classification, and leucoaraiosis, the prediction capacity of NCAM was lost.
PDGF along with its receptors PDGFRA and PDGFRB are involved not only in neural surviving pathways but also in neural and synaptic plasticity and neural regeneration.[27] Several mechanisms are involved in this protective process, such as increased activity of antioxidant enzymes, reducing the oxidative stress, the activation of the PI3-K/Akt pathway, or decreasing excitotoxicity. Moreover, the selective neuronal death is preceded by a dramatic decrease in PDGF-B, an isoform with antiapoptotic properties.[27] The protein expression was decreased after cerebral ischemia in the hippocampal CA1 region, while ischemic preconditioning protects neurons by increasing their vulnerability, probably due to PDGF-BB action against NMDA excitotoxicity.[28] A recent study regarding the role of PDGF in stroke revealed the association of PDFG-AB/BB in reducing the risk of recurrent events in stroke patients, with no other association with another outcome of interest.[29] Apart from the influence on neuronal protective aspects, some studies suggested the provasculogenic role and ROS generation of PDGF isoforms.[30,31] Another study showed the association between elevated levels of PDGF-AA and -AB at 24 and 72 h and an unfavorable outcome in patients having undergone thrombolytic therapy.[32] On the contrary, we found a favorable correlation between PDFG-AA and PDGF-AB/BB levels, and favorable outcome and a lower degree of functional dependency. Also, a strong correlation between PDGF, RANTES, and BDNF was found, suggesting the synergistic action of these neurotrophins. A possible explanation might be the fact that the PDGFRB receptors are involved in the interaction between BDNF and neural progenitors.[27]
BDNF is another neurotrophin involved in neural differentiation and protection, neurogenesis and angiogenesis, plasma values decreasing with age,[33,34] and reduced plasma levels being associated with cognitive regression even in the absence of an ischemic event.[35] Despite the fact that BDNF is associated with neuropathic pain after stroke,[36] higher peripheral levels are associated with favorable outcome.[37]
Overall, the results of the study emphasize the neuroprotective role of BDNF, PDGF-AA, and PDGF-AB/BB, partly not only by their intercorrelation but also by the positive association with a lower degree of functional dependency in daily life activities. Although the cutoff for BDNF and PDGF showed high sensitivity, there was a low specificity for poor functional prediction. There are scarce data across the literature regarding the cutoff point for BDNF levels in poststroke recovery or disability. A recent study revealed that a cutoff value of 20.7 ng/ml might be a good predictor for short-term poststroke recovery, with a sensitivity of 51.7% and specificity of 62.3%, although plasma BDNF was found to be correlated only statistically but not clinically with functional outcome.[38] Another study suggested a cutoff of 21.8 ng/ml as the optimal value for long-term recovery prediction.[39] The recovery after stroke or response to therapy is highly variable among patients, partly due to genetic polymorphism; (BDNF) Val66Met polymorphism is one of the genetic polymorphisms known to be associated with poorer recovery after ischemic stroke.[40]
Because both the BI and the mRS are the most frequently used measurements to assess the degree of functional dependence in stroke patients, we also assessed mRS at discharge, but none of the neurotrophic parameters were significantly associated with poor prognosis (mRS > 3) at discharge (data not shown). In a recent publication, we showed that the plasma level of neurofilament heavy chains (NfH), a possible marker of neuronal destruction, was significantly higher on day 5 after the onset of the disease, in a positive association with mRS ≥3 at discharge.[41] However, in the present analysis, significantly higher levels of NfH [median: 159.3 (IQR: 23.5–384.2) ng/ml] were found in patients with higher degree of disability in daily life activity (BI ≤80) compared with patients with BI >80 [median: 26.5 (IQR: 23.5–267.9) ng/ml] (data not shown).
Limitations
Although the relatively modest group size in relation to stroke incidence could be considered a potential limitation, the strength of the study is the dynamic measurement of the peripheral parameters and assessment of baseline biomarker projection on patient status 5 days after onset. Additionally, some of the above-discussed neurotrophins have been mostly studied in an experimental context and less in a clinical one. Further studies should investigate the impact of neurotrophic and deleterious markers on long-term outcome prediction, in larger group size, measuring both the degree of disability and functional independence in daily life activities.
CONCLUSIONS
BDNF, PDGF-AA, and PDGF-AB/BB plasma levels were significantly lower in severe stroke, being independent predictors for functional dependency in daily life activities, and thus, they may be useful prognostic markers in the assessment of ischemic stroke patients. xMAP technology allows multiple biomarker assessments, rendering a more comprehensive image on the multiple processes occurring during an acute ischemic stroke event.
Author contribution
A.H. designed the study, collected samples/data, analyzed samples, and drafted the manuscript. M.I. analyzed/interpreted data and drafted the manuscript. S.M. assessed the patients, collected clinical data, and drafted the abstract. R.B. assessed the patients and revised the manuscript. M.D. designed the study and revised the manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This study was partially funded by the Doctoral School affiliated with the University of Medicine, Pharmacy, Sciences and Technology of Tîrgu Mureş. Authors thank lecturer Adrian Năznean for his valuable assistance in reviewing English.
REFERENCES
- 1.Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, Kawase T, et al. Blood cell-derived RANTES mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia–reperfusion. Stroke. 2008;39:2560–70. doi: 10.1161/STROKEAHA.107.513150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y-C, Tang S-C, Liou H-C, Tu H-J, Kang K-H, Liou H-H, et al. Pathological Role of Two Chemokines RANTES and MIF in Ischemic Stroke. FASEB J. 2017;31:19. [Google Scholar]
- 3.Tokami H, Ago T, Sugimori H, Kuroda J, Awano H, Suzuki K, et al. RANTES has a potential to play a neuroprotective role in an autocrine/paracrine manner after ischemic stroke. Brain Res. 2013;1517:122–32. doi: 10.1016/j.brainres.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Huţanu A, Iancu M, Bălaşa R, Maier S, Dobreanu M. Predicting functional outcome of ischemic stroke patients in Romania based on plasma CRP, sTNFR-1, D-Dimers, NGAL and NSE measured using a biochip array. Acta Pharmacol Sin (Special Featur Circ Biomarkers Cardiovasc Dis) 2018;39:1228–36. doi: 10.1038/aps.2018.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn TJ, Langhorne P, Stott DJ. Barthel index for stroke trials: Development, properties, and application. Stroke. 2011;42:1146–51. doi: 10.1161/STROKEAHA.110.598540. [DOI] [PubMed] [Google Scholar]
- 6.Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke Definitions for use in a multicenter clinical trial TOAST Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 7.Klebanoff SJ. Myeloperoxidase: Friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 8.Eiserich JP, Baldus S, Brennan M-L, Ma W, Zhang C, Tousson A, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391 LP–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 9.Matthijsen RA, Huugen D, Hoebers NT, De Vries B, Peutz-Kootstra CJ, Aratani Y, et al. Myeloperoxidase is critically involved in the induction of organ damage after renal ischemia reperfusion. Am J Pathol. 2007;171:1743–52. doi: 10.2353/ajpath.2007.070184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly ES, Jr, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, et al. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest. 1996;97:209–16. doi: 10.1172/JCI118392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enzmann GU, Pavlidou S, Vaas M, Klohs J, Engelhardt B. ICAM-1null C57BL/6 mice are not protected from experimental ischemic stroke. Transl Stroke Res. 2018;9:608–21. doi: 10.1007/s12975-018-0612-4. [DOI] [PubMed] [Google Scholar]
- 12.Tay A, Tamam Y, Yokus B, Ustundag M, Orak M. Serum myeloperoxidase levels in predicting the severity of stroke and mortality in acute ischemic stroke patients. Eur Rev Med Pharmacol Sci. 2015;19:1983–8. [PubMed] [Google Scholar]
- 13.Ganz P, Amarenco P, Goldstein LB, Sillesen H, Bao W, Preston GM, et al. Association of osteopontin, neopterin, and myeloperoxidase with stroke risk in patients with prior stroke or transient ischemic attacks results of an analysis of 13 biomarkers from the stroke prevention by aggressive reduction in cholesterol levels trial. Stroke. 2017;48:3223–31. doi: 10.1161/STROKEAHA.117.017965. [DOI] [PubMed] [Google Scholar]
- 14.Wang JYJ, Zhou DHD, Li J, Zhang M, Deng J, Gao C, et al. Association of soluble intercellular adhesion molecule 1 with neurological deterioration of ischemic stroke: The Chongqing stroke study. Cerebrovasc Dis. 2006;21:67–73. doi: 10.1159/000090005. [DOI] [PubMed] [Google Scholar]
- 15.Simundic A-M, Basic V, Topic E, Demarin V, Vrkic N, Kunovic B, et al. Soluble adhesion molecules in acute ischemic stroke. Clin Invest Med. 2004;27:86–92. [PubMed] [Google Scholar]
- 16.Manolescu BN, Berteanu M, Dumitru L, Dinu H, Iliescu A, Fărcăsanu IC, et al. Dynamics of inflammatory markers in post-acute stroke patients undergoing rehabilitation. Inflammation. 2011;34:551–8. doi: 10.1007/s10753-010-9262-8. [DOI] [PubMed] [Google Scholar]
- 17.Yasar Yildiz S, Kuru P, Toksoy Oner E, Agirbasli M. Functional stability of plasminogen activator inhibitor-1. Scientific World Journal. 2014;2014:858293. doi: 10.1155/2014/858293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Praetner M, Zuchtriegel G, Holzer M, Uhl B, Schaubächer J, Laura M, et al. Plasminogen activator inhibitor-1 promotes neutrophil infiltration and tissue injury on ischemia–reperfusion. Arterioscler Thromb Vasc Biol. 2018;38:829–42. doi: 10.1161/ATVBAHA.117.309760. [DOI] [PubMed] [Google Scholar]
- 19.Balsara RD, Ploplis VA. Plasminogen activator inhibitor-1: The double-edged sword in apoptosis. Thromb Haemost. 2008;100:1029–36. [PMC free article] [PubMed] [Google Scholar]
- 20.Dohgu S, Takata F, Matsumoto J, Oda M, Harada E, Watanabe T, et al. Autocrine and paracrine up-regulation of blood–brain barrier function by plasminogen activator inhibitor-1. Microvasc Res. 2011;81:103–7. doi: 10.1016/j.mvr.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Kim JY, Kawabori M, Yenari MA. Innate inflammatory responses in stroke: Mechanisms and potential therapeutic targets. Curr Med Chem. 2014;21:2076–97. doi: 10.2174/0929867321666131228205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikolajczyk TP, Nosalski R, Szczepaniak P, Budzyn K, Osmenda G, Skiba D, et al. Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. FASEB J. 2016;30:1987–99. doi: 10.1096/fj.201500088R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weledji EP, Assob JC. The ubiquitous neural cell adhesion molecule (N-CAM) Ann Med Surg. 2014;3:77–81. doi: 10.1016/j.amsu.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An HM, Zhou LP, Yu Y, Fan H, Fan FM, Tan S, et al. Serum NCAM levels and cognitive deficits in first episode schizophrenia patients versus health controls. Schizophr Res. 2018;192:457–8. doi: 10.1016/j.schres.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Dubuisson N, Lycke J, Axelsson M, Giovanonni G, Gnanapavan S. CSF NCAM levels are modulated by highly-active DMTs. Eur J Neurol. 2017;24:596. [Google Scholar]
- 26.Hinkle CL, Diestel S, Lieberman J, Maness PF. Metalloprotease-induced ectodomain shedding of neural cell adhesion molecule (NCAM) J Neurobiol. 2006;66:1378–95. doi: 10.1002/neu.20257. [DOI] [PubMed] [Google Scholar]
- 27.Funa K, Sasahara M. The roles of PDGF in development and during neurogenesis in the normal and diseased nervous system. J Neuroimmune Pharmacol. 2014;9:168–81. doi: 10.1007/s11481-013-9479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JC, Kim YH, Lee TK, Kim IH, Cho JH, Cho GS, et al. Effects of ischemic preconditioning on PDGF-BB expression in the gerbil hippocampal CA1 region following transient cerebral ischemia. Mol Med Rep. 2017;16:1627–34. doi: 10.3892/mmr.2017.6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narasimhalu K, Ma L, De Silva DA, Wong MC, Chang HM, Chen C. Elevated platelet-derived growth factor AB/BB is associated with a lower risk of recurrent vascular events in stroke patients. Int J Stroke. 2015;10:85–9. doi: 10.1111/ijs.12358. [DOI] [PubMed] [Google Scholar]
- 30.Lange S, Heger J, Euler G, Wartenberg M, Piper HM, Sauer H. Platelet-derived growth factor BB stimulates vasculogenesis of embryonic stem cell-derived endothelial cells by calcium-mediated generation of reactive oxygen species. Cardiovasc Res. 2009;81:159–68. doi: 10.1093/cvr/cvn258. [DOI] [PubMed] [Google Scholar]
- 31.Kreuzer J, Viedt C, Brandes RP, Seeger F, Rosenkranz AS, Sauer H, et al. Platelet-derived growth factor activates production of reactive oxygen species by NAD (P) H oxidase in smooth muscle cells through Gi1,2. FASEB J. 2003;17:38–40. doi: 10.1096/fj.01-1036fje. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-González R, Blanco M, Rodríguez-Yáñez M, Moldes O, Castillo J, Sobrino T. Platelet derived growth factor-CC isoform is associated with hemorrhagic transformation in ischemic stroke patients treated with tissue plasminogen activator. Atherosclerosis. 2013;226:165–71. doi: 10.1016/j.atherosclerosis.2012.10.072. [DOI] [PubMed] [Google Scholar]
- 33.Oh H, Lewis DA, Sibille E. The role of BDNF in age-dependent changes of excitatory and inhibitory synaptic markers in the human prefrontal cortex. Neuropsychopharmacology. 2016;41:3080–91. doi: 10.1038/npp.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berretta A, Tzeng YC, Clarkson A. Post-stroke recovery: The role of activity-dependent release of brain-derived neurotrophic factor. Expert Rev Neurother. 2014;14:1335–44. doi: 10.1586/14737175.2014.969242. [DOI] [PubMed] [Google Scholar]
- 35.Navarro-Martínez R, Fernández-Garrido J, Buigues C, Torralba-Martínez E, Martinez-Martinez M, Verdejo Y, et al. Brain-derived neurotrophic factor correlates with functional and cognitive impairment in non-disabled older individuals. Exp Gerontol. 2015;72:129–37. doi: 10.1016/j.exger.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Siotto M, Aprile I, Simonelli I, Pazzaglia C, Ventriglia M, Santoro M, et al. An exploratory study of BDNF and oxidative stress marker alterations in subacute and chronic stroke patients affected by neuropathic pain. J Neural Transm. 2017;124:1557–66. doi: 10.1007/s00702-017-1805-9. [DOI] [PubMed] [Google Scholar]
- 37.Chan A, Yan J, Csurhes P, Greer J, McCombe P. Circulating brain derived neurotrophic factor (BDNF) and frequency of BDNF positive T cells in peripheral blood in human ischemic stroke: Effect on outcome. J Neuroimmunol. 2015;286:42–7. doi: 10.1016/j.jneuroim.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Luo W, Liu T, Li S, Wen H, Zhou F, Zafonte R, et al. The serum BDNF level offers minimum predictive value for motor function recovery after stroke Transl. Stroke Res. 2018 doi: 10.1007/s12975-018-0648-5. doi: 101007/s12975-018-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanne TM, Aberg ND, Nilsson S, Jood K, Blomstrand C, Andreasson U, et al. Low circulating acute brain-derived neurotrophic factor levels are associated with poor long-term functional outcome after ischemic stroke. Stroke. 2016;47:1943–5. doi: 10.1161/STROKEAHA.115.012383. [DOI] [PubMed] [Google Scholar]
- 40.Kim WS, Lim JY, Shin JH, Park HK, Tan SA, Park KU, et al. Effect of the presence of brain-derived neurotrophic factor val66met polymorphism on the recovery in patients with acute subcortical stroke. Ann Rehabil Med. 2013;37:311–9. doi: 10.5535/arm.2013.37.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huţanu A, Maier S, Bălaşa R, Oprea OR, Barbu Ş, Voidăzan S, et al. Plasma phosphorylated neurofilament heavy chains as a potential marker for ischemic stroke patients. Rev Rom Med Lab. 2018:26. doi: 102478/rrlm-2018-0004. [Google Scholar]


