ABSTRACT
Background
The existence of metabolic adaptation, following weight loss, remains a controversial issue. To our knowledge, no study has evaluated the role of energy balance (EB) in modulating metabolic adaptation.
Objectives
The aim of this study was to determine if metabolic adaptation, at the level of resting metabolic rate (RMR), is modulated by participants’ EB status. A secondary aim was to investigate if metabolic adaptation was associated with weight regain.
Methods
Seventy-one individuals with obesity (BMI: 34.6 ± 3.4 kg/m2; age: 45.4 ± 8.2 y; 33 men) enrolled in a 1000-kcal/d diet for 8 wk, followed by 4 wk of weight stabilization and a 9-mo weight loss maintenance program. Body weight/composition and RMR were measured at baseline, week 9 (W9), week 13 (W13), and 1 y (1Y). Metabolic adaptation was defined as a significantly different (lower or higher) measured compared with predicted RMR.
Results
Participants lost on average 14 kg by W9, followed by weight stabilization at W13, and regained 29% of their initial weight loss at 1Y. Metabolic adaptation was found at W9 (−92 ± 110 kcal/d, P < 0.001) and W13 (−38 ± 124 kcal/d, P = 0.011) but was not correlated with weight regain. A significant reduction in metabolic adaptation was seen between W9 and W13 (−53 ± 101 kcal/d, P < 0.001). In a subset of participants who gained weight between W9 and W13 (n = 33), no metabolic adaptation was seen at W13 (−26.8 ± 121.5 kcal/d, P = 0.214). In a subset of participants with data at all time points (n = 45), metabolic adaptation was present at W9 and W13 (−107 ± 102 kcal/d, P < 0.001 and −49 ± 128 kcal/d, P = 0.013) but not at 1Y (−7 ± 129, P = 0.701).
Conclusion
After weight loss, metabolic adaptation at the level of RMR is dependent on the EB status of the participants, being reduced to half after a period of weight stabilization. Moreover, metabolic adaptation does not predict weight regain at 1Y follow-up. These trials were registered at clinicaltrials.gov as NCT02944253 and NCT03287726.
Keywords: metabolic adaptation, adaptive thermogenesis, weight regain, resting metabolic rate, weight loss
See corresponding editorial on page 1157.
Introduction
Weight loss is accompanied by a significant reduction in total energy expenditure due to a decrease in both resting and nonresting energy expenditure (EE) (1). Some have argued that this reduction is greater than predicted, given the measured losses in both fat mass (FM) and fat-free mass (FFM) (1–6), a mechanism known as metabolic adaptation or adaptive thermogenesis. Metabolic adaptation would then correspond to an exaggerated reduction in EE, below predicted levels, and could be a barrier to successful weight loss maintenance. However, others have reported no evidence of metabolic adaptation when weight-stable individuals who had obesity and lost weight were compared with BMI-matched controls (7–12), and to our knowledge, no one has ever reported metabolic adaptation to be a risk factor for weight regain.
The existence, or lack of, and clinical relevance of metabolic adaptation, in response to underfeeding and weight loss, has been one of the most controversial issues in the obesity field (13–19). A careful examination of the available literature seems to suggest
that differences among studies derive from inconsistencies related with the status of energy balance (EB) and/or weight stability of the participants when measurements are taken. In fact, there is a trend for longitudinal studies to report metabolic adaptation (1–5), while cross-sectional studies, comparing individuals who lost weight with BMI-matched controls, do not tend to report metabolic adaptation (7–9, 20). However, cross-sectional studies suffer from interindividual variability in resting metabolic rate (RMR) and body composition. Comparing individuals with obesity who have lost weight with lean controls is therefore not as likely to demonstrate metabolic adaptation as carefully controlled longitudinal studies.
We have recently shown that when EE measurements are taken under conditions of weight stability, metabolic adaptation, at the level of RMR, is only ∼50 kcal/d after a 12-kg weight loss in previously overweight women (21). However, in that study, measurements were not taken immediately after weight loss, and as such, it is not possible to ascertain the role of weight stability in modulating metabolic adaptation. Therefore, the aim of the present secondary analysis was to determine if metabolic adaptation, at the level of RMR, was modulated by weight stability in a population of men and women with obesity by measuring RMR immediately after weight loss and after a 4-wk weight stabilization period. Secondary aims were to investigate the presence of metabolic adaptation at 1-y (1Y) follow-up and to determine if metabolic adaptation after weight loss was correlated with weight regain at 1Y follow-up. We hypothesized that metabolic adaptation would be reduced, or completely absent, when measurements were performed after a period of weight stabilization compared with immediately after weight loss (under conditions of negative EB). Moreover, we also hypothesized that metabolic adaptation would not be present at 1-y follow-up and that metabolic adaptation after weight loss [either week 9 (W9) or week 13 (W13)] would not be associated with weight regain at 1Y follow-up.
Methods
Participants
Participants in this analysis are part of a large weight loss study (ASKED—Ketosis and Appetite Suppression) that was then followed by a weight loss maintenance study. The primary aim of the studies was to identify the maximum carbohydrate (CHO) intake that is still associated with appetite suppression in a low-energy diet (LED) and to investigate the effect of probiotics (compared with placebo) on weight loss maintenance, respectively.
The original study included adult (aged 18–65 y) healthy volunteers, men and women, with obesity [BMI ≥30 (in kg/m2)], weight stable (<2-kg variation in weight within the past 3 mo), not currently dieting to lose weight, and not using any medications known to affect body weight, appetite, or metabolism. Given that both the RMR and appetite of normally ovulating women have been shown to vary across the menstrual cycle (22, 23) but not in those who take oral contraception (24), we included in this study postmenopausal women and premenopausal women taking oral contraceptives or with a normal menstrual cycle (28 ± 2 d) (but not those with an irregular menstrual cycle). This was done to make sure that measurements were taken in the same phase of the menstrual cycle.
The studies were both approved by the local ethical committee and were registered at clinicaltrials.gov as NCT02944253 and NCT03287726, respectively. All participants provided informed consent before participating in the study.
Study design
The weight loss study was a randomized controlled trial with repeated measurements conducted at the Regional Center for Obesity Research and Innovation (ObeCe) in Trondheim, Norway. All participants, both men and women, were randomly allocated to 3 isocaloric 1000-kcal/d LEDs for 8 wk containing varying amounts of CHO (70, 100, and 130 g in each group) and a fixed amount of protein (75 g/d), with fat counterbalancing the calories from CHO.
This was followed by a 4-wk controlled period of weight stabilization. At W9, participants were gradually reintroduced to consume normal foods while reducing the intake of LED products. An individualized dietary plan, aiming at weight stabilization, was prescribed to each participant following the Nordic Nutrition Recommendations consisting of 15–20% protein, 20–30% fat, and 50–60% CHO (25). Energy needs were estimated by multiplying RMR values at W9 by physical activity level (PAL) extracted from physical activity monitors (SenseWear). The consumption of LED products was discontinued by the end of week 10.
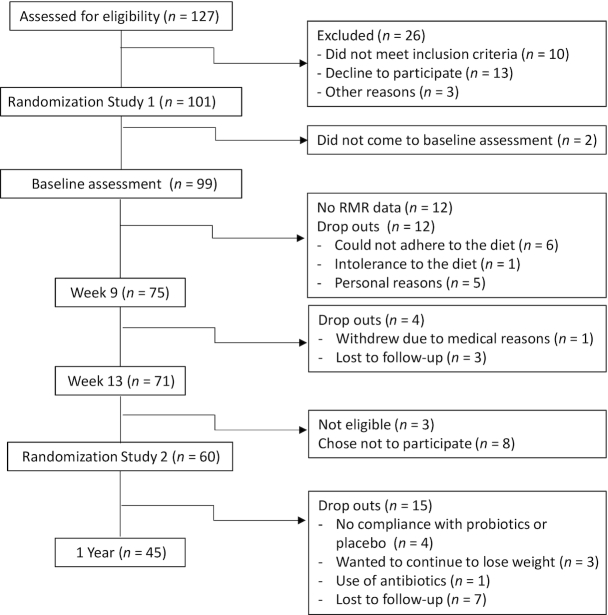
At W13, participants were randomly allocated (double blinded, placebo controlled) to take a multistrain probiotic (8 strains of lactobacilli and bifidobacteria) (Nycopro Ferie; Nycomed) or placebo twice daily (1 with lunch and 1 with dinner) over a period of 9 mo. Energy needs were recalculated at W13 by using RMR at W13 and PAL at week 12 (W12) and a new dietary plan prescribed to each participant, with the same macronutrient composition as for the 4-wk weight stabilization period. Participants had to attend follow-up meetings at ObeCe every month with a research nurse for weighing, discussion of potential side effects, and collection of probiotics dose for the following month. A flowchart of the study can be seen in Figure 1.
FIGURE 1.
Flowchart for the study.
Participants were asked not to change their physical activity (PA) levels during the first 12 wk of the study and to increase it afterward during the 9-mo weight maintenance phase.
Data collection
The following measurements were conducted at baseline, immediately after weight loss (day after the 8 wk of the 1000-kcal/d diet) (W9), after 4 wk of weight stabilization (W13), and at 1Y follow-up while the participants were in the fasting state and immediately after they had voided in the morning.
Body weight and composition
Body weight and composition were determined by whole-body air displacement plethysmography (ADP) (BOD POD; COSMED).
Intracellular water (ICW), as an indirect measure of glycogen storage (26), was measured by bioimpedance analysis (BIA) (InBody 720; Biospace).
Resting metabolic rate
RMR was measured by indirect calorimetry (Vmax Encore 29N; Care Fusion) using a canopy system and standard operating procedures (27).
Physical activity levels
Participants were asked to wear armbands (SenseWear) for a 7-d period at baseline; weeks 4, 8, and 12; and 12-mo follow-up. Data were considered valid if the participants wore the device for a minimum of 4 d, including at least 1 weekend day and >95% of the time (28). The following variables were analyzed: number of steps per day; time spent on sedentary [<1.5 metabolic equivalent of tasks (METs)], light (1.5–3 METs), moderate (3–6 METs), and vigorous to very vigorous (>6 METs) PA; and total PA (>1.5 METs) per day.
Statistical analysis
Only participants with RMR data available at baseline, W9, and W13 were included in this analysis. As no significant differences were seen in metabolic adaptation among randomly allocated groups, either at W9 or W13 (P = 0.921 and P = 0.952, respectively, from a one-factor ANOVA), all participants were analyzed together. Statistical analysis was performed with SPSS version 22 (SPSS, Inc.), data were presented as mean ± SD (except for PA data, which were presented as mean ± SEM), and statistical significance was set at P < 0.05. Changes in body weight/composition and RMR over time were assessed with a repeated-measures ANOVA, using Bonferroni correction for multiple comparisons. The presence of metabolic adaptation was tested by paired t tests, comparing measured RMR (RMRm) and predicted RMR (RMRp) at the same time points. An equation to predict RMR was derived from baseline data of all participants that were part of this analysis and included age, sex, FM, and FFM as predictors.
RMRp (kcal/d) = 505.945 + [110.894 × sex (1 for females and 2 for males)] + [0.402 × Age (years)]+ [5.616 × FM (kg)] + [15.213 × FFM (kg)].
 |
(1) |
This equation, derived from baseline data, was then used to predict RMR at W9, W13, and 1Y, by using FM and FFM at each specific time point.
Differences between metabolic adaptation at weeks 9 and 13 were evaluated by paired samples t test. Correlation analysis was performed between metabolic adaptation after weight loss (W9 and W13) and weight regain at 1Y (as a percentage of the initial weight lost) using Pearson or Spearman correlation coefficients, when appropriate.
Changes in PA over time were analyzed using a linear mixed model with repeated measures, with a restricted maximum likelihood estimation and fixed effects for time. A Bonferroni correction was applied for post hoc pairwise comparisons.
Results
Baseline characteristics of the study participants are shown in Table 1. Seventy-one adult participants (33 men) with obesity were included in the present analysis, with an average age of 45.4 ± 8.2 y and an average BMI of 34.6 ± 3.4.
TABLE 1.
Baseline characteristics of the study participants (N = 71)
| Characteristic | Value |
|---|---|
| Age, y | 45.4 ± 8.2 |
| Males, n (%) | 33 (47) |
| Anthropometrics | |
| BMI, kg/m2 | 34.6 ± 3.4 |
| Weight, kg | 104.0 ± 14.6 |
| Height, cm | 173.1 ± 8.9 |
| Fat mass, kg | 43.3 ± 9.1 |
| Fat mass, % | 41.7 ± 6.4 |
| Fat free mass, kg | 60.9 ± 10.9 |
| Fat free mass, % | 58.4 ± 6.4 |
Values are means ± SDs unless otherwise indicated.
Anthropometrics and RMR data, at baseline, W9, and W13, in all participants can be seen in Table 2. Average weight loss at W9 was 14.1 ± 0.4 kg (13.2% ± 2.8%), followed by maintenance between W9 and W13 (0.09 ± 0.22kg, P = 0.999). FM and FFM (kg) were significantly reduced at W9 and W13, compared with baseline (P < 0.001 for all comparisons), but a significant reduction in FM and a significant increase in FFM (kg) was seen between W9 and W13 (P < 0.001 for both). BIA data showed a significant reduction in ICW from baseline to W9 (P < 0.001), which returned to baseline at W13 (P = 0.126). RMRm was significantly lower than RMRp at both W9 and W13, resulting in a metabolic adaptation of −92 ± 110 (P < 0.001) and −38 ± 124 kcal/d (P = 0.011), respectively. A significant reduction in metabolic adaptation was seen from W9 to W13 (−53 ± 101 kcal/d, P < 0.001). In a subset of participants who gained weight between W9 and W13 (n = 33), RMRm−RMRp was −3.3 ± 119 kcal/d (P = 0.874) at baseline, −90.0 ± 94.5 kcal/d (P < 0.001) at W9, −26.8 ± 121.5 kcal/d (P = 0.214) at W13, and 6.4 ± 97.8 kcal/d (P = 0.769) at 1Y.
TABLE 2.
Anthropometrics and RMR at baseline, week 9, and week 13 in all participants (n = 71)
| P value | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Baseline | Week 9 | Week 13 | Baseline vs. week 9 | Baseline vs. week 13 | Week 9 vs. week 13 |
| Weight, kg | 104.0 ± 14.6 | 90.1 ± 11.6 | 90.0 ± 11.8 | <0.001 | <0.001 | 0.999 |
| FM, kg | 43.3 ± 9.1 | 32.4 ± 8.6 | 31.4 ± 8.4 | <0.001 | <0.001 | <0.001 |
| FFM, kg | 60.0 ± 10.9 | 57.6 ± 9.9 | 58.3 ± 10.1 | <0.001 | <0.001 | <0.001 |
| ICW, L | 28.4 ± 5.9 | 27.2 ± 5.2 | 27.9 ± 9 | <0.001 | 0.791 | 0.126 |
| RMRm, kcal/d | 1856 ± 249 | 1654 ± 204 | 1715 ± 238 | <0.001 | <0.001 | <0.001 |
| RMRp, kcal/d | 1856 ± 221 | 1746 ± 193 | 1754 ± 197 | <0.001 | <0.001 | 0.005 |
| RMRm−p, kcal/d | −0.01 ± 113 | −92 ± 110*** | −38 ± 124* | |||
Data presented as means ± SDs. Changes over time assessed with repeated-measures ANOVA, followed by post hoc comparisons between time points with Bonferroni adjustment. Asterisks denote significant differences between measured and predicted RMR at specific time points by paired sample t tests: *P = 0.011 and ***P < 0.001. FM, fat mass; FFM, fat-free mass; ICW, intracellular water; RMR, resting metabolic rate; RMRm, resting metabolic rate measured; RMRm−p, RMR measured minus RMR predicted; RMRp, resting metabolic rate predicted.
Anthropometrics and RMR data over time in a subgroup with data at all points (including 1Y) (n = 45, 33 males) can be seen in Table 3. Average weight loss in this subsample at W9 was 14.4 ± 0.6 kg (P < 0.001), followed by maintenance between W9 and W13 (−0.01 ± 0.3 kg, P = 0.999) and regain between W13 and 1Y (4.1 ± 1.2 kg, P < 0.001). Average weight regain at 1Y was 29.1% ± 52.1%. There was a significant metabolic adaptation at both W9 (−107 ± 102 kcal/d, P < 0.001) and W13 (−49 ± 128 kcal/d, P = 0.013), despite a significant reduction in metabolic adaptation between W9 and W13 (−57 ± 93 kcal/d, P < 0.001). No metabolic adaptation was seen at 1Y follow-up. Despite no significant differences in body weight between W9 and W13, at group level, there was a very large interindividual variation (range: −4.0 to +4.4 kg).
TABLE 3.
Anthropometrics and RMR data at all time points (baseline, week 9, week 13, and 1 y) in a subgroup of participants (n = 45)
| P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Baseline | Week 9 | Week 13 | 1 y | Baseline vs. week 9 | Baseline vs. week 13 | Baseline vs. 1 y | Week 9 vs. week 13 | Week 13 vs. 1 y |
| Weight, kg | 105.1 ± 14.0 | 90.7 ± 11.4 | 90.7 ± 11.5 | 94.8 ± 15.5 | <0.001 | <0.001 | <0.001 | 0.999 | 0.005 |
| FM, kg | 42.6 ± 9.1 | 31.4 ± 8.6 | 30.6 ± 8.3 | 35.4 ± 10.6 | <0.001 | <0.001 | <0.001 | 0.006 | <0.001 |
| FFM, kg | 62.7 ± 10.8 | 59.2 ± 9.8 | 60.1 ± 10.1 | 59.4 ± 11.5 | <0.001 | <0.001 | <0.001 | 0.001 | 0.999 |
| RMRm, kcal/d | 1884 ± 253 | 1665 ± 211 | 1732 ± 242 | 1790 ± 228 | <0.001 | <0.001 | <0.001 | <0.001 | 0.037 |
| RMRp, kcal/d | 1888 ± 216 | 1773 ± 190 | 1781 ± 195 | 1798 ± 224 | <0.001 | <0.001 | <0.001 | 0.088 | 0.999 |
| RMRm−p, kcal/d | −4 ± 122 | −107 ± 102*** | −49 ± 128* | −7 ± 129 | |||||
Data presented as means ± SDs. Changes over time assessed with repeated-measures ANOVA, followed by post hoc comparisons between time points with Bonferroni adjustment. Asterisks denote significant differences between measured and predicted RMR at specific timepoints by paired sample t tests: *P = 0.013 and ***P < 0.001. FM, fat mass; FFM, fat-free mass; ICW, intracellular water; RMR, resting metabolic rate; RMRm, resting metabolic rate measured; RMRm−p, RMR measured minus RMR predicted; RMRp, resting metabolic rate predicted.
Changes in body composition (FM and FFM) over time using data from BIA were of similar magnitude and significance as the changes previously reported based on ADP (data not shown).
Metabolic adaptation at W9 or W13 was not correlated with weight regain at 1Y follow-up (r = 0.034, P = 0.824, n = 45 and r = 0.106, P = 0.488, n = 45, respectively).
Changes in PA over time can be seen in Table 4. A significant overall effect of time was seen for steps/d (P = 0.006); sedentary (P < 0.001), light (P < 0.001), moderate (P = 0.13), and vigorous to very vigorous (P = 0.001) PA; and total PA duration (P = 0.001). A significant increase in steps/d was seen at 1Y compared with baseline (P = 0.03). Time spent on sedentary PA was significantly lower than baseline at week 8 (W8) (P < 0.001). Time spent on light PA was significantly higher than baseline at W8, week 12 (W12), and 1Y (P < 0.001 for all). No significant changes from baseline were seen for time spent on moderate PA. A significant increase in time spent on vigorous to very vigorous PA was seen at 1Y compared with baseline (P = 0.025). Time spent on total PA was significantly higher than baseline at W8, W12, and 1Y (P = 0.007, P < 0.001, and P < 0.001, respectively).
TABLE 4.
Physical activity levels over time (N = 71)
| Characteristic | Baseline | Week 4 | Week 8 | Week 12 | 1 y | P value |
|---|---|---|---|---|---|---|
| Steps/d | 6556 ± 285 | 6548 ± 286 | 6627 ± 287 | 7156 ± 295 | 7519 ± 340* | 0.006 |
| Sedentary time, min/d | 1184 ± 19 | 1177 ± 19 | 1071 ± 19*** | 1130 ± 20 | 1127 ± 24 | <0.001 |
| Light PA, min/d | 188 ± 7 | 202 ± 8 | 221 ± 7*** | 227 ± 8*** | 229 ± 9*** | <0.001 |
| Moderate PA, min/d | 55 ± 5 | 46 ± 5 | 54 ± 5 | 60 ± 5 | 59 ± 6 | 0.013 |
| Vigorous to very vigorous PA, min/d | 0.9 ± 0.4 | 0.5 ± 0.4 | 1.0 ± 0.4 | 1.8 ± 0.4 | 2.7 ± 0.5* | 0.001 |
| Total PA, min/d | 244 ± 10 | 249 ± 10 | 276 ± 10** | 289 ± 10*** | 290 ± 11*** | 0.001 |
Data presented as means ± SEMs. Changes over time assessed with linear mixed model, followed by post hoc comparisons between time points with Bonferroni adjustment. Asterisks denote significant differences from baseline: *P < 0.05 and ***P < 0.001. PA, physical activity.
Discussion
The present article examined if metabolic adaptation, at the level of RMR, was modulated by the energy balance status of the participants by measuring EE immediately after weight loss (under negative EB) and after 4 wk of weight stability. After a 14-kg (13%) weight loss, a metabolic adaptation of ∼90 kcal/d below predicted levels was found at W9, when participants were in negative EB, which then was significantly reduced to less than half (−38 kcal/d) after 4 wk of weight stabilization. Similar results were found when a subset of participants with data at all time points was analyzed, with a metabolic adaptation of ∼110 kcal/d immediately after weight loss at W9, which was then halved after 4 wk of weight stabilization at W13 (−49 kcal/d) and disappeared at 1Y follow-up.
We confirmed our hypothesis that metabolic adaptation is significantly reduced (in fact halved) when measurements are performed after weight stabilization, in comparison with immediately after weight loss (under negative EB). This reinforces previous research by our group showing a metabolic adaptation of ∼50 kcal/d after a 16% weight loss in women with overweight when measurements were done after a 4-wk weight stabilization phase (21).
Two reasons may explain why metabolic adaptation was still present after 4 wk of weight stability. The first is that 4 wk of weight stabilization may not be enough for metabolic adaptation to disappear. The second, and likely more plausible explanation, is that our participants, despite being weight stable, were probably in negative EB when measurements were performed at W13. Weight loss in the present study was induced by 1000-kcal/d diets with a CHO content of 70, 100, or 130 g/d. Analysis of β-hydroxybutyric acid plasma concentrations (a marker of ketosis) at W9 showed that participants were ketotic at W9 (0.76 ± 0.51 mmol/L), regardless of the diet, but not at W13 (0.11 ± 0.1 mmol/L). Ketosis is accompanied by glycogen depletion and with it water loss, while refeeding is followed by glycogen replenishment and with it increased water content. It has been estimated that glycogen stores are on average 400–500 g (29, 30), with 3–4 g of water bound to each gram of glycogen (29). This means that an increase in body weight between 1.6 and 2.5 kg, due to increased water content, should be expected when participants came out of ketosis (30). Even though we were unable to directly quantify changes in glycogen storage over time in the present study, data from BIA showed a significant reduction in ICW at W9 (P < 0.001), which returned to baseline values at W13. It has previously been shown that changes in ICW derived from BIA can be used as a proxy of changes in glycogen content due to CHO loading (26). This strongly suggests that our participants were in negative EB at W13, which may explain why despite a halving in metabolic adaptation from W9 to W13, metabolic adaptation was still present at W13. The fact that RMRm−RMRp was not significant in a subgroup of participants who gained weight between W9 and W13 adds further evidence to the fact that the residual metabolic adaptation seen at W13 in all participants is due to the fact that the participants were in negative EB at that time point and that if participants are in EB, no metabolic adaptation should be expected after weight loss.
Leibel et al. (1) reported in their 1995 landmark paper that the maintenance of a 10% weight loss (8 wk of a 800-kcal/d diet, followed by 2 wk of weight stabilization), in individuals with obesity, was followed by a reduction in RMR below predicted levels (metabolic adaptation) of 137 ± 305 kcal/d. This value is much higher (almost 3 times larger) than the metabolic adaptation reported in the present analysis after a 13% weight loss followed by 4 wk of weight stabilization. A potential reason for this discrepancy could be related to the length of the stabilization period, which was only 2 wk in Leibel et al. (1), while in the present analysis, it was 4 wk. Leibel et al. (1) also measured RMR immediately after weight loss and reported values to be significantly lower than after the 2-wk weight stabilization period (1598 ± 385 compared with 1747 ± 416 kcal/d, respectively, P = 0.043), but the difference between measured and predicted RMR was not reported. Even though predicted RMR can be presumed not to have changed significantly during weight stability, there was an average 1.6-kg weight gain over the 2-wk stabilization period. Moreover, it needs to be emphasized that the data from Leibel et al. (1) previously reported are derived from only 9 individuals with obesity, while the present analysis reflects changes in RMR in 71 individuals.
The findings of this study add to previous evidence from cross-sectional studies showing no metabolic adaptation at the level of RMR when weight-stable individuals with obesity who have lost weight are compared with never-obese BMI-matched controls (7–10) and reinforce the contention that metabolic adaptation in longitudinal studies (1, 4–6) is likely a result of measurements taken under negative EB. Importantly, and adding to previous evidence (4, 21), metabolic adaptation after weight loss (either at W9 or W13) was not correlated with weight regain at 1Y. If metabolic adaptation was part of a compensatory response that tries to bring body weight back to its original state and, therefore, a driver of weight regain, it would be expected that metabolic adaptation would be present after weight loss, regardless of the EB status of the participants, and that a larger metabolic adaptation would be associated with more weight regain in the long term. The present analysis refutes both of those premises.
It needs to be acknowledged that RMR contributes to only ∼60% of total energy expenditure in individuals with obesity (12). Metabolic adaptation could, therefore, also be present at the level of nonresting energy expenditure (NREE) due, supposedly, to increased exercise efficiency. Even though Leibel et al. (1) have reported that metabolic adaptation after a 10% weight loss, followed by 2 wk of weight stability, was associated with marked metabolic adaptation, particularly at the level of NREE (1), the study suffers from several methodologic limitations, some of them already previously highlighted. This includes a very small sample size and weight gain during the weight stabilization period. In line with the evidence previously discussed for RMR, it seems that the existence of metabolic adaptation at the level of NREE after weight loss is also likely to be modulated by the EB status of the individuals being measured. As such, in overweight premenopausal women who had lost 10–12 kg, no metabolic adaptation was found in NREE when measurements were done in controlled conditions of weight stability (31–34). More important, we could not identify a single study reporting increased exercise efficiency after weight loss to be associated with long-term weight regain. In reality, the opposite might be true, as improved locomotion economy/efficiency following exercise training has been shown to be associated with increased ease of locomotion (35–38), which in turn has been found to be associated with increased participation in free living physical activity and reduced weight regain (39–43).
Despite the potential minor role of metabolic adaptation as a driver of weight regain, the present findings have important clinical relevance and might explain why some individuals with obesity might experience resistance to further weight loss. If a larger than expected reduction in RMR offsets the prescribed energy restriction, no further weight loss will occur. In fact, there is a widespread dogma among dietitians that individuals with obesity who report not being able to lose further weight on a LED might continue losing weight under the same dietary prescription after a short period of overfeeding used to “switch off” metabolic adaptation. This fits well with the results described in the present article, by which metabolic adaptation was halved after 4 wk of weight stabilization and absent in those who did not lose weight between W9 and W13.
Our study has both strengths and limitations. The main strength is its design, with data collected immediately after weight loss, as well as after a 4-wk weight stabilization period. This allowed us to determine the role of weight stability and EB in modulating metabolic adaptation. Second, gold-standard methods were used for the measurements of RMR (indirect calorimetry) and body composition (BodPod). Third, it includes a heterogeneous sample of both males and females with obesity, with a wide range of BMI (30–43) and age (26–62 y), which is important for generalization purposes. However, we did not directly measure changes in glycogen storage over time and, as such, were not able to identify with certainty which participants were or were not in EB at W13. Nevertheless, ICW from BIA provided us with an indirect measure of glycogen and, as such, of EB status of the participants at W13.
In conclusion, metabolic adaptation at the level of RMR is reduced (halved) to ∼50 kcal/d when measurements are taken under conditions of weight stability compared with immediately after weight loss and is not sustained in the long term with weight regain. Moreover, metabolic adaptation does not predict relapse in the long term. Further research needs to address alternative mechanistic pathways that might contribute to relapse in obesity treatment.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows––CM, JR, and SS: conceived and designed the study; JR and SS: collected the data; CM: performed the statistical analysis; CM, JR, SS, BAG, and GRH: wrote the manuscript; CM: had primary responsibility for final content; and all authors: assisted with data interpretation and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Funding: This study was funded by the Norwegian University of Science and Technology (NTNU) (doctoral grant) and the Liaison Committee for Education, Research, and Innovation in Central Norway in partnership with NTNU (running costs). CM was supported by a sabbatical grant by the Liaison Committee for Education, Research, and Innovation in Central Norway and the NTNU.
Data sharing: Data described in the manuscript will be made available upon request pending approval by the local ethics committee.
Abbreviations used: ADP, air displacement plethysmography; BIA, bioelectric impedance analysis; CHO, carbohydrate; EB, energy balance; EE, energy expenditure; FM, fat mass; FFM, fat-free mass; ICW, intracellular water; LED, low-energy diet; MET, metabolic equivalent of task; NREE, nonresting energy expenditure; PA, physical activity; PA, physical activity level; RMR, resting metabolic rate; RMRm, resting metabolic rate measured; RMRp, resting metabolic rate predicted; W, week; Y, year.
Contributor Information
Catia Martins, Obesity Research Group, Department of Clinical and Molecular Medicine, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology (NTNU), Trondheim, Norway; Centre for Obesity and Innovation (ObeCe), Clinic of Surgery, St. Olav University Hospital, Trondheim, Norway; Department of Nutrition Sciences, University of Alabama, Birmingham, AL, USA.
Jessica Roekenes, Obesity Research Group, Department of Clinical and Molecular Medicine, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology (NTNU), Trondheim, Norway.
Saideh Salamati, Centre for Obesity and Innovation (ObeCe), Clinic of Surgery, St. Olav University Hospital, Trondheim, Norway.
Barbara A Gower, Department of Nutrition Sciences, University of Alabama, Birmingham, AL, USA.
Gary R Hunter, Department of Nutrition Sciences, University of Alabama, Birmingham, AL, USA.
References
- 1. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–8. [DOI] [PubMed] [Google Scholar]
- 2. Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88(4):906–12. [DOI] [PubMed] [Google Scholar]
- 3. Froidevaux F, Schutz Y, Christin L, Jequier E. Energy expenditure in obese women before and during weight loss, after refeeding, and in the weight-relapse period. Am J Clin Nutr. 1993;57(1):35–42. [DOI] [PubMed] [Google Scholar]
- 4. Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, Chen KY, Skarulis MC, Walter M, Walter PJ et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity. 2016;24(8):1612–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Camps SG, Verhoef SP, Westerterp KR. Weight loss, weight maintenance, and adaptive thermogenesis. Am J Clin Nutr. 2013;97(5):990–4. [DOI] [PubMed] [Google Scholar]
- 6. Johannsen DL, Knuth ND, Huizenga R, Rood JC, Ravussin E, Hall KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab. 2012;97(7):2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weinsier RL, Nagy TR, Hunter GR, Darnell BE, Hensrud DD, Weiss HL. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am J Clin Nutr. 2000;72(5):1088–94. [DOI] [PubMed] [Google Scholar]
- 8. Weinsier RL, Hunter GR, Zuckerman PA, Darnell BE. Low resting and sleeping energy expenditure and fat use do not contribute to obesity in women. Obes Res. 2003;11(8):937–44. [DOI] [PubMed] [Google Scholar]
- 9. Wyatt HR, Grunwald GK, Seagle HM, Klem ML, McGuire MT, Wing RR, Hill JO. Resting energy expenditure in reduced-obese subjects in the National Weight Control Registry. Am J Clin Nutr. 1999;69(6):1189–93. [DOI] [PubMed] [Google Scholar]
- 10. Larson DE, Ferraro RT, Robertson DS, Ravus E. Energy metabolism in weight-stable postobese individuals. Am J Clin Nutr. 1995;62:735–9. [DOI] [PubMed] [Google Scholar]
- 11. Ostendorf DM, Melanson EL, Caldwell AE, Creasy SA, Pan Z, MacLean PS, Wyatt HR, Hill JO, Catenacci VA. No consistent evidence of a disproportionately low resting energy expenditure in long-term successful weight-loss maintainers. Am J Clin Nutr. 2018;108(4):658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amatruda JM, Statt MC, Welle SL. Total and resting energy expenditure in obese women reduced to ideal body weight. J Clin Invest. 1993;92(3):1236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dulloo AG, Jacquet J, Montani JP, Schutz Y. Adaptive thermogenesis in human body weight regulation: more of a concept than a measurable entity?. Obes Rev. 2012;13(Suppl 2):105–21. [DOI] [PubMed] [Google Scholar]
- 14. Dulloo AG, Schutz Y. Adaptive thermogenesis in resistance to obesity therapies: issues in quantifying thrifty energy expenditure phenotypes in humans. Curr Obes Rep. 2015;4(2):230–40. [DOI] [PubMed] [Google Scholar]
- 15. Celi FS, Le TN, Ni B. Physiology and relevance of human adaptive thermogenesis response. Trends Endocrinol Metab. 2015;26(5):238–47. [DOI] [PubMed] [Google Scholar]
- 16. Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes. 2010;34(Suppl 1):S47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Major GC, Doucet E, Trayhurn P, Astrup A, Tremblay A. Clinical significance of adaptive thermogenesis. Int J Obes. 2007;31(2):204–12. [DOI] [PubMed] [Google Scholar]
- 18. Flatt JP. Exaggerated claim about adaptive thermogenesis. Int J Obes. 2007;31(10):1626; author reply 7–8. [DOI] [PubMed] [Google Scholar]
- 19. Kuchnia A, Huizenga R, Frankenfield D, Matthie JR, Earthman CP. Overstated metabolic adaptation after "The Biggest Loser" intervention. Obesity. 2016;24(10):2025. [DOI] [PubMed] [Google Scholar]
- 20. Larson DE, Ferraro RT, Robertson DS, Ravussin E. Energy metabolism in weight-stable postobese individuals. Am J Clin Nutr. 1995;62(4):735–9. [DOI] [PubMed] [Google Scholar]
- 21. Martins C, Gower BA, Hill JO, Hunter GR. Metabolic adaptation is not a major barrier to weight loss maintenance. Am J Clin Nutr. [epub ahead of print 9 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henry CJ, Lightowler HJ, Marchini J. Intra-individual variation in resting metabolic rate during the menstrual cycle. Br J Nutr. 2003;89(6):811–17. [DOI] [PubMed] [Google Scholar]
- 23. Brennan IM, Feltrin KL, Nair NS, Hausken T, Little TJ, Gentilcore D, Wishart JM, Jones KL, Horowitz M, Feinle-Bisset C. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am J Physiol Gastrointest Liver Physiol. 2009;297(3):G602–10. [DOI] [PubMed] [Google Scholar]
- 24. Curtis V, Henry CJ, Ghusain-Choueiri A. Basal metabolic rate of women on the contraceptive pill. Eur J Clin Nutr. 1996;50(5):319–22. [PubMed] [Google Scholar]
- 25. Nordic Council of Ministers Nordic nutrition recommendations. 5th ed Copenhagen: Narayana Press; 2012. [Google Scholar]
- 26. Shiose K, Yamada Y, Motonaga K, Sagayama H, Higaki Y, Tanaka H, Takahashi H. Segmental extracellular and intracellular water distribution and muscle glycogen after 72-h carbohydrate loading using spectroscopic techniques. J Appl Physiol. 2016;121(1):205–11. [DOI] [PubMed] [Google Scholar]
- 27. Compher C, Frankenfield D, Keim N, Roth-Yousey L. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106(6):881–903. [DOI] [PubMed] [Google Scholar]
- 28. Scheers T, Philippaerts R, Lefevre J. Patterns of physical activity and sedentary behavior in normal-weight, overweight and obese adults, as measured with a portable armband device and an electronic diary. Clin Nutr. 2012;31(5):756–64. [DOI] [PubMed] [Google Scholar]
- 29. Olsson KE, Saltin B. Variation in total body water with muscle glycogen changes in man. Acta Physiol Scand. 1970;80(1):11–18. [DOI] [PubMed] [Google Scholar]
- 30. Kreitzman SN, Coxon AY, Szaz KF. Glycogen storage: illusions of easy weight loss, excessive weight regain, and distortions in estimates of body composition. Am J Clin Nutr. 1992;56:292S–3S. [DOI] [PubMed] [Google Scholar]
- 31. Weinsier RL, Hunter GR, Zuckerman PA, Redden DT, Darnell BE, Larson DE, Newcomer BR, Goran MI. Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr. 2000;71(5):1138–46. [DOI] [PubMed] [Google Scholar]
- 32. Weinsier RL, Hunter GR, Desmond RA, Byrne NM, Zuckerman PA, Darnell BE. Free-living activity energy expenditure in women successful and unsuccessful at maintaining a normal body weight. Am J Clin Nutr. 2002;75(3):499–504. [DOI] [PubMed] [Google Scholar]
- 33. Newcomer BR, Larson-Meyer DE, Hunter GR, Weinsier RL. Skeletal muscle metabolism in overweight and post-overweight women: an isometric exercise study using (31)P magnetic resonance spectroscopy. Int J Obes. 2001;25(9):1309–15. [DOI] [PubMed] [Google Scholar]
- 34. Weinsier RL, Hunter GR, Schutz Y, Zuckerman PA, Darnell BE. Physical activity in free-living, overweight white and black women: divergent responses by race to diet-induced weight loss. Am J Clin Nutr. 2002;76(4):736–42. [DOI] [PubMed] [Google Scholar]
- 35. Borges JH, Carter SJ, Singh H, Hunter GR. Inverse relationship between changes of maximal aerobic capacity and changes in walking economy after weight loss. Eur J Appl Physiol. 2018;118(8):1573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carter SJ, Rogers LQ, Bowles HR, Norian LA, Hunter GR. Inverse association between changes in energetic cost of walking and vertical accelerations in non-metastatic breast cancer survivors. Eur J Appl Physiol. 2019;119(11–12):2547–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hunter GR, McCarthy JP, Bryan DR, Zuckerman PA, Bamman MM, Byrne NM. Increased strength and decreased flexibility are related to reduced oxygen cost of walking. Eur J Appl Physiol. 2008;104(5):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fisher G, McCarthy JP, Zuckerman PA, Bryan DR, Bickel CS, Hunter GR. Frequency of combined resistance and aerobic training in older women. J Strength Cond Res. 2013;27(7):1868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hunter GR, Byrne NM. Physical activity and muscle function but not resting energy expenditure impact on weight gain. J Strength Cond Res. 2005;19(1):225–30. [DOI] [PubMed] [Google Scholar]
- 40. Larew K, Hunter GR, Larson-Meyer DE, Newcomer BR, McCarthy JP, Weinsier RL. Muscle metabolic function, exercise performance, and weight gain. Med Sci Sports Exerc. 2003;35(2):230–6. [DOI] [PubMed] [Google Scholar]
- 41. Carter SJ, Hunter GR, Norian LA, Turan B, Rogers LQ. Ease of walking associates with greater free-living physical activity and reduced depressive symptomology in breast cancer survivors: pilot randomized trial. Support Care Cancer. 2018;26(5):1675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brock DW, Chandler-Laney PC, Alvarez JA, Gower BA, Gaesser GA, Hunter GR. Perception of exercise difficulty predicts weight regain in formerly overweight women. Obesity (Silver Spring). 2010;18(5):982–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hunter GR, Weinsier RL, Zuckerman PA, Darnell BE. Aerobic fitness, physiologic difficulty and physical activity in black and white women. Int J Obes. 2004;28(9):1111–17. [DOI] [PubMed] [Google Scholar]