ABSTRACT
Background
Despite the rising popularity of plant-based alternative meats, there is limited evidence of the health effects of these products.
Objectives
We aimed to compare the effect of consuming plant-based alternative meat (Plant) as opposed to animal meat (Animal) on health factors. The primary outcome was fasting serum trimethylamine-N-oxide (TMAO). Secondary outcomes included fasting insulin-like growth factor 1, lipids, glucose, insulin, blood pressure, and weight.
Methods
SWAP-MEAT (The Study With Appetizing Plantfood—Meat Eating Alternatives Trial) was a single-site, randomized crossover trial with no washout period. Participants received Plant and Animal products, dietary counseling, lab assessments, microbiome assessments (16S), and anthropometric measurements. Participants were instructed to consume ≥2 servings/d of Plant compared with Animal for 8 wk each, while keeping all other foods and beverages as similar as possible between the 2 phases.
Results
The 36 participants who provided complete data for both crossover phases included 67% women, were 69% Caucasian, had a mean ± SD age 50 ± 14 y, and BMI 28 ± 5 kg/m2. Mean ± SD servings per day were not different by intervention sequence: 2.5 ± 0.6 compared with 2.6 ± 0.7 for Plant and Animal, respectively (P = 0.76). Mean ± SEM TMAO concentrations were significantly lower overall for Plant (2.7 ± 0.3) than for Animal (4.7 ± 0.9) (P = 0.012), but a significant order effect was observed (P = 0.023). TMAO concentrations were significantly lower for Plant among the n = 18 who received Plant second (2.9 ± 0.4 compared with 6.4 ± 1.5, Plant compared with Animal, P = 0.007), but not for the n = 18 who received Plant first (2.5 ± 0.4 compared with 3.0 ± 0.6, Plant compared with Animal, P = 0.23). Exploratory analyses of the microbiome failed to reveal possible responder compared with nonresponder factors. Mean ± SEM LDL-cholesterol concentrations (109.9 ± 4.5 compared with 120.7 ± 4.5 mg/dL, P = 0.002) and weight (78.7 ± 3.0 compared with 79.6 ± 3.0 kg, P < 0.001) were lower during the Plant phase.
Conclusions
Among generally healthy adults, contrasting Plant with Animal intake, while keeping all other dietary components similar, the Plant products improved several cardiovascular disease risk factors, including TMAO; there were no adverse effects on risk factors from the Plant products.
This trial was registered at clinicaltrials.gov as NCT03718988.
Keywords: diet, meat, plant-based alternative meat, randomized controlled trial, cardiovascular disease risk factors, trimethylamine-N-oxide
See corresponding editorial on page 1151.
Introduction
Shifts to more plant-based diets, with fewer animal-based foods, have been widely recommended for health and environmental benefits (1–3). Yet changing dietary behaviors remains challenging owing to strongly held taste preferences, culinary traditions, and social and cultural norms (4). Although plant-based alternative meats (plant-meats)—i.e., vegetarian products designed to resemble the taste and appearance of traditional burgers, sausages, or other meats—have been available for years, their consumer popularity has increased rapidly in recent years (5). In North America, plant-meat sales grew by 37% from 2017 to 2019 (6). This rapid rise in popularity is due in part to producers better simulating the taste of animal-meat products, as well as increased marketing directed toward meat-eating consumers, rather than just vegetarians (5, 6).
The rapid increase in plant-meat consumption has raised scrutiny and criticism (7), namely the question of the overall health effects of plant-meat products compared with animal meat (8). Most plant-meat products meet the NOVA criteria for “ultra-processed foods” (9). A recent study found that ultra-processed food intake led to increased energy intake and weight gain relative to whole foods (10). Compared with fresh, minimally processed foods, many plant-meats are relatively high in saturated fat and sodium, which are metabolically linked to hypercholesterolemia and hypertension (11, 12). Notably, plant-meats also contain fiber, which is absent in animal meats, has been proven to lower LDL cholesterol (13), and is associated with reduced risk of cardiovascular disease (CVD) and obesity (14). There is a paucity of data on the net impact of differing amounts of saturated fat, sodium, and fiber on health factors for direct comparisons of plant with animal meat.
Two emerging risk factors for CVD and certain cancers include trimethylamine-N-oxide (TMAO) and insulin-like growth factor 1 (IGF-1). Animal-based foods, particularly red meat (e.g., beef and pork), have a relatively high content of carnitine and choline, which are precursors to TMAO (15). Recent trials have reported that red meat intake raises TMAO blood concentrations (15–17). In addition, vegans and vegetarians have been reported to have lower TMAO and IGF-1 than meat eaters (17–22). Some studies have suggested animal-meat consumption is associated with greater IGF-1 concentrations and may increase the risk of prostate and breast cancers (21, 22).
The objective of this randomized crossover trial [SWAP-MEAT (The Study With Appetizing Plantfood—Meat Eating Alternatives Trial)] was to compare the effects of consuming plant-based alternative meat (hereafter Plant) with those of consuming animal meat (hereafter Animal), primarily beef and pork, on emerging health risk factors, cardiometabolic risk factors, and the gut microbiome among generally healthy adults. The primary study outcome was differences in serum TMAO after 8 wk of Plant compared with Animal. Secondary outcomes were differences in fasting plasma IGF-1 concentrations, metabolic markers (blood lipids, glucose, and insulin), blood pressure, weight, and microbiota composition (Shannon diversity).
Methods
Procedures for this study were followed in accordance with the ethical standards from the Helsinki Declaration and were approved by the Stanford University Human Subjects Committee (Institutional Review Board). All study participants provided written informed consent.
Study design
SWAP-MEAT was a single-site, randomized crossover trial (NCT03718988) among adults assigned to 1 of 2 sequences: 8 wk of Plant followed by 8 wk of Animal, or vice versa. With the exception of the Plant compared with Animal exchange, participants were instructed to keep all other dietary habits similar across the phases. At the end of the first 8-wk phase, participants were encouraged to move directly into the second phase, without a washout period. However, a brief break between phases was allowed if participant scheduling conflicts would have otherwise prevented second-phase participation. Participant enrollment began on 5 December, 2018 and continued through 9 July, 2019. The date of final follow-up data collection was 5 December, 2019.
Participants
The target population was generally healthy omnivorous adults ≥18 y of age who reported typically consuming ≥1 serving of meat per day and were willing to consume ∼2 servings/d of both Plant and Animal. They were recruited from available e-mail lists among the Stanford community. Participants were required to have a stable dietary history, defined as neither introducing nor eliminating a major food group in their diet for at least the previous month.
Exclusion criteria were weighing <110 lbs (50 kg); having a BMI (in kg/m2) >40; LDL cholesterol >190 mg/dL; systolic blood pressure >160 mm Hg or diastolic blood pressure >90 mm Hg; clinically significant or unstable pulmonary, cardiovascular, gastrointestinal, hepatic, or renal functional abnormality; or pregnancy. Individuals were excluded or had their study start date delayed if within the past 2 mo they had taken systemic antibiotics, antifungals, antivirals or antiparasitics, corticosteroids, cytokines, methotrexate, or immunosuppressive cytotoxic agents known to affect the microbiome.
Randomization
Randomization to 1 of the 2 diet sequences (Plant→Animal or Animal→Plant) was performed in block sizes of 4 by an independent statistician. Pairs (spousal or parent–child) were randomly assigned in block sizes of 2. Participants did not learn of their diet sequence until they had completed all baseline measures and surveys. Laboratory technicians and study staff conducting the blood and stool analyses were blinded to the diet sequence.
Intervention
The study consisted of 2 phases, each lasting 8 wk: Plant and Animal. Participants were instructed to consume ≥2 servings of the phase-congruent type of meat product per day and instructed to match all non-study-provided foods as closely as possible between the 2 phases. Specific instructions included tracking items such as the types of burger buns and the garnishes and condiments used with burger items, and being consistent with these choices for both the Plant and Animal patties in the 2 phases. Eight weeks on each dietary phase was selected on the basis of previous dietary interventions that reported significant changes within just 4 wk for most of our primary and secondary outcomes (other than weight) (15, 23, 24). Participants were instructed to exclude other sources of plant-meat alternatives (i.e., tofu and tempeh) in both phases. Fish products were excluded in the Plant phase. However, to promote recruitment, a modest compromise was made to allow ≤8 oz (227 g) fish/wk in the Animal phase with the qualification that no fish was allowed in the 48 h before a study blood draw (17). All Plant products were supplied by Beyond Meat and distributed on-site at the research facility. All Animal products were supplied by a San Francisco–based organic foods delivery service; the red meat sources were grass-fed. The cut of ground beef purchased was “regular” (i.e., 80% lean, 20% fat), which is the type of ground beef most commonly purchased by US consumers (25). Notably, the saturated fat content of the animal-meat burgers was 9 g/burger compared with 6 g/burger for the Beyond Meat product. Participants purchased all other items and ingredients for their own meals and were encouraged to prepare meals themselves. They were allowed to eat out occasionally provided they followed study requirements. Tables 1 and 2 provide the study products’ nutrient profiles and ingredients. Supplemental Table 1 provides a weekly order overview.
TABLE 1.
Nutrient profiles for Plant and Animal products1
| Product | Serving size | Kcals | Carbohydrates, g | Protein, g | Total fat, g | Saturated fat, g | Fiber, g | Sodium, mg |
|---|---|---|---|---|---|---|---|---|
| Plant products | ||||||||
| Burger | 4 oz (113 g) | 250 | 3 | 20 | 18 | 6 | 2 | 390 |
| Beef crumbles | 1/2 cup (55 g) | 90 | 3 | 12 | 3 | <1 | 1 | 240 |
| Breakfast sausage | 1 patty (65 g) | 170 | 2 | 13 | 13 | 5 | 1 | 330 |
| Hot Italian sausage | 1 cooked link (76 g) | 190 | 5 | 16 | 12 | 5 | 3 | 500 |
| Brat sausage | 1 cooked link (76 g) | 190 | 5 | 16 | 12 | 5 | 3 | 500 |
| Grilled chicken strips | 3 oz (85 g) | 130 | 6 | 22 | 2 | 0 | 3 | 360 |
| Lightly seasoned chicken strips | 3 oz (85 g) | 130 | 5 | 20 | 3.5 | 0 | 3 | 340 |
| Animal products | ||||||||
| Burger | 3.6 oz (100 g) | 293 | 0 | 16 | 25 | 9 | 0 | 672 |
| Ground beef | 3.6 oz (100 g) | 293 | 0 | 16 | 25 | 9 | 0 | 672 |
| Good Morning pork breakfast sausage | 1 link (47 g) | 110 | 0 | 7 | 9 | 3 | 0 | 320 |
| Hot Italian sausage | 1 link (71 g) | 170 | 1 | 10 | 14 | 5 | 0 | 480 |
| Pork bratwurst | 1 link (57 g) | 230 | 4 | 8 | 21 | 9 | 0 | 400 |
| Chicken breast | 4 oz (113 g) | 140 | 0 | 26 | 3 | 0.5 | 0 | 1402 |
Some substitutions (≤10% of products) were necessary in the Animal phase because of limited availability; however, neither the amount, quality, nor the meat were altered. Animal, animal meat; Plant, plant-based alternative meat.
For raw meat products (beef and chicken), sodium and other seasonings were added by participants.
TABLE 2.
Ingredients of Plant and Animal products1
| Plant products | Animal products | ||
|---|---|---|---|
| Product | Ingredients | Product | Ingredients |
| Burger | Water, pea protein isolate, expeller-pressed canola oil, refined coconut oil, rice protein, natural flavors, cocoa butter, mung bean protein, methylcellulose, potato starch, apple extract, pomegranate extract, salt, potassium chloride, vinegar, lemon juice concentrate, sunflower lecithin, beet juice extract, carrot | Burger | Beef (seasonings added by participants) |
| Beef crumbles | Water, pea protein isolate, canola and sunflower oil, rice flour, spice, tomato powder, yeast extract, sugar, potassium chloride, tapioca maltodextrin, citric acid, salt, acacia gum, onion extract, natural flavor, garlic extract | Ground beef | Beef (seasonings added by participants) |
| Breakfast sausage | Water, pea protein isolate, expeller-pressed canola oil, refined coconut oil, natural flavors, rice protein, methylcellulose, sunflower protein, mung bean protein, nutritional yeast (dried yeast, niacin, pyridoxine hydrochloride, thiamin hydrochloride, riboflavin, folic acid, cyanocobalamin), apple extract, salt, vinegar, lemon juice concentrate, sunflower lecithin | Good Morning pork breakfast sausage | Pork, water, sea salt, organic herbs, spices |
| Hot Italian sausage | Water, pea protein isolate, refined coconut oil, sunflower oil, natural flavor, rice protein, fava bean protein, potato starch, salt, fruit juice, vegetable juice, apple fiber, methylcellulose, citrus extract, calcium alginate casing | Hot Italian sausage | Pork, water, organic spices, organic chili pepper, sea salt, organic evaporated cane syrup, organic garlic, organic paprika |
| Brat sausage | Water, pea protein isolate, refined coconut oil, sunflower oil, natural flavor, rice protein, fava bean protein, potato starch, salt, fruit juice, vegetable juice, apple fiber, methylcellulose, citrus extract, calcium alginate casing | Pork bratwurst | Pork shoulder, pork fatback, salt, milk powder, white and black pepper, ginger, mustard powder, nutmeg |
| Grilled chicken strips | Water, soy protein isolate, pea protein isolate, rice flour, canola and sunflower oil, soy fiber, yeast extract, carrot fiber, maltodextrin, natural flavor, spices, distilled vinegar, titanium dioxide, salt, sugar, molasses powder, potassium chloride, paprika | Chicken breast | Chicken (seasonings added by participants) |
| Lightly seasoned chicken strips | Water, soy protein isolate, pea protein isolate, rice flour, canola and sunflower oil, soy fiber, yeast extract, carrot fiber, maltodextrin, natural flavor, spices, distilled vinegar, titanium dioxide, salt, sugar, molasses powder, potassium chloride, paprika | ||
Animal, animal meat; Plant, plant-based alternative meat.
Measures
Self-reported sociodemographic data on age, gender, race/ethnicity, marital status, education, and employment status were collected during the enrollment phase.
Diet data
Three types of dietary data were collected. During eligibility screening, all potential participants completed a brief questionnaire about current habitual meat intake. During the study, participants logged all of their food intake for 3 d (2 weekdays and 1 weekend day) biweekly from week 0 to 16 using Cronometer (Cronometer Pro, Nutrition Tracking Software for Professionals; https://cronometer.com/pro). In addition, 2 unannounced 24-h multiple-pass diet recall interviews were administered by a dietitian at baseline and at the end of each 8-wk phase using Nutrition Data System for Research (NDS-R) (Nutrition Coordinating Center, University of Minnesota) (26).
Adherence to the protocol for consuming ≥2 servings/d was determined from a composite score with a 50:50 weighting of Cronometer data and the biweekly survey that asked how many servings were consumed that week.
Metabolic and anthropometric data
Fasting blood concentrations for TMAO, IGF-1, lipids, glucose, and insulin were determined from samples collected at baseline and weeks 2, 4, 8 (phase 1), 10, 12, and 16 (phase 2). TMAO was measured by LC with tandem MS (Cleveland HeartLab) (27). IGF-1, glucose, insulin, and blood lipid concentrations were analyzed by standard methodologies, all at the Core Laboratory for Clinical Studies (Washington University, St Louis, MO) (28–33). Height, body weight, and blood pressure data were collected at Stanford's Clinical and Translational Research Unit during clinic visits.
Physical activity
Participants’ physical activity was assessed at baseline and at 4 and 8 wk of each phase with the International Physical Activity Questionnaire short form (34).
Microbiome assessment
A detailed methodology for stool sample collection and 16S analysis is provided in the Supplemental Methods.
Process measures and poststudy diet preferences
A 5-point Likert scale was used to assess food satisfaction with both Plant and Animal products biweekly throughout the study. Gastrointestinal symptoms were assessed at baseline and biweekly using the Gastrointestinal Symptoms Rating Scale (35, 36).
Statistical analysis
Participant baseline characteristics are presented as means ± SDs or percentages. Nutrient comparisons between Plant and Animal phases were performed using NDS-R diet assessment data and paired t tests. Adherence rates for Plant compared with Animal during the 8-wk phases were contrasted using combined diet data from Cronometer and the biweekly self-reported data on daily study product servings consumed (paired t tests). For our primary outcome, we used a linear mixed-effects model to investigate if the change in TMAO values from baseline at the end of each phase was significantly different for Plant compared with Animal (“meat type”), adjusting for the fixed effect of diet order (e.g., study arm), phase, and the random effect of correlated observations for each participant from the 2 phases. The primary analysis was a complete case analysis and used participants’ last available laboratory values in each phase. Participants who did not complete both phases (i.e., crossover) were excluded from the primary analysis and accounted for in exploratory analysis. To investigate no difference in the change of TMAO values between diet types, a 2-sided likelihood ratio test was used. We set a significance level of 0.05 for all analyses. Data were analyzed in R version 3.5.1 (R Foundation for Statistical Computing; 2 July, 2018). The primary packages used for modeling were “lme4” and “lmerTest” (37–39).
Similarly, for our secondary outcomes, we used separate mixed-effects models to evaluate fasting plasma IGF-1, lipids, insulin, glucose, blood pressure, and weight for Plant compared with Animal, adjusting for the order of diet and repeated measures. A 2-sided likelihood ratio test was used to assess no difference between the diet types.
Sample size determination was based on available resources, rather than a formal power calculation. We performed an ad hoc power analysis for a paired t test, which assumed no order effect for the primary analysis aforementioned. With 38 participants, the trial had 80% power to detect a −2.5 mean difference in TMAO values for Plant compared with Animal, assuming an SD of 5.4 at a 5% significance level (15).
Results
Demographics
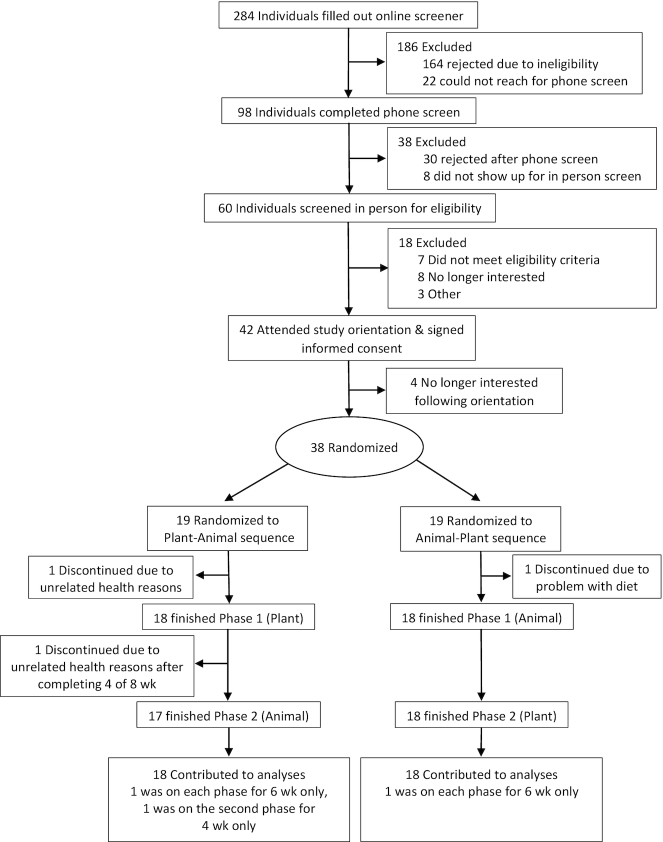
Table 3 presents baseline demographic, anthropometric, and metabolic characteristics. Of the 284 potential participants that completed an initial online screener for the study, 38 participants were randomly assigned to the intervention arms, including 2 pairs of same-household participants. Thirty-six participated in both phases of the intervention (Figure 1). One of the originally randomly assigned 38 participants dropped out owing to a lack of satisfaction with study products. Two of the remaining 37 participants dropped out mid-study owing to unrelated health issues; however, 1 provided complete data through the middle of the second phase, which was used as the end-phase endpoint for that 1 participant for the second phase, resulting in n = 36 for complete analyses. The participants were mostly female (67%), Caucasian (69%), and college educated (83%). BMI ranged from 18 to 39 kg/m2; age ranged from 21 to 75 y.
TABLE 3.
Study participants’ baseline sociodemographic, anthropometric, and metabolic characteristics1
| Plant–Animal | Animal–Plant | All | |
|---|---|---|---|
| Gender, n | |||
| Female | 15 | 9 | 24 |
| Male | 3 | 9 | 12 |
| Age, y | 49.3 ± 11.7 | 51.1 ± 16.0 | 50.2 ± 13.8 |
| Highest level of education achieved, n | |||
| Less than high school | 0 | 1 | 1 |
| High school degree | 1 | 0 | 1 |
| Some college | 0 | 4 | 4 |
| College degree | 7 | 7 | 14 |
| Some postgraduate school | 2 | 1 | 3 |
| Postgraduate degree | 8 | 5 | 13 |
| Race/ethnicity, n | |||
| Non-Hispanic white | 11 | 14 | 25 |
| Hispanic/Latinx | 3 | 0 | 3 |
| Asian | 3 | 2 | 5 |
| Black/African American | 1 | 0 | 1 |
| Other | 0 | 2 | 2 |
| Weight, kg | |||
| Women | 73.7 ± 16.8 | 71.5 ± 14.1 | 72.8 ± 15.6 |
| Men | 80.9 ± 7.0 | 93.2 ± 18.6 | 88.7 ± 17.1 |
| Both sexes | 74.9 ± 15.7 | 82.3 ± 19.5 | 78.0 ± 17.6 |
| BMI, kg/m2 | |||
| Women | 27.7 ± 5.7 | 27.0 ± 4.3 | 27.4 ± 5.1 |
| Men | 26.2 ± 2.2 | 29.7 ± 6.0 | 28.8 ± 5.4 |
| Both sexes | 27.4 ± 5.2 | 28.3 ± 5.3 | 27.9 ± 5.2 |
| Blood pressure, mm Hg | |||
| Systolic | 112 ± 13 | 116 ± 10 | 114 ± 11 |
| Diastolic | 67 ± 7 | 71 ± 9 | 69 ± 8 |
| Blood lipids, mg/dL | |||
| Total cholesterol | 212 ± 37 | 191 ± 41 | 201 ± 40 |
| HDL cholesterol | 60 ± 12 | 59 ± 15 | 60 ± 13 |
| LDL cholesterol | 130 ± 32 | 113 ± 35 | 122 ± 34 |
| Triglycerides | 107 ± 44 | 92 ± 32 | 100 ± 39 |
| Fasting glucose, mg/dL | 94 ± 7 | 99 ± 9 | 96 ± 8 |
| Fasting insulin, µIU/mL | 8.3 ± 5.3 | 9.4 ± 5.8 | 8.8 ± 5.5 |
| TMAO concentrations, µM | 3.5 ± 1.8 | 3.4 ± 2.1 | 3.4 ± 1.4 |
| IGF-1 concentrations, ng/mL | 154.6 ± 49.5 | 153.6 ± 62.9 | 154.1 ± 55.8 |
| Physical activity, metabolic equivalent minutes per week | 2578.1 ± 2120.3 | 4342.7 ± 3412.5 | 3460.4 ± 2939.6 |
Values are ns or means ± SDs. Animal, animal meat; IGF-1, insulin-like growth factor 1; Plant, plant-based alternative meat; TMAO, trimethylamine-N-oxide.
FIGURE 1.
Participant flowchart. Animal, animal meat; Plant, plant-based alternative meat.
Diet
For both treatment orders, servings per day over the 8-wk phases were similar (mean ± SD: 2.5 ± 0.6 and 2.6 ± 0.7 for Plant and Animal, respectively, P = 0.76).
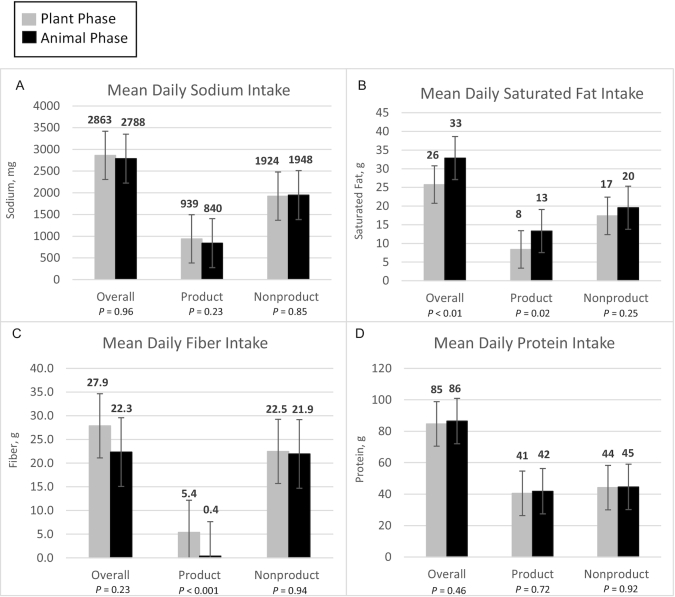
Figure 2 presents diet data for sodium, saturated fat, fiber, and protein, with a breakdown of plant compared with animal protein in Supplemental Figure 1. A more extensive profile of nutrient intake including vitamins, minerals, and carbohydrates as determined by NDS-R (2 d at the end of each phase) is provided in Supplemental Table 2, and as determined by Cronometer (≥3 d every other week) in Supplemental Table 3.
FIGURE 2.
Nutrient data for means of sodium (A), saturated fat (B), fiber (C), and protein (D) consumed daily between the 2 diet phases (n = 36). Product indicates the nutrients from only Plant or Animal, whereas Nonproduct indicates the nutrients from all other sources. Together they add up to the overall amounts of nutrients. Paired t tests were conducted for energy intake and each nutrient to assess for any differences between the Plant and Animal phases. Data are based on estimates determined using NDS-R. Animal, animal meat; NDS-R, Nutrition Data System for Research; Plant, plant-based alternative meat.
In Figure 2 selected nutrient intakes are presented in 3 categories: 1) overall (total), and then separately for 2) amounts contributed by study products being provided to participants, and 3) all other nonproducts that participants chose themselves. As expected from product nutrient profiles (Table 1) and the similar servings consumed, the Plant products were higher in fiber and plant protein, similar in sodium and total protein, and lower in saturated fat compared with the Animal products (Table 1, Figure 2). Energy intake was similar for Plant and Animal overall, and for products and nonproducts (Supplemental Figure 2). For nonproducts, across all 4 nutrients, the reported intake was similar for Plant and Animal. With 1 exception—fiber—the total intake contrasts mirrored those of the product differences: higher in plant protein; similar in calories, sodium, and total protein; and lower in saturated fat for Plant compared with Animal. For fiber, despite being significantly different in intake from products, the absolute mean difference of >5 g total fiber (27.9 compared with 22.3 g) for Plant compared with Animal was not statistically significant. As is evident in Figure 2, nutrient intakes in nonproducts were similar, and thus the differences in total intake, when present, were due primarily to the differences in the Plant and Animal products. The nutrient intakes were similar by order (Supplemental Figure 2).
Physical activity
No significant differences in physical activity levels were observed between Plant and Animal phases (Supplemental Table 4).
Primary and secondary outcomes
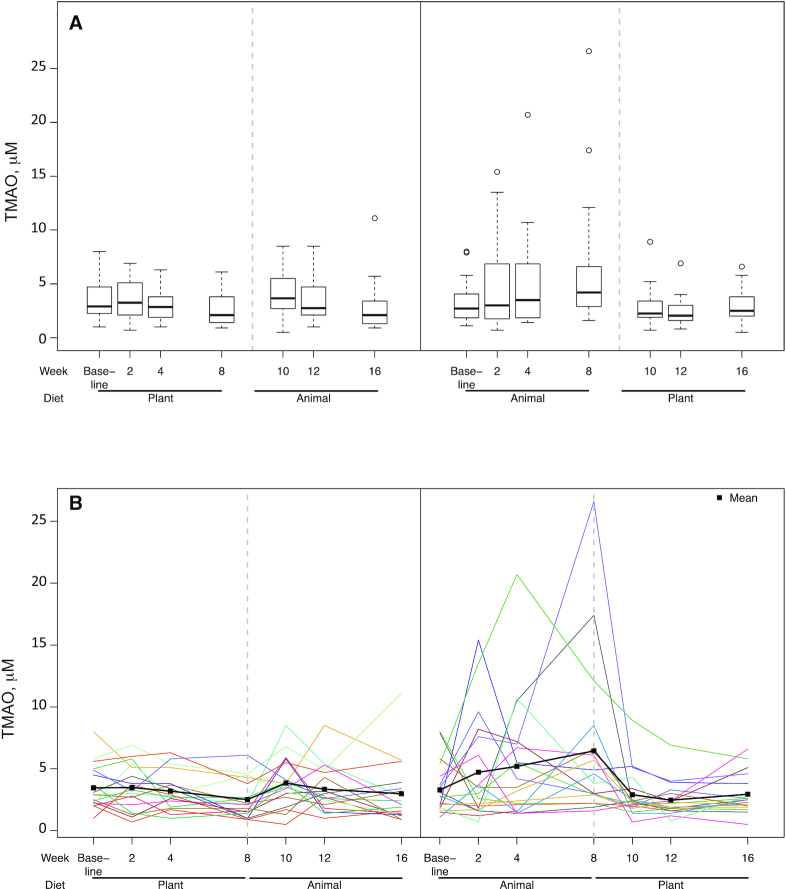
Overall, the difference in TMAO after 8 wk of Plant compared with Animal was statistically significant (P = 0.02) (Table 4). However, as Figure 3 presents, a significant order effect was observed. For the n = 18 assigned to receive the Plant first, mean ± SEM TMAO concentrations were not significantly different at weeks 8 and 16 (the end of the 8-wk intervention phases) (2.5 ± 0.4 and 3.0 ± 0.6 µM, respectively, P = 0.28, Wilcoxon test). For the n = 18 receiving Animal first, TMAO concentrations were significantly lower for the Plant than for the Animal phase (2.9 ± 0.4 and 6.4 ± 1.5 µM, respectively, P = 0.007).
TABLE 4.
Outcome levels at the end of the 8-wk phases1
| Outcome | Plant, mean ± SEM | Animal, mean ± SEM | Plant–Animal difference, mean (95% CI) | P value2 |
|---|---|---|---|---|
| Primary | ||||
| TMAO,3 µM | 2.7 ± 0.3 | 4.7 ± 0.9 | −2.0 (−3.6, −0.3) | 0.012 |
| Secondary | ||||
| IGF-1, ng/mL | 147.6 ± 7.5 | 152.3 ± 8.3 | −4.7 (−13.9, 4.5) | 0.30 |
| Weight, kg | 78.7 ± 3.0 | 79.6 ± 3.0 | −1.0 (−1.5, −0.5) | <0.001 |
| Insulin, µIU/mL | 9.2 ± 1.1 | 8.8 ± 0.9 | 0.4 (−0.7, 1.5) | 0.38 |
| Glucose, mg/dL | 94.9 ± 1.6 | 94.5 ± 1.4 | 0.5 (−1.8, 2.8) | 0.65 |
| Lipids, mg/dL | ||||
| LDL-C | 109.9 ± 4.5 | 120.7 ± 4.5 | −10.8 (−17.3, −4.3) | 0.002 |
| HDL-C | 62.5 ± 2.2 | 61.8 ± 2.5 | 0.7 (−2.4, 3.8) | 0.66 |
| Triglycerides | 99.7 ± 7.3 | 100.2 ± 7.0 | −0.6 (−10.5, 9.2) | 0.89 |
| Blood pressure, mm Hg | ||||
| Systolic | 114.5 ± 2.1 | 113.1 ± 1.9 | 1.2 (−1.4, 4.1) | 0.31 |
| Diastolic | 70.0 ± 1.4 | 68.8 ± 1.2 | 1.1 (−0.8, 3.2) | 0.20 |
n = 36. Animal, animal meat; HDL-C, HDL cholesterol; IGF-1, insulin-like growth factor 1; LDL-C, LDL cholesterol; Plant, plant-based alternative meat; TMAO, trimethylamine-N-oxide.
Likelihood ratio test from mixed-effects model evaluating change from baseline for each product type (Plant compared with Animal), adjusting for order and phase.
Significant order effect in model (P = 0.023).
FIGURE 3.
TMAO concentrations at 7 time points (baseline and biweekly during the 16-wk protocol) by randomization order: Plant→Animal (n = 18) and Animal→Plant (n = 18). (A) Boxplots with medians, IQRs, 5th and 95th percentiles, and extreme values. (B) Spaghetti plots of each participant's data across the full protocol; means are represented by filled squares. Significant differences in TMAO concentrations after 8 wk of Plant compared with Animal were observed (P = 0.01). However, a significant order effect was observed (P = 0.02). For participants assigned to the Plant group first, TMAO concentrations were not significantly different at weeks 8 and 16 (mean ± SEM: 2.5 ± 0.4 and 3.0 ± 0.6 µM, respectively, P = 0.28, Wilcoxon test). For participants assigned to the Animal group first, TMAO concentrations were significantly lower during the Plant than during the Animal phase (mean ± SEM: 2.9 ± 0.4 and 6.4 ± 1.5 µM, respectively, P = 0.007). Animal, animal meat; Plant, plant-based alternative meat; TMAO, trimethylamine-N-oxide.
LDL cholesterol and weight were significantly lower for the Plant than for the Animal phase. Fasting concentrations of IGF-1, insulin, glucose, HDL cholesterol, and triglycerides, and blood pressure, were not significantly different between the Plant and Animal phases. No order effects were observed among secondary outcomes. Supplemental Table 5 provides outcome data by sequence order.
Microbiome
Analysis of stool 16S rRNA profiles did not reveal associations of overall gut microbiota composition with diets, diet order, or TMAO production. Some specific taxa were associated with dietary interventions, but none were associated with TMAO production. Consistent with the gut microbiome being highly individualized, the vast majority of variance in the 16S rRNA profiles was derived from participants (80.1%). It is possible that given a larger study cohort or function-focused analysis of the microbiome, differences between diets, order effects, or individualized TMAO-production associations could be identified. The Supplemental Results and Supplemental Figures 3 and 4 present an extended results description and figures related to gut microbiota profiling.
Product satisfaction and gastrointestinal issues
Satisfaction with products was generally high. Most products scored a mean of ≥3.5 on a 5-point rating scale (5 being highest). Four of the 6 matched products (e.g., Plant compared with Animal patties) scored almost identically; 2 of the 6 Plant products scored <3.0 on average, and lower than their matched Animal products (Supplemental Table 6).
Participants reported negligible study changes for most of the 13 gastrointestinal symptoms queried for both product types. The most notable changes, although modest, were increases in abnormal abdominal distention in both phases, and a decrease in daily bowel movements during the Animal phase (Supplemental Table 7).
Discussion
This randomized crossover-design trial compared the effect of consuming Plant as opposed to Animal products on emerging health risk factors, cardiometabolic risk factors, and the gut microbiome in a population of generally healthy adults after 8 wk of consuming ≥2 servings/d of each product type. TMAO, the primary outcome, improved significantly in the plant-based phase relative to the Animal phase. However, an intervention order effect was identified. Participants who consumed Plant first had TMAO concentrations that were not significantly different from Animal at the end of that phase; the significantly higher TMAO concentrations after the Animal phase were observed only among the participants who consumed Animal first and Plant second. In addition, LDL-cholesterol concentrations and body weight were lower in the Plant phase.
Consistent with our findings, several studies have previously reported that red meat intake raises TMAO concentrations (15–17). Our finding of an order effect is particularly interesting. Among the group getting Animal first and Plant second, the observed mean increase in TMAO in the Animal phase decreased back to baseline concentrations within 2 wk during the Plant phase, and remained stable through 8 wk, suggesting no carryover effect. In stark contrast, those assigned to the Plant phase first had no mean increase in TMAO, and no apparent effect to carry over, and yet this group was observed to have no mean increase in TMAO when shifted to the Animal phase. Although some may take issue with the lack of a washout period in the study design, in this case it appears the absence of a washout may have been important in revealing the significant order effect. Koeth et al. (40) investigated the effect on TMAO of feeding l-carnitine, abundant in red meat, to vegetarians. They reported no increase in TMAO, speculating that this may be due to their “vegetarian” microbiomes. This finding suggests that those in the current study who were assigned to Plant first, and were therefore following a vegetarian diet for 8 wk, may have differentially altered their microbiomes compared with those who consumed Animal first, in such a way as to prevent the production of TMAO when the Animal phase came second. Although the 16S microbiome analysis conducted in the current study was unable to identify these changes, it is difficult to know if the findings reflect a true null effect. The data suggest that the plant-first group may have suppressed trimethylamine (TMA)-producing taxa leading to less TMAO in the meat-second phase (TMA is a precursor to TMAO). However, this is merely a hypothesis that requires in-depth analysis to investigate fully. Therefore, it is possible that even if there is an effect on TMA production by the dietary interventions, it is not detectable by 16S analysis (e.g., if the taxa that underlie TMA production differ between individuals). It is possible that such changes are only detectable with additional in-depth microbiome investigation focused on genes and encoded functions relevant to TMAO generation. Although we expect that differences in fiber content may influence the microbiota, how such changes would interact with TMA production is currently unclear. We also have observed in another intervention focused on fiber, currently in preparation, that the effect of fiber on the microbiota over a similar duration is individualized and more modest than anticipated (Carter MM, Topf MA, Wastyk HC, Sonnenburg ED, Sonnenburg JL and Gardner CD, unpublished results, 2020). Future studies of potential microbiome changes that could be determined by metagenomic or other function-targeted analyses are warranted.
We also observed considerable variability among participants in TMAO concentration changes; 2 of the 18 participants in the Animal→Plant group had particularly large excursions of TMAO during the Animal phase, whereas others had very little changes from baseline. We assessed baseline red meat consumption, baseline TMAO concentrations, and adherence as possible explanations for these response differences, but were unable to identify meaningful differences between the responders and the nonresponders.
Although plant-based meat products could support individuals in adhering to national and global health recommendations to reduce red meat consumption (3, 41–43), concerns have been raised about their overall health effects (5). Some of these products fall under the NOVA category of ultra-processed food, which have been reported to lead to weight gain compared with whole foods (9, 10). However, the finding in the current study was that weight was modestly but statistically significantly lower after 8 wk on the Plant than on the Animal phase. Notably, this was observed despite no differences in reported total energy intake or physical activity levels between each phase.
The other cardiometabolic benefit for the Plant phase was lower concentrations of LDL cholesterol. This was consistent with the lower saturated fat and higher fiber and plant protein during the Plant phase, all of which have been established to lower LDL cholesterol (11, 14, 44). The observed lack of differences in blood pressure between the Plant and Animal phases was consistent with similar sodium intakes during the 2 study phases, which were similar in terms of products, nonproducts, and total sodium (12). It has been reported that IGF-1 concentrations are higher in vegetarians than omnivores, but no IGF-1 differences were observed in the current study (21, 22). In addition, no differences were observed in other secondary outcomes, although there were no specific a priori hypotheses as to why glucose, insulin, HDL cholesterol, or triglycerides might be differentially affected by the Plant and Animal phases—the balances of carbohydrates and fats were relatively similar overall.
Beyond its potential benefits for human health, others have advocated for the transition to more plant-based diets because of the broader environmental, animal, and societal benefits of displacing red meat (2, 5, 45–48). Plant-based meats may play an important role in facilitating a global shift toward a more sustainable food system based on more plant-based and fewer animal-based foods (45). Although this study did not assess these impacts, Eshel et al. (47) found that by replacing meat with protein-conserving plant alternatives, Americans could satisfy key nutritional requirements while eliminating pastureland use and reducing cropland, nitrogen fertilizer usage, and greenhouse gas emissions. In addition, Gardner et al. (2) reported significant reductions in greenhouse gasses when modeling a 25% shift from animal to plant protein. Notably, both the Plant and Animal products in the current study accounted for ∼25% of total calories and half of total protein.
This study included several strengths. First was the crossover design that allowed each participant to serve as their own control. Second were various aspects of internal validity, including high participant retention and minimal missing data. A third strength was a high treatment fidelity supported by providing Plant and Animal products, and the high and comparable participant satisfaction with the 2 types of products. A fourth strength was the collection of multiple sources of dietary intake data: NDS-R, Cronometer, and food survey data. Finally, a database lock, predetermined database quality check, and third-party data analysis reduced bias.
This study's potential limitations include its crossover design without a second baseline measurement and the allowance for some participants to take a break between the 2 phases (12 of the 36 participants took a 1- to 7-wk break). However, a close examination of the data from those individuals suggested no apparent pattern of differences from the 24 participants that transitioned without a break, and the primary testing between phases remained focused on contrasting the end of each 8-wk phase. The potential issues with the lack of washout period—both limitations and opportunities—were described previously. Another limitation may have been the inclusion and allowance of chicken and fish in the study, because both of these have different effects on TMAO than red meat, making the Plant/Animal contrast less controlled. However, only small amounts of chicken or fish were consumed by participants, fish was not allowed within 48 h of a blood sampling which should have eliminated its effect on serum TMAO concentrations, and the allowance of these small amounts facilitated recruitment and retention. Whereas the plant-meat and animal-meat products were provided, the remainder of the diet was self-selected, thus limiting the ability to control the intake of other foods and nutrients. Although this limited the rigor of dietary control, it increased the generalizability of the findings. Finally, this study used 1 of the many different types of plant-meat formulations, and 1 set of matched meat products; results could have differed for a different type of plant-based meat and for other cuts of meat. For example, the “regular” ground beef used in the study was 80:20 (lean:fat), with 9 g saturated fat/burger compared with 6 g saturated fat/burger for the comparable Beyond Meat products. Other options for ground beef include lean (85:15) and extra lean (90:10), both with lower saturated fat content. Of the different lean:fat types, regular is the most commonly consumed type in the United States (25).
In light of growing, consistent recommendations to reduce red meat intake for optimal cardiovascular health, plant-based meats offer a potentially healthy alternative (5, 48). Notably, their growing popularity among consumers has been coupled with rising critiques of their ultra-processed composition and potential adverse health consequences. Until now, there has been a paucity of data upon which to evaluate these claims. This study found several beneficial effects and no adverse effects from the consumption of plant-based meats. The interesting order effect observed for TMAO reinforces prior findings about the microbiome's potential personalization effects, and warrants further study.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge many individuals that contributed meaningfully to the study. Jennifer Robinson oversaw the project and staff. Carrie McKinley, Lindsey Durand, Tania Davila, and Diane Demis conducted the diet assessments. Alexandra Garrity assisted with data collection and study intervention materials. Dalia Perelman, Heyjun Park, and Claire Bladier provided feedback and critical review of the manuscript.
The authors’ responsibilities were as follows—CDG: designed the research project and contributed to writing both the initial and final drafts of the manuscript; AC: wrote the initial and final drafts of the manuscript; SS, CP, TS, KC, JL, PF-S, MMC, MAT, HCW, EDS, and JLS: provided feedback and critical revisions of the manuscript; KC and JL: conducted the statistical analyses; and all authors: read and approved the final manuscript. CDG received funding for the study from Beyond Meat in the form of an unrestricted research gift made to Stanford University. All other authors report no conflicts of interest.
Notes
Supported by a research gift from Beyond Meat Inc. (to CDG), National Heart, Lung, and Blood Institute at the NIH grant T32HL007034 (to CDG), and Stanford Clinical and Translational Science Award to Spectrum NIH UL1 TR001085 (to CDG).
Funding for this study was provided by Beyond Meat. In an effort to reduce any influences on the outcomes of this study, a statistical analysis plan was submitted to ct.gov. The main analysis was conducted by a third-party individual who had no involvement with the study design or collection of data, and was blinded to all study participants.
Supplemental Tables 1–7, Supplemental Figures 1–4, Supplemental Methods, and Supplemental Results are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article, code book, and analytic code will be made available upon request pending application and approval by the corresponding author.
Abbreviations used: Animal, animal meat; CVD, cardiovascular disease; CVDRFs, Cardiovascular Disease Risk Factors; IGF-1, insulin-like growth factor 1; NDS-R, Nutrition Data System for Research; Plant, plant-based alternative meat; SWAP-MEAT, The Study With Appetizing Plantfood—Meat Eating Alternatives Trial; TMA, trimethylamine; TMAO, trimethylamine-N-oxide.
Contributor Information
Anthony Crimarco, Stanford Prevention Research Center, Stanford University School of Medicine, Stanford, CA, USA.
Sparkle Springfield, Stanford Prevention Research Center, Stanford University School of Medicine, Stanford, CA, USA.
Christina Petlura, Stanford Prevention Research Center, Stanford University School of Medicine, Stanford, CA, USA.
Taylor Streaty, Stanford Prevention Research Center, Stanford University School of Medicine, Stanford, CA, USA.
Kristen Cunanan, Quantitative Sciences Unit, Stanford University School of Medicine, Stanford, CA, USA.
Justin Lee, Quantitative Sciences Unit, Stanford University School of Medicine, Stanford, CA, USA.
Priya Fielding-Singh, Stanford Prevention Research Center, Stanford University School of Medicine, Stanford, CA, USA.
Matthew M Carter, Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA.
Madeline A Topf, Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA.
Hannah C Wastyk, Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA; Department of Bioengineering, Stanford University School of Medicine, Stanford, CA, USA.
Erica D Sonnenburg, Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA; Center for Human Microbiome Studies, Stanford University, Stanford, CA, USA.
Justin L Sonnenburg, Microbiology and Immunology, Stanford University School of Medicine, Stanford, CA, USA; Center for Human Microbiome Studies, Stanford University, Stanford, CA, USA; Chan Zuckerberg Biohub, San Francisco, CA, USA.
Christopher D Gardner, Stanford Prevention Research Center, Stanford University School of Medicine, Stanford, CA, USA.
References
- 1. Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, Clinton S, Hu F, Nelson M, Neuhouser ML et al. The 2015 Dietary Guidelines Advisory Committee scientific report: development and major conclusions. Adv Nutr. 2016;7(3):438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gardner CD, Hartle JC, Garrett RD, Offringa LC, Wasserman AS. Maximizing the intersection of human health and the health of the environment with regard to the amount and type of protein produced and consumed in the United States. Nutr Rev. 2019;77(4):197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. US Department of Health Human Services (DHHS) and USDA 2015–2020 Dietary Guidelines for Americans. [Internet] 8th ed Washington (DC): US DHHS and USDA; 2015; [cited 30 April, 2020] Available from: https://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 4. Sanchez-Sabate R, Sabaté J. Consumer attitudes towards environmental concerns of meat consumption: a systematic review. Int J Environ Res Public Health. 2019;16(7):1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu FB, Otis BO, McCarthy G. Can plant-based meat alternatives be part of a healthy and sustainable diet?. JAMA. 2019;322(16):1547–8. [DOI] [PubMed] [Google Scholar]
- 6. Olayanju JB. Plant-based meat alternatives: perspectives on consumer demands and future directions. [Internet] Jersey City, NJ: Forbes; 2019; [cited 22 September, 2019] Available from: https://www.forbes.com/sites/juliabolayanju/2019/07/30/plant-based-meat-alternatives-perspectives-on-consumer-demands-and-future-directions/#2873b2136daa. [Google Scholar]
- 7. Sweeney E. Are beyond meat and impossible burgers better for you? Nutritionists weigh in. [Internet] New York: HuffPost; 2019; [cited 22 September, 2019] Available from: https://www.huffpost.com/entry/beyond-meat-impossible-burger-healthy_l_5d164ad1e4b07f6ca57cc3ed. [Google Scholar]
- 8. Hemler EC, Hu FB. Plant-based diets for cardiovascular disease prevention: all plant foods are not created equal. Curr Atheroscler Rep. 2019;21(5):18. [DOI] [PubMed] [Google Scholar]
- 9. Monteiro CA, Cannon G, Moubarac J-C, Levy RB, Louzada MLC, Jaime PC. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, Chung ST, Costa E, Courville A, Darcey V et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010;7(3):e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cogswell ME, Mugavero K, Bowman BA, Frieden TR. Dietary sodium and cardiovascular disease risk—measurement matters. N Engl J Med. 2016;375(6):580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anderson JW, Baird P, Davis RH, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fiber. Nutr Rev. 2009;67(4):188–205. [DOI] [PubMed] [Google Scholar]
- 14. Veronese N, Solmi M, Caruso MG, Giannelli G, Osella AR, Evangelou E, Maggi S, Fontana L, Stubbs B, Tzoulaki I. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. Am J Clin Nutr. 2018;107(3):436–44. [DOI] [PubMed] [Google Scholar]
- 15. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40(7):583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park JE, Miller M, Rhyne J, Wang Z, Hazen SL. Differential effect of short-term popular diets on TMAO and other cardio-metabolic risk markers. Nutr Metab Cardiovasc Dis. 2019;29(5):513–17. [DOI] [PubMed] [Google Scholar]
- 17. Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, Sutter JL, Caudill MA. Trimethylamine‐N‐oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017;61(1):1600324. [DOI] [PubMed] [Google Scholar]
- 18. Djuric Z, Mitchell CM, Davy KP, Neilson AP. A Mediterranean diet does not alter plasma trimethylamine N-oxide concentrations in healthy adults at risk for colon cancer. Food Funct. 2019;10(4):2138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Randrianarisoa E, Lehn-Stefan A, Wang X, Hoene M, Peter A, Heinzmann SS, Zhao X, Königsrainer I, Königsrainer A, Balletshofer B et al. Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci Rep. 2016;6:26745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–28. [DOI] [PubMed] [Google Scholar]
- 21. Allen NE, Appleby PN, Davey GK, Kaaks R, Rinaldi S, Key TJ. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1441–8. [PubMed] [Google Scholar]
- 22. Allen N, Appleby P, Davey G, Key T. Hormones and diet: low insulin-like growth factor-I but normal bioavailable androgens in vegan men. Br J Cancer. 2000;83(1):95–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gardner CD, Coulston A, Chatterjee L, Rigby A, Spiller G, Farquhar JW. The effect of a plant-based diet on plasma lipids in hypercholesterolemic adults: a randomized trial. Ann Intern Med. 2005;142(9):725–33. [DOI] [PubMed] [Google Scholar]
- 24. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. [DOI] [PubMed] [Google Scholar]
- 25. Krebs A. Popular and versatile – ground beef reigns!. [Internet] Boca Raton, FL: PerishableNews.com; 2019; [cited 20 June, 2020] Available from: https://www.perishablenews.com/meatpoultry/popular-and-versatile-ground-beef-reigns/. [Google Scholar]
- 26. Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30(1):47–57. [DOI] [PubMed] [Google Scholar]
- 27. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bystrom C, Sheng S, Zhang K, Caulfield M, Clarke NJ, Reitz R. Clinical utility of insulin-like growth factor 1 and 2; determination by high resolution mass spectrometry. PLoS One. 2012;7(9):e43457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peterson JI, Young DS. Evaluation of the hexokinase/glucose-6-phosphate dehydrogenase method of determination of glucose in urine. Anal Biochem. 1968;23(2):301–16. [DOI] [PubMed] [Google Scholar]
- 30. Morgan CR, Lazarow A. Immunoassay of insulin: two antibody system: plasma insulin levels of normal, subdiabetic and diabetic rats. Diabetes. 1963;12(2):115–26. [Google Scholar]
- 31. Rambaldi DC, Reschiglian P, Zattoni A, Johann C. Enzymatic determination of cholesterol and triglycerides in serum lipoprotein profiles by asymmetrical flow field-flow fractionation with on-line, dual detection. Anal Chim Acta. 2009;654(1):64–70. [DOI] [PubMed] [Google Scholar]
- 32. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 33. Miller WG, Myers GL, Sakurabayashi I, Bachmann LM, Caudill SP, Dziekonski A, Edwards S, Kimberly MM, Korzun WJ, Leary ET et al. Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures. Clin Chem. 2010;56(6):977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. [DOI] [PubMed] [Google Scholar]
- 35. Svedlund J, Sjödin I, Dotevall G. GSRS—a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Digest Dis Sci. 1988;33(2):129–34. [DOI] [PubMed] [Google Scholar]
- 36. Winham DM, Hutchins AM. Perceptions of flatulence from bean consumption among adults in 3 feeding studies. Nutr J. 2011;10(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. R Development Core Team R: a language and environment for statistical computing. [Internet] Vienna, Austria: R Foundation for Statistical Computing; 2020; [cited 18 May, 2020] Available from: https://www.R-project.org/. [Google Scholar]
- 38. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Soft. 2015;67(1):1–48. [Google Scholar]
- 39. Kuznetsova A, Brockhoff PB, Christensen RH. lmerTest package: tests in linear mixed effects models. J Stat Soft. 2017;82(13):1–26. [Google Scholar]
- 40. Koeth RA, Lam-Galvez BR, Kirsop J, Wang Z, Levison BS, Gu X, Copeland MF, Bartlett D, Cody DB, Dai HJ et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Invest. 2018;129(1):373–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Miller NH, Hubbard VS, Lee I-M, Lichtenstein AH, Loria CM, Millen BE et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Part B):2960–84. [DOI] [PubMed] [Google Scholar]
- 42. World Health Organization Q&A on the carcinogenicity of the consumption of red meat and processed meat. [Internet] Geneva: WHO; 2015; [cited 8 April, 2020] Available from: https://www.who.int/features/qa/cancer-red-meat/en/. [Google Scholar]
- 43. Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K; International Agency for Research on Cancer Monograph Working Group . Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599–600. [DOI] [PubMed] [Google Scholar]
- 44. Jenkins DJ, Kendall CW, Mehling CC, Parker T, Rao AV, Agarwal S, Novokmet R, Jones PJ, Raeini M, Story JA et al. Combined effect of vegetable protein (soy) and soluble fiber added to a standard cholesterol-lowering diet. Metabolism. 1999;48(6):809–16. [DOI] [PubMed] [Google Scholar]
- 45. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, Garnett T, Tilman D, DeClerck F, Wood A et al. Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393(10170):447–92. [DOI] [PubMed] [Google Scholar]
- 46. Poore J, Nemecek T. Reducing food's environmental impacts through producers and consumers. Science. 2018;360(6392):987–92. [DOI] [PubMed] [Google Scholar]
- 47. Eshel G, Stainier P, Shepon A, Swaminathan A. Environmentally optimal, nutritionally sound, protein and energy conserving plant based alternatives to U.S. meat. Sci Rep. 2019;9:10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Godfray HCJ, Aveyard P, Garnett T, Hall JW, Key TJ, Lorimer J, Pierrehumbert RT, Scarborough P, Springmann M, Jebb SA. Meat consumption, health, and the environment. Science. 2018;361(6399):eaam5324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.