ABSTRACT
Background
The association between accelerometer-assessed physical activity and risk of diabetes remains unclear, especially among US Hispanic/Latino adults who have lower levels of physical activity and a higher diabetes burden compared with other racial/ethnical populations in the country.
Objectives
To examine the association between accelerometer-assessed physical activity and incident diabetes in a US Hispanic/Latino population.
Methods
We included 7280 participants of the Hispanic Community Health Study/Study of Latinos who aged 18–74 y and free of diabetes at baseline. Data on moderate-to-vigorous physical activity (MVPA) were collected using a 7-d accelerometer measurement. Incident diabetes was assessed after a mean ± SD of 6.0 ± 0.8 y using standard procedures including blood tests. RRs and 95% CIs of diabetes associated with MVPA were estimated using survey Poisson regressions. The associations of MVPA with 6-y changes in adiposity measures were also examined.
Results
A total of 871 incident cases of diabetes were identified. MVPA was inversely and nonlinearly associated with risk of diabetes (P-nonlinearity = 0.006), with benefits accruing rapidly at the lower end of MVPA range (<30 min/d) and leveling off thereafter. The association differed by population age (P-interaction = 0.006). Higher MVPA was associated with lower risk of diabetes among individuals older than 50 y (RRQ4 versus Q1 = 0.50; 95% CI: 0.35, 0.73; P-trend < 0.001) but not among younger individuals (RRQ4 versus Q1 = 0.98; 95% CI: 0.66, 1.47; P-trend = 0.92). An inverse association between MVPA and 6-y gain in waist circumference was also limited to the older group (P-interaction with age < 0.001).
Conclusions
Among US Hispanic/Latino adults, baseline accelerometer-derived MVPA was inversely associated with incident diabetes only among individuals aged 50 y and older. Further studies are needed to confirm our findings and to clarify potential mechanisms underlying the possible age differences in the MVPA–diabetes association. This study was registered at clinicaltrials.gov as NCT02060344.
Keywords: accelerometers, diabetes, Hispanic American, physical activity, weight gain
Introduction
Hispanics/Latinos are the largest and the fastest-growing minority in the United States. They constitute 17% of the nation's population, and the proportion is expected to be 30% by 2050 (1). Compared with other US racial/ethnic groups, Hispanics/Latinos often have distinct socioeconomic, lifestyle, and genetic characteristics that may contribute to their disproportionately high burden of metabolic disorders such as obesity and diabetes (2–4). For example, in a study including nationally representative samples of US adults, Hispanics were found to have a lower rate of meeting the recommended levels of physical activity (5) than other racial/ethnic populations in each year during 2007–2016 (6).
Lifestyle interventions, including counseling for maintaining optimal physical activity, diets, and body weight, have been demonstrated to be effective for diabetes reduction among high-risk adults (7). Higher physical activity levels have been extensively associated with lower risk of diabetes in a large body of cohort studies conducted in populations of different world regions (8). This evidence has led to several national organizations (5, 9), including the American Diabetes Association (9), to recommend regular physical activity of moderate-to-vigorous intensities for preventing or delaying the onset of diabetes or other chronic diseases in adults. Notably, such a recommendation is largely driven by findings from studies of middle-aged and older, non-Hispanic populations, and whether it is similarly applicable to younger populations or populations of Hispanic/Latino heritages remains open for investigation. Another concern related to previous studies on physical activity and diabetes is the sole use of self-reported physical activity measures, which generally overestimate an individual's physical activity [e.g., due to reporting biases arising from imprecise recall or social desirability (10, 11)].
The Hispanic Community Health Study/Study of Latinos (HCHS/SOL), a population-based cohort study that recruited individuals aged 18–74 y who represented the diverse Hispanic/Latino backgrounds in the United States (12, 13), provides a unique opportunity to fill this knowledge gap. Thus, we evaluated the association between moderate-to-vigorous physical activity (MVPA) as assessed by accelerometers and incident diabetes in a US Hispanic/Latino population covering the adult life span. Because habitual physical activity may reduce risk of diabetes by preventing or attenuating weight gain (5), we further examined the association of MVPA with longitudinal changes in adiposity. We hypothesized that higher levels of accelerometer-assessed MVPA would be associated with lower risk of diabetes.
Methods
Study design and population
The HCHS/SOL is a prospective population-based study of 16,415 Hispanic/Latino adults aged 18–74 y at recruitment who were living in 4 US metropolitan areas (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA). Recruitment was designed to occur in stable communities to facilitate follow-up and reexaminations. As described previously (12, 13), participants were recruited by using a 2-stage probability sample design. Following standard protocols, a comprehensive battery of interviews and a clinical assessment with fasting blood draw were conducted by trained and certified staff at in-person clinic visits during 2008–2011 (visit 1). The second visit (visit 2) period started in October 2014 and concluded in December 2017. In visit 2, 11,623 cohort members were reexamined to collect data predictive of cardiopulmonary outcomes and the onset of diabetes. The study was approved by the institutional review boards at all participating institutions, and all participants gave written informed consent.
Assessment of physical activity
During the baseline examination, participants were instructed to wear an Actical accelerometer (version B-1; model 198–0200–03; Respironics) for 7 d, positioned above the iliac crest, with removal only for swimming, showering, and sleeping. Acceptable technical reliability for counts has been shown for the accelerometer (14), and detailed information concerning accelerometer performance and adherence in the HCHS/SOL has been described elsewhere (15). The accelerometer was programmed to capture accelerations in counts of 1-min epochs. Using the Choi algorithm (16), nonwear time was defined as ≥90 consecutive min of zero counts, with allowance of 1 or 2 min of nonzero counts if no counts were detected in a 30-min window upstream and downstream of the 90-min period. An adherent day was defined as ≥10 h of wear time, and ≥3 adherent days were required for inclusion in the current analysis. As compared with individuals not adherent to the accelerometer wear protocols, adherent individuals were older, were more likely to be employed and be never smokers or moderate alcohol drinkers, and had a higher diet quality score and lower adiposity measures (Supplementary Table 1). Differences in the prevalence of diabetes between adherent and nonadherent individuals were nonsignificant (P-difference = 0.066). We used accelerometer counts previously calibrated among adults of a wide age range (18–59 y) to define sedentary behavior (<100 counts/min) and MVPA (≥1535 counts/min) (17, 18). In addition, we explored an alternative definition of MVPA (≥1065 counts/min) (19).
Ascertainment of outcomes
At both visits 1 and 2, participants were asked to fast for ≥8 h before the examination and consume only water and necessary medications. Venous blood samples were collected and analyzed. Details on the laboratory collection, processing, and analysis are reported elsewhere (3, 20). Plasma glucose was measured using a hexokinase enzymatic method (Roche Diagnostics); hemoglobin A1c (HbA1c) was measured in EDTA-anticoagulated whole blood using a Tosoh G7 automated, nonporous ion-exchange high-performance liquid chromatography analyzer (Tosoh Bioscience) (3, 20). Diabetes cases were identified according to the following American Diabetes Association criteria applied to centrally measured laboratory tests: 1) fasting plasma glucose ≥126 mg/dL, 2) post–oral glucose tolerance test plasma glucose concentration ≥200 mg/dL, and 3) HbA1c ≥6.5%. In addition to laboratory test criteria, information on self-reported physician diagnosis or current use of antidiabetic medications was also used to identify additional diabetes cases. Based on these criteria, participants free of diabetes at visit 1 who were identified as having diabetes at visit 2 were deemed to be incident diabetes cases (21).
Measurements of anthropometric indexes and body composition were performed at both visits 1 and 2. Waist circumference at the top of the iliac crest and standing height were measured to the nearest centimeter, and body weight was measured to the nearest 0.1 kg. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Body fat mass was estimated from bioimpedance using a Total Body Composition Analyzer (model TBF-300A; Tanita Corporation). Longitudinal changes in adiposity measures, including changes in BMI, waist circumference, and body fat mass, were computed by subtracting the measures collected at visit 1 from those at visit 2.
Assessment of covariates
The definitions and methods used for measurements of other covariates at visit 1 have been reported elsewhere (12). Briefly, bilingual interviewer-administered questionnaires were used to collect information on socioeconomic and demographic characteristics, including Hispanic/Latino background, diet and lifestyle factors, and medical and family histories. To ascertain Hispanic/Latino background, participants were asked which of the following groups best described their heritage: Central American, Cuban, Dominican, Mexican, Puerto Rican, South American, >1 background, or other. Dietary energy and the Alternative Healthy Eating Index (AHEI)–2010 score were computed by using the National Cancer Institute methodology, based on data collected by two 24-h dietary recalls with ∼1 mo apart in addition to a food propensity questionnaire (22).
Statistical analysis
Among the 11,623 reexamined participants, 7280 (2703 men and 4577 women) were eligible for the analysis of MVPA and incident diabetes (primary outcome) after excluding participants who had prevalent diabetes at visit 1 (n = 2541), participants missing information on diabetes diagnosis at either study visit (n = 11), and those who did not adhere to the accelerometer protocol according to the criteria described above (n = 1791) (Supplementary Figure 1). The sample sizes were slightly smaller for the analyses of changes in adiposity measures (secondary outcomes), including changes in BMI (n = 7055), waist circumference (n = 7036), and body fat mass (n = 6620) (Supplementary Figure 1).
All analyses incorporated HCHS/SOL complex study design and sampling weights to account for visit 2 nonresponse and oversampling of specific population subgroups. Weights were trimmed and calibrated to 2010 US Census characteristics by age, sex, and Hispanic/Latino background in each field center's target population (12, 13). Additional adjustment was made to account for missing or incomplete accelerometer data using inverse probability weighting (23, 24). As a result, the final weight used in our analysis was the multiplicative product of the inverse probability weighting weight (to weight the results for the compliant subset back to the whole HCHS/SOL sample) and the HCHS/SOL sampling weight (to further weight the results back to the Hispanic/Latino population in the target areas).
Sex-specific weighted quartiles of MVPA were modeled to account for the substantial differences in MVPA levels between men and women. Age-adjusted descriptive characteristics of the study population across quartiles of MVPA were computed as means (95% CI) for continuous variables and the data were compared using a survey linear regression; and as percentages (95% CI) for categorical variables and the data were compared using a χ2 test. We used survey Poisson regression models that offset the lag time between visits 1 and 2 to estimate RRs with 95% CIs of diabetes across MVPA quartile, using the lowest quartile as reference. Age, sex (where appropriate), study field center, and Hispanic/Latino background were included in the minimally adjusted model. The second model further included the following potential confounders: education, annual household income, employment status, smoking, alcohol consumption, AHEI-2010 score, energy intake, history of cardiovascular disease, number of days wearing accelerometer, and family history of diabetes. The third (full) model also included sedentary time. Further exploratory analyses were performed by including baseline BMI, waist circumference, or body fat mass in the full model to assess the potential mediation of adiposity on the MVPA–diabetes association.
Potential nonlinear relation between MVPA and risk of diabetes was examined using restricted cubic splines with 3 knots at the 10th, 50th, and 90th percentiles of the distribution. We also evaluated potential interactions of MVPA with baseline age (≤50 compared with >50 y), sex, BMI (≤30 compared with >30), Hispanic background (Mexican compared with non-Mexican), and study field center on risk of diabetes. Additional sensitivity analyses were performed by 1) excluding self-known incident diabetes cases (including cases identified solely by blood testing), 2) using 4 convenient categories of MVPA (≤5, >5–15, >15–30, and >30 min/d) instead of MVPA quartile, and 3) using an alternate cut-point for accelerometer counts (≥1065 counts/min) to define MVPA (19).
We next examined associations between MVPA and longitudinal changes in adiposity measures by using a survey linear regression. Models were adjusted for covariates as described above and further adjusted for baseline level of the tested adiposity measure as well as the lag time between visits 1 and 2. The generated results were plotted as marginal means (95% CI) of changes in adiposity according to MVPA quartile. Statistical analyses were performed using Stata 15.1 (StataCorp).
Results
Population characteristics
Weighted median MVPA at baseline was 19.6 (IQR: 8.0–38.5) min/d for the study population, 25.6 (IQR: 12.5–48.0) min/d for men, and 14.0 (IQR: 5.7–29.2) min/d for women. The largest Hispanic/Latino group was Mexican (35.4%), followed by Cuban (22.8%), Puerto Rican (15.8%), Central or South American (12.4%), Dominican (9.6%), and other or multiple backgrounds (3.9%). Age-adjusted characteristics of the study population according to sex-specific quartile of MVPA are shown in Table 1. Differences in MVPA distributions according to study center and Hispanic/Latino background were evident. Higher MVPA was associated with younger age and lower levels of total energy intake, sedentary time, and adiposity measures at baseline. Individuals with higher MVPA were more likely to be never smokers and less likely to have cardiovascular disease or prediabetes at baseline.
TABLE 1.
Age-adjusted baseline population characteristics according to quartile of MVPA in US Hispanic/Latino adults1
| Quartile for MVPA2 | ||||||
|---|---|---|---|---|---|---|
| Characteristic | All | Q1 | Q2 | Q3 | Q4 | P value |
| Number of participants | 7280 | 2059 | 1783 | 1798 | 1640 | |
| Age, y | 45.9 (45.6, 46.2) | 50.0 (49.4, 50.5) | 45.9 (45.3, 46.4) | 44.4 (43.9, 45.0) | 42.6 (42.0, 43.3) | <0.001 |
| Male sex, % | 47.8 (46.2, 49.4) | 48.2 (44.9, 51.5) | 48.2 (44.9, 51.6) | 48.4 (44.9, 51.8) | 49.4 (45.8, 53.0) | 0.55 |
| Study field center, % | ||||||
| Bronx | 28.3 (25.4, 31.3) | 14.6 (11.7, 18.1) | 22.5 (19.2, 26.2) | 34.7 (30.2, 40.0) | 44.8 (40.4, 49.4) | <0.001 |
| Chicago | 14.5 (12.6, 16.5) | 13.9 (11.4, 16.9) | 16.6 (14.2, 19.5) | 14.1 (11.9, 16.6) | 13.6 (11.5, 16.1) | |
| Miami | 32.0 (28.0, 36.4) | 45.9 (40.4, 51.5) | 34.0 (29.1, 39.2) | 24.2 (19.5, 29.6) | 19.3 (15.4, 23.8) | |
| San Diego | 25.2 (22.1, 28.6) | 25.6 (21.4, 30.5) | 26.9 (23.1, 31.2) | 27.0 (22.6, 31.8) | 22.3 (18.7, 26.4) | |
| Hispanic/Latino background, % | ||||||
| Central/South American | 12.4 (10.9, 14.1) | 10.7 (8.9, 12.8) | 13.0 (10.8, 15.5) | 12.9 (10.7, 15.5) | 13.7 (11.1, 16.9) | <0.001 |
| Cuban | 22.9 (19.8, 26.2) | 36.0 (30.9, 41.5) | 24.4 (20.3, 28.9) | 16.1 (12.6, 20.3) | 10.3 (7.9, 13.3) | |
| Dominican | 9.6 (8.3, 11.1) | 5.5 (4.2, 7.4) | 8.2 (6.5, 10.4) | 11.2 (9.0, 13.9) | 14.2 (11.7, 17.2) | |
| Mexican | 35.4 (32.3, 38.6) | 32.1 (27.9, 36.5) | 37.6 (33.6, 41.9) | 37.9 (33.6, 42.4) | 35.1 (31.0, 39.4) | |
| Puerto Rican | 15.8 (14.1, 17.6) | 11.7 (9.2, 14.7) | 13.7 (11.4, 16.4) | 18.3 (15.5, 21.4) | 21.7 (18.6, 25.1) | |
| Other/>1 heritage | 3.9 (3.1, 4.7) | 3.9 (2.6, 5.9) | 3.1 (2.0, 4.9) | 3.7 (2.5, 5.2) | 5.0 (3.5, 7.1) | |
| Above high school education, % | 42.0 (40.1, 44.0) | 41.8 (38.2, 45.4) | 44.0 (40.0, 48.0) | 42.7 (39.2, 46.2) | 37.6 (34.2, 41.1) | 0.16 |
| Annual household income, % | ||||||
| ≤$25,000 | 52.1 (50.0, 54.3) | 51.7 (48.2, 55.2) | 50.7 (46.9, 54.7) | 51.2 (47.1, 55.3) | 57.0 (53.4, 60.6) | 0.043 |
| >$25,000 | 38.2 (36.0, 40.4) | 36.7 (33.2, 40.4) | 41.6 (36.9, 44.4) | 40.1 (36.3, 44.0) | 34.4 (31.3, 37.6) | |
| Not reported | 9.7 (8.4, 11.1) | 11.6 (9.2, 14.5) | 8.8 (6.9, 11.0) | 8.7 (6.7, 11.2) | 8.6 (6.5, 11.4) | |
| Employment status, % | ||||||
| Not employed | 47.7 (46.0, 49.5) | 52.4 (48.7, 56.1) | 46.0 (42.8, 49.2) | 46.2 (43.0, 49.4) | 45.2 (42.2, 48.3) | <0.001 |
| Employed part-time (≤35 h/wk) | 17.5 (16.3, 18.7) | 14.7 (12.4, 17.3) | 17.0 (14.8, 19.5) | 17.6 (15.3, 20.2) | 20.1 (17.6, 22.8) | |
| Employed full-time (>35 h/wk) | 34.8 (33.3, 36.4) | 32.9 (29.5, 36.6) | 37.0 (34.2, 39.8) | 36.2 (33.1, 39.5) | 34.7 (31.7, 37.8) | |
| Smoking status, % | ||||||
| Never | 61.8 (60.1, 63.4) | 57.5 (54.2, 61.7) | 64.9 (61.8, 68.0) | 60.5 (67.1, 63.8) | 62.5 (58.9, 66.0) | <0.001 |
| Former | 18.1 (16.8, 19.5) | 21.0 (18.4, 23.7) | 17.6 (15.3, 20.2) | 18.6 (16.1, 21.5) | 17.8 (15.1, 20.7) | |
| Current | 20.1 (18.5, 21.8) | 21.5 (18.4, 25.0) | 17.5 (14.9, 20.4) | 20.9 (18.1, 24.0) | 19.7 (16.9, 23.0) | |
| Alcohol consumption, % | ||||||
| None | 48.7 (46.6, 50.7) | 48.6 (45.1, 52.1) | 49.3 (45.2, 53.4) | 47.1 (43.4, 50.8) | 46.1 (42.2, 50.1) | 0.040 |
| Light to moderate3 | 45.9 (43.9, 47.9) | 44.1 (40.7, 47.6) | 46.2 (42.2, 50.3) | 46.8 (43.2, 50.5) | 48.9 (45.1, 52.8) | |
| Heavier | 5.4 (4.6, 6.4) | 7.3 (5.3, 10.0) | 4.5 (3.3, 6.1) | 6.1 (4.4, 8.2) | 5.0 (3.6, 6.9) | |
| Total energy intake, kcal/d | 2020 (1996, 2043) | 2073 (2026, 2120) | 2054 (2012, 2096) | 1995 (1952, 2039) | 1956 (1914, 1999) | <0.001 |
| AHEI-2010 score | 47.0 (46.6, 47.4) | 46.2 (45.6, 46.7) | 47.4 (46.9, 48.0) | 47.3 (46.7, 47.9) | 47.1 (46.5, 47.6) | 0.033 |
| Accelerometer wearing days | 5.1 (5.06, 5.15) | 4.9 (4.8, 5.0) | 5.1 (5.0, 5.2) | 5.2 (5.1, 5.3) | 5.2 (5.1, 5.3) | <0.001 |
| Sedentary time, h/d | 11.7 (11.5, 11.8) | 12.1 (11.9, 12.3) | 11.7 (11.5, 11.9) | 11.7 (11.5, 11.9) | 11.2 (11.0, 11.4) | <0.001 |
| History of CVD, % | 5.4 (4.6, 6.3) | 6.5 (4.7, 8.9) | 5.5 (4.1, 7.3) | 4.3 (2.9, 6.3) | 4.6 (3.2, 6.4) | <0.001 |
| Family history of diabetes, % | 36.8 (35.0, 38.6) | 36.8 (33.8, 40.0) | 36.3 (33.1, 40.1) | 38.2 (34.4, 42.0) | 36.6 (33.1, 40.1) | 0.43 |
| Baseline prediabetes,4 % | 47.2 (45.8, 48.7) | 49.2 (46.6, 51.7) | 46.6 (43.5, 49.8) | 46.2 (42.9, 49.4) | 43.3 (39.9, 46.8) | <0.001 |
| Baseline BMI, kg/m2 | 28.9 (28.7, 29.2) | 29.7 (29.2, 30.1) | 29.3 (28.8, 29.8) | 28.8 (28.3, 29.4) | 27.5 (27.5, 28.3) | <0.001 |
| Baseline waist circumference, cm | 96.1 (95.5, 96.6) | 97.9 (96.8, 99.0) | 97.0 (95.8, 98.1) | 95.9 (94.8, 97.0) | 93.4 (92.4, 94.5) | <0.001 |
| Baseline body fat mass, kg | 26.0 (25.5, 26.6) | 28.0 (26.9, 29.0) | 26.8 (25.6, 28.0) | 25.7 (24.7, 26.7) | 23.7 (22.8, 24.5) | <0.001 |
Data are age-adjusted mean (95% CI) for continuous variables or percentage (95% CI) for categorical variables. All results except for age and number of participants were weighted for survey design, nonresponse, and noncompliance with accelerometer wear protocols. AHEI, Alternative Healthy Eating Index; CVD, cardiovascular disease; MVPA, moderate-to-vigorous physical activity; Q, quartile.
Quartiles are sex specific due to the substantially different MVPA levels in men and women; MVPA ranges across quartiles are ≤12.4, 12.5–25.5, 25.6–47.9, and ≥48.0 min/d in men and ≤5.7, 5.8–13.9, 14.0–29.1, and ≥29.2 min/d in women.
Alcohol consumption of ≤1 drink/d in women or ≤2 drinks/d in men.
Prediabetes was defined as 1) fasting glucose 100–125 mg/dL, or 2) post–oral glucose tolerance test plasma glucose concentration 140–199 mg/dL, or 3) 5.7% ≤ hemoglobin A1c <6.5%.
MVPA and incident diabetes
During a mean ± SD follow-up of 6.0 ± 0.8 y, 871 incident cases of diabetes (339 in men and 532 in women) were identified. Age-adjusted incidence rates (per 1000 person-years) were 17.7 (95% CI: 16.0, 19.4) for the whole population included, 19.3 (95% CI: 16.7, 21.8) in men, and 16.3 (95% CI: 14.2, 18.4) in women.
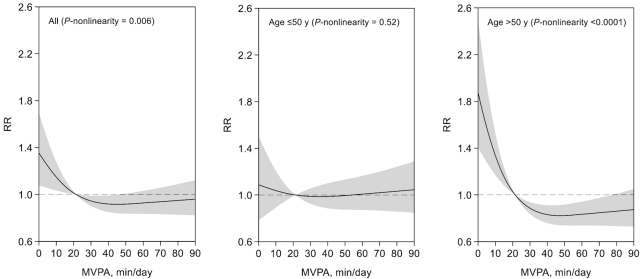
After the full adjustment for demographic, social-economic, and lifestyle factors including sedentary time (model 3), as shown in Figure 1, there was a significant, nonlinear, and inverse association between MVPA and risk of diabetes (P-nonlinearity = 0.006). Diabetes risk decreased sharply with increasing MVPA up to the level of ∼30 min/d, with risk reduction leveling off thereafter. Compared with the first MVPA quartile, the multivariable-adjusted RRs of diabetes across the second to fourth quartiles of MVPA were 0.76 (0.59, 0.97), 0.67 (0.51, 0.89), and 0.78 (0.57, 1.05).
FIGURE 1.
Restricted cubic splines examining nonlinear relation between MVPA and risk of diabetes. All data were included in the analyses and results for MVPA <90 min/d (the 95th percentile for the MVPA of the study population) are shown for presentation purpose. MVPA of (at least) 21.4 min/d (or 150 min/wk) recommended by the “2018 Physical Activity Guidelines for Americans” was used as the reference. Results were from multivariable survey Poisson regression models that were weighted for survey design, nonresponse, and noncompliance with accelerometer wear protocols and further adjusted for covariates listed for model 3 in Table 2. The analysis included 4420 individuals aged ≤50 y and 2860 individuals aged >50 y. MVPA, moderate-to-vigorous physical activity.
We performed stratified analyses with multivariable adjustment (model 3 in Table 2). The association of MVPA with risk of diabetes did not vary significantly by sex, BMI, Hispanic background, or study field center (P-interaction > 0.20). However, there was evidence that the association was modified by age (P-interaction = 0.006). MVPA was not associated with risk of diabetes among younger individuals (≤50 y; 418 incident cases; RRQ4 versus Q1 = 0.98; 95% CI: 0.66, 1.47; P-trend = 0.92). Among older individuals (>50 y; 453 incident cases), there was a strong nonlinear and inverse association (P-nonlinearity < 0.001) (Figure 1), with those in the highest MVPA quartile having 50% lower risk of diabetes as compared with those in the lowest quartile (RR = 0.50; 95% CI: 0.35, 0.73; P-trend < 0.001) (Table 2). The age-specific difference in the associations of MVPA with risk of diabetes appeared more evident in men than in women (P-interaction with age: 0.006 in men and 0.41 in women) (Table 2). Furthermore, the inverse association in the older group was attenuated only slightly in an exploratory analysis with additional adjustment for baseline waist circumference (model 4 in Table 2) or BMI or body fat mass (data not shown).
TABLE 2.
Association between MVPA and incident diabetes according to age groups in US Hispanic/Latino adults1
| Quartile for MVPA2 | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P-trend | |
| Men and women | |||||
| ≤50 y | |||||
| Cases/participants | 113/974 | 107/1119 | 94/1164 | 104/1163 | |
| Incidence rate3 | 13.5 (10.1, 16.9) | 13.1 (9.3, 17.0) | 13.5 (8.9, 18.1) | 16.3 (11.1, 21.5) | |
| Model 1, RR (95% CI) | 1.00 (reference) | 0.85 (0.59, 1.23) | 0.77 (0.52, 1.14) | 0.92 (0.64, 1.33) | 0.67 |
| Model 2, RR (95% CI) | 1.00 (reference) | 0.83 (0.57, 1.21) | 0.76 (0.52, 1.12) | 0.94 (0.66, 1.33) | 0.72 |
| Model 3, RR (95% CI) | 1.00 (reference) | 0.85 (0.59, 1.23) | 0.78 (0.53, 1.16) | 0.98 (0.66, 1.47) | 0.92 |
| Model 4, RR (95% CI) | 1.00 (reference) | 0.88 (0.61, 1.28) | 0.81 (0.55, 1.21) | 1.12 (0.73, 1.69) | 0.68 |
| >50 y | |||||
| Cases/participants | 207/1085 | 96/664 | 86/634 | 64/477 | |
| Incidence rate3 | 34.9 (28.9, 40.8) | 22.1 (16.3, 28.0) | 22.7 (16.1, 29.3) | 20.6 (13.1, 28.0) | |
| Model 1, RR (95% CI) | 1.00 (reference) | 0.62 (0.45, 0.87) | 0.54 (0.39, 0.76) | 0.47 (0.33, 0.66) | <0.001 |
| Model 2, RR (95% CI) | 1.00 (reference) | 0.65 (0.48, 0.89) | 0.59 (0.42, 0.80) | 0.49 (0.35, 0.68) | <0.001 |
| Model 3, RR (95% CI) | 1.00 (reference) | 0.66 (0.48, 0.90) | 0.59 (0.42, 0.84) | 0.50 (0.35, 0.73) | <0.001 |
| Model 4, RR (95% CI) | 1.00 (reference) | 0.70 (0.51, 0.96) | 0.65 (0.45, 0.92) | 0.56 (0.38, 0.82) | <0.001 |
| Men | |||||
| ≤50 y | |||||
| Cases/participants | 45/392 | 39/417 | 34/431 | 48/437 | |
| Incidence rate3 | 14.4 (8.8, 19.9) | 11.6 (6.0, 17.2) | 16.4 (8.0, 24.8) | 22.3 (13.8, 30.7) | |
| Model 1, RR (95% CI) | 1.00 (reference) | 0.73 (0.41, 1.28) | 0.75 (0.43, 1.32) | 1.02 (0.57, 1.82) | 0.81 |
| Model 2, RR (95% CI) | 1.00 (reference) | 0.75 (0.42, 1.33) | 0.76 (0.45, 1.26) | 1.04 (0.62, 1.76) | 0.76 |
| Model 3, RR (95% CI) | 1.00 (reference) | 0.75 (0.42, 1.33) | 0.76 (0.43, 1.32) | 1.04 (0.55, 1.96) | 0.84 |
| Model 4, RR (95% CI) | 1.00 (reference) | 0.75 (0.42, 1.36) | 0.77 (0.44, 1.34) | 1.15 (0.59, 2.24) | 0.64 |
| >50 y | |||||
| Cases/participants | 83/398 | 37/237 | 29/215 | 24/176 | |
| Incidence rate3 | 42.2 (32.4, 52.0) | 22.6 (13.9, 31.4) | 22.9 (11.9, 33.8) | 24.7 (12.4, 37.0) | |
| Model 1, RR (95% CI) | 1.00 (reference) | 0.52 (0.32, 0.85) | 0.46 (0.27, 0.77) | 0.44 (0.26, 0.75) | <0.001 |
| Model 2, RR (95% CI) | 1.00 (reference) | 0.54 (0.35, 0.82) | 0.50 (0.30, 0.84) | 0.48 (0.28, 0.81) | 0.001 |
| Model 3, RR (95% CI) | 1.00 (reference) | 0.52 (0.34, 0.81) | 0.48 (0.27, 0.86) | 0.45 (0.25, 0.80) | 0.003 |
| Model 4, RR (95% CI) | 1.00 (reference) | 0.57 (0.37, 0.89) | 0.54 (0.30, 0.97) | 0.50 (0.28, 0.88) | 0.001 |
| Women | |||||
| ≤50 y | |||||
| Cases/participants | 68/582 | 68/702 | 60/733 | 56/726 | |
| Incidence rate3 | 13.5 (9.0, 18.1) | 14.5 (9.9, 19.0) | 11.1 (7.2, 15.0) | 10.7 (6.6, 14.8) | |
| Model 1, RR (95% CI) | 1.00 (reference) | 0.98 (0.60, 1.59) | 0.77 (0.47, 1.28) | 0.77 (0.48, 1.24) | 0.20 |
| Model 2, RR (95% CI) | 1.00 (reference) | 0.98 (0.62, 1.56) | 0.76 (0.46, 1.25) | 0.75 (0.47, 1.17) | 0.13 |
| Model 3, RR (95% CI) | 1.00 (reference) | 1.01 (0.64, 1.61) | 0.80 (0.48, 1.31) | 0.81 (0.48, 1.39) | 0.31 |
| Model 4, RR (95% CI) | 1.00 (reference) | 1.17 (0.74, 1.85) | 0.84 (0.49, 1.45) | 0.99 (0.57, 1.72) | 0.65 |
| >50 y | |||||
| Cases/participants | 124/687 | 59/427 | 57/419 | 40/301 | |
| Incidence rate3 | 29.3 (22.2, 36.4) | 21.7 (14.3, 29.2) | 22.8 (15.4, 30.2) | 15.8 (9.5, 22.1) | |
| Model 1, RR (95% CI) | 1.00 (reference) | 0.74 (0.48, 1.12) | 0.64 (0.42, 0.97) | 0.48 (0.29, 0.77) | 0.002 |
| Model 2, RR (95% CI) | 1.00 (reference) | 0.73 (0.48, 1.09) | 0.63 (0.43, 0.93) | 0.46 (0.28, 0.73) | 0.001 |
| Model 3, RR (95% CI) | 1.00 (reference) | 0.77 (0.50, 1.17) | 0.69 (0.46, 1.03) | 0.53 (0.32, 0.89) | 0.012 |
| Model 4, RR (95% CI) | 1.00 (reference) | 0.82 (0.54, 1.24) | 0.76 (0.50, 1.13) | 0.61 (0.36, 1.02) | 0.053 |
Results were from multivariable survey Poisson regression models that were weighted for survey design, nonresponse, and noncompliance with accelerometer wear protocols. Model 1 included study field center, baseline age, sex (where appropriate), and Hispanic/Latino background. Model 2 included the covariates in model 1 and education (no high school, at most high school, greater than high school), annual household income (≤ 10,000;
10,000;  10,001–
10,001–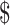 25,000;
25,000; 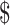 25,001–
25,001–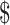 50,000;
50,000;  50,001–
50,001–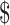 75,000; ≥
75,000; ≥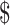 75,001), employment status (retired and not employed, not retired and not employed, part-time employed, full-time employed), smoking (never, former, current ≤10 pack-years, current >10 pack-years), alcohol consumption (never, former, current ≤2 drinks/d in men or ≤1 drink/d in women, current >2 drinks/d in men or >1 drink/d in women), Alternative Healthy Eating Index–2010 score (continuous), total energy intake (kcal/d), history of cardiovascular disease (yes, no), number of days wearing accelerometer, and family history of diabetes (yes, no). Model 3 included the covariates in model 2 and sedentary time (h/d). Model 4 included the covariates in model 3 and baseline waist circumference (cm). MVPA, moderate-to-vigorous physical activity; Q, quartile.
75,001), employment status (retired and not employed, not retired and not employed, part-time employed, full-time employed), smoking (never, former, current ≤10 pack-years, current >10 pack-years), alcohol consumption (never, former, current ≤2 drinks/d in men or ≤1 drink/d in women, current >2 drinks/d in men or >1 drink/d in women), Alternative Healthy Eating Index–2010 score (continuous), total energy intake (kcal/d), history of cardiovascular disease (yes, no), number of days wearing accelerometer, and family history of diabetes (yes, no). Model 3 included the covariates in model 2 and sedentary time (h/d). Model 4 included the covariates in model 3 and baseline waist circumference (cm). MVPA, moderate-to-vigorous physical activity; Q, quartile.
Quartiles are sex specific due to the substantially different MVPA levels in men and women. MVPA ranges across quartiles are ≤12.4, 12.5–25.5, 25.6–47.9, and ≥48.0 min/d in men and ≤5.7, 5.8–13.9, 14.0–29.1, and ≥29.2 min/d in women, respectively. P values for interaction between MVPA quartile and age on diabetes risk were 0.006 for the whole study population, 0.006 for men, and 0.41 for women based on model 3, and the corresponding values based on model 4 were 0.014, 0.008, and 0.53.
Age-adjusted incidence rate per 1000 person-years.
The age-specific association between MVPA and risk of diabetes was observed for both individuals of Mexican background and non-Mexican populations (Supplementary Table 2). When 4 convenient categories instead of MVPA quartile were used, the association was similarly age dependent (Supplementary Table 3). After excluding 388 cases who were aware of their diabetes diagnosis before the follow-up visit, MVPA remained inversely associated with risk of diabetes in the older group (249 incident cases; RRQ4 versus Q1 = 0.60; 95% CI: 0.37, 0.97; P-trend = 0.011) but not in the younger group (234 incident cases; RRQ4 versus Q1 = 1.04; 95% CI: 0.64, 1.70; P-trend = 0.69). We performed an additional analysis based on 4 age groups. Age-adjusted incidence rates (per 1000 person-years) were 9.4 (95% CI, 7.3, 11.5), 22.9 (95% CI: 17.9, 27.8), 27.3 (95% CI: 22.0, 32.6), and 28.6 (95% CI: 22.5, 34.7) among individuals aged ≤40, 41–50, 51–60, and >60 y, respectively. Higher MVPA was associated with substantially lower risk of diabetes in the 2 subgroups of older individuals (P-trend ≤ 0.008), but there were no associations among either group including younger individuals (P-trend > 0.70) (P-interaction with age = 0.017) (Supplementary Table 4).
We performed an additional analysis in which MVPA was defined by an alternate accelerometer count (≥1065 counts/min). Correlation between the original and the new MVPA variables was substantial (Pearson r = 0.93). Weighted medians for the new MVPA were 32.8 (IQR: 16.8–57.6) min/d for the study population, 43.3 (IQR: 24.0–70.2) min/d for men, and 24.8 (IQR: 12.4–43.5) min/d for women. Similar to the original MVPA variable, the new MVPA variable was associated with lower risk of diabetes among individuals older than 50 y (RRQ4 versus Q1 = 0.53; 95% CI: 0.35, 0.79; P-trend < 0.001) but not among younger individuals (RRQ4 versus Q1 = 1.01; 95% CI: 0.66, 1.56; P-trend = 0.92) (P-interaction with age = 0.006) (Supplementary Table 5).
MVPA and changes in adiposity
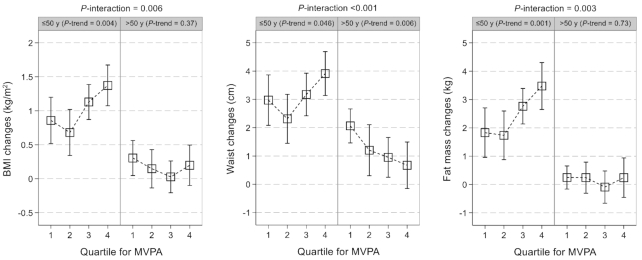
During the 6-y follow-up, on average, the study population experienced an increment of 0.85 (95% CI: 0.72, 0.99) in BMI, 2.76 (95% CI: 2.41, 3.11) cm in waist circumference, and 2.03 (95% CI: 1.67, 2.39) kg in body fat mass. Younger (≤50 y) relative to older individuals had greater increases in BMI [1.03 (95% CI: 0.87, 1.19) compared with 0.20 (95% CI: 0.05, 0.34)], waist circumference [3.13 (95% CI: 2.71, 3.55) compared with 1.41 (95% CI: 1.03, 1.78) cm], and body fat mass [2.51 (95% CI: 2.07, 2.95) compared with 0.18 (95% CI: −0.09, 0.44) kg].
Informed by the substantial differences in the associations of MVPA with weight change by age (all P-interaction with age ≤0.006), age-specific associations for the secondary outcomes of adiposity changes were plotted (Figure 2). For the younger group, higher MVPA was significantly associated with larger increases in BMI (P-trend = 0.004), waist circumference (P-trend = 0.046), and body fat mass (P-trend = 0.001). Conversely, MVPA was not associated with changes in BMI or body fat mass but was significantly associated with a smaller waist circumference gain (P-trend = 0.006) in the older group. Such age-specific differences in the association of MVPA with adiposity changes appeared more evident in men than in women (Supplementary Figure 2) and were observed for both Mexican- and non-Mexican-heritage groups (Supplementary Figure 3). In an additional analysis based on the aforementioned 4 age groups, MVPA tended to be positively associated with adiposity changes in the 2 groups of younger individuals, but the associations appeared inverse or null in the other 2 groups of older individuals (P-interaction with age ≤0.011) (Supplementary Figure 4).
FIGURE 2.
Association between MVPA and prospective changes in adiposity according to age group. Results were from multivariable survey linear regression models that were weighted for survey design, nonresponse, and noncompliance with accelerometer wear protocols. In addition to the covariates listed in the model 3 of Table 2, results were further adjusted for lag time between the 2 study visits and baseline level of the examined adiposity measure. The numbers of individuals by age group (≤50/>50 y) were 4272/2783 (BMI changes), 4261/2775 (waist changes), or 4077/2543 (fat mass changes). MVPA, moderate-to-vigorous physical activity.
Discussion
In this population-based cohort of US Hispanic/Latino adults, we initially found a nonlinear inverse association between accelerometer-derived MVPA at baseline and risk of diabetes after 6 y of follow-up, with the reduction of diabetes risk accruing rapidly at the lower end of the MVPA range (<30 min/d). Such a nonlinear pattern, which has been frequently depicted in other racial/ethnic populations (25), agrees with the statement by the 2018 Physical Activity Guidelines for Americans that “there is no threshold (of MVPA) that must be exceeded before benefits begin to occur” (5). Making use of the broad age range of our study, further analyses revealed substantial differences in the association of MVPA with risk of diabetes by age, with a strong inverse association in older individuals (age >50 y) but no association in younger individuals (age ≤50 y).
To our knowledge, this is the first study examining accelerometer-derived physical activity in relation to incident diabetes. While previous cohort studies have extensively documented an inverse association between self-reported physical activity and risk of diabetes (8), they included mostly middle-aged and older, non-Hispanic populations. In 2 prospective studies of US multiethnic populations, higher levels of self-reported physical activity were associated with a lower risk of diabetes for Hispanics in the Women's Health Initiative study (26) but not in the Multi-Ethnic Study of Atherosclerosis (27), although the sample size of Hispanics in the latter study was relatively small.
There have been limited cohort studies assessing associations of physical activity with diabetes risk by different age groups, and results were not clear (28, 29). Results from some intervention trials, including high-risk participants, have indicated that lifestyle interventions may be particularly effective for relatively older people in terms of diabetes prevention (30–32). In the Finnish Diabetes Prevention Study (30), intensive lifestyle intervention with advice to exercise for >4 h/wk significantly reduced diabetes risk by 51% among participants aged 51–61 y and by 64% among those older than 61 y, respectively, whereas the intervention conferred no benefits for those younger than 51 y (P-interaction with age = 0.013). Similar age-varying effects of intensive lifestyle on diabetes reduction were observed in another trial conducted among the US population (Diabetes Prevention Program) (31, 32). However, because both healthful diets and regular physical activity were emphasized in the trials, it is unclear whether the stronger benefits for older individuals could be partially attributable to their greater adherence to the recommendations on physical activity.
In our study, the associations between baseline MVPA and longitudinal changes in adiposity also appeared age dependent. Higher MVPA was associated with a smaller waist circumference gain in the older group but was unexpectedly associated with larger gains in all the examined adiposity measures in the younger group. Several previous studies (33–38) that had a smaller sample size and shorter duration assessed how accelerometer-derived MVPA may be associated with adiposity changes. Most of these studies included relatively young individuals [i.e., ≤50 y (33–36, 38)] and suggested no association despite few exceptions (33, 38).
Our findings may not refute the beneficial roles of physical activity in the prevention of diabetes in the younger population but instead may reflect challenges in capturing usual physical activity by accelerometers among younger adults. Despite assessing physical activity objectively, accelerometer measurement has limitations in terms of determining certain physical activity types that are more common among younger individuals (39). For example, physical activity in water such as swimming was not recorded. Other types of physical activity that would be expected to be poorly captured by the hip-worn accelerometer include work-related exertion (e.g., lifting and using tools), resistance training, and cycling. It is also uncertain whether the study participants continued to wear the accelerometer during team athletics events such as soccer, during which device wearing may become inconvenient.
Reproducibility of usual physical activity assessed by a single 7-d accelerometer measurement has been shown to be acceptable in older individuals (40, 41) but remains uncertain in younger adults. Owning to the major life events (e.g., starting job, getting married, and having children) as well as apparent weight gains across early to middle adulthood, younger individuals may have an intermittent pattern of physical activity that is inaccurately captured by a single measurement (31, 42).
It is also known that, in the absence of other lifestyle changes such as improved diet, the benefits of being physically active alone on long-term weight loss are typically modest (if not null) (43–45). Thus, the age-varying association of MVPA with risk of diabetes or with changes in adiposity may also involve age-specific differences in other physical activity–associated lifestyle behaviors (e.g., caloric restriction and diet quality) that play critical roles in diabetes prevention and weight regulation (44, 46). Finally, age-related biologic variation may have contributed to the stronger association of MVPA with risk of diabetes observed for the older group (47). Aging is accompanied by elevated abdominal and visceral fat, leading to various metabolic abnormalities, including insulin resistance and hyperinsulinemia (47). Because regular physical activity may improve insulin sensitivity and glycemic control beyond habitual diet and weight loss (47, 48), being active may be particularly favorable for diabetes prevention for relatively older people who are prone to metabolic dysfunctions, even in the absence of weight reduction.
Strengths of our study include the representative population sample of US Hispanics and Latinos across the adult life span, the adjustment for both nonresponse in the study recruitment or follow-up phases and noncompliance with accelerometer wear protocols, and the ascertainment of diabetes according to multiple standard procedures. Some limitations to our study need to be acknowledged. Despite the relatively comprehensive measurements of and statistical adjustment for a wide range of covariates, potential influence of unmeasured/unknown confounding on our results cannot be completely excluded. One may argue that the lack of association between MVPA and risk of diabetes among younger individuals may be attributable to the much lower incidence of diabetes in this group. However, the lack of association remained among individuals aged 41–50 y who had a much higher incidence of diabetes than those aged ≤40 y (Supplementary Table 4). Other limitations of our study include the low ability of the accelerometer to collect specific physical activity data and the single 7-d accelerometer measurement. Future studies using repeated accelerometer data will shed further light on how sustained physical activity over a long period may affect health risk, including risk of diabetes, especially among younger individuals.
In summary, our study of US Hispanic/Latino adults suggests an age-varying association between baseline accelerometer-derived MVPA and risk of diabetes, with a strong inverse association limited to individuals aged 50 y and older. Additional studies of accelerometer-derived physical activity are needed to confirm our findings and to clarify potential mechanisms underlying the possible age differences in the MVPA–diabetes association.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the staff and participants of HCHS/SOL for their important contributions. A complete list of HCHS/SOL staff and investigators can be found in Ann Epidemiol 2010;20:642–9 or at http://sites.cscc.unc.edu/hchs/.
The authors’ responsibilities were as follows––G-CC, QQ, and XX: designed the research and developed the analytical plan; G-CC, SH, and XX: performed the statistical analyses; G-CC: prepared tables and figures and had primary responsibility for writing the manuscript; DS-A and KRE: defined and derived the MVPA variables; QQ, RCK, XX, and YM-R: directed the study; and all authors: contributed to the interpretation of data and critically reviewed and revised the manuscript. QQ is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors report no conflicts of interest.
Notes
Sources of support: The Hispanic Community Health Study/Study of Latinos is a collaborative study supported by contracts from the NIH National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (HHSN268201300001I/N01-HC-65233), University of Miami (HHSN268201300004I/N01-HC-65234), Albert Einstein College of Medicine (HHSN268201300002I/N01-HC-65235), University of Illinois at Chicago (HHSN268201300003I/N01-HC-65236 Northwestern University), and San Diego State University (HHSN268201300005I/N01-HC-65237).
The following institutes/centers/offices have contributed to the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Neurological Disorders and Stroke, and NIH Institution–Office of Dietary Supplements.
QQ was supported by the NHLBI (K01HL129892, R01HL060712, and R01HL140976) and by the NIDDK (R01DK119268 and R01DK120870). RCK and RSV were supported by NHLBI (R01HL136266). RSV was also supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine. YM-R, DS-A, and KRE were supported by the NHLBI (R01HL136266) and by the National Institute on Aging (R01AG055527). This work was also partially supported by the New York Regional Center for Diabetes Translation Research (P30 DK111022).
Availability of data: The data and computer code used for this analysis reside at Albert Einstein College of Medicine. The HCHS/SOL fully supports data sharing with outside investigators through processes internal to the study, based upon reasonable request in addition to a Data and Materials Distribution Agreement to protect the confidentiality and privacy of the HCHS/SOL participants and their families. Alternatively, deidentified HCHS/SOL data are publicly available at BioLINCC (https://biolincc.nhlbi.nih.gov/home/) and dbGaP (https://www.ncbi.nlm.nih.gov/gap/) for the subset of the study cohort that authorized general use of their data at the time of informed consent.
Supplementary Tables 1–5 and Supplementary Figures 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AHEI, Alternative Healthy Eating Index; HbA1c, hemoglobin A1c; HCHS/SOL, Hispanic Community Health Study/Study of Latinos; MVPA, moderate-to-vigorous physical activity.
Contributor Information
Guo-Chong Chen, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Qibin Qi, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Simin Hua, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Jee-Young Moon, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Nicole L Spartano, Sections of Preventative Medicine and Epidemiology, and Cardiology, Department of Medicine, Boston University School of Medicine, Boston, MA, USA; The Whitaker Cardiovascular Institute, Boston University School of Medicine, Boston, MA, USA.
Ramachandran S Vasan, Departments of Medicine and Epidemiology, Boston University Schools of Medicine and Public Health, Boston, MA, USA.
Daniela Sotres-Alvarez, Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Sheila F Castaneda, Department of Psychology, San Diego State University, San Diego, CA, USA.
Kelly R Evenson, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Krista M Perreira, Department of Social Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Linda C Gallo, Department of Psychology, San Diego State University, San Diego, CA, USA.
Amber Pirzada, Institute for Minority Health Research, University of Illinois at Chicago, IL, USA.
Keith M Diaz, Center for Behavioral Cardiovascular Health, Department of Medicine, Columbia University Medical Center, New York, NY, USA.
Martha L Daviglus, Institute for Minority Health Research, University of Illinois at Chicago, IL, USA.
Marc D Gellman, Behavioral Medicine Research Center, Department of Psychology, University of Miami, Miami, FL, USA.
Robert C Kaplan, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA; Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Xiaonan Xue, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Yasmin Mossavar-Rahmani, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
References
- 1. Ennis SR, Rios-Vargas M, Albert NG. The Hispanic Population: 2010. [Internet]. [cited 2019 May 13]. Available from: http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf. [Google Scholar]
- 2. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021–9. [DOI] [PubMed] [Google Scholar]
- 3. Schneiderman N, Llabre M, Cowie CC, Barnhart J, Carnethon M, Gallo LC, Giachello AL, Heiss G, Kaplan RC, LaVange LM et al. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care. 2014;37(8):2233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aguayo-Mazzucato C, Diaque P, Hernandez S, Rosas S, Kostic A, Caballero AE. Understanding the growing epidemic of type 2 diabetes in the Hispanic population living in the United States. Diabetes Metab Res Rev. 2019;35(2):e3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. US Department of Health and Human Services Physical Activity Guidelines for Americans. 2nd edition [Internet]. 2018 [cited 2019 May 13]. Available from: https://health.gov/paguidelines/secondedition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. [Google Scholar]
- 6. Du Y, Liu B, Sun Y, Snetselaar LG, Wallace RB, Bao W. Trends in adherence to the physical activity guidelines for Americans for aerobic activity and time spent on sedentary behavior among US adults, 2007 to 2016. JAMA Netw Open. 2019;2(7):e197597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haw JS, Galaviz KI, Straus AN, Kowalski AJ, Magee MJ, Weber MB, Wei J, Narayan KMV, Ali MK. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2017;177(12):1808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, Veerman JL, Delwiche K, Iannarone ML, Moyer ML et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Diabetes Association 3. Prevention or delay of type 2 diabetes: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S32–S6. [DOI] [PubMed] [Google Scholar]
- 10. Olds TS, Gomersall SR, Olds ST, Ridley K. A source of systematic bias in self-reported physical activity: the cutpoint bias hypothesis. J Sci Med Sport. 2019;22(8):924–8. [DOI] [PubMed] [Google Scholar]
- 11. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):1. [DOI] [PubMed] [Google Scholar]
- 12. Lavange LM, Kalsbeek WD, Sorlie PD, Aviles-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Welk GJ, Schaben JA, Morrow JR Jr. Reliability of accelerometry-based activity monitors: a generalizability study. Med Sci Sports Exerc. 2004;36(9):1637–45. [PubMed] [Google Scholar]
- 15. Evenson KR, Sotres-Alvarez D, Deng YU, Marshall SJ, Isasi CR, Esliger DW, Davis S. Accelerometer adherence and performance in a cohort study of US Hispanic adults. Med Sci Sports Exerc. 2015;47(4):725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong SL, Colley R, Connor Gorber S, Tremblay M. Actical accelerometer sedentary activity thresholds for adults. J Phys Act Health. 2011;8(4):587–91. [DOI] [PubMed] [Google Scholar]
- 18. Colley RC, Tremblay MS. Moderate and vigorous physical activity intensity cut-points for the Actical accelerometer. J Sports Sci. 2011;29(8):783–9. [DOI] [PubMed] [Google Scholar]
- 19. Hooker SP, Feeney A, Hutto B, Pfeiffer KA, McIver K, Heil DP, Vena JE, Lamonte MJ, Blair SN. Validation of the actical activity monitor in middle-aged and older adults. J Phys Act Health. 2011;8(3):372–81. [DOI] [PubMed] [Google Scholar]
- 20. Aviles-Santa ML, Hsu LL, Arredondo M, Menke A, Werner E, Thyagarajan B, Heiss G, Teng Y, Schneiderman N, Giachello AL et al. Differences in hemoglobin A1c between Hispanics/Latinos and non-Hispanic whites: an analysis of the Hispanic Community Health Study/Study of Latinos and the 2007–2012 National Health and Nutrition Examination Survey. Diabetes Care. 2016;39(6):1010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen GC, Chai JC, Yu B, Michelotti GA, Grove ML, Fretts AM, Daviglus ML, Garcia-Bedoya OL, Thyagarajan B, Schneiderman N et al. Serum sphingolipids and incident diabetes in a US population with high diabetes burden: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Am J Clin Nutr. 2020;112(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siega-Riz AM, Sotres-Alvarez D, Ayala GX, Ginsberg M, Himes JH, Liu K, Loria CM, Mossavar-Rahmani Y, Rock CL, Rodriguez B et al. Food-group and nutrient-density intakes by Hispanic and Latino backgrounds in the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr. 2014;99(6):1487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qi Q, Strizich G, Merchant G, Sotres-Alvarez D, Buelna C, Castaneda SF, Gallo LC, Cai J, Gellman MD, Isasi CR et al. Objectively measured sedentary time and cardiometabolic biomarkers in US Hispanic/Latino adults: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Circulation. 2015;132(16):1560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moon JY, Wang T, Sofer T, North KE, Isasi CR, Cai J, Gellman MD, Moncrieft AE, Sotres-Alvarez D, Argos M et al. Objectively measured physical activity, sedentary behavior, and genetic predisposition to obesity in U.S. Hispanics/Latinos: results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes. 2017;66(12):3001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia. 2016;59(12):2527–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma Y, Hebert JR, Manson JE, Balasubramanian R, Liu S, Lamonte MJ, Bird CE, Ockene JK, Qiao Y, Olendzki B et al. Determinants of racial/ethnic disparities in incidence of diabetes in postmenopausal women in the U.S.: the Women's Health Initiative 1993–2009. Diabetes Care. 2012;35(11):2226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joseph JJ, Echouffo-Tcheugui JB, Carnethon MR, Bertoni AG, Shay CM, Ahmed HM, Blumenthal RS, Cushman M, Golden SH. The association of ideal cardiovascular health with incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Diabetologia. 2016;59(9):1893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Demakakos P, Hamer M, Stamatakis E, Steptoe A. Low-intensity physical activity is associated with reduced risk of incident type 2 diabetes in older adults: evidence from the English Longitudinal Study of Ageing. Diabetologia. 2010;53(9):1877–85. [DOI] [PubMed] [Google Scholar]
- 29. Lucke J, Waters B, Hockey R, Spallek M, Gibson R, Byles J, Dobson A. Trends in women's risk factors and chronic conditions: findings from the Australian Longitudinal Study on Women's Health. Womens Health (Lond). 2007;3(4):423–32. [DOI] [PubMed] [Google Scholar]
- 30. Lindstrom J, Peltonen M, Eriksson JG, Aunola S, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Uusitupa M, Tuomilehto J; Finnish Diabetes Prevention Study Group . Determinants for the effectiveness of lifestyle intervention in the Finnish Diabetes Prevention Study. Diabetes Care. 2008;31(5):857–62. [DOI] [PubMed] [Google Scholar]
- 31. Diabetes Prevention Program Research Group, Crandall J, Schade D, Ma Y, Fujimoto WY, Barrett-Connor E, Fowler S, Dagogo-Jack S, Andres R. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61(10):1075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Drenowatz C, Hill JO, Peters JC, Soriano-Maldonado A, Blair SN. The association of change in physical activity and body weight in the regulation of total energy expenditure. Eur J Clin Nutr. 2017;71(3):377–82. [DOI] [PubMed] [Google Scholar]
- 34. Dugas LR, Kliethermes S, Plange-Rhule J, Tong L, Bovet P, Forrester TE, Lambert EV, Schoeller DA, Durazo-Arvizu RA, Shoham DA et al. Accelerometer-measured physical activity is not associated with two-year weight change in African-origin adults from five diverse populations. PeerJ. 2017;5:e2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barone Gibbs B, Pettee Gabriel K, Carnethon MR, Gary-Webb T, Jakicic JM, Rana JS, Reis JP, Siddique J, Sternfeld B, Lewis CE. Sedentary time, physical activity, and adiposity: cross-sectional and longitudinal associations in CARDIA. Am J Prev Med. 2017;53(6):764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Staiano AE, Martin CK, Champagne CM, Rood JC, Katzmarzyk PT. Sedentary time, physical activity, and adiposity in a longitudinal cohort of nonobese young adults. Am J Clin Nutr. 2018;108(5):946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ekelund U, Kolle E, Steene-Johannessen J, Dalene KE, Nilsen AKO, Anderssen SA, Hansen BH. Objectively measured sedentary time and physical activity and associations with body weight gain: does body weight determine a decline in moderate and vigorous intensity physical activity?. Int J Obes. 2017;41(12):1769–74. [DOI] [PubMed] [Google Scholar]
- 38. Shook RP, Hand GA, Drenowatz C, Hebert JR, Paluch AE, Blundell JE, Hill JO, Katzmarzyk PT, Church TS, Blair SN. Low levels of physical activity are associated with dysregulation of energy intake and fat mass gain over 1 year. Am J Clin Nutr. 2015;102(6):1332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee IM, Shiroma EJ. Using accelerometers to measure physical activity in large-scale epidemiological studies: issues and challenges. Br J Sports Med. 2014;48(3):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keadle SK, Shiroma EJ, Kamada M, Matthews CE, Harris TB, Lee IM. Reproducibility of accelerometer-assessed physical activity and sedentary time. Am J Prev Med. 2017;52(4):541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saint-Maurice PF, Sampson JN, Keadle SK, Willis EA, Troiano RP, Matthews CE. Reproducibility of accelerometer and posture-derived measures of physical activity. Med Sci Sports Exerc. 2020;52(4):876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Corder K, Ekelund U, Steele RM, Wareham NJ, Brage S. Assessment of physical activity in youth. J Appl Physiol. 2008;105(3):977–87. [DOI] [PubMed] [Google Scholar]
- 43. Ekelund U, Lee IM. Will new physical activity guidelines prevent weight gain?. Nat Rev Endocrinol. 2019;15(3):131–2. [DOI] [PubMed] [Google Scholar]
- 44. Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012;125(9):1157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilks DC, Besson H, Lindroos AK, Ekelund U. Objectively measured physical activity and obesity prevention in children, adolescents and adults: a systematic review of prospective studies. Obes Rev. 2011;12(5):e119–29. [DOI] [PubMed] [Google Scholar]
- 46. Chin SH, Kahathuduwa CN, Binks M. Physical activity and obesity: what we know and what we need to know. Obes Rev. 2016;17(12):1226–44. [DOI] [PubMed] [Google Scholar]
- 47. Ryan AS. Insulin resistance with aging: effects of diet and exercise. Sports Med. 2000;30(5):327–46. [DOI] [PubMed] [Google Scholar]
- 48. Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med. 2017;2(1):e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.