Abstract
Aim:
The aim is to compare the effects of diode laser, GC tooth mousse, and sodium fluoride (NaF) varnish on dentinal hypersensitivity by scanning electron microscopic (SEM) evaluation.
Materials and Methods:
Forty extracted human maxillary first premolar teeth were selected to prepare dentin disc of 2 mm thickness. The specimens were divided into four groups of 10 discs each-Group 1: Negative control, Group 2: Diode laser, Group 3: GC tooth mousse, and Group 4: NaF varnish. Diode laser and NaF was exposed on dentin disc for three consecutive days. GC tooth mousse was applied twice a day for 1 week. All the specimens were examined under SEM at a magnification of ×1500. The standardized SEM microphotographs were analyzed according to the scoring criteria.
Statistical Analysis:
Friedman test with post hoc Wilcoxon sign rank test for pair wise comparison at different time intervals within the group was computed using SPSS version 20. A P < 0.05 was regarded as statistically significant.
Results:
The results showed that the use of diode laser was able to achieve complete occlusion of dentinal tubules followed by the use of GC tooth mousse and that of NaF varnish while control group showed completely open tubules.
Conclusions:
Irradiation of dentinal tubules using diode laser could be effective for routine clinical treatment of dentinal hypersensitivity compared to NaF and GC tooth mousse.
Keywords: Dentinal hypersensitivity, diode laser, GC tooth mousse, scanning electron microscope, sodium fluoride
INTRODUCTION
Dentinal hypersensitivity (DH) has been defined as “pain derived from exposed dentin in response to chemical, thermal, tactile, or osmotic stimuli which cannot be explained as arising from any other dental defect or disease.”[1] This is one among the most common problems a dentist comes across in daily practice. Dentinal hypersensitivity (DH), Dentin hypersensitivity/cervical hypersensitivity/root hypersensitivity/cemental hypersensitivity/sensitivity all convey the same clinical representation and can be used interchangeably.[2,3]
In literature, several hypotheses were formulated to explain the cause of DH such as Direct Innervation Theory, Odontoblast Receptor Theory, and fluid movement/hydrodynamic theory.[4] Brannstorm in 1964, proposed the theory of DH, which is the most widely accepted theory. This theory explains that DH is due to the movement of the fluid inside the dentinal tubules which further stimulates the baroreceptors, causing neural discharge.[3] Some stimuli such as cooling, drying, evaporation, and hypertonic chemical cause the dentinal fluid to flow away from the dentin-pulp complex and leads to an increase in pain such as while others such as heat, cause the fluid to flow towards the pulp.[4]
Reducing dentin permeability and reducing the intradental nerve response to fluid shifts are the two main mechanisms to desensitize the dentin. There are many products in the market which reduce DH by reducing the permeability such as laser therapy and application of different materials such as fluoride, hydroxyapatite, strontium chloride, zinc chloride, potassium chloride as well as dental adhesive, glass ionomer cement, oxalate, bioglass, portland cement, and casein phosphopeptide-amorphous calcium phosphate.[5]
Based on its active medium, wavelength, power density as well as the optical properties of the target tissue, a laser produces different changes in the tissue. Lasers used for the treatment of DH can be divided into two categories:[6]
-
Low output power (low-level) lasers
- Helium-neon and gallium/aluminum/arsenide (diode lasers)
-
Middle output power lasers
- Neodymium-doped yttrium aluminum garnet and carbon dioxide lasers.
The laser works by two mechanisms on sensitive teeth. One is by melting the dentin and occluding the dentinal tubules. The second mechanism is by dentin desiccation after laser irradiation, which can bring some temporary relief of DH.[6] Diode laser has shown a reduction in DH equal or superior to other conventional treatments.[7]
At-home treatment, like the application of GC tooth mousse, is another reliable treatment option. Demineralizing agents like GC tooth mousse contains a high concentration of neutral sodium fluoride (NaF), stannous fluoride, and strontium fluoride, which can form mineral precipitates. These precipitates physically seal dentinal tubules with a plug that resists normal pulpal pressures and acid challenge and reduces dentinal fluid flow.[8]
Another commonly advised method is the application of NaF varnish. Dental varnishes remain on the tooth surface for hours, which allows the varnish base to penetrate deep into the dentinal tubules and obstruct tubule fluid movements. It further releases high concentrations of fluoride ions from time to time forming fluorapatite and calcium fluoride (CaF2).[9,10]
In clinical settings, the commonly used topical agents for DH are GC tooth mousse and varnishes. The aim of this in vitro study is to compare the effects of diode laser, GC tooth mousse, and NaF varnish on DH by scanning electron microscopic (SEM) evaluation.
MATERIALS AND METHODS
Forty healthy human maxillary first premolar teeth extracted for orthodontic purposes were collected for this study. Specimens with the following findings (if any) were excluded from the study: Crown or root surface caries, restorations or fillings, tooth crack or fractures, history of pulp or periapical pathology, and dentin sclerosis. Teeth were cleaned thoroughly, disinfected in 5% sodium hypochlorite solution for 1 h and stored in distilled water. A dentin disc of two mm thickness was obtained using a diamond disc from the crown. Subsequently, the dentin discs were polished using a carbide abrasive paper. For smear layer removal, all the specimens were dipped in 17% ethylenediaminetetraacetic acid for 1 min.
An exposed surface was marked on each disc, and other parts of the disc were covered with protective tape. The specimens were divided into four groups of ten discs each:
Group 1: (negative control): No treatment material was applied
Group 2: Specimens were treated with diode laser
Group 3: Specimens were treated with GC tooth mousse. (GC, Melbourne, Australia)
Group 4: NaF 5% varnish was applied (Fluor protector by Ivoclar Vivadent).
The present study simulates practical clinical application of each agent. In general, pastes are instructed to be applied twice a day but laser exposure and varnish application can only be done in a clinical setting with the usual number of exposures and applications being three. Therefore, GC tooth mousse was applied twice a day for 3 min with 12 h interval for a week. Diode laser exposure and varnish application were done on dentin disc for 3 consecutive days. The specimens were kept in distilled water after each treatment.
All the specimens were washed with distilled water, dried in a desiccator for 2 days, and sputter-coated with a thin gold layer. All the specimens were examined under SEM (Zeiss sigma VP, Zeiss Oberkochen, Germany) at a magnification of ×1500. SEM images were categorized as follows:
Occluded (100% of tubules occluded)
Mostly occluded (50% to <100% of tubules occluded)
Partially occluded (25% to <50% of tubules occluded)
Mostly unoccluded (<25% of tubules occluded)
Unoccluded (0%, no tubule occlusion).
The standardized SEM microphotographs were scored using above criteria by two blind observers.
Statistical analysis
Descriptive statistics such as mean, standard deviation was calculated. Inferential statistics like Kruskal–Wallis was computed to find the difference among the groups; Friedman test with post hoc Wilcoxon sign-rank test for pairwise comparison at different time intervals within the group was computed using SPSS (Statistical Package for Social Sciences) version 20 (IBM Corp., Armonk, N.Y., USA). A P < 0.05 was regarded as statistically significant.
RESULTS
The present study evaluated the occlusion of dentinal tubules by diode laser, NaF varnish, and GC tooth mousse. The average scores by the two observers were selected and considered in this study [Figure 1].
Figure 1.
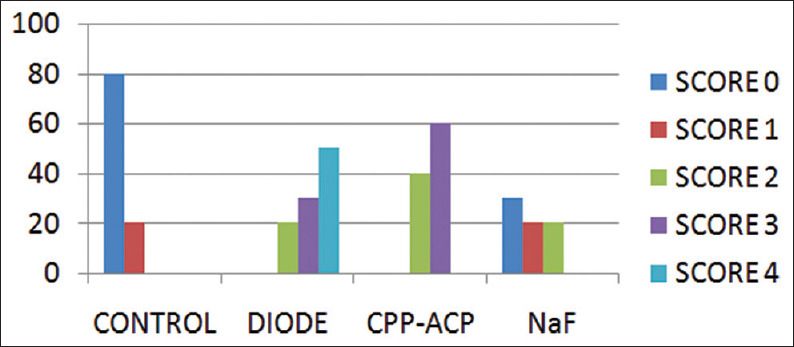
Comparison of occlusion of dentinal tubules scored by 2 blind observers for four Groups
Group 1 (negative control group) showed 80% samples with score 0 and 20% with score 1 [Figure 2].
Figure 2.
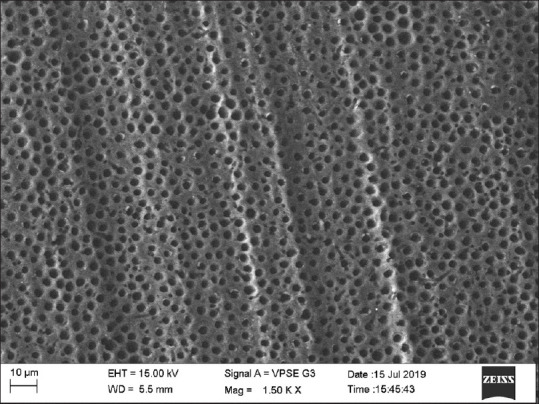
Scanning electron microscopic image of control group
Group 2 (diode group) showed 20% samples with score 3, 30% with score 4, and 50% samples with score 4 [Figure 3].
Figure 3.
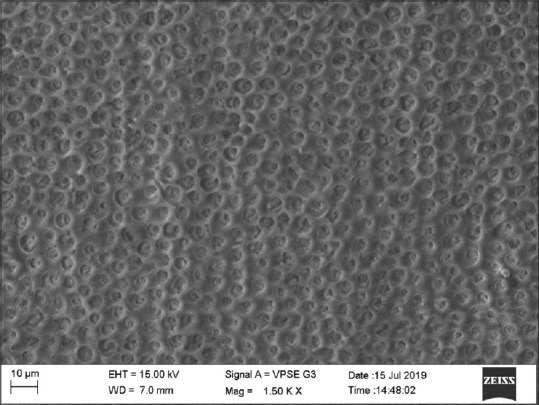
Scanning electron microscopic image of diode group
Group 3 (GC tooth mousse) showed 40% samples showing score 0 and 60% showing score 1 [Figure 4].
Figure 4.
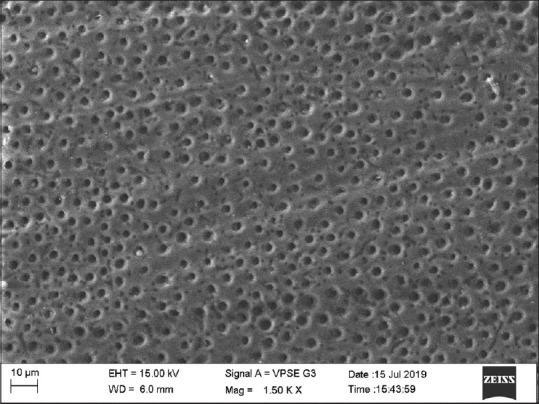
Scanning electron microscopic image of casein phosphopeptide-amorphous calcium phosphate group
Group 4 (NaF) showed 30% samples with score 0, 50% with score 1, and 20% samples with score 2 [Figure 5].
Figure 5.
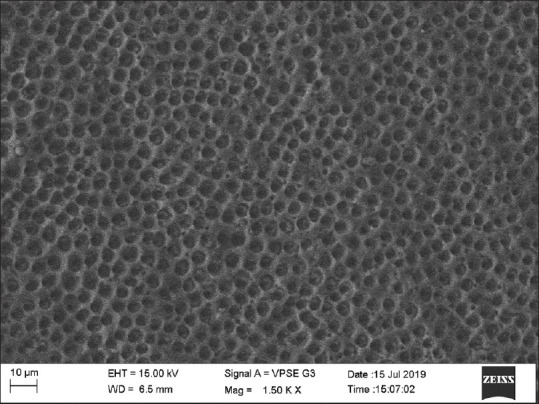
Scanning electron microscopic image of NaF group
The results showed that the use of diode laser was able to achieve complete occlusion of dentinal tubules followed by the use of GC tooth mousse and that of NaF varnish while control group showed completely open tubules.
DISCUSSION
DH is a common clinical situation.[4] Irrespective of the location (Buccal/lingual/palatal/occlusal), any exaggerated pain experienced by the patient to certain stimuli is termed as DH.[11,12] True hypersensitivity can develop due to pulpal inflammation, which shows the clinical features of irreversible pulpitis, i.e., severe and persistent pain, as compared to a typical short sharp pain of DH.[13]
DH develops in two events: Lesion localization and lesion initiation. Loss of enamel through attrition, abrasion, erosion, abfraction, gingival recession due to toothbrush abrasion, pocket reduction surgery, tooth preparation for a crown, excessive flossing, or secondary to periodontal diseases removes the protective covering of dentin, thereby exposing it to the external environment. This causes lesion localization, thereby exposing and opening the dentinal tubules by removing the protective shield of smear layer.[4,14,15]
According to Brannstrom's hydrodynamic theory, a stimulus applied to open tubules of dentin increases the flow of dentinal tubular fluid,[16] which causes mechanical deformation of the nerves, i.e., A delta fibers[17] located at the inner ends of the tubules or in the outer layers of the pulp.[18]
The dentin, which is sensitive to certain stimuli, is permeable through its thickness; any treatment that reduces dentinal permeability must reduce DH but sometimes DH remains the same even after effective sealing of the tubules, indicating that further mechanisms are involved in nerve activation instead of or in addition to the hydrodynamic mechanism.[19]
In Group 1, no agents were applied to the specimens. After etching, the SEM images showed completely open tubules. This was considered as the control group for comparison. All the specimens in this group showed completely opened tubules, which were scored by the observers as score 4.
According to Asnaashari and Moeiniand Aranha and Eduardo Cde low power laser therapy gives biomodulatory effects, minimizes pain, and reduces inflammatory processes.[20,21] Diode laser acts on DH by two mechanisms. First, by a melting effect with the crystallization of dentin's inorganic component and the coagulation of fluids contained in the dentinal tubules. Second, by reducing the pulpal nerve's pain threshold.[22] Among lasers, diode lasers are the most studied and gave the best results in several clinical protocols even in high-grade DH cases.[19] In our study, based on observers scoring, the laser group showed scores 0 and 1 suggesting complete occlusion of tubules, which can reduce DH drastically.
Remineralizing agents can promote the partial occlusion of open dentinal tubules by deposition of mineral aggregates, for example, high concentration neutral NaF, stannous fluoride, and strontium fluoride gels.[23] The peptides of the Recaldent bind to the dentin surface facilitating mineral deposits to occlude dentin tubules. This process reduces the diameter of dentinal tubules.[8]
In the present study, we used FV (Fluor protector by Ivoclar Vivadent), which was originally developed in 1975 by Arends and Schuthof. The first and most commonly used therapy for DH is the use of fluorides in the form of pastes, rinses, varnishes, etc.[24] Flourides are generally described as a caries preventive agent but it is also a treatment option for DH. It forms precipitates of CaF2 crystals inside the dentinal tubules. Various fluoride formulations include NaF, stannous fluoride, sodium monofluorophosphate, fluorosilicates, and fluoride combined with iontophoresis.[14]
Similar study was conducted by Jayaram et al. evaluating diode laser along with 1.23% acidulated phosphate fluoride gel on dentinal tubule occlusion and found diode laser to be more effective.[25] Ghoneim and Aboelsaad evaluated the efficacy of diode laser combined with two in-office desensitizing agents 8% arginine-calcium carbonate and 1.23 NaF varnish on reducing DH. It was concluded that desensitizing agents plus soft laser irradiation reduced DH.[26] Several studies have reported that the application of Diode laser could be used safely in endodontic treatment and in root canal disinfection. However, few studies have documented the interaction of diode laser energy with the dentin surface and the ensuing structural alterations. More studies are required to determine whether diode laser can treat DH effectively.[27] As described by Lutin ND et al. the properties of an ideal desensitizing technique/material, are easy application, nonirritating to the pulp tissue, painless, consistent and effective in long term, quick acting, and produce no discoloration.[28] Therefore, in the present study, diode laser irradiation which is becoming increasingly popular in dentistry and GC tooth mousse and NaF which are clinically available and easy to use are compared.
However, several limitations of this methodology are still present and acknowledged. First, the depth of penetration of the agents into the dentinal tubules was not estimated, as increased penetration leads to more lasting relief from DH. The mechanism of action of laser in occluding dentinal tubules was also not assessed. Only a surface examination of dentinal tubules was performed following diode laser application by two blind observers. The resistance of these occluded tubules to acid challenge was not evaluated.[25] Another limitation of this study was that distilled water was used to store the samples instead of solutions such as artificial saliva, phosphate-buffered saline which better replicate clinical conditions. Nevertheless, significant dentinal tubule occlusion was seen in all three groups despite use of distilled water.
CONCLUSIONS
Under the limitations of the present in vitro study, it can be concluded that the application of laser followed by GC tooth Mousse and fluoride varnish is effective in the reduction of dentin permeability.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69:221–6. [PubMed] [Google Scholar]
- 2.Porto IC, Andrade AK, Montes MA. Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci. 2009;51:323–32. doi: 10.2334/josnusd.51.323. [DOI] [PubMed] [Google Scholar]
- 3.Davari A, Ataei E, Assarzadeh H. Dentin hypersensitivity: Etiology, diagnosis and treatment; A literature review. J Dent (Shiraz) 2013;14:136–45. [PMC free article] [PubMed] [Google Scholar]
- 4.Miglani S, Aggarwal V, Ahuja B. Dentin hypersensitivity: Recent trends in management. J Conserv Dent. 2010;13:218–24. doi: 10.4103/0972-0707.73385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghafournia M, Tehrani MH, Nekouei A, Faghihian R, Mohammadpour M, Feiz A. In vitro evaluation of dentin tubule occlusion by three bioactive materials: A scanning electron microscopic study. Dent Res J (Isfahan) 2019;16:166–71. [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Saud LM, Al-Nahedh HN. Occluding effect of Nd: YAG laser and different dentin desensitizing agents on human dentinal tubules in vitro: A scanning electron microscopy investigation. Oper Dent. 2012;37:340–55. doi: 10.2341/10-188-L. [DOI] [PubMed] [Google Scholar]
- 7.Jain PR, Naik GD, Uppor SA, Kamath DG. Diode laser and fluoride varnish in the management of dentin hypersensitivity. J Interdiscip Dentistry. 2015;5:71–4. [Google Scholar]
- 8.Walsh LJ. The effects of GC tooth mousse on cervical dentinal sensitivity: A controlled clinical trial. S Afr dent J. 2010;12:4–12. [Google Scholar]
- 9.Petersson LG. The role of fluoride in the preventive management of dentin hypersensitivity and root caries. Clin Oral Investig. 2013;17(Suppl 1):S63–71. doi: 10.1007/s00784-012-0916-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George G, Ranjini MA, Pai VS, Darsan J, Nadig RR. Evaluation of dentinal tubule occlusion using a desensitizing toothpaste and mouthwash for a period of 60 days: Scanning electron microscopy analysis. J Interdiscip Dentistry. 2018;8:96–101. [Google Scholar]
- 11.Addy M. In: Tooth Wear and Sensitivity. London: Martin Dunitz; 2000. Dentin hypersensitivity: Definition, prevalence distribution and aetiology; pp. 239–48. [Google Scholar]
- 12.Addy M. Etiology and clinical implications of dentine hypersensitivity. Dent Clin North Am. 1990;34:503–14. [PubMed] [Google Scholar]
- 13.Trowbridge HO. Mechanism of pain induction in hypersensitive teeth. In: Rowe NH, editor. Hypersensitive Dentin: Origin and Management. Ann Arbor, USA: University of Michigan; 1985. pp. 1–0. [Google Scholar]
- 14.Orchardson R, Gillam DG. Managing dentin hypersensitivity. J Am Dent Assoc. 2006;137:990–8. doi: 10.14219/jada.archive.2006.0321. [DOI] [PubMed] [Google Scholar]
- 15.Dababneh RH, Khouri AT, Addy M. Dentine hypersensitivity-An enigma.A review of terminology, mechanisms, aetiology and management? Br Dent J. 1999;187:606–11. doi: 10.1038/sj.bdj.4800345. [DOI] [PubMed] [Google Scholar]
- 16.Matthewsand B, Vongsavan N. “Interactions between neural and hydrodynamic mechanisms in dentin and pulp”. Arch Oral Biol. 1994;39:87–95. doi: 10.1016/0003-9969(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 17.Ladalardo TC, Pinheiro A, Campos RA, Brugnera Júnior A, Zanin F, Albernaz PL, Weckx LL. Laser therapy in the treatment of dentin hypersensitivity. Braz Dent J. 2004;15:144–50. doi: 10.1590/s0103-64402004000200011. [DOI] [PubMed] [Google Scholar]
- 18.Brannstrom M. “A hydrodynamic mechanism in the transmission of pain-producing stimuli through dentin,” in sensory mechanism in dentin. In: Anderson DJ, editor. J Oxford. UK: Pergamon; 1963. pp. 73–9. [Google Scholar]
- 19.Umberto R, Claudia R, Gaspare P, Gianluca T, Alessandro del V. Treatment of dentine hypersensitivity by diode laser: A clinical study. Int J Dent. 2012:1–8. doi: 10.1155/2012/858950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asnaashari M, Moeini M. Effectiveness of lasers in the treatment of dentin hypersensitivity. J Lasers Med Sci. 2013;4:1–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Aranha AC, Eduardo Cde P. Effects of Er: YAG and Er, Cr: YSGG lasers on dentine hypersensitivity.Short-term clinical evaluation. Lasers Med Sci. 2012;27:813–8. doi: 10.1007/s10103-011-0988-9. [DOI] [PubMed] [Google Scholar]
- 22.Kimura Y, Wilder-Smith P, Yonaga K, Matsumoto K. Treatment of dentin hypersensitivity by lasers: A review. J Clin Periodontol. 2002;27:715–21. doi: 10.1034/j.1600-051x.2000.027010715.x. [DOI] [PubMed] [Google Scholar]
- 23.Sensabaugh C, Sagel ME. Stannous fluoride dentifrice with sodium hexametaphosphate: Review of laboratory, clinical and practice-based data. J Dent Hyg. 2009;83:70–8. [PubMed] [Google Scholar]
- 24.Aghanashini S, Puvvalla B, Nadiger S, Mundinamanae DB, Bhat D, Andavarapu S. Comparative evaluation of diode laser and fluoride varnish for treatment of dentin hypersensitivity: A clinical stud. J Interdiscip Dentistry. 2018;8:110–7. [Google Scholar]
- 25.Jayaram P, Coutinho AO, Bhadranna A, Chatterjee A, Raghunathan V, Imran F. Evaluation of Diode laser along with 1.23% acidulated phosphate fluoride gel on dentinal tubule occlusion: Anin vitro study. J Indian Soc Periodontol. 2020;24:253–8. doi: 10.4103/jisp.jisp_341_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghoneim MM, Aboelsaad N. Treatment of dentinal hypersensitivity using desensitizing agents plus soft laser irradiation.A randomized comparative clinical trial. Egypt Dent J. 2020;66:397–403. [Google Scholar]
- 27.Sameera U, Bilichodmath S, Paul P. Evaluation of the efficacy of strontium chloride, biodentin® and biodentin® in combination with diode laser in the management of dentinal hypersensitivity-An in vitro SEM Study. J Int Acad Periodontol. 2019;22:74–81. [PubMed] [Google Scholar]
- 28.Lutins ND, Greco GW, McFall WT., Jr Effectiveness of sodium fluoride on tooth hypersensitivity with and without iontophoresis. J Periodontol. 1984;55:285–8. doi: 10.1902/jop.1984.55.5.285. [DOI] [PubMed] [Google Scholar]


