Abstract
We describe the creation of a mass spectral library of acylcarnitines and conjugated acylcarnitines from the LC-MS/MS analysis of six NIST urine reference materials. To recognize acylcarnitines, we conducted in-depth analyses of fragmentation patterns of acylcarnitines and developed a set of rules, derived from spectra in the NIST17 Tandem MS Library and those identified in urine, using the newly developed hybrid search method. Acylcarnitine tandem spectra were annotated with fragments from carnitine and acyl moieties as well as neutral loss peaks from precursors. Consensus spectra were derived from spectra having similar retention time, fragmentation pattern and the same precursor m/z and collision energy. The library contains 157 different precursor masses, 586 unique acylcarnitines, and 4,332 acylcarnitine consensus spectra. Furthermore, from spectra that partially satisfied the fragmentation rules of acylcarnitines, we identified 125 conjugated acylcarnitines represented by 987 consensus spectra, which appear to originate from Phase II biotransformation reactions. To our knowledge, this is the first report of conjugated acylcarnitines. The mass spectra provided by this work may be useful for clinical screening of acylcarnitines as well as for studying relationships among fragmentation patterns, collision energies, structures, and retention times of acylcarnitines. Further, these methods are extensible to other classes of metabolites.
Keywords: Acylcarnitine, conjugated acylcarnitine, metabolite, mass spectral library
Graphical Abstract
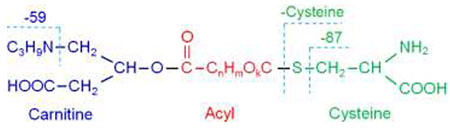
A generalized structure of cysteinylated acylcarnitines.
1. Introduction
The identification of small-molecule metabolites in biological samples is of importance in the diagnosis, treatment, and monitoring of human diseases. For a single metabolite mass with a given elemental composition, many isomers may exist, including structural, positional, stereo isomers as well as chromatographically inseparable enantiomers and separable diastereomers. The high degree of heterogeneity of metabolite structures and inherent difficulties in deriving chemical structures from mass spectra are major impediments in metabolite identification.
In metabolomics studies, acylcarnitines have been widely used to diagnose a variety of inherited and acquired diseases2–6. This is due to their biological functions of transferring long-chain fatty acids into mitochondria for energy production and removing potential toxic excesses of acyl groups.1–6
Although MS analyses of underivatized and derivatized acylcarnitines in samples of newborns and patients have been performed since the 1980s,7–22, acquiring a full profile of this chemical class from bio-samples remains a major challenge. Two studies have attempted to generate such a full profile. One was published in 2011 by Zuniga and Li23. Their method involved a solid phase extraction (SPE) technique for selective analyte extraction, an ultra-HPLC separation, and a targeted tandem MS analysis with information-dependent acquisition and selected reaction monitoring. The authors reported 355 unique acylcarnitines in a urine sample pooled from six healthy individuals. The authors also noted that certain very hydrophilic and hydrophobic acylcarnitines were not detectable in their experiments. This work presented valuable methods for analyzing acylcarnitines, but had an important limitation in that the spectra were acquired on a low mass resolution instrument. The other study was published in 2018 by Yu and co-authors14. They adopted a two-step method for acquiring all possible acylcarnitine spectra. The first step was to detect all acylcarnitine precursors based on characteristic fragments, resulting in 298 precursor ions. The second step was to acquire acylcarnitine spectra with “multirun LC-full scan MS combined with parallel reaction monitoring acquisition” using the identified precursors24. Due to limited sampling speed, a single run covered only 30 precursor ions, requiring 10 runs for the 298 precursor ions. With a mass spectrometer and three collision energies, the authors detected 241, 515, and 222 acylcarnitines from samples of human plasma (pooled from 36 healthy adults), human urine (24 healthy adults), and rat liver tissue, respectively. In total, they reported 733 different acylcarnitines.
The abovementioned targeted studies demonstrated that many acylcarnitines are detectable in biological samples. However, currently, there is no efficient way of identifying these compounds in complex biological samples. Providing such a method through mass spectral library searching is a key objective of this work. This is done using HCD mass spectra acquired with nine collision energies from an untargeted LC-MS/MS analysis of urine reference materials and a metabolite-class targeting method for making identifications. The major strategies used in this work include: (1) establish rules for detecting acylcarnitines based on the analyses of reference spectra in the NIST17 Tandem MS Library25 and spectra retrieved by the recently developed “hybrid” search method;26–28 (2) annotate acylcarnitine spectra by fragments of carnitine and acyl moieties, as well as by neutral loss fragments; (3) exclude artifacts, such as in-source ion spectra and acylcarnitine-contaminated spectra. We provide the resulting mass spectral library for 586 acylcarnitines and 125 conjugated acylcarnitines with freely available search software.
2. Materials and Experiment
Six pooled NIST standard reference materials (SRM)29 served as the urine samples analyzed in this study. The details are provided in Table S1 of the supplemental file AcylcarnitineInfo.docx.
The experimental procedure was previously reported,29,30 and is also provided in the part 1 of the supplementary file AcylcarnitineInfo.docx. Briefly, this involves four steps: (1) urine samples were thawed from −80 °C to room temperature; (2) diluted with methanol (1:1, v/v) and centrifuged; (3) the supernatant was collected and subjected to evaporation under a gentle nitrogen stream; (4) the dried sample was reconstituted with 10 % ACN and 0.1 % formic acid.
Reverse phase separations were made on a waters ACQUITY UPLC CSH-C18 column using of a Dionex Ultimate 3000 liquid chromatograph. LC-ESI MS and tandem spectra were recorded by orbitrap Fusion Lumos (Thermo Scientific, USA). The MS1 scan resolution was 120,000. To acquire HCD spectra, data dependent sampling was performed using an exclusion duration of 18 seconds, an isolation window of ±0.75 m/z, and a resolution of 30,000. The HCD spectra of each sample were obtained in duplicates under 9 normalized collision energies (NCE, 10, 15, 20, 25, 30, 40, 50, 60, and 80). At each collision energy, 12 runs were made (2 runs per sample). The dataset used in this study includes 108 individual runs in total. These runs were part of a larger series used to develop a general ‘recurrent spectral library’.29
3. Data analysis methods
3.1. Extraction of acylcarnitine feature fragments and fragmentation patterns
(a). From spectra in the NIST metabolite library.
Acylcarnitine feature fragments and fragmentation patterns were derived from spectra in the NIST17 Tandem MS Library, which contains mass spectra of commercially available metabolites at multiple collision energies. For this study, only protonated high-resolution acylcarnitine HCD Orbitrap library spectra were considered, which included 308 spectra of 27 acylcarnitines. These spectra were used to extract acylcarnitine fragmentation features. See the table ES1 in the supplemental file AcylcarnitineInfo.xlsx for these acylcarnitines.
(b). From the Hybrid search.
The NIST Hybrid Search, a recently developed algorithm applicable to unidentified spectra,26–28 was used to detect a larger diversity of putative acylcarnitines. While only 20 acylcarnitines could be identified by a direct mass spectral library matching program in a urine run against the NIST17 Tandem MS Library, hybrid searching provided 150 candidates with match scores larger than 500 (the highest score is 999). This large number of candidates provided a diversity of examples on which to base more general rules for recognizing acylcarnitine spectra. The hybrid search algorithm performs an analysis that determines whether unmatched fragment ions can be explained as a consequence of the library and query compound differing by a chemical group or atom that has no major effect on the fragmentation mechanism. The hybrid search program used in this study is NIST MS PepSearch31, developed for batch processing of both proteomics and metabolomics datasets.
3.2. Recognition of acylcarnitine
We characterize acylcarnitines based on four spectral feature fragments, i.e, C4H5O2+ (m/z: 85.0284); TMAI (trimethylamine ion C3H10N+, m/z: 60.0808); neutral loss fragment TMA (trimethylamine, C3H9N) or (TMA + H2O); and C7H14NO2+ (carnitine – H2O + H+) or P – C7H13NO2 under typical collision energies (NCE 20 to 40). After an acylcarnitine and its precursor m/z and retention time (RT) were determined, all spectra acquired at the same m/z and retention time (deviation < 7 s) under other collision energies were assigned to the same acylcarnitine, without requiring the presence of all the four feature fragments in these spectra.
Determination of acylcarnitine ion formula and m/z data.
We manually confirmed the formula and m/z value of each unknown acylcarnitine on the basis of its precursor and spectral fragment m/z values. Because acylcarnitine masses are relatively small (< 600 Da), and the resolving power we used is high (120,000), chemical formulas could be readily derived for almost all ions. An acceptance threshold was set at 5 ppm.
Calculation and assignment of ion retention time and abundance.
In LC-MS, each ionized compound is associated with a set of MS1 (LC-MS) centroided peaks with associated isotopes, abundances and retention times. This set of peaks is denoted as an ion cluster. The key properties of a cluster include monoisotopic m/z, charge state, abundance, and RT. In this work, each ion has a cluster RT, which is calculated as the time when the accumulated abundance of the most abundant isotope reaches half of its total abundance. All spectra belonging to the same ion are assigned with the same cluster RT. Two abundance values for each ion were derived in this study: the extracted ion chromatogram (XIC) and the largest peak of an ion cluster. All cluster properties of the 108 urine runs were processed. The programs performing these calculations were described previously as the NIST ProMS and NIST MSQC pipeline.32–33
3.3. Annotation of acylcarnitine fragments
Our fragmentation analysis found that acylcarnitine spectra can be annotated using three types of fragments:
Fragments from decomposition of the carnitine moiety, such as TMAI (m/z: 60.0808) and C4H5O2+ (m/z: 85.0284); a list of all the relevant fragments is provided in Table S2 of the supplemental file AcylcarnitineInfo.docx;
Fragments from decomposition of the acyl moiety, including losses of CO, H2O, C2H4, C2H2O, CH2O2, C2H4O2, etc.;
Neutral losses from the acylcarnitine precursor ion, such as TMA, H2O, CH2O2, C2H4O2 and C7H13NO2, or a combination of two or more neutral losses, such as TMA + H2O, TMA + 2H2O, TMA + CH4O3, etc.
For more descriptions of the annotations, see part 3 of the supplemental file AcylcarnitineInfo.docx.
4. Result and Discussion
4.1. Acylcarnitine fragmentation patterns, feature and neutral loss fragments
An acylcarnitine is an esterified product of carnitine and a fatty acid, which is represented in solution as a zwitterion. A protonated form is presented in Structure 1.
Structure 1.
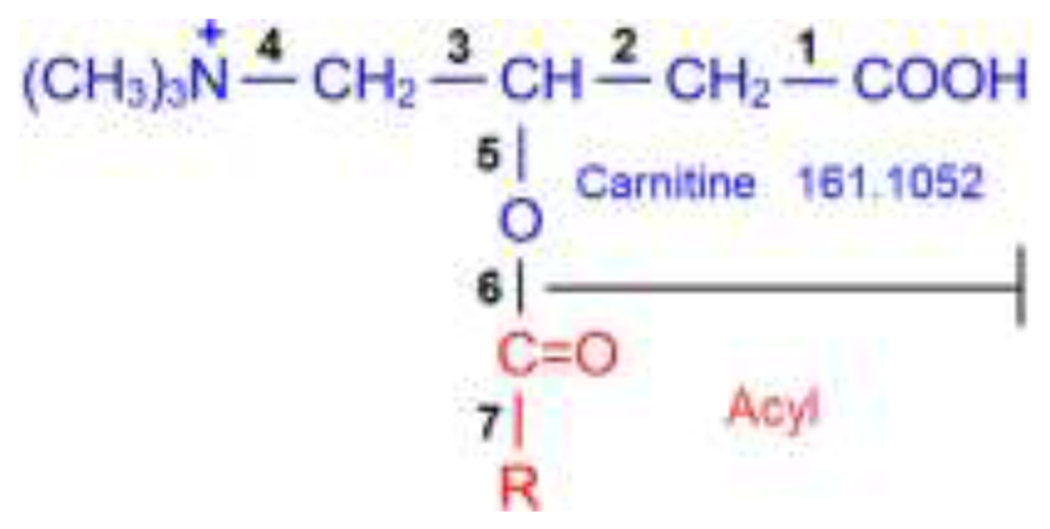
A representative structure of a protonated acylcarnitine. C(=O)R is derived from its precursor fatty acid.
Under typical collision energies, such as from NCE 20 to 40, the acylcarnitine spectra usually contain fragments from carnitine and acyl moieties as well as neutral losses from both. These fragments provide not only information for recognizing acylcarnitines, but also clues of their structures. A brief description of these fragments is given in the following section (see part 4 of the supplemental file AcylcarnitineInfo.docx for more information).
Carnitine moiety related fragments
Acylcarnitine mass spectra commonly contain three well-known feature fragments,7,8,12 i.e., C4H5O2+, TMAI, and neutral loss of TMA or (TMA + H2O). Acylcarnitine HCD spectra in the NIST17 Tandem MS Library at multiple collision energies indicate that fragments C4H5O2+ (m/z: 85.0284) and TMAI (m/z: 60.0808) can always be observed, except for spectra of larger mass acylcarnitines under low collision energies. For example, spectra of acylcarnitine ion C23H38NO4 (m/z 392.279, RT around 1335 s) have observable fragments P – TMA only at NCE 10, have both C4H5O2+ and P – TMA at NCE 15 and have all the three fragments at NCE 20.
Fragment C4H5O2+ is the most significant feature peak of acylcarnitine spectra. Its abundance, under commonly used collision energies, is consistently the largest or among the most abundant peaks, but may decrease at higher collision energies, such as when NCE > 80. Figure 1 shows the relationships between abundances of the major carnitine fragments and collision energies for (R)-butyrylcarnitine. The ion C4H5O2+ is not unique to acylcarnitine; many non-acylcarnitines can exhibit this fragment. For example, among the total 80396 HCD spectra in the NIST17 Tandem MS Library, 3874 (4.8 %) spectra contain fragment C4H5O2+, far exceeding the number of acylcarnitine spectra.
Figure 1.
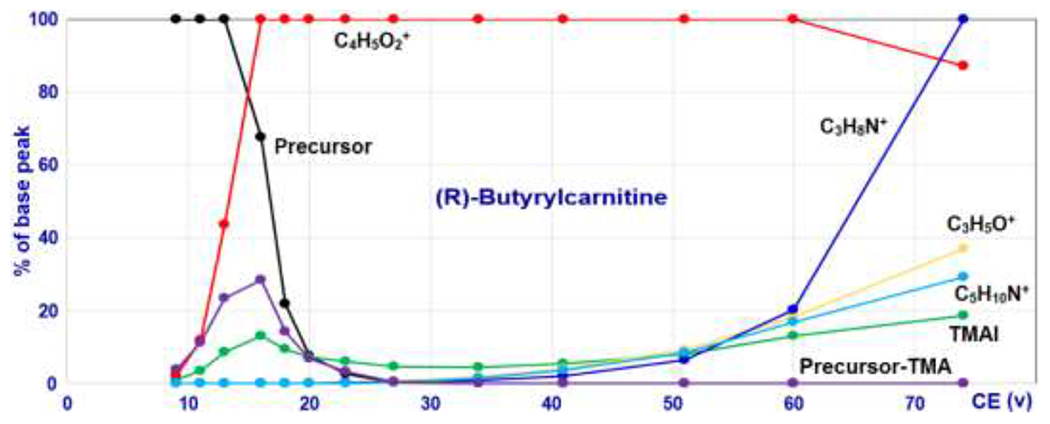
Intensities of the major carnitine moiety-related fragments of (R)-Butyrylcarnitine at different collision energies. Data source: NIST17 Tandem MS Library.
The second feature fragment is TMAI. Its intensities at different collision energies show a non-monotonic pattern (green line in Figure 1), which is unique in comparison with the other fragments in the acylcarnitine spectra. TMAI co-maximizes with C4H5O2+, and then decreases with the increase in collision energy. At higher collision energies, TMAI intensities gradually increase again, generated by secondary decomposition of other ions. Although this ion is a key diagnostic fragment of acylcarnitine, it also occurs in spectra of other compounds, such as betaines, choline, N-trimethyllysine and their derivatives.
The third feature fragment is the neutral loss peak of TMA (mass: 59.0735) from the precursor. This fragment is relatively abundant at low collision energies, and sometimes even more abundant than the ion C4H5O2+. A mass spectrum obtained at a lower collision energy lacking this characteristic can be confidently rejected. This fragment is not stable at higher collision energy. It maximizes as does TMAI, then, its abundance decreases quickly and disappears at higher collision energies.
A valuable usage of the neutral loss of TMA fragment is to recognize ‘contaminated’ spectra. This is because non-acylcarnitine spectra can contain acylcarnitine fragments due to their coelution and co-fragmentation with acylcarnitines. The figure S9 (see part 7.2 of the supplemental file AcylcarnitineInfo.docx) is an example, which shows that a non-acylcarnitine spectrum with precursor at m/z 344.2283 contains abundant acylcarnitine fragments. This spectrum can be determined as non-acylcarnitine because it does not have the neutral loss peak of TMA at m/z: 285.1548 (= 344.2283 – 59.0735). Further analysis revealed that this spectrum was contaminated by a coeluting acylcarnitine at m/z 344.2076. Such co-fragmentation presents a serious concern in developing libraries derived from complex materials.
Another useful diagnostic fragment is C7H14NO2+ (= carnitine – H2O, m/z = 144.1019), which arises from the cleavage of the bond 5 in Structure 1. Zuniga and Li discussed the formation of this fragment.23 Generally, its abundance is low, less than 20 % of the base peak. It does not appear in Figure 1 because its relative abundance is less than 4 %. But it is relatively stable and can be detected even up to 70 V or higher for larger acylcarnitines.
Acyl moiety related fragments
The acyl moiety peak is generated when the ester bond between carnitine and acyl moiety (the bond 6 in Structure 1) is broken. As collision energy increases, the intensity of this ion rises from undetectable to low, then high, and then decreases to undetectable at higher collision energy. For most spectra collected in this study, the acyl moiety can be detected at normalized collision energies from 20 to 40. There is a small percentage of acylcarnitines for which no acyl peaks can be detected in their spectra due to more favorable pathways to other stable ions, such as fragments of acyl – CO (from the cleavage of the bond 7 in Structure 1), and acyl – H2O (corresponding to the neutral loss of carnitine and a loss of H2O from hydroxylated or carboxylated acyl moiety23).
The carnitine moiety has a well-defined structure, and its fragments can generally be described based on carnitine and acylcarnitine spectra in the NIST17 Tandem MS Library. In contrast, acyl structures are diverse, making it challenging to annotate their fragments. The most common neutral losses from acyl moiety are CO and C2H2O. Other acyl-related neutral losses vary with the individual acyl structures, for example, neutral losses of H2O for a hydroxylated acyl, and CH2O2 and CO2 for a carboxylated acyl.
4.2. Retention time consistency of metabolites from different runs
Consistent RT values of compounds are critical to link isomers across different runs. For examining the RT consistency of the urine data, a set of 92 reference chemicals was selected from the urine LC-MS/MS data (see table ES2 in the supplemental file AcylcarnitineInfo.xlsx). Good reproducibility and no or few isomers are the key criteria in selecting these reference chemicals. Then, RT consistencies of metabolites in all different runs were evaluated with these reference chemicals. Multiple datasets were examined, revealing one dataset of highly consistent RT among its 108 replications. For this dataset, the RT median standard deviations of the same compounds in different runs are less than 5 s. This dataset was then used exclusively for producing consensus spectra.
4.3. Detection of acylcarnitines
The following four criteria were used to qualify an ion as acylcarnitine. 1. The ion spectra must contain the four diagnostic fragments: a. C4H5O2+, b. TMAI, c. P – TMA or P – TMA – H2O, and d. C7H14NO2+ (carnitine – H2O + H+) or P – C7H13NO2. 2. The fragment C4H5O2+ is among the most abundant peaks. 3. The fragments TMAI and P – TMA are attributable to the structural element trimethylamine (CH3)3N, not elements like -CH2CH2CH2NH2, because the later can also form fragments C3H10N+ and P – C3H9N, which are the same as TMAI and P – TMA in mass. The differences between their spectra are that the group -CH2CH2CH2NH2 can form fragments P – NH3, P – CH3NH2, etc., but TMA, i.e., (CH3)3N cannot. 4. The major fragments of an acylcarnitine spectrum can be explained by the methods described in the section 3.3. Note: fewer than 3% of spectra do not satisfy all of these rules, due either to low ion abundance, or acyls having more than two oxygen atoms.
Based on these criteria and the derived ion properties (ion m/z, RT and abundance values), 586 unique acylcarnitines were identified from 30746 spectrum replicates extracted from the 108 urine runs (6 samples, 2 replicates for each sample, and 9 collision energies). These detected acylcarnitine spectra contain clues for structure elucidation, although by themselves these clues do not generally enable the complete assignment of isomer structures.
4.4. Conjugated acylcarnitines
During the process of recognizing acylcarnitines and filtering false positives, a set of spectra that partially satisfied the rules for acylcarnitines was further analyzed. This analysis revealed 125 unexpected species that were identified as conjugated acylcarnitines (see Table 1). These compounds have not been reported previously. Compounds in the first five rows (92 compounds) of Table 1 can be explained as products of Phase I and II biotransformation reactions.35–40 The sixth row denotes 30 acylcarnitines that have N-containing acyls (the N atom is not in an amino acid). The seventh row denotes three Cl-containing acylcarnitines. A list of these conjugated acylcarnitines can be found from table ES5 in the supplemental file AcylcarnitineInfo.xlsx. Table 2 shows that these conjugates can have abundances comparable to their corresponding non-conjugated species.
Table 1.
A list of conjugated acylcarnitines and their possible biotransformation phases.
| No. | Conjugators | Counts | Phases |
|---|---|---|---|
| 1 | Cysteine | 31 | II |
| 2 | Acetylcysteine | 20 | II |
| 3 | CH4S, CH4OS, SO2, SO3 and thiocysteine | 14 | I and II |
| 4 | Taurine | 4 | II |
| 5 | Glucuronic acid | 23 | II |
| 6 | N-containing groups | 30 | |
| 7 | Cl | 3 | |
| Sum | 125 |
Table 2.
The most abundant conjugated acylcarnitines and their corresponding acylcarnitines with their median abundances from 18 analyses of the standard reference sample 3667.29
| Unconjugated acylcarnitines | Conjugated acylcarnitines | ||
|---|---|---|---|
| Acylcarnitine | Abundance | Conjugated Acylcarnitine | Abundance |
| C4:1 | 1.18e+8 |
C4:1 + Cysteine | 4.31e+8 |
| C4:1 + Acetylcysteine | 1.44e+7 | ||
| C4:1 + SO2 | 5.84e+7 | ||
| C5:1 | 5.93e+8 | C5:1 + Cysteine | 3.64e+8 |
| C5:1 + CH4OS | 2.08e+7 | ||
| C5:1 + Acetylcysteine | 7.29e+6 | ||
| C6:1 | 6.85e+7 | C6:1 + CH4S | 1.00e+8 |
| C8:1 | 8.74e+9 | C8:1 + Cysteine | 2.03e+7 |
| C8:1 + Acetylcysteine | 2.32e+7 | ||
| C10:2 | 8.81e+8 | C10:2 + Cysteine | 1.04e+8 |
| C10:2 + Acetylcysteine | 1.56e+8 | ||
As an example, a spectrum of acetylcysteine (ACYS) conjugated acylcarnitine at NCE 30 is shown in Figure 2. This spectrum has two acylcarnitine characteristic peaks, C4H5O2+ and TMAI, but does not contain a peak corresponding to neutral loss TMA, typical of acylcarnitines. The most abundant fragment in this spectrum is the peak at m/z 185.0808, which appears to be the result of two successive neutral losses, i.e., TMA and ACYS. This is consistent with the initial loss of TMA followed by the rapid loss of ACYS. For acetylcysteine conjugates, key fragments include P – 129 (i.e. P – (ACYS – H2S)), P – C2H2O (acetyl group), P – CH2O2, and fragment at m/z 130 (ACYS – H2S + H+)37,38. Figure 2 shows multiple ACYS related fragments, providing evidence of the presence of ACYS and a tentatively assigned structure of this conjugated acylcarnitine.
Figure 2.
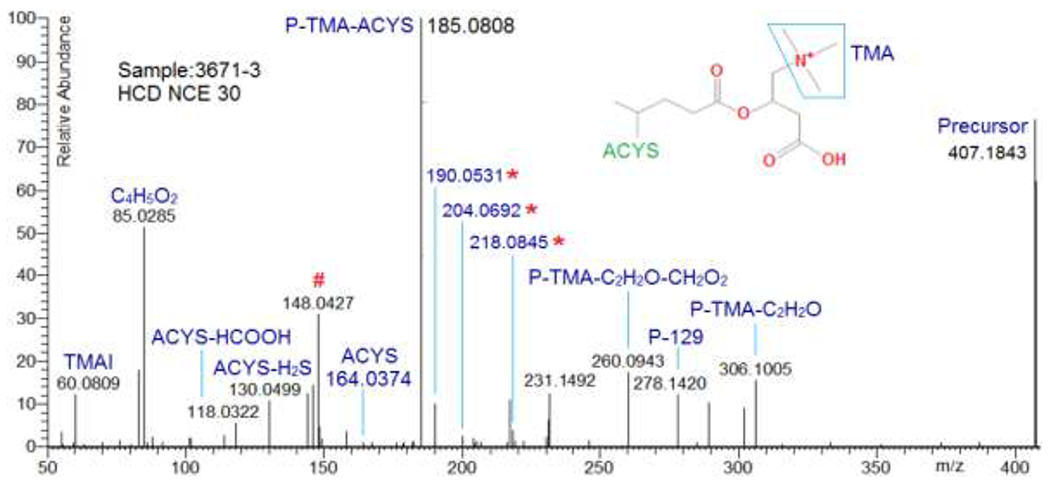
An HCD NCE 30 spectrum of acetylcysteinylated acylcarnitine with a tentatively assigned structure. Here, ACYS is acetylcysteine, P is precursor, and P – 129 is P – (ACYS – H2S). # Denotes an ion ACYS - acetyl group + a part of acyl moiety. * Denotes fragments arising from ACYS + a part of the acyl moiety, i.e., 190.0531 corresponds to ACYS + C2H2, 204.0692 to ACYS + C3H4, and 218.0845 to ACYS + C4H6.
Criteria used to determine conjugated acylcarnitines were similar to those for acylcarnitines, with three exceptions: 1. Fragment C4H5O2+ may not be among the most abundant peaks. 2. P – conjugator, P – TMA – conjugator, and N/S-containing acyl fragments should be among the most abundant peaks; 3. P – TMA or TMAI or C7H14NO2+ may be missing.
4.5. In-source artifacts associated with conjugated acylcarnitines
Misidentification of artifacts as natural metabolites is of general concern in the analysis of LC-MS/MS data. It is expected that ESI processes can produce the in-source ions TMAI, C4H5O2+ and P – TMA, because these fragments are present in low energy spectra. While metabolite analysis should correctly identify them, these in-source ions are not artifact acylcarnitines. For confidently filtering artifact acylcarnitines, a systematic inspection was made by examining each low energy acylcarnitine MS2 spectrum (NCEs 10 and 15) to see (a) if the spectrum contains fragments (RA > 0.01) that have the same m/z as that of an acylcarnitine ion, or (b) if such fragments are found, are there corresponding acylcarnitine MS1 peaks co-eluting at the same retention time as that of the spectrum? This inspection did not find any such spectra.
While there are no in-source acylcarnitines generated from acylcarnitines with higher masses, this inspection did detect 11 acylcarnitines, which were coeluting with corresponding conjugated acylcarnitines (CAC) and could be neutral loss products of CACs, see Table 3. Retention times of these ACs were significantly earlier than those of identified AC ions with the same formula (Table 3). Consequently, the 11 ACs are considered to be artifacts and not included in the library.
Table 3.
Eleven CACs and their in-source artifacts, including formula and m/z values for the CACs, coeluting artifact formula, difference in formula (Form Diff) between each CAC and its artifact AC, and their retention time (RT/s). Also shown are RT ranges of identified acylcarnitine isomers (RT/s), and the abundance ratio of artifact AC to CAC.
| No. | CAC | Artifact AC | Identified AC | Ab ratio | |||
|---|---|---|---|---|---|---|---|
| Formula | m/z | Formula | Form Diff | RT/s | RT/s | ||
| 1 | C13H26NO5S+ | 308.1526 | C13H22NO4+ | CH4OS | 204 | 368 - 455 | 1.16 |
| 2 | C19H38NO8S+ | 440.2313 | C19H38NO5+ | SO3 | 1207 | 1225 - 1243 | 1.92 |
| 3 | C15H29N2O6S+ | 365.1741 | C12H22N2O4+ | C3H7NO2S* | 131 | 368 - 455 | 0.04 |
| 4 | C18H35N2O6S+ | 407.2210 | C15H28N2O4+ | C3H7NO2S* | 673 | 1006 - 1190 | 0.10 |
| 5 | C18H35N2O6S+ | 407.2210 | C15H28N2O4+ | C3H7NO2S* | 708 | 1006 - 1190 | 0.12 |
| 6 | C20H35N2O6S+ | 431.2210 | C17H28N2O4+ | C3H7NO2S* | 791 | 1170 - 1208 | 0.14 |
| 7 | C20H37N2O6S+ | 433.2367 | C17H30N2O4+ | C3H7NO2S* | 859 | 1191 - 1238 | 0.20 |
| 8 | C20H37N2O6S+ | 433.2367 | C17H30N2O4+ | C3H7NO2S* | 960 | 1191 - 1238 | 0.30 |
| 9 | C20H39N2O6S+ | 435.2523 | C17H32N2O4+ | C3H7NO2S* | 1130 | 1226 - 1260 | 0.12 |
| 10 | C20H39N2O6S+ | 435.2523 | C17H32N2O4+ | C3H7NO2S* | 1156 | 1226 - 1260 | 0.15 |
| 11 | C24H45N2O7S+ | 505.2942 | C21H38N2O5+ | C3H7NO2S* | 1146 | 1249 - 1301 | 0.51 |
C3H7NO2S is for formula of cysteine.
This study found few in-source acylcarnitine adducts. For example, no observable adducts in the LC-MS peaks of the three most abundant acylcarnitines were found, except for a sodiated AC with relative abundance less than 0.001 parent (see Figures S6–S8 in the supplementary file AcylcarnitineInfo.docx).
4.6. Consensus spectral library for acylcarnitines and conjugated acylcarnitines
For each acylcarnitine and conjugated acylcarnitine, replicate spectra with the same precursor m/z and collision energy as well as similar RT (within 7 s of each other) were used to generate a consensus spectrum. Spectra of the same m/z and similar retention times but different spectral patterns were separated using the method described in the part 5.1 of the supplemental file AcylcarnitineInfo.docx. When generating a consensus spectrum from replicate spectra, fragments with replicates present in fewer than one-third of the number of replicates were excluded. Each library entry is accompanied by ion formula, m/z, RT, acquired NCE, number of replicate spectra, and annotated spectral fragments. This library contains 5,328 mass spectra, derived from 586 acylcarnitines having 157 different masses, and 125 conjugated acylcarnitines having 85 different masses (Table 4). A list of unique masses of these acylcarnitine ions is provided in Table ES3 of the supplemental file AcylcarnitineInfo.xlsx; lists of chemical formulas, retention times and relative abundances of acylcarnitines and conjugated acylcarnitines are presented in Tables ES4 and ES5 of the supplemental file, respectively. For more information about the library spectra, see part 5.2 (Description of the acylcarnitine spectra) in the supplemental file AcylcarnitineInfo.docx. Spectral data processed in this study is provided to researchers in two ways: (1) a NIST library, (2) an ASCII text file - both are downloadable from https://chemdata.nist.gov/dokuwiki/doku.php?id=peptidew:lib:acylcarnitine.
Table 4.
Unique masses, ions, and spectra of acylcarnitines and conjugated acylcarnitines in the library.
| No. | Metabolites | Masses | Ions | Spectra |
|---|---|---|---|---|
| 1 | Carnitine | 1 | 1 | 9 |
| 2 | Acylcarnitines | 157 | 586 | 4,332 |
| 3 | Conjugated acylcarnitines | 85 | 125 | 987 |
| Sum | 243 | 713 | 5,328 |
4.7. Examples of isomer analysis
While mass spectrometry is the most sensitive method for identifying compounds, it is often unable to establish structures of isomers. However, fragmentation details and the energy dependence of these spectra may provide clues for differentiating isomers having similar spectra. We provide two examples of using fragmentation details for establishing structures. The first involves four isomers, among them two can be identified by matching spectra in the NIST17 Tandem MS Library, while structures of the other two are not known, but can be inferred from their fragment ions. The second example demonstrates that methylmalonyl carnitine and its unbranched isomer succinyl carnitine can be distinguished based on their product ions.
Example 1:
The first example involves four acylcarnitine isomers (m/z = 290.1598, ion formula is C13H24NO6+). These isomers eluted around 280 s, 300 s, 360 s, and 392 s, and are denoted as isomers A, B, C, and D, respectively. A spectral search against the NIST17 Library indicated that the isomer A is adipoyl-L-carnitine (Figure 3A) and isomer B is 3-methylglutaryl carnitine (Figure 3B).
Figure 3.
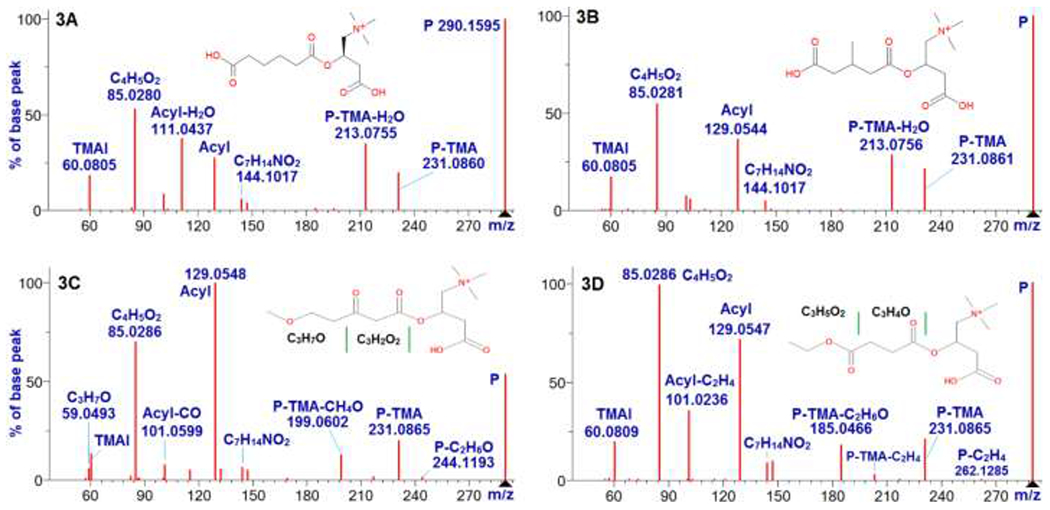
HCD spectra of four acylcarnitine isomers A, B, C and D (m/z: 290.1598, ion formula: C13H24NO6+). 3A is the spectrum of adipoyl-L-carnitine at 20 V in NIST17 Tandem MS Library. 3B is the spectrum of 3-methylglutary-L-carnitine at 20 V in NIST17 Tandem MS Library. 3C is the spectrum of isomer C at NCE 25. 3D is the spectrum of isomer D at NCE 25. In the spectra, the code P means precursor.
Spectra of isomers C and D, see Figures 3C and 3D, do not have neutral loss of H2O or CH2O2, indicating that the acyls of the two late eluting isomers are not carboxylated. Isomer C has a fragment at m/z 59.0491 (C3H7O+, i.e., acyl – C3H2O2), which increased with increased collision energies, and became the most abundant one at NCE 80 (data not shown). Isomers A and B exhibit low abundance for peak C3H7O+, while isomer D does not have this ion. Furthermore, isomer C also exhibits another unique fragment at m/z 199.0601, which is a result of neutral loss of (TMA + CH4O) from precursor. These unique fragments and the delayed RT of isomer C imply that its acyl may have a methoxy and a carbonyl group separated by two CH2. A putative strucuture of isomer C is presented in Figure 3C.
Isomer D exhibits four unique feature fragments at m/z 73.0284 (C3H5O2+, i.e., acyl – C3H4O), 101.0233 (C4H5O3+, acyl – C2H4), m/z 185.0444 (C8H9O5+, precursor – TMA – C2H6O), and m/z 203.055 (C8H11O6+, precursor – TMA – C2H4). The intensity of the fragment C3H5O2+ at m/z 73.0287 increased with collision energy, and relative abundance reached to 0.36 at NCE 80. These feature fragments suggest that the isomer D could be an ester. A putative structure of isomer D is provided in Figure 3D.
Example 2:
The second example concerns the differentiation of methylmalonyl carnitine (C3:M-DC, Figure 4A) from its unbranched isomer succinyl carnitine (C4:DC, Figure 4B). Figure 4A shows that because methylmalonyl carnitine has feature fragments at m/z 159.0652 (C7H11O4+) and m/z 218.1388 (neutral loss peak of CO2), it can be confidently distinguished from isomer succinyl carnitine. The feature fragment at m/z 159.0652 relates to a phenomenon, i.e., spectra of acylcarnitines with a hydroxy in the third position of their acyl groups have a fragment at m/z 145.0495 (C6H9O4+)10, or m/z 159.0652 if there is a methylation at the second position.11 Figure 4A indicates that the hydroxy on a 2-COOH group can also facilitate this rearrangement. Actually, the neutral loss peak of CO2 is a better indicator of methylmalonyl carnitine, because it is more abundant than that of the fragment at m/z 159.0652. This is also observed in malonyl carnitine spectra, see Figure S11 in the supplemental file AcylcarnitineInfo.docx.
Figure 4.

Spectra of isomers (m/z = 262.1285) of 2-methylmalonyl carnitine (C3:M-DC) and succinyl carnitine (C4:DC) under HCD NCE 25. Figure 4A is the spectrum of C3:M-DC sampled at RT 98.7 s. The fragments at m/z 218.1388 and 159.0655 indicate that it is 2-methylmalonyl carnitine. Figure 4B is the spectrum of C4:DC sampled at RT = 82.5 s.
Illustration of library use:
Figure 5 shows results for a LC-MS/MS run of a cancer patient urine sample,29 generated by an orbitrap Fusion Lumos mass spectrometer. It employs our acylcarnitine spectral library and NIST17 Library using the MSPepSearch software.31 The acylcarnitine library identified 248 ions with a score 500 or higher and deviation less than 0.001 m/z. After removing redundant identifications, a total of 242 unique acylcarnitine spectra were confirmed, including 223 acylcarnitines and 19 conjugated acylcarnitines. Against NIST17 Tandem MS Library, 20 acylcarnitines were matched. A list of the 242 identifications and their ion m/z, formulas, retention times, match scores and abundances can be found from the supplementary table ES6 in AcylcarnitineInfo.xlsx. For researchers to have a better understanding of these identifications, a supplementary file, Spectra242.txt, is provided. It contains 242 raw spectra, from which the 242 identifications were made.
Figure 5.
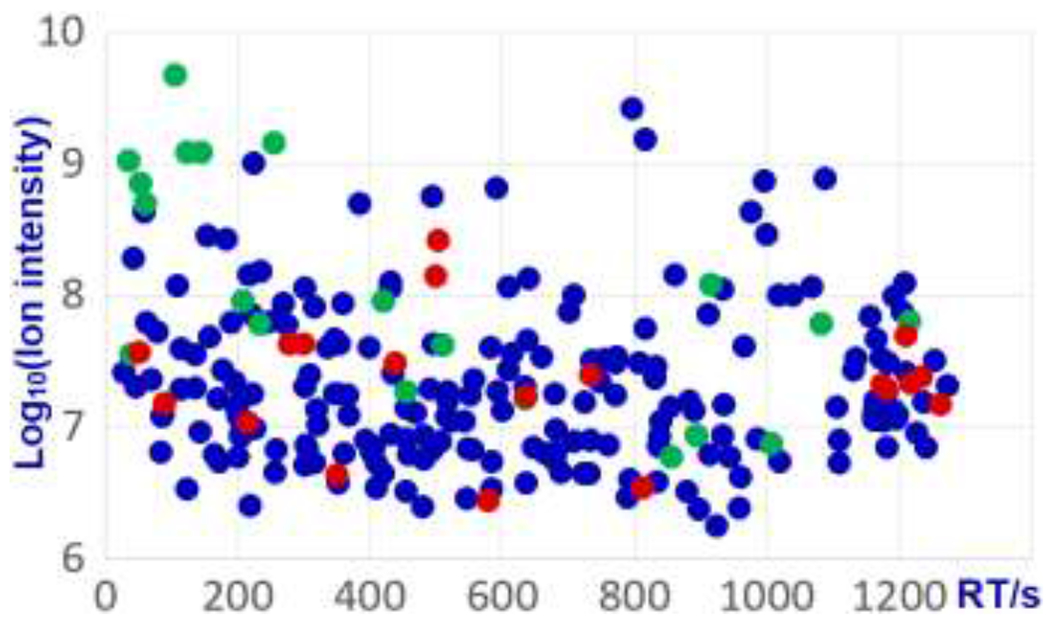
Acylcarnitines identified by matching the library developed in this study to spectra of a single urine analysis29, 19 are conjugated acylcarnitines (red) and 223 are acylcarnitines (blue), among the 223 hits, 20 were also identified by searching NIST 17 library (green).
Criteria described in section 4.3 are also appropriate to confirm identifications of acylcarnitines from library search results. Identifications with high scores suggest an exact match of the library compound, while low scores suggest that the identified ions may be isomers of the library compounds, or the candidate spectra are of low quality due to low ion abundances or contaminants. By aligning retention times of metabolites with our data, identification confidence can be increased further. The compounds in tables ES2, ES4 and ES5 in the supplemental file AcylcarnitineInfo.xlsx contain such retention times.
4.8. Summary
With the pure compound spectra in the NIST17 Tandem MS Library and spectra found in the analysis of urine by the hybrid search method, we developed rules for identifying and annotating acylcarnitines, as well as for excluding artifacts. Using these rules, 586 unique acylcarnitine ions were identified. In the course of this analysis, 125 novel acylcarnitine species were also identified. They appeared to primarily result from the conjugation of acylcarnitines with hydrophilic compounds, such as cysteine, taurine, glucuronic acid, and SO3. Significantly, these conjugated acylcarnitines can have comparable abundances to their non-conjugated acylcarnitines. Based on these studies, a spectral library of acylcarnitines and conjugated acylcarnitines was created and has been made available from https://chemdata.nist.gov/dokuwiki/doku.php?id=peptidew:lib:acylcarnitine. This resource is a searchable spectral library containing thousands of mass spectra, each of which is associated with a molecular formula, precursor ion m/z, retention time, collision energy, and annotated fragment ions. This library is intended as an aid in identifying acylcarnitines in biological samples as well as for studying structure-fragmentation-retention relationships. Moreover, the methods reported appear capable of being extended to identify other classes of metabolites.
Supplementary Material
Acknowledgments
We thank Drs. Yamil Simón-Manso, Karl K. Irikura, Lisa E. Kilpatrick, Rebecca A. Zangmeister, Pawel Jaruga, Oleg V. Toropov, Dmitrii V. Tchekhovskoi of the National Institute of Standards and Technology for helps provided to us in accomplishing of this work.
Abbreviation:
- AB
abundance
- ACYS
acetylcysteine
- CYS
cysteine
- ESI
electrospray ionization
- HCD
higher-energy collision dissociation
- HPLC
high-performance liquid chromatography
- LC-MS
liquid chromatography-mass spectrometry
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MS1
full MS scan
- MS2
tandem MS scan
- NCE
normalized collision energy
- NIST
National Institute of Standards and Technology
- P
precursor
- RA
relative abundance
- RT
retention time
- s
second
- TMA
trimethylamine (C3H9N)
- TMAI
positive ion of trimethylamine (C3H10N+)
- XIC
extracted ion chromatogram
Footnotes
Supporting Information Available:
1. Materials and Experiment, complementary description of the rules to detect acylcarnitines, annotation steps of MS2 fragments, major neutral losses, descriptions of spectra in the acylcarnitine library, brief discussions on collision energy and retention time, and artifact analyses (PDF).
2. Standard spectra of 27 acylcarnitines in the NIST17 Tandem MS Library, 92 chemicals used to analyze retention time consistency of different runs, 157 acylcarnitine masses, carnitine and 586 acylcarnitine ions, 125 conjugated acylcarnitines, and 242 identifications reported in the application section (XLSX).
3. Spectra242.txt contains the spectra 242 raw spectra from which 242 identifications were made (TXT).
3. Spectra242.txt refers to section 4.7 (Illustration of library use) and contains the spectra from which the 242 identifications were made (TXT).
This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Publisher's Disclaimer: Disclaimer
Certain commercial equipment, instruments, or materials are identified in this paper in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose.
Reference
- 1.Fritz IB; Yue KTN Long-chain carnitine acyltransferase and the role of acylcarnitine derivatives in the catalytic increase of fatty acid oxidation induced by carnitine. J. Lipid Res 1963, 4, 279–288. [PubMed] [Google Scholar]
- 2.Bremer J Carnitine - metabolism and functions. Physiol. Rev 1983, 63, 1420–1480. [DOI] [PubMed] [Google Scholar]
- 3.Rebouche CJ; Paulson DJ Carnitine metabolism and function in humans. Ann. Rev. Nutr 1986, 6, 41–66. [DOI] [PubMed] [Google Scholar]
- 4.Bieber LL Carnitine. Annu. Rev. Biochem 1988, 57, 261–283. [DOI] [PubMed] [Google Scholar]
- 5.Kelly BM; Rose ME; Wycherley D; Preece SW Electrospray mass spectra of medium-chain and long-chain acylcarnitines. Org. Mass Spectrom 1992, 27, 924–926. [Google Scholar]
- 6.Indiveri C; Iacobazzi V; Tonazzi A; Giangregorio N; Infantino V; Convertini P; Console L; & Palmieri F The mitochondrial carnitine/acylcarnitine carrier: function, structure and physiopathology. Mol. Aspects Med 2011, 32, 223–233. [DOI] [PubMed] [Google Scholar]
- 7.Millington DS; Roe CR; Maltby DA Application of high resolution fast atom bombardment and constant B/E ratio linked scanning to the identification and analysis of acylcarnitines in metabolic disease. Biomed. Mass. Spectrom 1984, 11, 236–241. [DOI] [PubMed] [Google Scholar]
- 8.Gaskell SJ; Guenat C; Millington DS; Maltby DA; Roe CR Differentiation of isomeric acylcarnitines using tandem mass spectrometry. Anal. Chem 1986, 58, 2801–2805. [DOI] [PubMed] [Google Scholar]
- 9.Maeda Y; Ito T; Ohmi H; Yokoi K; Nakajima Y; Ueta A; Kurono Y; Togari H; Sugiyama N Determination of 3-hydroxyisovalerylcarnitine and other acylcarnitine levels using liquid chromatography–tandem mass spectrometry in serum and urine of a patient with multiple carboxylase deficiency. J. Chromatogr., B 2008, 870, 154–159. [DOI] [PubMed] [Google Scholar]
- 10.Su X; Han X; Mancuso DJ; Abendschein DR; Gross RW Accumulation of long-chain acylcarnitine and 3-hydroxy acylcarnitine molecular species in diabetic myocardium: identification of alterations in mitochondrial fatty acid processing in diabetic myocardium by shotgun lipidomics. Biochemistry. 2005, 44, 5234–5245. [DOI] [PubMed] [Google Scholar]
- 11.Peng M; Fang X; Huang Y; Cai Y; Liang C; Lin R; Liu L Separation and identification of underivatized plasma acylcarnitine isomers using liquid chromatography–tandem mass spectrometry for the differential diagnosis of organic acidemias and fatty acid oxidation defects. J. Chromatogr. A 2013, 1319, 97–106. [DOI] [PubMed] [Google Scholar]
- 12.Möder M; Kiessling A; Löster H; Brüggemann L The pattern of urinary acylcarnitines determined by electrospray mass spectrometry: a new tool in the diagnosis of diabetes mellitus. Anal. Bioanal. Chem 2003, 375, 200–210. [DOI] [PubMed] [Google Scholar]
- 13.Millington DS; Kodo N; Norwood NL; Roe CR Tandem mass spectrometry: A new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J. Inherit. Metab. Dis 1990, 13, 321–324. [DOI] [PubMed] [Google Scholar]
- 14.Rashed MS; Ozand PT; Bucknall MP; Little D Diagnosis of inborn errors of metabolism from blood spots by acylcarnitines and amino acids profiling using automated electrospray tandem mass spectrometry. Pediatr Res, 1995, 38, 324–331. [DOI] [PubMed] [Google Scholar]
- 15.Rizzo C; Boenzi S; Wanders RJ; Duran M; Caruso U; Dionisi-Vici C Characteristic acylcarnitine profiles in inherited defects of peroxisome biogenesis: a novel tool for screening diagnosis using tandem mass spectrometry. Pediatr Res. 2003, 53, 1013–1018. [DOI] [PubMed] [Google Scholar]
- 16.Abdenur JE; Chamoles NA; Guinle AE; Schenone AB; Fuertes ANJ Fuertes. Diagnosis of isovaleric acidaemia by tandem mass spectrometry: False positive result due to pivaloylcarnitine in a newborn screening programme. J. Inherit. Metab. Dis 1998, 21, 624–630. [DOI] [PubMed] [Google Scholar]
- 17.Minkler PE; Stoll MSK; Ingalls ST; Hoppel CL Correcting false positive medium-chain acyl-CoA dehydrogenase deficiency results from newborn screening; synthesis, purification, and standardization of branched-chain C8 acylcarnitines for use in their selective and accurate absolute quantitation by UHPLC–MS/MS. Mol Genet Metab. 2017, 120, 363–369. [DOI] [PubMed] [Google Scholar]
- 18.Soeters MR; Serlie MJ; Sauerwein HP; Duran M; Ruiter JP; Kulik W; Ackermans MT; Minkler PE; Hoppel CL; Wanders RJ; Houten SM Characterization of D-3-hydroxybutyrylcarnitine (ketocarnitine): an identified ketosis-induced metabolite. Metabolism. 2012, 61, 966–973. [DOI] [PubMed] [Google Scholar]
- 19.Minkler PE; Stoll MS; Ingalls ST; Yang S; Kerner J; Hoppel CL Quantification of carnitine and acylcarnitines in biological matrices by HPLC electrospray ionization–mass spectrometry. Clin. Chem 2008, 54, 1451–1462. [DOI] [PubMed] [Google Scholar]
- 20.Maeda Y; Ito T; Suzuki A; Kurono Y; Ueta A; Yokoi K; Sumi S; Togari H; Sugiyama N Simultaneous quantification of acylcarnitine isomers containing dicarboxylic acylcarnitines in human serum and urine by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007, 21, 799–806. [DOI] [PubMed] [Google Scholar]
- 21.Minkler PE; Stoll MS; Ingalls ST; Kerner J; Hoppel CL. Quantitative acylcarnitine determination by UHPLC-MS/MS--Going beyond tandem MS acylcarnitine “profiles”. Mol Genet Metab. 2015. 116, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Hooft JJ; Ridder L; Barrett MP; Burgess KE Enhanced acylcarnitine annotation in high-resolution mass spectrometry data: fragmentation analysis for the classification and annotation of acylcarnitines. Front Bioeng. Biotechnol 2015, 3, article 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuniga A; Li L Ultra-high-performance liquid chromatography tandem mass spectrometry for comprehensive analysis of urinary acylcarnitines. Anal. Chim. Acta 2011, 689, 77–84. [DOI] [PubMed] [Google Scholar]
- 24.Yu D; Zhou L; Xuan Q; Wang L; Zhao X; Lu X; Xu G Strategy for comprehensive identification of acylcarnitines based on liquid chromatography–high-resolution mass spectrometry. Anal. Chem 2018, 90, 5712–5718. [DOI] [PubMed] [Google Scholar]
- 25. https://chemdata.nist.gov/
- 26.Burke MC; Mirokhin YA; Tchekhovskoi DV; Markey SP; Heidbrink Thompson J; Larkin C; Stein SE The Hybrid Search: A mass spectral library search method for discovery of modifications in proteomics. J. Proteome Res 2017, 16, 1924–1935. [DOI] [PubMed] [Google Scholar]
- 27.Moorthy AS; Wallace WE; Kearsley AJ; Tchekhovskoi DV; Stein SE Combining fragment-ion and neutral-loss matching during mass spectral library searching: a new general purpose algorithm applicable to illicit drug identification. Anal. Chem 2017, 89, 13261–13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper BT; Yan X; Simón-Manso Y; Tchekhovskoi DV; Mirokhin YA; Stein SE Hybrid search: a method for identifying metabolites absent from tandem mass spectrometry libraries. Anal. Chem 2019, 91, 13924–13932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simón-Manso Y; Marupaka R; Yan X; Liang Y; Telu KH; Mirokhin YA; Stein SE Mass spectrometry fingerprints of small-molecule metabolites in biofluids: building a spectral library of recurrent spectra for urine analysis. Anal. Chem, 2019, 91, 12021–12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Telu KH; Yan X; Wallace WE; Stein SE; Simón-Manso Y Analysis of human plasma metabolites across different liquid chromatography/mass spectrometry platforms: Cross-platform transferable chemical signatures. Rapid Commun Mass Spectrom. 2016, 30 (5), 581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. https://chemdata.nist.gov/dokuwiki/doku.php?id=peptidew:mspepsearch.
- 32.Dong Q; Liang Y; Yan X; Markey SP; Mirokhin YA; Tchekhovskoi DV; Bukhari TH; Stein SE The NISTmAb tryptic peptide spectral library for monoclonal antibody characterization. mAbs. 2018, 10, 354–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong Q; Yan X; Liang Y; Stein SE In-depth characterization and spectral library building of glycopeptides in the tryptic digest of a monoclonal antibody using 1D and 2D LC–MS/MS. J. Proteome Res 2016, 15, 1472–1486. [DOI] [PubMed] [Google Scholar]
- 34.Testa B; Kramer SD The biochemistry of drug metabolism – an introduction. Part 4. Reactions of conjugation and their enzymes. Chem. Biochem 2008, 5, 2171–2336. [DOI] [PubMed] [Google Scholar]
- 35.Parkinson A; Ogilvie BW Biotransformation of xenobiotics Chapter 6 (page 161) in Casarett and Doull’s Toxicology - the Basic Science of Poisons (editor: Klaassen Curtis D.), Seventh edition, McGraw-Hill, New York, 2008. [Google Scholar]
- 36.Kind T; Fiehn O Advances in structure elucidation of small molecules using mass spectrometry. Bioanal. Rev 2010, 2, 23–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levsen K; Schiebel H-M; Behnke B; Dotzer R; Dreher W; Elend M; Thiele H Structure elucidation of phase II metabolites by tandem mass spectrometry: an overview. J. Chromatogr., A 2005, 1067, 55–72. [DOI] [PubMed] [Google Scholar]
- 38.Xu C; Li CY; Kong AN Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005, 28, 249–268. [DOI] [PubMed] [Google Scholar]
- 39.Ionescu C; Caira MR Drug Metabolism. 2005, Springer, printed in the Netherlands. [Google Scholar]
- 40.Demarque DP; Crotti AEM; Vessecchi R; Lopes JLC; Lopes NP Fragmentation reactions using electrospray ionization mass spectrometry: an important tool for the structural elucidation and characterization of synthetic and natural products. Nat. Prod. Rep 2016, 33, 432–455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


