Abstract
Chronic inflammation in women diagnosed with breast cancer is critically linked with tumor progression, metastasis and survival. C-reactive protein (CRP)—a circulating marker of inflammation—is an important prognostic marker for cancer-related outcomes in breast cancer survivors (e.g. recurrence, fatigue). Psychological stress, which increases circulating markers of inflammation following sympathetic nervous system (SNS) activation, may modulate tumor-relevant inflammatory processes. However, little is known about neural mechanisms that might link stress and downstream SNS-initiated proinflammatory processes, such as elevated CRP. Past work suggests that threat-related neural regions, such as the amygdala, may be key in translating psychological stress into SNS activity and subsequent peripheral inflammation. Thus, we examined amygdala reactivity to socially threatening stimuli in association with perceived stress and plasma CRP levels to further elucidate neuro-immune pathways of social threat processing within breast cancer survivors (N = 37). Significant positive correlations were found between left amygdala reactivity in response to socially threatening stimuli (e.g. angry/fearful faces vs happy faces) and perceived stress in the previous month (r = 0.32, P = 0.025) and between left amygdala reactivity and CRP (r = 0.33, P = 0.025). This work builds on prior research implicating the amygdala as a key structure in crosstalk between threat-related neural circuitries and peripheral inflammation, particularly within cancer survivors.
Keywords: CRP, perceived stress, amygdala, social threat, breast cancer
Introduction
Chronic inflammation is critically linked with clinical outcomes such as mortality in those diagnosed with breast cancer (Pierce et al., 2009). Circulating markers of peripheral inflammation, such as C-reactive protein (CRP), are considered important prognostic markers tied to a variety of cancer-related outcomes in breast cancer survivors, including fatigue, tumor progression, cancer recurrence and overall mortality (Pierce et al., 2009; Orre et al., 2011; Kaur et al., 2019). Experiences of both acute and chronic psychological stress have been shown to be related to elevated levels of circulating markers of inflammation (Danese et al., 2007; Steptoe et al., 2007). Thus, psychological stress may play a role in driving tumor-relevant inflammatory processes (Moreno-Smith et al., 2010). Despite the identified prognostic value of inflammatory markers for breast cancer survivors, the neural mechanisms that might translate experiences of psychological stress into downstream proinflammatory processes remain poorly understood. Here, we examine whether the amygdala, a neural region that may be critical in neuro-immune crosstalk, might play a role in the link between stress and inflammation in a sample of breast cancer survivors.
Inflammation, psychological stress and cancer
Chronic inflammation is a key regulator of cancer development and tumor progression (Coussens and Werb, 2002; de Visser et al., 2006). The role of inflammation within cancer is extensive, such that inflammatory cytokines and cells have been identified as key contributors to all stages of tumor progression and proliferation (Coussens and Werb, 2002; Wu and Zhou, 2009). Thus, circulating markers of inflammation, such as CRP, are now considered clinically valuable markers that can supplement traditional screening methods for important cancer-related outcomes. For example, CRP has been linked with various outcomes related to breast cancer, the most prevalent cancer in women worldwide. Elevated CRP is associated with cancer metastasis (Kaur et al., 2019), as well as increased overall mortality (Pierce et al., 2009), in those diagnosed with breast cancer. Even within breast cancer survivors who are considered disease-free, CRP remains linked with cancer-related clinical and behavioral outcomes. Elevated CRP is associated with increased likelihood of cancer recurrence (Pierce et al., 2009; Kaur et al., 2019), as well as greater levels of fatigue (Orre et al., 2011). Thus, CRP is associated with a variety of outcomes related to breast cancer, particularly post-treatment, and is thus an important prognostic marker for breast cancer survivors in particular.
Experiences of acute and chronic psychological stressors, via activation of the sympathetic nervous system (SNS), have been shown to increase circulating markers of inflammation, including CRP (Steptoe et al., 2007; Gouin et al., 2012). Given that CRP has been consistently linked with cancer-related outcomes such as tumor progression, psychological stress (and the associated SNS response) may play a role in driving tumor-relevant inflammatory processes (Moreno-Smith et al., 2010). In line with this, more chronic or prolonged psychological stress has been linked with tumor growth and metastasis, which contributes to reduced survival rates in those diagnosed with breast cancer (Chida et al., 2008; Moreno-Smith et al., 2010). Additionally, SNS activation is more broadly known to promote tumor progression and metastasis on a molecular level via changes in gene expression (Cole et al., 2015). This link between SNS activation and tumor progression has been demonstrated in various animal models of cancer (Palermo-Neto et al., 2003; Thaker et al., 2006), including breast cancer (Sloan et al., 2007; Campbell et al., 2012). However, little is known about the neural mechanisms that might translate psychological stress into these downstream SNS-initiated proinflammatory processes, such as elevated CRP, that ultimately have implications for survivors.
Threat-related amygdala activity and SNS responding
Neural regions involved in signaling potential stress or threats, such as the amygdala (Whalen et al., 2001; Mobbs et al., 2009; Eisenberger, 2012), are promising candidates for exploring potential neural mechanisms that might contribute to SNS-initiated proinflammatory processes that occur in response to stress. Supporting the amygdala’s role in SNS responding, stimulating the amygdala leads to increases in blood pressure, while lesions to the amygdala have the opposite effect, attenuating SNS responses to aversive stimuli (Delgado et al., 2006). The amygdala is also associated with physiological responses during fear conditioning, such that greater amygdala activity in response to aversive stimuli is associated with greater skin conductance responses (SCRs), an index of SNS activity (Phelps et al., 2004). In addition, the amygdala has strong, efferent projections to regions that are integral in activating the SNS following a potential stressor (e.g. hypothalamus, brainstem) (LeDoux et al., 1988; Delgado et al., 2006). Finally, animal work has shown that threat-induced disease outcomes (e.g. inflammatory-related gastric ulcers) may be attenuated following lesions to the amygdala (Henke, 1982), underscoring the clinical relevance of threat-related amygdala activity.
Thus, the amygdala may be a key neural structure supporting the bidirectional link between psychological stress and inflammation, particularly for cancer survivors. In line with this idea, greater amygdala reactivity to social threat is associated with elevated inflammation in breast cancer survivors (Muscatell et al., 2016a). Interestingly, this work highlights that associations between amygdala reactivity and inflammation appear stronger in cancer survivors relative to a healthy control group, suggesting that crosstalk between threat-related neural circuitries and peripheral inflammation may be amplified within cancer populations (Muscatell et al., 2016a). However, such findings linking threat reactivity and inflammation in breast cancer survivors remain preliminary, as this previous work utilized a small sample of breast cancer survivors (N = 15). Further, very few studies to date have probed neuro-immune interactions in breast cancer survivors. Thus, this work will serve to replicate and extend such findings in a larger, independent sample of breast cancer survivors.
The current study
Based on this past research, we examined whether the amygdala might play a role in translating experiences of prolonged psychological stress into heightened levels of inflammation. To do this, female breast cancer survivors completed a neuroimaging study in which they viewed socially threatening stimuli (fearful and angry faces), a task known to activate the amygdala (Morris et al., 1996; Hariri et al., 2002; Mattavelli et al., 2014). We then examined (i) whether greater levels of perceived stress over the past month were associated with greater activity in the amygdala in response to the socially threatening images and (ii) whether greater activity in the amygdala in response to socially threatening images was associated with higher circulating levels of CRP.
Methods
Participants
Participants consisted of 45 female breast cancer survivors. Seven participants were excluded from analyses for various reasons: did not complete the neuroimaging task (n = 2), severe signal dropout in the neuroimaging data due to technical issues related to the scanner (n = 3), neuroimaging session ended prematurely due to overheating concerns (n = 1) and missing CRP data due to not completing the blood draw (n = 1). One additional participant was deemed a multivariate outlier (described below) and was also removed from analyses. Thus, the final sample consisted of 37 participants.
Participants were recruited via the University of California Los Angeles (UCLA) Tumor Registry, newspaper advertisements, re-contacting participants from past research studies and word-of-mouth referrals. In order to be eligible for the study, participants had to be 18 years of age or older, fluent in English and have no current diagnosis of major psychiatric illness. Additionally, individuals must have been diagnosed with early stage breast cancer (stages 0–IIIA), completed any radiation or chemotherapy between 3 months and 10 years ago and not currently have cancer of any type. Finally, due to restrictions for the neuroimaging portion of the study, participants were not eligible if they had any non-removable metallic implants, were pregnant or trying to become pregnant, had severe claustrophobia or were left-handed. Eligibility was confirmed through a telephone screening prior to their session. The UCLA IRB approved all study procedures, and all participants provided written informed consent.
Procedures
Overview
After confirming eligibility, sessions were scheduled between 8:00 AM and 12:00 PM. Upon arriving to UCLA, participants provided a blood sample for a circulating inflammatory marker, which were collected by venipuncture into tubes containing ethylenediaminetetraacetic acid, placed on ice, centrifuged for acquisition of plasma and stored at −80°C for subsequent batch testing. Next, participants underwent a functional magnetic resonance imaging (fMRI) scan while they completed a threat reactivity task designed to elicit amygdala activation. Following the scan, participants completed the Perceived Stress Scale (Cohen et al., 1983), a questionnaire measure of perceptions of stress. Participants were then debriefed and dismissed.
Threat-reactivity task and image acquisition
To examine amygdala reactivity, participants underwent an fMRI scan while they completed a standard threat-reactivity task that is widely used in the affective neuroscience literature to elicit amygdala activation. Specifically, participants viewed blocks of threatening facial expressions (e.g. fearful, angry) from a standardized stimulus set (Tottenham et al., 2009) and viewed blocks of non-threatening facial expressions (e.g. closed-mouth happy, hereafter simply referred to as ‘happy’), which served as the comparison condition (Morris et al., 1996; Phillips et al., 1998; Inagaki et al., 2012). Each block lasted 30 s, followed by 12 s of fixation crosshair. During each block, participants were instructed to passively view 15 faces for 1.5 s each. Participants completed two blocks of each facial expression (fearful, angry, happy), in one of two pseudo-randomized orders.
Imaging data were acquired using a Siemens Prisma 3.0 Tesla MRI scanner at the UCLA Brain Mapping Center. First, we acquired a T1-weighted MPRAGE anatomical image for functional image registration and normalization (slice thickness = 0.9 mm, 192 slices, TR = 2300 ms, TE = 2.32 ms, flip angle = 8°, matrix = 256 × 256, FOV = 240 mm). Then, we acquired 175 functional T2-weighted EPI volumes (slice thickness = 3 mm, gap = 1 mm, TR = 2000 ms, TE = 24 ms, flip angle = 90°, matrix = 64 × 64, FOV = 200 mm). EPI volumes were collected within a single run.
Perceptions of stress
To measure the degree to which participants appraised situations in everyday life as stressful, we administered the Perceived Stress Scale (Cohen et al., 1983). This 10-item scale (α = 0.911) asked participants to report on their subjective experiences of stress over the last month (e.g. ‘In the last month, how often have you found that you could not cope with all the things that you had to do?’, ‘In the last month, how often have you felt difficulties were piling up so high that you could not overcome them?’). Items were answered on a 0 (never) to 4 (very often) scale with higher scores indicating greater perceived stress.
Inflammatory assessment
Plasma CRP levels were determined by enzyme-linked immunosorbent assays (R&D Systems Human Quantikine ELISA; Minneapolis, MN) according to the manufacturer’s instructions but with a sample dilution of 1:500 and an extended standard curve to yield a lower limit of detection of 0.2 mg/l. All samples were run in duplicate, with intra- and inter-assay coefficients of variation of <4%, based on an internal quality control sample included on every assay plate.
Data analysis
Neuroimaging data were pre-processed and analyzed using statistical parametric mapping (SPM12; Wellcome Department of Cognitive Neurology, London, UK). Pre-processing included image realignment to correct for head motion and co-registration of the structural MPRAGE to the mean functional image. Images were then normalized to Montreal Neurological Institute space using diffeomorphic anatomical registration through exponentiated lie algorithms (resampled at 3 × 3 × 3 mm) and spatially smoothed using a 5 mm Gaussian kernel, full width at half maximum, to increase signal-to-noise ratio.
Following pre-processing, a general linear model was constructed for each participant, in which activation during each 30 s block of the task was convolved with a canonical hemodynamic response function. Three regressors were coded for the type of block (angry, fearful, happy faces), and we included the six motion parameters as covariates. In cases where motion of more than 1 mm from one volume to the next was detected, individual nuisance regressors were added to remove such images from analyses. For each model, the time series was high-pass filtered using a 128 Hz function and serial autocorrelation was modeled as a first-order autoregressive process. Following estimation, we computed linear contrasts for each participant that compared blood oxygenation level dependent (BOLD) signal during the threat reactivity trials (e.g. angry faces + fearful faces) to BOLD signal during non-threat trials (e.g. happy faces). Contrast images for each participant were then entered into random effects analyses at the group level for statistical inference.
We focused specifically on this threat vs non-threat contrast in order to isolate the activity related to social threat, over and above activity that may be involved in a more general processing of face stimuli or social information. Although separately comparing each face condition (threat, non-threat) to implicit baseline may seem to provide clarity on which face condition may be driving effects, we find the interpretation of such comparisons problematic. In the present study, the implicit baseline condition reflects activity while viewing a fixation cross, and it is unclear what activity during this condition reflects. Most importantly, such baseline comparisons would not allow us to examine the activity specific to social threat (controlling for more basic processes, such as viewing face stimuli). Thus, we focus on the threat vs non-threat comparison, given that the tighter control condition of non-threatening faces (as opposed to fixation cross) allows us to better isolate the phenomena of interest, as well as provide meaningful interpretations of results.
Given our a priori hypotheses regarding the associations between inflammation and neural activity in the amygdala, we conducted region-of-interest (ROI) analyses focusing on the left and right amygdala. Amygdala ROIs were defined anatomically based on the automated anatomical labeling atlas. Mean parameter estimates were extracted from the amygdala ROIs for each participant and entered into SPSS 25 for further analysis. Due to directional hypotheses and convention, all statistical tests investigating the activity within ROIs are thresholded at P < 0.05, one-tailed.
One participant was a multivariate outlier when examining perceived stress and CRP in combination with each amygdala ROI (Mahalanobis distance, P < 0.001). This participant was thus excluded from analyses, leaving 37 participants for all reported analyses.
Given known associations between CRP and body mass index (BMI), all results involving CRP are reported controlling for BMI. For the small number (n = 2) of samples with CRP concentrations below the limit of detection (0.2 mg/l), a value equal to one-half the lower limit (0.1 mg/l) was assigned. Inflammatory data were positively skewed so raw values were natural log-transformed to normalize the distribution prior to statistical testing.
We examined associations between perceived stress, amygdala reactivity and CRP by conducting Pearson correlations. To examine potential differences between associations with the left and right amygdala activity, we utilized Fisher’s z for dependent correlations (Lee and Preacher, 2013).
Results
Participants (N = 37) were, on average, 52.5 years of age (s.d. = 8.5; range, 32–65 years), and the majority of women (81.1%) identified as Caucasian. Over half (64.9%) of the participants were employed at the time of the study, and an additional 10.8% were retired. Further demographic and treatment-related information for the final sample is summarized in Table 1 and Table 2, respectively.
Table 1.
Demographic information
| Characteristic | N | % |
|---|---|---|
| Age (years) | ||
| 30–39 | 3 | 8.1 |
| 40–49 | 11 | 29.7 |
| 50–59 | 11 | 29.7 |
| 60–69 | 12 | 32.4 |
| Ethnicity | ||
| Caucasian | 30 | 81.1 |
| Latina | 3 | 8.1 |
| African American | 2 | 5.4 |
| Asian | 2 | 5.4 |
| Hispanic | 6 | 16.2 |
| Employment status | ||
| Employed | 24 | 64.9 |
| Retired | 4 | 10.8 |
| Other | 9 | 24.3 |
| Family incomea | ||
| ≤$29 999 | 3 | 8.1 |
| $30 000–$49 999 | 4 | 10.8 |
| $50 000–$69 999 | 5 | 13.5 |
| $70 000–$99 999 | 9 | 24.3 |
| $100 000–$149 999 | 4 | 10.8 |
| ≥$150 000 | 10 | 27.0 |
Note. N = 37 unless otherwise noted.
aTwo participants declined to report income.
Table 2.
Treatment-related information
| Characteristic | N | % |
|---|---|---|
| Stagea | ||
| 0 | 5 | 13.5 |
| I | 9 | 24.3 |
| II | 17 | 45.9 |
| III | 5 | 13.5 |
| Radiationb | 18 | 48.6 |
| Endocrine therapyb | 21 | 56.8 |
| Herceptinb | 6 | 16.2 |
| Chemotherapyc | 19 | 51.4 |
Note. N = 37 unless otherwise noted. Reported numbers and percentages reflect those who indicated they underwent radiation, endocrine therapy or chemotherapy, or took Herceptin, during the course of their breast cancer treatment.
aData missing/unknown for one participant.
bData missing/unknown for six participants.
cData missing/unknown for seven participants.
First, we examined the main effects of amygdala activity in response to the two conditions of interest, threatening faces and non-threatening faces. Both the threat and non-threat conditions resulted in significant amygdala activity relative to implicit baseline [threat: left amygdala, t(36) = 3.78 and P < 0.001; right amygdala, t(36) = 6.52 and P < 0.001; non-threat: left amygdala, t(36) = 2.74 and P = 0.005; right amygdala, t(36) = 3.70 and P < 0.001]. Overall amygdala activity was not significantly different when viewing threatening (vs non-threatening) facial expressions [left amygdala, t(36) = −0.03 and P = 0.49; right amygdala, t(36) = 0.52, P = 0.30]. Although there were no overall differences between amygdala activity to threatening vs non-threatening faces, our primary interest in the present study was to examine whether threat-related amygdala activity (in response to threatening vs non-threatening faces) was associated with perceived stress and CRP. It should be noted that age was positively correlated with threat-related amygdala activity [left: r(35) = 0.274, P = 0.050; right: r(35) = 0.335, P = 0.021). Additionally, employment status (employed vs retired/other) was significantly related to both perceived stress [t(18.52) = 2.154, P = 0.045] and threat-related amygdala activity [left: t(35) = 1.620, P = 0.057; right: t(35) = 2.355, P = 0.012], such that employed participants had lower stress and lower amygdala activity. However, controlling for age and employment status in analyses did not change results. (No other demographic or socioeconomic variables collected were related to perceived stress or threat-related amygdala activity.) Thus, uncontrolled results are presented below (except where controlling for BMI in analyses involving CRP).
We next examined whether perceived stress over the previous month was associated with amygdala activity while viewing threatening vs non-threatening faces. Correlational analyses revealed a significant, positive correlation between perceived stress and left amygdala reactivity to threatening (vs non-threatening) faces, r(35) = 0.323, P = 0.025 (Figure 1). A similar pattern was found for right amygdala activity, such that greater perceived stress was associated with greater right amygdala activity in response to threat. However, for right amygdala, this association did not reach statistical significance, r(35) = 0.205, P = 0.111.
Fig. 1.
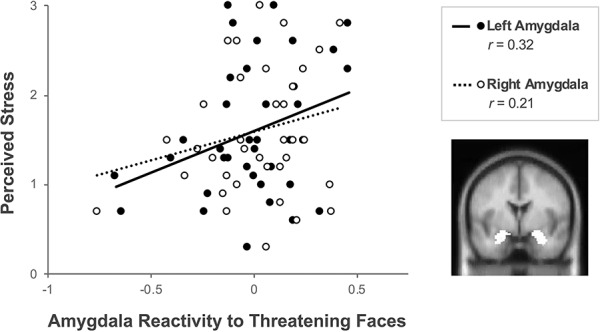
Perceived stress in breast cancer survivors is positively correlated with amygdala reactivity to threat. Correlations are shown between perceived stress over the previous month and parameter estimates for left amygdala reactivity (P = 0.025) and right amygdala reactivity (P = 0.111) to threatening (vs non-threatening) faces. Brain image depicts a highlighted cross-section of the amygdala ROI.
Since left amygdala activity in particular was significantly associated with perceived stress, we performed exploratory analyses to examine whether the associations with left amygdala were significantly stronger than those with right amygdala, as similar patterns have been reported previously (Muscatell et al., 2015; Muscatell et al., 2016a). However, the strength of the association between perceived stress and left amygdala compared with that of perceived stress and right amygdala was not significantly different (Fisher’s z = 1.27, two-tailed P = 0.21).
Next, we examined circulating levels of inflammation, as indexed by plasma CRP. On average, the women in the present sample had CRP levels of 2.20 mg/l (s.d. = 2.94 mg/l), falling within the range considered average inflammation and average cardiac risk (1–3 mg/l). However, there was a wide range in the values of plasma CRP within the sample (0.1–13 mg/l). Just over half of the sample (54%, n = 20) had CRP levels <1 mg/l (corresponding to low inflammation), 19% (n = 7) of women had CRP levels between 1 and 3 mg/l (average inflammation) and 27% (n = 10) had CRP levels >3 mg/l (high inflammation). This ample variability allowed us to examine associations with neural activity across a wide range of CRP levels.
We then examined whether amygdala activity was associated with circulating levels of inflammation, as indexed by plasma CRP. In line with hypotheses, greater left amygdala activity in response to threat (vs non-threatening faces) was associated with greater levels of CRP, r(34) = 0.329, P = 0.025 (Figure 2). A similar, but trending, pattern was found for the right amygdala activity and CRP, r(34) = 0.240, P = 0.079. As with perceived stress, associations between amygdala activity and CRP were not significantly different between the left and right amygdala (Fisher’s z = 1.01, two-tailed P = 0.32).
Fig. 2.

Circulating CRP level in breast cancer survivors is positively correlated with amygdala reactivity to threat. Correlations are shown between plasma levels of CRP and parameter estimates for left amygdala reactivity (P = 0.025) and right amygdala reactivity (P = 0.079) to threatening (vs non-threatening) faces. CRP values were natural log-transformed for analysis and plotting, but the vertical axis is labeled with non-transformed values for ease of interpretation. Brain image depicts a highlighted cross-section of the amygdala ROI.
Given that perceived stress was positively correlated with amygdala activity, which was in turn positively associated with CRP, we then considered testing whether heightened amygdala activity may serve as a mediator. However, perceived stress was not significantly associated with CRP, r(34) = 0.087, P = 0.615. Given this null association between predictor and outcome, we could not explore amygdala activity as a potential mediator of the relation between perceived stress and CRP.
Discussion
The primary goal of the present work was to examine amygdala reactivity to social threat in breast cancer survivors, focusing on associations with perceived stress, as well as associations with a circulating marker of inflammation, CRP. Results indicated that breast cancer survivors who reported greater levels of perceived stress in the preceding month showed greater amygdala reactivity to social threat, particularly in the left amygdala. Additionally, those who showed greater amygdala reactivity to social threat demonstrated higher levels of CRP, in line with the idea that, within breast cancer survivors in particular, neural activity in threat-related regions may be linked to peripheral inflammation.
Associations between amygdala activity in response to social threat and CRP have been demonstrated in prior work in a small sample (N = 15) of breast cancer survivors. Thus, in addition to replicating this finding in a larger, independent sample, the current findings extend this line of work by additionally linking perceived stress in the past month with such heightened amygdala activity in response to threat. This adds to growing evidence that the amygdala appears to be a key region involved in neuro-immune crosstalk. Specifically, the amygdala may be involved in translating psychological experiences of stress into downstream outcomes such as peripheral inflammation, which ultimately have been linked with important clinical outcomes within cancer populations.
In our results, left amygdala activity was more strongly tied to perceived stress and CRP, relative to right amygdala. Although exploratory follow-up analyses determined that the strength of the correlations for the left and right amygdala were not significantly different from each other, past work has found similar trends. For example, left amygdala activity in response to threat correlates with SNS-related responses (e.g. SCR), while right amygdala activity is not significantly correlated with SNS threat responses (Phelps et al., 2001). Additionally, left (but not right) amygdala activity correlates with inflammatory responses to stress (Muscatell et al., 2015). Within breast cancer survivors, Muscatell et al. (2016a) reported significant associations between amygdala reactivity and CRP for left amygdala, while associations for right amygdala generally did not reach significance.
However, it should be noted that some past work in undergraduate samples has not been consistent in this regard. For example, Swartz et al. (2017) found that in undergraduate men, but not women, threat-related amygdala activity was associated with higher levels of CRP. Although this study identified more robust right amygdala effects, significant effects in the left amygdala were also found when the sample was restricted to men without psychiatric diagnoses (matching an exclusion criterion that was utilized in the present study). Women in this previous undergraduate sample may not have shown the same associations as in the present sample due to differences in several demographic and health factors (e.g. age, cancer status, range of observed CRP values, presence of psychiatric diagnoses).
In sum, our findings are consistent with the past literature in breast cancer survivors, which shows that although associations between right amygdala and inflammatory markers are sometimes weaker, left and right amygdala typically show similar trends with inflammatory markers. Taken together, these findings may suggest that left amygdala activity in particular may be more tightly linked with SNS stress responding and a key region involved in modulating stress-induced peripheral inflammation. However, future work will be key in clarifying the role of amygdala lateralization in relation to experiences of stress and downstream inflammatory outcomes.
Data from this study should be interpreted in light of some important limitations. Due to the correlational and cross-sectional nature of the present work, inferences about the directionality of our reported findings cannot be made with confidence. We have focused on the hypothesis that psychological stress, via the SNS response, may lead to heightened amygdala reactivity in the course of initiating a stress response, ultimately leading to elevated levels of CRP. However, the perceived stress, amygdala reactivity and CRP metrics were derived from a single session, and the bidirectionality of neuro-immune pathways is well documented. While inflammation can occur following psychological distress or heightened threat-related neural activity, inflammation may also act as a precursor to both distress and threat-related neural activity. For example, increases in inflammation have been linked with greater subsequent feelings of social disconnection and depressed mood (Eisenberger et al., 2010). Past work has also demonstrated that experimentally heightened inflammation can lead to increased threat-related amygdala reactivity (Inagaki et al., 2012; Muscatell et al., 2016b). Thus, heightened inflammation, as represented here by plasma CRP, may act as both a cause and consequence of perceived stress and heightened threat-related neural activity. However, given that the present measure of perceived stress was retrospective and captured levels of perceived stress within the preceding month, we suspect that greater perceived stress may precede our observed heightened threat-related amygdala reactivity to social threats. Finally, additional variables that were not measured in the present sample, such as contraceptive use, may have influenced CRP levels (Dreon et al., 2003; Williams et al., 2004).
Despite these limitations, results from the present study add to a growing literature that suggests an important linkage between neural and immune outcomes, particularly within cancer survivors. This work builds on prior research that implicates the amygdala as a key structure in threat-related neural circuitries and peripheral inflammation, specifically linking perceived stress to amygdala reactivity to social threat. Furthermore, we demonstrate associations between perceptions of stress and heightened amygdala activity in response to threat, as well as replicating prior work linking CRP—an inflammatory marker critically linked with cancer-related outcomes—and heightened social threat-related amygdala reactivity, within a sample of breast cancer survivors. In sum, data from the present study suggest that breast cancer survivors who experience greater levels of psychological stress may be further sensitized to social threats, as demonstrated by heightened amygdala activity. Further, such heightened amygdala activity may coincide with greater levels of inflammation (e.g. CRP), which is likely to have implications for breast cancer recurrence and survivorship.
Funding
This work was supported by funds from the National Cancer Institute Network on Biobehavioral Pathways in Cancer (contract no. HHSN261200800001E, RFP no. 16X164). This publication was made possible by the Eunice Kennedy Shriver National Institute of Child Health and Human Development-funded predoctoral fellowships to C.J.L. (1T32HD091059, 1F31HD100144).
Conflict of interest
None declared.
Contributor Information
Carrianne J Leschak, Department of Psychology, University of California, Los Angeles, CA 90095, USA.
Janine M Dutcher, Department of Psychology, Carnegie Mellon University, Pittsburgh, PA 15213, USA.
Kate E Byrne Haltom, Department of Psychology, University of California, Los Angeles, CA 90095, USA.
Elizabeth C Breen, Semel Institute for Neuroscience and Human Behavior, University of California Los Angeles, Los Angeles, CA 90095, USA; Cousins Center for Psychoneuroimmunology, University of California Los Angeles, Los Angeles, CA 90095, USA.
Julienne E Bower, Department of Psychology, University of California, Los Angeles, CA 90095, USA; Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA 90095, USA; Jonsson Comprehensive Cancer Center, University of California Los Angeles, Los Angeles, CA 90095, USA.
Naomi I Eisenberger, Department of Psychology, University of California, Los Angeles, CA 90095, USA.
References
- Campbell J.P., Karolak M.R., Ma Y., et al. (2012). Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biology, 10(7), e1001363 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y., Hamer M., Wardle J., Steptoe A. (2008). Do stress-related psychosocial factors contribute to cancer incidence and survival? Nature Clinical Practice Oncology, 5(8), 466–75 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 386–96. [PubMed] [Google Scholar]
- Cole S.W., Nagaraja A.S., Lutgendorf S.K., Green P.A., Sood A.K. (2015). Sympathetic nervous system regulation of the tumour microenvironment. Nature Reviews Cancer, 15(9), 563–72 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L.M., Werb Z. (2002). Inflammation and cancer. Nature, 420(6917), 860–7 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., Pariante C.M., Caspi A., Taylor A., Poulton R. (2007). Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences, 104(4), 1319–24 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R., Olsson A., Phelps E.A. (2006). Extending animal models of fear conditioning to humans. Biological Psychology, 73(1), 39–48 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dreon D.M., Slavin J.L., Phinney S.D. (2003). Oral contraceptive use and increased plasma concentration of C-reactive protein. Life Sciences, 73(10), 1245–52 10.1016/S0024-3205(03)00425-9. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I. (2012). The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nature Reviews Neuroscience, 13(6), 421–34 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Inagaki T.K., Mashal N.M., Irwin M.R. (2010). Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, Behavior, and Immunity, 24(4), 558–63 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J.P., Glaser R., Malarkey W.B., Beversdorf D., Kiecolt-Glaser J. (2012). Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychology, 31(2), 264–8 10.1037/a0025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Tessitore A., Mattay V.S., Fera F., Weinberger D.R. (2002). The amygdala response to emotional stimuli: a comparison of faces and scenes. NeuroImage, 17(1), 317–23. [DOI] [PubMed] [Google Scholar]
- Henke P.G. (1982). The telencephalic limbic system and experimental gastric pathology: a review. Neuroscience and Biobehavioral Reviews, 6(3), 381–90. [DOI] [PubMed] [Google Scholar]
- Inagaki T.K., Muscatell K.A., Irwin M.R., Cole S.W., Eisenberger N.I. (2012). Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage, 59(4), 3222–6 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R.P., Rubal Banipal R.P.S., Vashistha R., Dhiman M., Munshi A. (2019). Association of elevated levels of C-reactive protein with breast cancer, breast cancer subtypes, and poor outcome. Current Problems in Cancer, 43(2), 123–9 10.1016/j.currproblcancer.2018.05.003. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E., Iwata J., Cicchetti P., Reis D.J. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neuroscience, 8(7), 2517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.A., Preacher K.J. (2013). Calculation for the test of the difference between two dependent correlations with one variable in common [Computer software]. Available: http://quantpsy.org/corrtest/corrtest2.htm (January 23, 2019).
- Mattavelli G., Sormaz M., Flack T., et al. (2014). Neural responses to facial expressions support the role of the amygdala in processing threat. Social Cognitive and Affective Neuroscience, 9(11), 1684–9 10.1093/scan/nst162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Marchant J.L., Hassabis D., et al. (2009). From threat to fear: the neural organization of defensive fear systems in humans. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29(39), 12236–43 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Smith M., Lutgendorf S.K., Sood A.K. (2010). Impact of stress on cancer metastasis. Future Oncology, 6(12), 1863–81 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.S., Frith C.D., Perrett D.I., et al. (1996). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature, 383, 812–5. [DOI] [PubMed] [Google Scholar]
- Muscatell K.A., Dedovic K., Slavich G.M., et al. (2015). Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain, Behavior, and Immunity, 43, 46–53 10.1016/J.BBI.2014.06.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Eisenberger N.I., Dutcher J.M., Cole S.W., Bower J.E. (2016a). Links between inflammation, amygdala reactivity, and social support in breast cancer survivors. Brain, Behavior, and Immunity, 53, 34–8 10.1016/j.bbi.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Moieni M., Inagaki T.K., et al. (2016b). Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain, Behavior, and Immunity, 21–9 10.1016/J.BBI.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orre I.J., Reinertsen K.V., Aukrust P., et al. (2011). Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. Journal of Psychosomatic Research, 71(3), 136–41 10.1016/J.JPSYCHORES.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Palermo-Neto J., de Oliveira Massoco C., Robespierre de Souza W. (2003). Effects of physical and psychological stressors on behavior, macrophage activity, and Ehrlich tumor growth. Brain, Behavior, and Immunity, 17(1), 43–54. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., Delgado M.R., Nearing K.I., LeDoux J.E. (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron, 43(6), 897–905 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., O’Connor K.J., Gatenby J.C., Gore J.C., Grillon C., Davis M. (2001). Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience, 4(4), 437–41 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Young A.W., Scott S.K., et al. (1998). Neural responses to facial and vocal expressions of fear and disgust. Proceedings of the Royal Society B, 265, 1809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce B.L., Ballard-Barbash R., Bernstein L., et al. (2009). Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. Journal of Clinical Oncology, 27(21), 3437–44 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan E.K., Capitanio J.P., Tarara R.P., Mendoza S.P., Mason W.A., Cole S.W. (2007). Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. The Journal of Neuroscience, 27(33), 8857–65 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A., Hamer M., Chida Y. (2007). The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, Behavior, and Immunity, 21(7), 901–12 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Swartz J.R., Prather A.A., Hariri A.R. (2017). Threat-related amygdala activity is associated with peripheral CRP concentrations in men but not women. Psychoneuroendocrinology, 78, 93–6 10.1016/j.psyneuen.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker P.H., Han L.Y., Kamat A.A., et al. (2006). Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature Medicine, 12(8), 939–44 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., et al. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168(3), 242–9 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser K.E., Eichten A., Coussens L.M. (2006). Paradoxical roles of the immune system during cancer development. Nature Reviews Cancer, 6(1), 24–37 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Shin L.M., McInerney S.C., Fischer H., Wright C.I., Rauch S.L. (2001). A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion (Washington, D.C.), 1(1), 70–83. [DOI] [PubMed] [Google Scholar]
- Williams M.J.A., Williams S.M., Milne B.J., Hancox R.J., Poulton R. (2004). Association between C-reactive protein, metabolic cardiovascular risk factors, obesity and oral contraceptive use in young adults. International Journal of Obesity and Related Metabolic Disorders, 28(8), 998–1003 10.1038/sj.ijo.0802713. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhou B.P. (2009). Inflammation: a driving force speeds cancer metastasis. Cell Cycle, 8(20), 3267–73 10.4161/cc.8.20.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]


