Abstract
There is a growing appreciation for the health benefits of giving support, though variability in such behavior exists. Based on the possibility that the dorsomedial (DMPFC) default network subsystem is associated with social thinking and behavior, integrity of this subsystem may facilitate giving support to others. The current study tested associations between DMPFC subsystem connectivity at rest and tendencies related to giving support. During a functional magnetic resonance imaging session, 45 participants completed an emotional social cues task, a resting-state scan and self-report measures of social support. Supportive behavior during the month following the scan was also assessed. Greater DMPFC subsystem connectivity at rest was associated with greater support giving (though not receiving or perceiving support) at the time of the scan and one month later. Results held after adjusting for extraversion. In addition, greater resting-state DMPFC subsystem connectivity was associated with attenuated dorsal anterior cingulate cortex, anterior insula and amygdala activity to others’ negative emotional social cues, suggesting that DMPFC subsystem integrity at rest is also associated with the dampened withdrawal response proposed to facilitate care for others in need. Together, results begin to hint at an additional role for the ‘default’ social brain: giving support to others.
Keywords: resting state, default network, social cognition, giving social support, social support
Introduction
Giving nurturing, supportive care to other people is an emerging predictor of health and well-being (Inagaki, 2018). For instance, giving support is associated with greater longevity (Brown et al., 2003; Poulin et al., 2013) and greater self-reported mental and physical health (Lum & Lightfoot, 2005). The health benefits of giving support suggest that people might be particularly inclined to engage in such other-focused behavior. Indeed, relative to other mammalian species, humans show some of the highest levels of nurturing and supportive behavior (American Time Use Survey, 2016), and many individuals care for others on a daily basis (Clark et al., 1987). However, there is variability in how much support and care people give to others (Clark et al., 1987), and this variability has implications for the health outcomes related to giving support (e.g. Piferi & Lawler, 2006). How and why some individuals are inclined to nurture and give support to others and the neurobiological mechanisms associated with this other-focused facet of sociality remain open for inquiry.
Dorsomedial prefrontal cortex subsystem connectivity and giving support to others
Insight into these questions may come from the observation that the same portions of the brain that engage when participants are instructed to think about others also spontaneously coordinate by default during rest. The dorsomedial prefrontal cortex (DMPFC), tempoparietal junction (TPJ), lateral temporal cortex (LTC) and temporal poles (TPs) increase activation when participants consider other people’s thoughts, emotions and traits (Saxe & Kanwisher, 2003; Mitchell et al., 2004; Frith & Frith, 2006; Van Overwalle, 2009; Spunt et al., 2011). These regions also comprise the dorsomedial (DMPFC) subsystem of the default network, showing preferential coordination during ‘resting-state scans’ (Andrews-Hanna et al., 2010). That is, the default network comprises multiple brain regions whose spontaneous fluctuations at rest are correlated (Greicius et al., 2003; Fox et al., 2005; Fransson, 2005; Vincent et al., 2007). However, these brain regions can be further organized into three constellations: the core subsystem, associated with self-referential processing (Gusnard & Raichle, 2001; Denny et al., 2012; Lieberman et al., 2019); the medial temporal lobe (MTL) subsystem, associated with episodic memory (Squire and Zola-Morgan, 1991); and the DMPFC subsystem, associated with thinking about others (Saxe & Kanwisher, 2003; Mitchell et al., 2004; Frith & Frith, 2006; Van Overwalle, 2009; Spunt et al., 2011). Importantly, there is variability in the extent to which individuals spontaneously engage the default network in general and these subsystems in particular during rest (Greicius, 2008; Reineberg et al., 2015; Waytz et al., 2015; Gratton et al., 2018; Meyer et al., 2019). Thus, individual differences in social tendencies relevant to these systems may be traceable to variation in these networks at rest.
Relevant to nurturing, supportive behavior, recent research suggests that the tendency to engage the DMPFC subsystem at rest may promote understanding and connecting with others. Engaging these regions during rest helps us learn new information about others (Meyer et al., 2019), step outside of our personal point of view (Meyer et al., 2019) and facilitate mental state and trait processing (Spunt et al., 2015; Meyer & Lieberman, 2018). For example, greater DMPFC subsystem activation during brief rest periods (6–9 s) predicts faster reaction time on subsequent trials that require reasoning about people’s intentions and personalities (Spunt et al., 2015; Meyer & Lieberman, 2018), suggesting that activating this system primes, or nudges, consideration of others’ points of view. Given that engaging the DMPFC subsystem at rest is associated with understanding others, coupled with evidence that thinking about others is associated with more supportive behavior (Batson et al., 1997; Masten et al., 2011; Waytz et al., 2012), individuals with the greatest tendency to engage the DMPFC subsystem at rest may be most inclined to give supportive, nurturing responses to others in need.
Here, we examine the possibility that variation in support giving is related to the functional integrity of the DMPFC subsystem at rest. Specifically, we test whether individuals who demonstrate the greatest functional connectivity in the DMPFC subsystem at rest also give the most support to others. Consistent with this possibility, prior research has shown that greater task-induced activity in the medial prefrontal cortex (MPFC), including DMPFC, as well as TPJ, is associated with greater support-giving behavior (Masten et al., 2011; Waytz et al., 2012; Zanon et al., 2014). These results suggest that individual differences in the tendency to activate portions of the DMPFC subsystem while considering others and giving support to them are linked. However, these results capture instances when participants are instructed to think about others. Whether integrity of the DMPFC subsystem as a whole, when left unprompted at rest, relates to the tendency to engage in supportive behavior remains unknown.
DMPFC subsystem connectivity and brain activity to social targets in need of support
If engaging the DMPFC subsystem at rest is associated with support giving, we would also expect that functional connectivity in this subsystem at rest corresponds with less dorsal anterior cingulate cortex (DACC), anterior insula (AI) and amygdala activity in response to others’ negative experiences, a salient signal that they may be in need. Indeed, it has been proposed that less activity in these regions in response to targets in need may facilitate social approach, allowing an individual to nurture and care for them (Brown & Brown, 2006; Numan, 2007; Preston, 2013; Inagaki & Orehek, 2017; Inagaki, 2018). In support of this hypothesis, greater self-reports of giving support to others in need are associated with less activity in the DACC, AI and amygdala to negative emotional social cues, including negative facial expressions (Inagaki et al., 2016; Inagaki & Ross, 2018). Relatedly, those suffering from social anxiety, a disorder characterized by reduced social approach, show greater activity in the DACC, AI and amygdala to negative emotional social cues (relative to non-anxious or less anxious groups; Amir et al., 2005; Phan et al., 2006; Stein et al., 2007). Less AI and amygdala activity to the same stimuli are also associated with less anxiety about interacting with others. Finally, machine-learning classifiers applied to functional magnetic resonance imaging (fMRI) data suggest that activity in the DACC and AI reflects personal distress to those in need (Ashar et al., 2017), which has also been shown to thwart supportive responses (Batson et al., 1987). Therefore, reduced activity in these regions may facilitate giving support.
The current study was a first step toward testing whether engaging the DMPFC subsystem at rest might be associated with (i) individual differences in giving support and (ii) neural mechanisms that contribute to giving support to others. Based on the importance of the DMPFC subsystem for understanding others, we hypothesized that individuals with greater integrity of the DMPFC subsystem, as evidenced by stronger functional connectivity within this network at rest, would report greater support giving in their daily life. In addition, we hypothesized that greater functional connectivity within the DMPFC subsystem at rest would correlate with attenuated DACC, AI and amygdala activity to others’ negative emotional social cues, a response that may facilitate providing support to others in need (Inagaki, 2018). Finally, we further assessed whether DMPFC connectivity at rest preferentially relates to giving support, relative to other similar, health-relevant individual differences in social tendencies, such as receiving support, perceiving support and extraversion.
Methods
Participants
Forty-eight individuals who met the inclusion criteria for MRI scanning (i.e. right handed, metal free, not claustrophobic) were recruited for a larger study on the neural correlates of giving social support to others. Other findings from the study have been reported elsewhere (Inagaki & Ross, 2018), but the data presented here have not previously been reported. Participants completed procedures under the oversight of the University of Pittsburgh’s Human Research Projection Office and were paid $50 for their participation. Prior to entering the scanner, participants were screened for current physical or mental illness, medication use other than birth control and pregnancy with a urine pregnancy test at the time of the scan.
Our goal was to obtain a complete data set of at least 40 individuals with usable data; thus, 48 individuals were run to guard against data loss due to motion artifacts, attrition and technical errors. Three participants were excluded from final analyses for either brain abnormalities (n = 2) or noncompliance with study screening criteria (previously undisclosed psychiatric medication; n = 1). Final imaging analyses are based on 45 participants (M age = 21.978 years, s.d. = 3.286, 31 were female).
Procedure
Participants completed three tasks in the MRI scanner: an emotional social cues task, a resting-state scan and a charitable giving task (reported separately; Inagaki & Ross, 2018). After the scan, participants completed self-report measures of social behavior. In addition, approximately 1 month later, participants completed a brief follow-up survey to assess giving support behavior outside of the experimental setting since the time of the scanning session.
Neuroimaging measures
Emotional social cues task
The current hypotheses are based on the theory that reducing social withdrawal from targets in need facilitates the subsequent provision of support. Critically, brain activity in the DACC, AI and amygdala to negative facial expressions has previously been related to social withdrawal (e.g. Stein et al., 2007), and negative facial expression is one of many cues that can signal the need for support. Therefore, DACC, AI and amygdala activity to emotional facial expressions was assessed in the current study.
Participants viewed two blocks each of angry and fearful emotional facial expressions from the NimSim set of Facial Expressions (Tottenham et al., 2009). Fearful and angry faces were evaluated because they map onto situations that could induce withdrawal or approach to provide support. As noted above, seeing a negative and high arousal facial expression is related to social withdrawal (Stein et al., 2007). Alternatively, a person expressing fear or anger could be consoled, which would help them calm down. For this reason, negative, high arousal emotion displays are well suited to test the dampened withdrawal response needed for and characteristic of support provision (Brown & Brown, 2006; Numan, 2007; Inagaki & Eisenberger, 2012; Preston, 2013). As a control, participants also viewed two blocks of neutral facial expressions. Two blocks of happy facial expressions were included as an additional control to test the parent study’s hypotheses (Inagaki & Ross, 2018), but were not examined here because we are principally interested in brain activity in response to individuals who might be in need of social support (those experiencing fear and anger). Each block included 20 facial expressions presented for 1.5 s each, and each block was separated by 12 s of a fixation crosshair. To encourage engagement with the task, participants were instructed to press a button whenever a new face appeared on the screen. Data from one participant were lost due to a technical error. Therefore, the emotional social cues task is based on data from 44 participants.
Resting state
Each scanning session included a resting-state scan. Participants were instructed to view a fixation crosshair on the screen as they relaxed and let their minds wander, but were asked to keep their eyes open and not to fall asleep. Rest scans were 8 min, 24 s, following past work (Tambini et al., 2010; Meyer et al., 2018).
Post-scan self-report measures
Giving and receiving support
After exiting the scanner, participants completed three social support measures in order to characterize perceptions of both forms of support: the two-way Social Support Scale (two-way SSS; Shakespeare-Finch & Obst, 2011), the Communal Orientation Scale (Clark et al., 1987) and the Social Provisions Scale (SPS; Cutrona & Russell, 1987).
The two-way SSS measures perceptions of both giving and receiving social support. Based on the theory that social support serves distinct functions (Wills, 1985), the scale divides into four subscales: giving emotional support (M = 4.236, s.d. = 0.743,  = 0.816), giving instrumental support (M = 3.693, s.d. =.722,
= 0.816), giving instrumental support (M = 3.693, s.d. =.722,  = 0.560), receiving emotional support (M = 4.567, s.d. = 0.670,
= 0.560), receiving emotional support (M = 4.567, s.d. = 0.670, 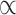 = 0.857) and receiving instrumental support (M = 4.300, s.d. = 0.852,
= 0.857) and receiving instrumental support (M = 4.300, s.d. = 0.852, 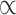 = 0.846). Participants used a 0–5 scale (anchored by ‘not at all’ and ‘always’) to items such as ‘I give others a sense of comfort during times of need’ and ‘I give financial assistance to people in my life,’ as examples from the giving emotional support subscale and giving instrumental support subscale, respectively. One item from the receiving emotional support subscale was mistakenly omitted, and so average ratings, as opposed to the sum, were calculated for each subscale.
= 0.846). Participants used a 0–5 scale (anchored by ‘not at all’ and ‘always’) to items such as ‘I give others a sense of comfort during times of need’ and ‘I give financial assistance to people in my life,’ as examples from the giving emotional support subscale and giving instrumental support subscale, respectively. One item from the receiving emotional support subscale was mistakenly omitted, and so average ratings, as opposed to the sum, were calculated for each subscale.
The communal orientation scale is similar to giving emotional support in that questions assess how important individuals find others’ needs and feelings, as well as how often one should give support to others and care for those in need. For the communal orientation scale, participants used a 1 (extremely uncharacteristic of me) to 5 (extremely characteristic of me) scale to respond to items, such as ‘I often go out of my way to help another person’ and ‘When people get emotionally upset, I tend to avoid them (reversed).’ Items were averaged such that higher numbers reflect greater communal orientation (M = 3.811, s.d. = 0.475,  = 0.734).
= 0.734).
The SPS is one of the most widely used measures of perceived social support (Cutrona & Russell, 1987) and has previously been associated with reduced amygdala activity to emotional social cues (Muscatell et al., 2016). Because this measure focuses on perceived social support, it gives us an additional way to test whether any observed associations are unique to giving support. Using a 1–4 scale, anchored by ‘strongly disagree’ and ‘strongly agree,’ participants reported on the extent to which they perceive different types of support (e.g. ‘There are people I can depend on to help me if I really need it.’). Responses were averaged prior to analyses (M = 3.558, s.d. = 0.337,  = 0.877).
= 0.877).
Extraversion
The health effects of giving or receiving support may be driven by other, well-known, individual differences in social behavior, such as extraversion (Roberts et al., 2007). Thus, extraversion was measured with the extraversion subscale of the Eysenck Personality Questionnaire (Eysenck & Eysenck, 1975) and was also evaluated as a covariate to assess the specificity of any associations with social support. Participants responded to 12 items with either a yes or no (e.g. do you enjoy meeting new people? do you like mixing with people?). Responses were summed such that higher numbers reflect higher extraversion (M = 8.422, s.d. = 3.792,  = 0.903).
= 0.903).
One month follow-up
Approximately 1 month after the scanning session, self-reported giving support was collected to assess giving support behavior since leaving the scanner. Four participants were unresponsive to follow-up requests, leaving a sample of 41 participants for the follow-up survey. We note that this sample size is still above our target to collect complete data on at least 40 participants to test the current aims.
Participants reported on the frequency with which they gave support to one of their own close others. Using a 1 (not at all) to 7 (a great deal) scale, participants identified a close other and then responded to the question ‘Over the past month, how often have you generally helped this person (e.g. gave them advice, gave them a shoulder to cry on?).’ In this sample, participants reported moderately high levels of giving to close others (M = 4.951, s.d. = 1.687).
Giving emotional support, as reported at the time of the scan, and frequency of giving support, as assessed 1 month later, were correlated (r = 0.438, P = 0.004), suggesting that the two measures were assessing similar but separate indices of giving behavior. Likewise, communal orientation was also associated with frequency of giving support outside of the scanner (r = 0.384, P = 0.007).
Brain imaging data acquisition
fMRI scanning took place at the University of Pittsburgh’s Neuroscience Imaging Center on a Siemens 3 T MAGNETOM Allegra MRI Scanner. Scans began with a Magnetization Prepared Rapid Gradient Echo scan [MP-RAGE; repetition time/echo time (TR/TE)
= 1540/3.04 ms; flip angle = 8 degrees; 256 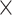 256 matrix; 192 sagittal slices; field of view (FOV) = 256; 1 mm thick] followed by functional scans. For the current hypotheses, participants completed a run of the emotional social cues task (5 min, 56 s) and a resting-state scan (8 min, 24 s; T2*-weighted gradient echo covering 36 axial slices; TR/TE = 2000/25 ms; flip angle
70 degrees; 64
256 matrix; 192 sagittal slices; field of view (FOV) = 256; 1 mm thick] followed by functional scans. For the current hypotheses, participants completed a run of the emotional social cues task (5 min, 56 s) and a resting-state scan (8 min, 24 s; T2*-weighted gradient echo covering 36 axial slices; TR/TE = 2000/25 ms; flip angle
70 degrees; 64  64 matrix; FOV = 200 mm; 3 mm thick).
64 matrix; FOV = 200 mm; 3 mm thick).
Data analyses
Brain imaging data analysis
Brain imaging data were analyzed with Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, London, UK). Data were realigned, normalized to the MP-RAGE, warped into Montreal Neurologic Institute space and smoothed with an 8-mm full-width half-maximum Gaussian kernel with the DARTEL procedure. For the resting-state scans, data were high-pass filtered with a 111 s cutoff to remove low frequencies below 0.009 Hz (Fox et al., 2005; Vincent et al., 2007; Tambini et al., 2010). Nuisance variables for each subject were created for the six motion parameters and their temporal derivatives from the realignment step. Next, a general linear model was created for each subject that included their nuisance variables as regressors. Brain activity during rest, controlling for activation due to motion (i.e. residual images from the first level analysis modeling nuisance variables), was saved and analyzed for group-level analyses.
For the emotional social cues task, the general linear model was used to estimate first-level effects for the contrast negative (fear and angry) vs neutral emotional social cues, which was then brought to group-level analyses. This contrast allowed us to examine individual differences in DACC, AI and amygdala activity in response to other people’s negative emotional social cues.
Region-of-interest analyses
Based on graph analytic approaches that delineate three distinct subsystems within the default network (Andrews-Hanna et al., 2010), resting-state analyses examined connectivity in the DMPFC, core and MTL subsystems separately. For the main hypotheses, DMPFC subsystem connectivity, comprising the DMPFC, TPJ, LTC and TP (Fig. 1), was examined by extracting time course data for each region from each resting-state scan. Next, the simple Pearson correlation (r) between time courses for each pair of regions within the DMPFC subsystem (DMPFC–TPJ, DMPFC–LTC, DMPFC–TP, TPJ–LTC, TPJ–TP, LTC–TP) was calculated. Correlation values were then Fisher z transformed to a single measure and used in subsequent statistical tests relating DMPFC subsystem connectivity to support-related responses (see below). In addition, the core and MTL subsystems were examined in the same manner as the DMPFC subsystem. The core subsystem included regions of interest (ROIs) of the MPFC and posterior cingulate cortex (PCC), and the MTL subsystem included the hippocampal formation, parahippocampal cortex, retrosplenial cortex, posterior inferior parietal lobule and ventromedial prefrontal cortex.
Fig. 1.

ROIs. Panel A shows regions that make up the DMPFC subsystem of the default network based on graph analytic approaches (Andrews-Hanna et al., 2010). Panel B shows the DACC, AI and amygdala ROIs evaluated in response to emotional social cues.
To examine associations between individual differences in DMPFC subsystem connectivity at rest and giving support to others, a tiered approach was taken to limit the number of comparisons. Pearson correlations between functional connectivity of the DMPFC subsystem and scores from the four subscales of the two-way SSS (giving emotional support, giving instrumental support, receiving emotional support, receiving instrumental support), communal orientation, SPS and follow-up measure of giving support were run separately in SPSS v.24. Next, to assess the specificity of associations, follow-up correlations with the core and MTL subsystems were run as well as partial correlations adjusting for extraversion for any significant correlations. Of note, there was a restricted range on the giving emotional support subscale of the two-way SSS and the follow-up measure of giving support. Therefore, Spearman rank-order correlations (rs) between DMPFC subsystem connectivity and these two scales were also run. Results from both Pearson correlations and Spearman correlations are reported.
Our corollary hypothesis is that DMPFC subsystem activity at rest will also be associated with less DACC, AI and amygdala activity to negative emotional social cues. Therefore, analyses for the emotional social cues task were constrained to activity in these three regions. All three regions have previously been shown to relate to giving support to others (Inagaki et al., 2016; Inagaki & Ross, 2018) and have known connections to physical health-relevant outcomes (Eisenberger & Cole, 2012). ROIs were structurally defined using the Automated Anatomical Labeling Atlas (Tzourio-Mazoyer et al., 2002). The DACC ROI was further constrained at 32 < y < 0 (Vogt et al., 2003). The insula was divided at y = 8, the approximate boundary between the dysgranular and granular sectors, to examine the anterior portion. To reduce the number of comparisons run to test the current hypotheses, regions were combined to create one mask. Parameter estimates from the mask were then extracted using MarsBar (http://marbar.sourceforge.net) from the negative > neutral emotional social cues contrast.
To examine associations between connectivity of the DMPFC subsystem at rest and DACC, AI and amygdala activity to the emotional social cues task, Pearson correlations between DMPFC connectivity and parameter estimates from the mask were run in SPSS v.24. For all analyses, 95% confidence intervals (CI) were estimated using the bias corrected and accelerated percentile bootstrap method (BCa) with 10 000 random samples with replacement. Because we had specific hypotheses, statistical tests of correlation strength were one-tailed.
Results
Individual-differences in DMPFC subsystem connectivity and giving support to others
Consistent with hypotheses, greater DMPFC subsystem connectivity at rest was associated with higher self-reports of giving emotional support to others at the time of the scan (r = 0.317, P = 0.017; BCa 95% CI, 0.024–0.552; rs = 0.244, P = 0.053; Fig. 2) and higher communal orientation (r = 0.272, P = 0.036; BCa 95% CI, 0.005–0.495). The association between functional connectivity of the core subsystem and giving emotional support was also significant (r = 0.272, P = 0.035; BCa 95% CI, 0.008–0.505), but there were no meaningful associations between connectivity of the MTL subsystem and giving emotional support (r = 0.172, P = 0.130; 95% BCa CI, −0.202 to 0.519) or between connectivity of the core or MTL subsystems at rest and communal orientation (Table 1).
Fig. 2.

Association between DMPFC subsystem connectivity at rest and social support measures at the time of the scan. Greater DMPFC subsystem connectivity at rest was associated with higher self-reports of giving emotional support to others and communal orientation (A), but not with receiving support or perceived support (B). Associations between DMPFC subsystem connectivity and the giving support measures hold when adjusting for extraversion. DMPFC subsystem connectivity values are Fisher z-transformed correlation values.
Table 1.
Default network connectivity at rest and self-reported social support: Pearson correlation coefficients (n = 45). Note that default network subsystem connectivity variables were Fisher z transformed
| Self-reported social support | |||||||
|---|---|---|---|---|---|---|---|
| Giving emotional support | Giving instrumental support | Receiving emotional support | Receiving instrumental support | Communal orientation | SPS | ||
| Resting-state connectivity | DMPFC subsystem | 0.317* | 0.145 | −0.006 | −0.031 | 0.272* | 0.007 |
| Core subsystem | 0.272* | 0.217 | 0.009 | 0.107 | 0.088 | 0.031 | |
| MTL subsystem | 0.172 | 0.177 | −0.024 | 0.075 | 0.092 | −0.021 | |
* P < 0.05.
As evidence for the specificity of DMPFC connectivity at rest to giving emotional, nurturing support, there were no associations between resting-state connectivity and the other subscales (i.e. giving instrumental support, receiving emotional support, receiving instrumental support) of the two-way SSS or between resting-state connectivity and the SPS (Table 1). The association between DMPFC subsystem connectivity at rest and giving emotional support was statistically different from the same associations with receiving and perceiving support, further suggesting a preferential association with giving support (receiving emotional support: z = 2.319, P = 0.010; receiving instrumental support: z = 2.287, P = 0.011; SPS: z = 2.186, P = 0.014). The association between DMPFC subsystem connectivity at rest and communal orientation was marginally different from the other associations (receiving emotional support: z = 1.561, P = 0.059; receiving instrumental support: z = 1.545, P = 0.061; SPS: z = 1.523, P = 0.064).
In addition to completing the social support measures at the time of the scan, participants also reported on their support given to a close other over the course of the month following their scan. DMPFC connectivity at rest was once again related to the frequency of giving support to a close other outside of the scanner over this 1 month period. Thus, greater DMPFC connectivity at rest was associated with a greater frequency of giving support since leaving the scanning session, conceptually replicating and extending the patterns reported above to real-world experience (r = 0.313, P = 0.023; BCa 95% CI, 0.036–0.570; rs = 0.382, P = 0.012; Fig. 3). However, connectivity of the core (r = 0.035, P = 0.413; BCa 95% CI, −0.247 to 0.327) and MTL (r = 0.185, P = 0.124; BCa 95% CI, −0.186 to 0.585) subsystems was not associated with frequency of giving support.
Fig. 3.

DMPFC subsystem connectivity at rest and giving support, as assessed 1 month after the scanning session. Greater DMPFC subsystem connectivity at rest was associated with greater reports of giving support to a close other since leaving the scanner the month prior. DMPFC subsystem connectivity values are Fisher z-transformed correlation values.
It is possible that associations between DMPFC connectivity at rest and the giving support measures reflect a greater tendency to be social, or simply interact with others more generally rather than give emotional support per se. Indeed, greater DMPFC connectivity at rest was also associated with higher extraversion (r = 0.367, P = 0.007; BCa 95% CI, 0.028–0.607). Thus, correlations were run again, adjusting for extraversion. The association between DMPFC connectivity at rest and giving emotional support remained after adjusting for extraversion (r = 0.295, P = 0.032; BCa 95% CI, 0.006–0.528), but the association with communal orientation reduced to marginal (r = 0.217, P = 0.089; BCa CI, −0.078 to 0.468). The association between DMPFC connectivity at rest at the time of the scan and frequency of giving support to a close other 1 month later also held after adjusting for extraversion (r = 0.363, P = 0.011; BCa 95% CI, 0.093–0.594).
Individual differences in DMPFC subsystem connectivity and DACC, AI and amygdala activity to emotional social cues
DACC, AI and amygdala activity to other people’s negative emotional social cues may have implications for giving support to others (Inagaki, 2018). Consistent with this notion and in a conceptual replication of previous correlational findings (Inagaki & Ross, 2018), greater reports of giving emotional support, taken at the time of the scan, were associated with less DACC, AI and amygdala activity to negative (vs neutral) emotional social cues (r = −0.252, P = 0.050; BCa 95% CI, −0.508 to −0.014). Furthermore, communal orientation was also associated with brain activity to the emotional social cues task such that those reporting higher communal orientation showed less DACC, AI and amygdala activity to negative (vs neutral) emotional social cues (r = −0.312, P = 0.020; BCa 95% CI, −0.593 to −0.017).
To test our second hypothesis, correlations between DMPFC connectivity at rest and DACC, AI and amygdala activity to emotional social cues were examined. In support of the hypothesis, greater functional connectivity of the DMPFC subsystem at rest was associated with lower activity in the DACC, AI and amygdala to negative (vs neutral) emotional social cues (r = −0.282, P = 0.032; BCa 95% CI, −0.533 to −0.011; Fig. 4). The association between connectivity of the core subsystem and DACC, AI and amygdala activity was trending in a similar direction (r = −0.213, P = 0.083; 95% CI, −0.534 to 0.124). In contrast, connectivity of the MTL subsystem at rest was not strongly correlated with DACC, AI and amygdala activity (r = −0.172, P = 0.132; BCa 95% CI, −0.435 to 0.094).
Fig. 4.

DMPFC subsystem connectivity during rest and brain activity to the emotional social cues task. Greater connectivity of the DMPFC subsystem at rest (Fisher z-transformed correlation values) was associated with lower DACC, AI and amygdala activity to negative (vs neutral) emotional social cues.
Discussion
The health relevance of social support has long been appreciated. However, a relatively new perspective is that giving support to others, in addition to any support one receives, also contributes to health in positive ways (Brown & Brown, 2006; Inagaki, 2018). Thinking about and understanding others, a process linked with the DMPFC default network subsystem, is theorized to be a major function of our inherent, ‘default state’ (Mitchell, 2006; Schilbach et al., 2008; Lieberman, 2013; Meyer, 2019). Here, we show that this default state may have implications for giving support to others. The current study examined whether individual differences in DMPFC subsystem resting-state functional connectivity are related to the inclination to give support to others. Indeed, greater functional connectivity within the DMPFC subsystem at rest was associated with giving emotional support to others across three different measures of giving emotional support and after adjusting for extraversion. Moreover, receiving and perceiving support from others was not associated with DMPFC subsystem connectivity at rest, further suggesting that functional connectivity in this system at rest may preferentially promote giving supportive care.
Results further show that DMPFC subsystem resting-state connectivity was negatively correlated with DACC, AI and amygdala activity to negative emotional social cues. It has been proposed that giving effective support relies on dampening DACC, AI and amygdala responding in order to approach and care for others (Taylor et al., 2000; Brown & Brown, 2006; Numan, 2007; Preston, 2013; Inagaki & Orehek, 2017). In animals, lesions to the amygdala, one of the neural regions examined in the present study, result in greater approach toward and parenting of offspring in otherwise naïve, virgin animals that do not normally display such forms of supportive behavior (Fleming et al., 1980; Sheehan et al., 2000). Furthermore, in humans, heightened DACC and AI to emotional stimuli has been shown in those struggling with social anxiety (Amir et al., 2005; Phan et al., 2006; Stein et al., 2007), suggesting that heightened activity in these regions may be a barrier to social approach. However, directional interpretations are made with caution due to the correlational nature of the present results. Future work that directly manipulates DACC, AI and amygdala responding to social cues (e.g. via pharmacological means; Inagaki et al., 2012) or manipulates opportunities to rest may help clarify the causal pathways linking DMPFC subsystem connectivity at rest and attenuated DACC, AI and amygdala responses to social cues.
In the context of physical health, the DACC, AI and amygdala are part of a network of regions that have anatomical connections with downstream, peripheral sympathetic nervous system (SNS) responding (Eisenberger & Cole, 2012; Muscatell & Eisenberger, 2012). Should approaching others in order to give them support involve the reduction of DACC, AI and amygdala activity to cues of those in need, giving support may also reduce downstream SNS responding. Consistent with this hypothesis, giving support reduces systolic blood pressure responses to a laboratory social threat (vs a control condition where no support is given; Inagaki & Eisenberger, 2016). Giving to others (vs giving to the self) also reduces resting systolic and diastolic blood pressure (Whillans et al., 2016), and proinflammatory gene expression (Nelson-Coffey et al., 2017), which are peripheral markers of physical health.
Neuroanatomical and experimental evidence further suggests that portions of the MPFC, including the DMPFC, have bidirectional links between the social world and peripheral autonomic responding (Eisenberger & Cole, 2012; Gianaros & Wager, 2015; Muscatell et al., 2015). Indeed, a recent study found that resting-state connectivity between DMPFC and other portions of the default network are negatively correlated with DACC activity at rest, as well as circulating plasma levels of interleukin 6, a key inflammatory mediator implicated in chronic illness (Marsland et al., 2017). Such findings are consistent with the notion that resting-state DMPFC engagement has implications for downstream physiological responding and ultimately physical health. Whether and how resting-state connectivity in the DMPFC subsystem contributes to the link between giving support and health in a causal way may be an interesting avenue for future inquiry into the health effects accrued from giving support.
The current study is not without limitations. First, results are correlational and, as noted above, require additional studies to establish the causal role of DMPFC subsystem resting-state connectivity in giving support to others in need. Second, although our hypotheses were specific to the DMPFC subsystem, resting-state connectivity in the core default subsystem (MPFC and PCC), which is hypothesized to promote self-focused (Gusnard & Raichle, 2001; Denney et al., 2012; Lieberman et al., 2019) rather than other-focused processing, also correlated with self-reports of giving emotional support. However, core subsystem connectivity was not related to the other measures of giving support, namely, communal orientation and giving support over the course of the subsequent month. Therefore, we have less confidence in the core default subsystem’s relationship to giving support. Future work may clarify whether and how the core default system relates to giving emotional support, as well as the extent to which our findings are specific to the DMPFC subsystem. Second, resting-state scans in the current study were acquired after the emotional social cues task, making it challenging to rule out the possibility that neural responses to the emotional social cues task impacted subsequent resting-state connectivity. Additional research with resting-state scans placed before the task or presented in counterbalanced order is needed to replicate the current findings. Third, while the current study has implications for the neural mechanisms that link giving support with health, no health measures were collected. Future research that integrates measures more proximal to health (e.g. blood pressure, measures of systemic inflammation) would clarify the implications of the current findings for health.
Given the importance of giving support for mental and physical health, the brain may have mechanisms in place that promote such behavior. The current results provide initial evidence consistent with this notion, showing associations between DMPFC subsystem connectivity at rest and giving nurturing, support-related tendencies (at both the levels of self-reported subjective experience in and outside of the laboratory and brain activity in response to tasks in the scanner). Collectively, these results suggest that engaging the DMPFC subsystem at rest relate to and thus may promote an important part of human sociality, namely, giving nurturing support to others in need.
Acknowledgments
We thank Lauren Ross and Laura Hazlett for their assistance in running the study.
Contributor Information
Tristen K Inagaki, Department of Psychology, University of Pittsburgh, Pittsburgh, PA 15260, USA.
Meghan L Meyer, Department of Psychological and Brain Sciences, Dartmouth College, Hanover, NH 03755, USA.
References
- American Time Use Survey, Bureau of Labor Statistics, U.S. Department of Labor Time spent in primary activities for the civilian population by age, sex, race, Hispanic or Latino ethnicity, marital status, and educational attainment, 2018 annual averages. www.bls.gov/news.release/atus.t03.htm. July 11, 2019.
- Amir N., Klumpp H., Elias J., Bedwell J.S., Yanasak N., Miller L.S. (2005). Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biological Psychiatry, 57, 975–81. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. (2010). Functional-anatomic fractionation of the brain’s default network. Neuron, 65, 550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashar Y.K., Andrews-Hanna J.R., Dimidjian S., Wager T.D. (2017). Empathic care and distress: predictive brain markers and dissociable brain systems. Neuron, 94, 1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson C.D., Fultz J., Schoenrade P.A. (1987). Distress and empathy: two qualitatively distinct vicarious emotions with different motivational consequences. Journal of Personality, 55, 19–39. [DOI] [PubMed] [Google Scholar]
- Batson C.D., Sager K., Garst E., Kang M., Rubchinsky K., Dawson K. (1997). Is empathy-induced helping due to self-other merging? Journal of Personality and Social Psychology, 73, 495. [Google Scholar]
- Brown S.L., Brown R.M. (2006). Selective investment theory: recasting the functional significance of close relationships. Psychological Inquiry, 17, 1–29. [Google Scholar]
- Brown S.L., Nesse R.M., Vinokur A.D., Smith D.M. (2003). Providing social support may be more beneficial than receiving it: results from a prospective study of mortality. Psychological Science, 14, 320–7. [DOI] [PubMed] [Google Scholar]
- Clark M.S., Oullette R., Powell M.C., Milberg S. (1987). Recipient’s mood, relationship type, and helping. Journal of Personality and Social Psychology, 53, 94–103. [DOI] [PubMed] [Google Scholar]
- Cutrona C.E., Russell D. (1987). The provisions of social relationships and adaptation to stress In: Jones P., editor. Advances in Personal Relationships, Vol. 1, Greenwich, CT: JAI Press, pp. 37–67. [Google Scholar]
- Denny B.T., Kober H., Wager T.D., Ochsner K.N. (2012). A meta-analysis of functional neuroimaging studies of self-and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience, 24, 1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Cole S.W. (2012). Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nature Neuroscience, 15, 669–74. [DOI] [PubMed] [Google Scholar]
- Eysenck H.J., Eysenck S.B.G. (1975). Manual of the Eysenck Personality Questionnaire (Junior and Adult), Kent, UK: Hodder & Stoughton. [Google Scholar]
- Fleming A.S., Vaccarino F., Luebke C. (1980). Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiology & Behavior, 25, 731–43. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102, 9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. (2005). Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Human Brain Mapping, 26, 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C.D., Frith U. (2006). The neural basis of mentalizing. Neuron, 50, 531–4. [DOI] [PubMed] [Google Scholar]
- Gianaros P.J., Wager T.D. (2015). Brain–body pathways linking psychological stress and physical health. Current Directions in Psychological Science, 24, 313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C., Laumann T.O., Nielsen A.N., et al. (2018). Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron, 98, 439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. (2008). Resting-state functional connectivity in neuropsychiatric disorders. Current Opinion in Neurology, 21, 424–30. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100, 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D.A., Raichle M.E. (2001). Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience, 2, 685–94. [DOI] [PubMed] [Google Scholar]
- Inagaki T.K. (2018). Neural mechanisms of the link between giving social support and health. Annals of the New York Academy of Sciences, 1428, 33–50. [DOI] [PubMed] [Google Scholar]
- Inagaki T.K., Eisenberger N.I. (2012). Neural correlates of giving support to a loved one. Psychosomatic Medicine, 74, 3–7. [DOI] [PubMed] [Google Scholar]
- Inagaki T.K., Eisenberger N.I. (2016). Giving support to others reduces sympathetic nervous system-related responses to stress. Psychophysiology, 53, 427–35. [DOI] [PubMed] [Google Scholar]
- Inagaki T.K., Orehek E. (2017). On the benefits of giving social support: when, why, and how support providers gain by caring for others. Current Directions in Psychological Science, 26, 109–13. [Google Scholar]
- Inagaki T.K., Ross L.P. (2018). Neural correlates of giving social support: differences between giving targeted versus untargeted support. Psychosomatic Medicine, 80, 724–32. [DOI] [PubMed] [Google Scholar]
- Inagaki T.K., Muscatell K.A., Irwin M.R., Cole S.W., Eisenberger N.I. (2012). Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage, 59, 3222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T.K., Byrne Haltom K.E., Suzuki S., et al. (2016). The neurobiology of giving versus receiving support: the role of stress-related and social reward-related neural activity. Psychosomatic Medicine, 78, 443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D. (2013). Social: Why Our Brains Are Wired to Connect, New York: Crown. [Google Scholar]
- Lieberman M.D., Straccia M.A., Meyer M.L., Du M., Tan K.M. (2019). Social, self, (situational), and affective processes in medial prefrontal cortex (MPFC): causal, multivariate, and reverse inference evidence. Neuroscience & Biobehavioral Reviews, 99, 311–28. [DOI] [PubMed] [Google Scholar]
- Lum T.Y., Lightfoot E. (2005). The effects of volunteering on the physical and mental health of older people. Research on Aging, 27, 31–55. [Google Scholar]
- Marsland A.L., Kuan D.C.H., Sheu L.K., et al. (2017). Systemic inflammation and resting state connectivity of the default mode network. Brain, Behavior, and Immunity, 62, 162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten C.L., Morelli S.A., Eisenberger N.I. (2011). An fMRI investigation of empathy for ‘social pain’and subsequent prosocial behavior. NeuroImage, 55, 381–8. [DOI] [PubMed] [Google Scholar]
- Meyer M.L. (2019). Social by default: characterizing the social functions of the resting brain. Current Directions in Psychological Science. doi: 10.1177/0963721419857759. [DOI] [Google Scholar]
- Meyer M.L., Lieberman M.D. (2018). Why people are always thinking about themselves: medial prefrontal cortex (MPFC) activity during rest primes self-referential processing. Journal of Cognitive Neuroscience, 30, 714–21. [DOI] [PubMed] [Google Scholar]
- Meyer M.L., Davachi L., Ochsner K.N., Lieberman M.D. (2018). Evidence that default network connectivity during rest consolidates social information. Cerebral Cortex, 29, 1910–20. [DOI] [PubMed] [Google Scholar]
- Meyer M., Waytz A.G., Hershfield H.E., Mildner J., Tamir D.I. (2019). Creative expertise is associated with transcending the here and now. Journal of Personality and Social Psychology, 116, 483–94. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P. (2006). Mentalizing and Marr: an information processing approach to the study of social cognition. Brain Research, 1079, 66–75. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P., Macrae C.N., Banaji M.R. (2004). Encoding-specific effects of social cognition on the neural correlates of subsequent memory. Journal of Neuroscience, 24, 4912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Eisenberger N.I. (2012). A social neuroscience perspective on stress and health. Social and Personality Psychology Compass, 6, 890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Dedovic K., Slavich G.M., et al. (2015). Greater amygdala activity and dorsomedial prefrontal–amygdala coupling are associated with enhanced inflammatory responses to stress. Brain, Behavior, and Immunity, 43, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Eisenberger N.I., Dutcher J.D., Cole S.W., Bower J.E. (2016). Links between inflammation, amygdala reactivity, and social support in breast cancer survivors. Brain, Behavior, and Immunity, 53, 34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson-Coffey S.K., Fritz M.M., Lyubomirsky S., Cole S.W. (2017). Kindness in the blood: a randomized controlled trial of the gene regulatory impact of prosocial behavior. Psychoneuroendocrinology, 81, 8–13. [DOI] [PubMed] [Google Scholar]
- Numan M. (2007). Motivational systems and the neural circuitry of maternal behavior in the rat. Developmental Psychobiology, 49, 12–21. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Tancer M.E. (2006). Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry, 59, 424–9. [DOI] [PubMed] [Google Scholar]
- Piferi R.L., Lawler K.A. (2006). Social support and ambulatory blood pressure: an examination of both receiving and giving. International Journal of Psychophysiology, 62, 328–36. [DOI] [PubMed] [Google Scholar]
- Poulin M.J., Brown S.L., Dillard A.J., Smith D.M. (2013). Giving to others and the association between stress and mortality. American Journal of Public Health, 103, 1649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston S.D. (2013). The origins of altruism in offspring care. Psychological Bulletin, 139, 1305. [DOI] [PubMed] [Google Scholar]
- Reineberg A.E., Andrews-Hanna J.R., Depue B.E., Friedman N.P., Banich M.T. (2015). Resting-state networks predict individual differences in common and specific aspects of executive function. NeuroImage, 104, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B.W., Kuncel N.R., Shiner R., Caspi A., Goldberg L.R. (2007). The power of personality: the comparative validity of personality traits, socioeconomic status, and cognitive ability for predicting important life outcomes. Perspectives on Psychological Science, 2, 313–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. (2003). People thinking about thinking people: the role of the temporo-parietal junction in ‘theory of mind’. NeuroImage, 19, 1835–42. [DOI] [PubMed] [Google Scholar]
- Schilbach L., Eickhoff S.B., Rotarska-Jagiela A., Fink G.R., Vogeley K. (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the ‘default system’ of the brain. Consciousness and Cognition, 17, 457–67. [DOI] [PubMed] [Google Scholar]
- Shakespeare-Finch J., Obst P.L. (2011). The development of the 2-Way Social Support Scale: a measure of giving and receiving emotional and instrumental support. Journal of Personality Assessment, 93, 483–90. [DOI] [PubMed] [Google Scholar]
- Sheehan T.P., Cirrito J., Numan M.J., Numan M. (2000). Using c-Fos immunocytochemistry to identify forebrain regions that may inhibit maternal behavior in rats. Behavioral Neuroscience, 114, 337. [DOI] [PubMed] [Google Scholar]
- Spunt R.P., Satpute A.B., Lieberman M.D. (2011). Identifying the what, why, and how of an observed action: an fMRI study of mentalizing and mechanizing during action observation. Journal of Cognitive Neuroscience, 23, 63–74. [DOI] [PubMed] [Google Scholar]
- Spunt R.P., Meyer M.L., Lieberman M.D. (2015). The default mode of human brain function primes the intentional stance. Journal of Cognitive Neuroscience, 27, 1116–24. [DOI] [PubMed] [Google Scholar]
- Squire L.R., Zola-Morgan S. (1991). The medial temporal lobe memory system. Science, 253, 1380–5. [DOI] [PubMed] [Google Scholar]
- Stein M.B., Simmons A.N., Feinstein J.S., Paulus M.P. (2007). Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. American Journal of Psychiatry, 164, 318–27. [DOI] [PubMed] [Google Scholar]
- Tambini A., Ketz N., Davachi L. (2010). Enhanced brain correlations during rest are related to memory for recent experiences. Neuron, 65, 280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.E., Klein L.C., Lewis B.P., Gruenewald T.L., Gurung R.A.R., Updegraff J.A. (2000). Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological Review, 107, 411–29. [DOI] [PubMed] [Google Scholar]
- Tomasello M. (2016). A Natural History of Human Morality, Cambridge, MA: Harvard University Press. [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., et al. (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168, 242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15, 273–89. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. (2009). Social cognition and the brain: a meta-analysis. Human Brain Mapping, 30, 829–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J.L., Patel G.H., Fox M.D., et al. (2007). Intrinsic functional architecture in the anaesthetized monkey brain. Nature, 447, 83. [DOI] [PubMed] [Google Scholar]
- Vogt B.A., Berger G.R., Derbyshire S.W. (2003). Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience, 18, 3134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waytz A., Zaki J., Mitchell J.P. (2012). Response of dorsomedial prefrontal cortex predicts altruistic behavior. Journal of Neuroscience, 32, 7646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waytz A.G., Hershfield H.E., Tamir D.I. (2015). Mental simulation and meaning in life. Journal of Personality and Social Psychology, 108, 336–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whillans A.V., Dunn E.W., Sandstrom G.M., Dickerson S.S., Madden K.M. (2016). Is spending money on others good for your heart? Health Psychology, 35, 574–83. [DOI] [PubMed] [Google Scholar]
- Wills T.A. (1985). Supportive functions of interpersonal relationships In: Cohen S., Syme L., editors. Social Support and Health, Orlando, FL: Academic Press, pp. 61–82. [Google Scholar]
- Zanon M., Novembre G., Zangrando N., Chittaro L., Silani G. (2014). Brain activity and prosocial behavior in a simulated life-threatening situation. NeuroImage, 98, 134–46. [DOI] [PubMed] [Google Scholar]


