Abstract
Systemic inflammation is increasingly appreciated as a predictor of health and well-being. Further, inflammation has been shown to influence and be influenced by affective experiences. Although prior work has substantiated associations between inflammatory and affective processes, fewer studies have investigated the neurobiological correlates that underlie links between systemic, low-grade inflammation and affective reactivity. Thus, the current study examined whether markers of systemic inflammation (i.e. interleukin-6, C-reactive protein) are associated with differential patterns of neural activation and connectivity in corticolimbic regions in response to affective images. We investigated this question in a sample of 66 adults (44 women, M age = 54.98 years, range = 35–76) from the Midlife in the United States study. Higher levels of inflammation were associated with lower activity in limbic regions (i.e. amygdala, hippocampus, anterior insula, temporal pole) when viewing positive (vs neutral) images. Higher levels of inflammation were also associated with greater connectivity between the hippocampus and the medial prefrontal cortex in response to positive images. Inflammatory markers were not associated with significant differences in activation or connectivity to negative images. These findings highlight the utility of health neuroscience approaches in demonstrating that physiological processes such as inflammation are related to how our brains respond to affective information.
Keywords: inflammation, affect, functional connectivity, hippocampus, amygdala, interleukin-6
Systemic inflammation, a component of the innate immune system, is increasingly appreciated for its role in the pathophysiology of chronic disease and psychopathology (Dantzer et al., 2008; Liu et al., 2017; Bennett et al., 2018). The inflammatory response is primarily an adaptive defense mechanism that activates to harmful pathogens and promotes healing; however, chronic and uncontrolled inflammation can have negative consequences for physical and mental health (Gabay, 2006; Franceschi and Campisi, 2014; Furman et al., 2019). Interestingly, a growing literature in psychoneuroimmunology shows that, in addition to its role in both acute infection and chronic disease, systemic inflammation both affects and is affected by psychological experiences (Dickerson et al., 2009; Irwin and Cole, 2011). The purpose of the present study was to investigate the association between systemic, low-grade inflammation and one such psychological experience, affective reactivity. Specifically, we examined the relationship between markers of inflammation and neural responses to positive and negative images, compared to neutral images, to further our nascent understanding of the bidirectional links between the innate immune system and brain function.
What do we currently know about links between affective reactivity and inflammation? Most prior work in this area has focused on the links between negative affect, both chronic (e.g. depression) and acute (e.g. in response to a psychological stressor), and inflammation. Meta-analytic evidence suggests that elevated depressive symptoms are associated with higher levels of systemic inflammation (Howren et al., 2009) and that acute stressors elicit increases in markers of systemic inflammation (Marsland et al., 2017b). A handful of functional MRI (fMRI) studies have investigated the neural correlates of negative affect-inducing experiences and inflammation, showing that both social evaluation (Muscatell et al., 2016b) and grief elicitation among recently bereaved individuals (O’Connor et al., 2009b) are associated with greater activation in the medial prefrontal cortex, amygdala and anterior cingulate cortex and with greater levels of inflammation.
Some work has also investigated the ‘bottom-up’, afferent influence of inflammation on negative affective processes. This area of work demonstrates that experimentally induced increases in markers of inflammation (via inflammatory challenge studies utilizing lipopolysaccharide or typhoid vaccination) are associated with higher depressive symptoms and greater feelings of social disconnection (Eisenberger et al., 2009, 2010b; Harrison et al., 2009). Complementary neuroimaging work has investigated the influence of peripheral inflammation on neural reactivity to negative affect-related stimuli, such as threatening faces (Inagaki et al., 2012), negative social feedback (Muscatell et al., 2016b), social exclusion (Eisenberger et al., 2009) and threatening images (Kullmann et al., 2013). Together, these studies show that higher levels of inflammation are associated with increased activity in limbic (e.g. amygdala, hippocampus) and cortical (e.g. medial prefrontal cortex, cingulate cortex) regions in response to negative stimuli. Thus, a growing literature shows that systemic inflammation both affects and is affected by negative emotional experiences and stimuli, via alterations in subcortical (i.e. amygdala) and cortical (i.e. ACC, insula, dmPFC) neural activity.
Fewer studies have explored the bidirectional links between positive affect and levels of inflammation, especially as they relate to neural functioning. This paucity of work is surprising, considering that several studies have found that both dispositional positive affect (Marsland et al., 2006; Stellar et al., 2015; Hartanto et al., 2019) and momentary positive affective states (Steptoe et al., 2005) are associated with lower levels of inflammation. To date, no known neuroimaging studies have examined the efferent pathway or how the induction of a positive affective experience influences levels of inflammation. Correlational studies show that activity in the medial prefrontal cortex in response to positive stimuli (e.g. favorite actor; positive autobiographical memories) is related to better innate immune system functioning (i.e. natural killer cell count; Matsunaga et al., 2008) and lower inflammation (i.e. interferon-γ; Matsunaga et al., 2013), respectively, suggesting that greater neural responses to positive stimuli might be related to lower levels of systemic inflammation. A more substantial literature has examined how manipulating inflammation results in changes in neural responses to positive affective stimuli. Most of the work examining this afferent pathway has focused on documenting inflammation-related changes in neural reactivity to monetary reward tasks. These studies generally find that inflammation causes a decrease in neural activity in reward-related regions (i.e. ventral striatum) in response to monetary gain (Eisenberger et al., 2010a; Capuron et al., 2012; Moieni et al., 2019, c.f. Inagaki et al., 2015; Muscatell et al., 2016b). Together, these studies suggest that higher levels of inflammation might be associated with decreased activity in regions within the basal ganglia in response to positive stimuli. Generally, a growing literature demonstrates that systemic inflammation can both affect and be affected by positive experiences and stimuli, via alterations in subcortical (i.e. ventral striatum) and cortical (i.e. medial prefrontal cortex) neural activity, although less work has been conducted in this area.
Interestingly, there is substantial overlap in the brain regions that are implicated in inflammatory processes reviewed previously and in regions that show significant activation to positive and negative stimuli. For example, recent meta-analytic work has revealed that activity in several corticolimbic regions [e.g. dorsal medial prefrontal cortex (dmPFC), amygdala, hippocampus, striatum, insula] is consistently associated with levels of peripheral inflammation (Kraynak et al., 2018). In another meta-analysis that examined the brain basis of affective processing (Lindquist et al., 2016), similar limbic (e.g. amygdala, insula, striatum) and cortical regions (e.g. dmPFC and dACC) were also implicated in the processing of positive and negative information. Findings from these two meta-analyses converge to suggest that corticolimbic regions are involved in both affective and inflammatory processes. Thus, these corticolimbic regions may be important in facilitating cross-talk between the brain and the innate immune system in response to affective information.
Although prior research has identified associations between peripheral inflammation and corticolimbic activity in response to affective experiences, numerous gaps in our knowledge still exist. For example, most studies have utilized acute inflammatory challenge manipulations to study links between inflammation and neural activity; as such, we have limited knowledge about the association between chronic, low-grade inflammation and corticolimbic activity to affective stimuli. Further, most studies focus on monetary rewards as a proxy for positive experiences and angry/fearful faces for negative stimuli, leaving gaps in our knowledge of the associations between inflammation and neural responses to other types of affective stimuli (e.g. positive and negative scenes). Finally, although several psychoneuroimmunological studies have examined the associations between inflammation and functional connectivity while individuals are at rest (Felger et al., 2016; Lekander et al., 2016; Marsland et al., 2017a; Mehta et al., 2018; Kraynak et al., 2019; Nusslock et al., 2019), few known studies have examined how markers of systemic inflammation might relate to functional connectivity while participants are engaged in a dynamic affective reactivity task. In several studies, task-based connectivity has been shown to outperform resting-state models for detecting relationships between neural activity and individuals’ differences in behavior (Greene et al., 2018; Jiang et al., 2020), suggesting that investigations of associations between inflammation and task-based connectivity are warranted. Thus, the present study was designed to fill these gaps in our knowledge by exploring associations between low-grade peripheral inflammation and neural reactivity and connectivity in response to viewing affective images.
To accomplish this, we examined associations between markers of systemic inflammation and neural reactivity/connectivity to affective images in a sample of 66 adults from the Midlife in the United States (MIDUS) study. Specifically, we examined the relationship between levels of interleukin-6 (IL-6) and C-reactive protein (CRP) and corticolimbic responsivity and connectivity to positive and negative images. IL-6 and CRP are two commonly measured markers of inflammation in psychoneuroimmunology research. IL-6 is an inflammatory cytokine that is released into circulation in response to both physical and psychological threats to help facilitate communication among immune cells, among other functions. IL-6 also stimulates the production of CRP, an acute-phase protein produced by the liver that plays several roles during an inflammatory response. Elevated levels of IL-6 and CRP in the absence of acute infection are often conceptualized as representing chronic, low-grade inflammation (O’Connor et al., 2009a).
Methods
Participants
Data for this project were drawn from the Midlife in the United States (MIDUS) study, a national longitudinal study that examines biopsychosocial factors influencing health across later life. A subset of individuals from the MIDUS cohort completed the Neuroscience Project, beginning in 2007. Participants were eligible for this sub-study if they completed the Biomarker Project 4 visit, met MRI inclusion criteria (e.g. no metal implants, no claustrophobia, not currently pregnant) and had no prior history of a neurological disorder. Of the 72 total individuals enrolled in the Neuroscience Project, for the present paper, we excluded six: two for excessive head motion, one due to incomplete fMRI data and four due to levels of CRP greater than 10 mg/l which likely indicates a current or recent infection (Jaye and Waites, 1997). The 66 participants included in the analyses had a mean age of 54.98 years (s.d. = 10.76; range = 35–76) and consisted of 44 women (66.67%). See Table 1 for additional demographic information. Participants in the fMRI subsample were of similar age and exhibited comparable values of CRP and IL-6 to those in the larger MIDUS study, such that there were no significant differences between the samples for these characteristics (P’s > 0.42).
Table 1.
Demographic and biomarker summary of the study sample
| Variable | Count (N) | Percentage (%) |
|---|---|---|
| Sex (female, %) | 44 | 66.7 |
| Race/ethnicity | ||
| Black | 19 | 28.8 |
| Native American or Aleutian Islander | 1 | 3 |
| White | 45 | 68.2 |
| Other | 1 | 3 |
| Educational attainment | ||
| Less than high school | 27 | 40.9 |
| High school | 21 | 31.8 |
| Bachelor’s degree | 8 | 12.1 |
| Master’s degree | 10 | 15.2 |
| Mean (SD) | Range | |
| Age | 54.98 (10.76) | 35–76 |
| BMI | 29.52 (5.67) | 19.51–46.78 |
| IL-6 (pg/ml) | 2.88 (2.87) | 0.16–18.40 |
| IL-6 (natural log) | 0.74 (0.81) | −1.83–2.91 |
| CRP (ug/ml) | 2.21 (1.98) | 0.16–7.62 |
| CRP (natural log) | 0.38 (0.96) | −1.83–2.03 |
Procedures and materials
Overview
Participants in this study first completed the Biomarker Project 4 visit. The visit was an overnight session in which participants completed questionnaires and provided urine, blood and saliva samples to assess biological indicators of physiological functioning and health status, including markers of inflammation. Following the biomarker collection, participants then completed an fMRI scan.
IL-6 and CRP
After overnight fasting, participants provided blood samples later assayed for levels of IL-6 and CRP. Assays were conducted using commercially available kits according to manufacturer instructions. Both CRP and IL-6 assays showed acceptable inter-assay CVs (2.1–12.3%). More details are provided in Supplemental Materials. Both IL-6 and CRP values were natural log-transformed to adjust for the positive skew in the data. Finally, given the significant correlation between IL-6 and CRP in the present sample (r = 0.63, P < 0.001) and the known physiological association (i.e. IL-6 can stimulate the production of CRP), the natural log values of IL-6 and CRP were standardized and averaged to create a composite inflammation score to assess the combined associations between these inflammatory markers and neural activity. See Supplementary Materials for exploratory analyses separated by inflammatory markers.
fMRI task
For the fMRI task (data from which are also published in Heller et al., 2013; van Reekum et al., 2018), participants viewed 60 positive, 60 negative and 60 neutral pseudo-randomized images selected from the International Affective Picture System (IAPS; Lang et al., 2008) for five runs (see Supplementary Materials for list of IAPS images used). The stimuli were matched across valence categories for complexity, social content, arousal and luminosity. Each trial progressed as follows: a fixation crosshair was displayed for 1 s, followed by an IAPS image presented for 4 s, and then a blank screen intertrial interval (ITI) was displayed (M length = 8.89 s, range = 5.5–17.6 s). Participants were instructed to indicate on a button box the valence (i.e. positive, negative, or neutral) of the image presented. After 40 out of 60 trials in each valence category, a neutral male face was presented for 0.5 s after the IAPS image was displayed. Because the current project focused on examining neural responses to the affective images, the face stimuli were coded as regressors of no-interest in analyses. Across runs, the order the valence of the images presented was consistent across participants, though the specific stimuli presented within each valence category were randomized across participants.
fMRI data collection
Neuroimaging data for the current study were collected on a GE SIGNA 3.0 Tesla high-speed MRI scanner with a standard clinical whole-head transmit–receive quadrature head coil. The blood oxygen level-dependent (BOLD) signal was acquired using a T2*-weighted gradient-echo echo-planar imaging (EPI) pulse sequence across five runs of approximately 8 min each. Each EPI acquired 30 sagittal slices that used the following parameters: TR = 2000 ms, TE = 30 ms, flip angle = 60°, field of view = 240 mm, acquisition matrix = 64 × 64 and 4 mm slice thickness with 1 mm gap. A T1-weighted anatomical image was also collected using a T1-weighted inversion recovery fast gradient echo with the following parameters: acquisition matrix = 256 × 256 and a field of view = 240 mm, with 124 × 1.1 mm axial slices.
Data analysis
fMRI preprocessing and analysis
Neuroimaging data were preprocessed utilizing an in-house pipeline. The fsl_motion_outliers program (Jenkinson et al., 2012) was used to identify artifacts and excessive motion. Motion spikes were included in each person-level general linear model (GLM) to control for motion exceeding 2 mm. Further, runs with 2 mm of framewise displacement for greater than 20% of volumes acquired were excluded (N = 10; 0.03% of total runs). Next, a four-dimensional registration algorithm utilizing NiPy to conduct spatio-temporal transformations that simultaneously motion and slice-time corrected (Roche, 2011) was implemented. In two steps, this algorithm aligned all five functional images to a mean image computed after initial realignment. FSL’s FLIRT algorithm coregistered T2*-weighted images to the T1-weighted images, which were then anatomically coregistered to each individual’s high-resolution structural image. Images were nonlinearly registered to the Montreal Neurologic Institute’s (MNI) standard space utilizing the Advanced Normalization Tools (ANTs) software (Avants et al., 2011). Finally, spatial smoothing was applied with a Gaussian kernel of 5-mm full width at half maximum.
fMRI data were analyzed using FSL’s FMRI Expert Analysis Tool (FEAT) Version 6.00. A general linear model (GLM) was constructed for each run per individual. The GLMs included regressors modeling the positive, negative and neutral events as well as the nuisance regressors of motion (i.e. each individual’s six motion parameters and their first derivatives and single-point motion outliers) and the face events. For each run, a high-pass filter (100 Hz) was applied to remove low-frequency drifts. Higher-level analyses were conducted, utilizing FLAME stage 1 (Woolrich et al., 2004), a fixed-effects GLM approach, to combine BOLD activation and differences in variance across runs. The two contrasts of interests were negative images vs neutral images and positive images vs neutral images. The whole-brain main effects for both contrasts (cluster-based threshold at z > 2.3, P < 0.05) are reported in the Results section.
Region-of-interest construction
A mask was constructed by combining meta-analytic maps of neural activation related to affective processing (Lindquist et al., 2016) and peripheral inflammation (Kraynak et al., 2018). The binary maps derived from the meta-analyses were multiplied using fslmaths to create a mask that encompassed overlapping voxels from both of the meta-analyses. The regression analyses conducted in this study were restricted to the a priori limited search space represented by the combined meta-analytic mask by specifying this as the pre-threshold mask in FEAT. Thus, regression analyses searched for significant clusters of activity within the search space that were associated with systemic inflammation while controlling for covariates (see below for additional details).
Next, this combined meta-analytic mask was used to guide the selection of ROIs for the functional connectivity analysis. Corticolimbic regions present in the mask included the amygdala, insula, hippocampus, thalamus, striatum, pallidum and mPFC. The amygdala, insula, hippocampus, pallidum and thalamus ROIs for connectivity analyses were derived from the Harvard–Oxford subcortical structural atlas (Desikan et al., 2006). The striatum mask was generated using the Oxford–Imanova striatal structural atlas (Tziortzi et al., 2014). The mPFC mask was generated using the Sallet Dorsal Frontal connectivity–parcellation atlas (Sallet et al., 2013). ROI clusters 3 and 4, which consisted of Brodmann Areas 9 and 10 (Lieberman et al., 2019), were combined to create an mPFC mask.
Regression analyses associating levels of inflammation with neural activity
Two general linear models were employed to assess the relationship between inflammation (composite of CRP and IL-6) and activity in clusters encompassed within the combined meta-analytic mask when participants viewed positive (vs neutral) and negative (vs neutral) images. Consistent with prior work in this area (O’Connor et al., 2009a), group-level regression models controlled for age and gender. Considering adipose tissue’s role in systemic inflammation (Mohamed-Ali et al., 1997), body mass index (computed via measures of height and weight) was also included as a covariate. The higher-level models conducted for these analyses utilized cluster-based thresholding at z > 2.3, P < 0.05. In conjunction with FSL FLAME 1, the correction parameters used in this study have been found to effectively decrease type II errors (Eklund et al., 2016).
Functional connectivity analyses
Finally, functional connectivity analyses were conducted utilizing the functional connectivity toolbox (CONN-Toolbox v.18.b; Whitfield-Gabrieli and Nieto-Castanon, 2012). The CONN toolbox was used to perform ROI-to-ROI regression analyses to examine associations between inflammation and corticolimbic ROI connectivity during the two contrasts of interest. Trial onsets and durations were imported into the toolbox to implement the generalized psychophysiological interaction procedure (gPPI; McLaren et al., 2012). Following the standard CONN denoising pipeline, a simultaneous linear regression and temporal band-pass filtering procedure was conducted to remove the influence of non-neural variability in the data (Hallquist et al., 2013). The pipeline implemented an anatomical component-based noise correction process (aCompCor) to remove the first five principal noise components from white matter and cerebrospinal fluid. Twelve motion parameters, outlier scans, constant linear session effects and constant task-related effects were also included as regressors. Finally, temporal frequencies above 0.09 Hz and below 0.008 Hz were removed to minimize further the influence of physiological and motion sources of noise.
While controlling for age, gender and BMI, two separate models examined the association between inflammation and functional connectivity between all possible combinations of the seven ROIs while participants viewed negative (vs neutral images) and positive (vs neutral images). An analysis-wise false discovery rate (FDR) at P < 0.05 was implemented to correct for multiple comparisons.
Results
Negative vs neutral images
Overall neural reactivity
Results from the whole-brain analysis for the negative vs neutral contrast identified three significant clusters (z > 2.3, P < 0.05). Two clusters extended from the lateral occipital cortex, through the middle temporal gyrus, to the inferior temporal gyrus within both hemispheres (z = 5.98, k = 2794, P = 0.0001; z = 6.65, k = 2615, P = 0.0002). The final cluster extended from the left and right amygdala through to the right thalamus (z = 4.87, k = 2728, P = 0.0001). See Supplementary Table S1 for full details.
Inflammation and neural reactivity
To examine the relationship between inflammation and neural reactivity to negative images, we ran regression analyses looking for clusters of activity within our search space mask to negative (vs neutral) images that were significantly associated with the composite measure of inflammation, controlling for age, gender and BMI. Contrary to hypotheses, we found no significant associations between levels of inflammation and activity in any clusters within the mask when participants viewed negative (vs neutral) images.
Inflammation and functional connectivity
Next, we conducted an ROI-to-ROI regression analysis to examine the relationship between inflammation and connectivity between the corticolimbic regions of interest in response to negative images. While controlling for age, gender and BMI, inflammation was not significantly associated with connectivity between any of the ROIs (p-FDR > 0.05).
Positive vs neutral images
Overall neural reactivity
Results from the whole-brain analysis for the positive vs neutral contrast identified three significant clusters. One large cluster encompassed regions in the occipital cortex extending into the putamen, hippocampus and amygdala (z = 7.49, k = 13 949, P < 0.001). Another cluster was found in the vmPFC (z = 5.37, k = 1726, P = 0.0019). A final cluster extended from the cingulate gyrus through to the precuneus (z = 4.13, k = 1180, P = 0.016). See Supplementary Table S2 for full details.
Inflammation and neural reactivity
Next, we ran regression analyses looking for clusters of activity within our search space mask to positive (vs neutral) images that were significantly associated with the composite measure of inflammation, controlling for age, gender and BMI. There was a negative association between inflammation and activation in one cluster, such that higher levels of inflammation were associated with lower levels of activity in a cluster encompassing voxels in the anterior insula, amygdala, hippocampus and temporal pole (peak coordinate: x = 30, y = −10, z = ; z = 3.74, k = 515, P = 0.0134; see Figure 1). See Table 2 for more information regarding the regions encompassing the cluster.
Fig. 1.
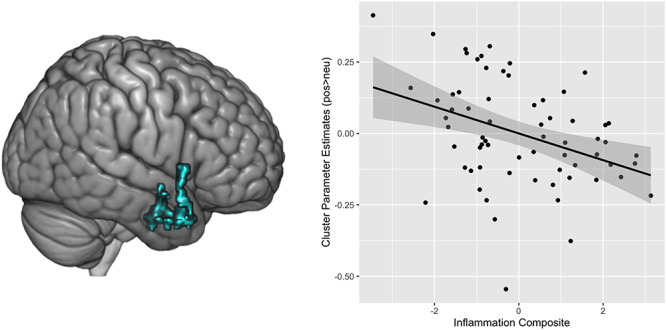
The rendered image on the left depicts the cluster of voxels that showed a significant negative association between inflammation and neural activation to positive (vs neutral) images while controlling for age, gender and BMI. The image on the right illustrates a scatterplot of the negative association between composite inflammation and activation in that cluster to the positive (vs neutral) images.
Table 2.
Local maxima within significant cluster negatively associated with inflammation during positive > neutral images
| Region | x (mm) | y (mm) | z (mm) | Z statistic |
|---|---|---|---|---|
| Parahippocampal gyrus | 30 | −10 | −32 | 3.74 |
| Insular cortex | 44 | 14 | 0 | 3.6 |
| Inferior temporal gyrus | 44 | 2 | −32 | 3.51 |
| Temporal pole | 34 | 12 | −32 | 3.29 |
| Right amygdala | 26 | −3 | −23 | 2.83 |
Inflammation and functional connectivity
Next, we conducted an ROI-to-ROI regression analysis to examine the relationship between inflammation and connectivity between the corticolimbic regions of interest in response to positive images. While controlling for age, gender and BMI, the composite inflammation score was positively associated with bilateral hippocampus–mPFC connectivity (t(61) = 3.68, p-FDR = 0.028; see Figure 2).
Fig. 2.
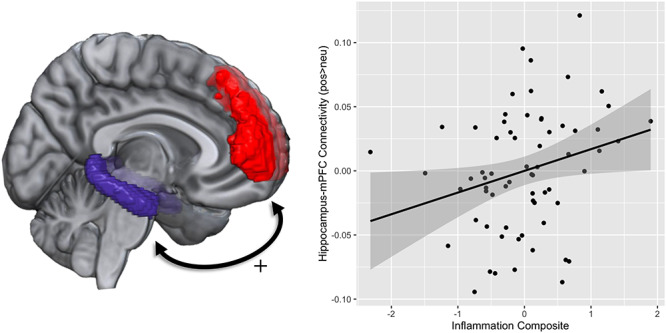
The image on the left depicts the ROI masks of the hippocampus and medial prefrontal cortex that were used for the analyses. The scatterplot on the right illustrates the positive association between inflammation and hippocampus–mPFC connectivity while viewing positive (vs neutral) images.
Discussion
Results from the present study suggest that levels of peripheral inflammation are associated with differences in neural reactivity and connectivity while processing positive affective information among mid- to late-life adults. First, higher levels of peripheral inflammation were associated with lower activation in the amygdala, hippocampus, anterior insula and temporal pole in response to positive images (vs neutral). There were no associations between markers of inflammation and neural reactivity to negative images (vs neutral). Second, the present study also found that greater inflammation was associated with stronger connectivity between the hippocampus and medial prefrontal cortex in response to positive images (vs neutral). Together, these results add to a growing literature in health neuroscience documenting associations between peripheral inflammation and neural responses to social and affective information. The present results extend past findings by looking at an older sample of individuals and novel affective reactivity paradigm while also exploring the associations between inflammation and task-based functional connectivity.
Our first set of findings showing that greater inflammation is associated with lower neural activity in the amygdala, hippocampus, insula and temporal pole activity in response to positive stimuli is consistent with a growing literature documenting associations between inflammation and blunted neural reactivity to positive stimuli (Capuron et al., 2012; Eisenberger et al., 2010a; Moieni et al., 2019). Lower reactivity to positive images in canonical regions implicated in the detection of and attention to salient stimuli suggests that less sensitivity to positive stimuli may be linked to higher low-grade inflammation. One possible psychological interpretation of these findings is that inflammation may blunt neural sensitivity to positive experiences, which may generally reduce one’s interest in positive information and motivation to engage with positive stimuli (Eisenberger et al., 2017), perhaps in an effort to conserve metabolic resources. Although we do not see inflammation-related differences in activity in the regions implicated in processing reward (e.g. basal ganglia) that other studies have found (Capuron et al., 2012; Eisenberger et al., 2010a; Felger and Miller, 2012), our findings are consistent with the general idea that inflammation is related to lower levels of neural activation to positive stimuli. Further, the present findings extend previous literature in this area, which has focused almost exclusively on neural responses to monetary reward tasks (c.f., Inagaki et al., 2015; Muscatell et al., 2016b), to document that inflammation is also associated with lower levels of activity in temporal-lobe regions in response to a wider variety of positive stimuli (i.e. pictures of positive scenes). Thus, the present results are consistent with prior research showing that inflammation is associated with reduced neural responsivity to positive stimuli.
Surprisingly, we did not find an association between levels of peripheral inflammation and corticolimbic activation to negative images. This lack of association is inconsistent with prior research, indicating that reactivity to negative stimuli is positively associated with inflammation (e.g. Inagaki et al., 2012; Gianaros et al., 2014; Muscatell et al., 2016a). Though null findings should be interpreted with caution, differences between the current study and past work in this area provide potential explanations for this lack of association. For example, others have found that neural activity to negative stimuli varies as a function of participant age (Mather, 2012), and the present study utilized a mid- to later-later life sample, whereas most other work on associations between inflammation and neural responses to negative stimuli have utilized younger samples (Gianaros et al., 2014; Swartz et al., 2017). Thus, age differences in participants may partially explain the divergence between the present findings and past work in this area. Additionally, others have found that inflammation differentially influences neural activation to social vs non-social stimuli (Inagaki et al., 2012) and most of the work in this area examines neural responses to negative social stimuli (e.g. threatening faces, negative social feedback; Inagaki et al., 2012; Muscatell et al., 2016a,b; c.f. Gianaros et al., 2014). To conserve power, neural activity to social and non-social images were collapsed in this study, which may also explain the lack of expected associations. Additional work is needed to examine whether there is indeed no association between low-grade inflammation and neural reactivity to negative information broadly or if specific characteristics of the present sample or task contribute to the lack of association observed in the current analysis.
In response to the positive stimuli, inflammation was positively associated with connectivity between the hippocampus and the medial prefrontal cortex. These findings are consistent with past literature, showing that inflammation is associated with differential corticolimbic connectivity (Felger et al., 2016; Kraynak et al., 2018; Kraynak et al., 2019), although the specific pattern of positive associations between inflammation and hippocampal-cortical connectivity conflicts with findings from several resting-state studies. Specifically, among healthy volunteers, markers of inflammation have been shown to be negatively associated with connectivity among corticolimbic regions (Kraynak et al., 2019) and regions in the emotion regulation, central executive and default mode networks (Dev et al., 2017; Marsland et al., 2017a; Nusslock et al., 2019). Thus, there may be differential associations between inflammation and corticolimbic connectivity, depending upon if the connectivity is measured at rest or in response to a task.
Not only does the current study suggest the need for studies exploring task-based functional connectivity and inflammation, but it also expands our understanding of the neuro-immune influences on affective reactivity. Results from the current study support the preclinical and clinical studies that implicate the hippocampus as a critical node in neuroinflammatory processes (Colasanti et al. 2016; Williamson and Bilbo 2013). Inflammation has been shown to alter hippocampal neurogenesis (Giannakopoulou et al., 2013), and synaptic plasticity (Nisticò et al., 2013), which may extend to the inflammation-related differences in hippocampal activity and connectivity observed in the current study. Additionally, prior work suggests that hippocampus–mPFC connectivity is critical for cognitive and emotion regulation as well as spatial and emotional memory processes (Jin and Maren, 2015). Further, inflammation has also been implicated in emotion and cognition-related impairment (Appleton et al., 2013; Patki et al., 2013). Though speculative, these results suggest the possibility of a neuro-immune pathway whereby affective and memory-related disruptions relate to inflammatory processes via differences in hippocampal–medial prefrontal connectivity. Although this study provides initial evidence regarding task-based corticolimbic connectivity and inflammation, future studies should explore the links between task-based neural connectivity, inflammation and the behavioral sequelae to expand our understanding of the neuro-immune influences on social and affective processes.
Multiple bidirectional physiological pathways provide plausible mechanisms for the observed links between systemic inflammation and neural activity/connectivity (Irwin and Cole, 2011). First, neural activity can alter peripheral inflammation via ‘top-down’, efferent pathways. Corticolimbic activity in response to negative stimuli can elicit the activation of the sympathetic nervous system and release of catecholamines, which can then lead to greater inflammation (Sternberg, 2006; Nusslock and Miller, 2016). Likewise, more positive affect has been linked with increases in cardiac vagal tone (Kok et al., 2013), and the vagus nerve can dampen pro-inflammatory responses (Pavlov and Tracey, 2012; Metz and Tracey, 2005). Second, peripheral inflammation can alter neurotransmitter, neuron and cerebral microvasculature functioning via a ‘bottom-up’, afferent pathway. Cytokines such as IL-6 can reach the central nervous system through active transport, binding to receptors on peripheral nerves (e.g. the vagus nerve), and by crossing the blood–brain barrier in areas of increased permeability (Dantzer et al., 2000; Dantzer et al., 2008). As such, systemic inflammation may affect corticolimbic function by entering the central nervous system to alter neurotransmitter (e.g. dopamine) and neuron functioning (Capuron et al., 2012; Felger and Treadway, 2017; Menard et al., 2017). Considering the multiple differing routes by which inflammation and neural activity relate, the precise mechanism linking inflammation and neural responses in this study is unknown. Future work is needed to gain clarity on the specific pathways linking inflammation and neural reactivity to affective information.
The present findings should be interpreted in the context of the study’s limitations. First and foremost, the current study was cross-sectional, which precludes concluding the direction of the association between neural responses and peripheral inflammation. Second, as with many fMRI studies, our project has a relatively small sample size (N = 66), and thus future work with larger samples is needed to replicate the findings observed here. Finally, only two markers of systemic inflammation (i.e. CRP and IL-6) were explored in this analysis. Other studies that explore how neural activation varies as a function of a diverse set or pattern of inflammatory markers would be an important contribution to future literature.
In sum, the present project utilized publicly available data from the MIDUS study to bring together methods from psychoneuroimmunology and affective neuroscience to explore a question at the core of health neuroscience research (Erickson et al. 2014): How are physiological processes implicated in disease development associated with neural functioning? The results are consistent with theorizing on the neuro-immune network (Nusslock and Miller, 2016), suggesting that inflammation in the periphery is associated with neural activity in and connectivity between regions that are critical for supporting successful social behavior and emotional functioning. More broadly, the present findings highlight the utility of health neuroscience approaches to map the connections between the brain and the body, showing that physiological processes such as inflammation are related to how our brains respond to affective information. As such, physiologic functioning may represent an often-overlooked contributor to and consequence of social and affective processes that social cognitive and affective neuroscience should work to incorporate into future empirical work and theoretical models of functioning within the social brain.
Supplementary Material
Contributor Information
Gabriella M Alvarez, Department of Psychology & Neuroscience, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-3270, USA.
Daniel A Hackman, USC Suzanne Dworak-Peck School of Social Work, University of Southern California, Los Angeles, CA 90089, USA.
Adam Bryant Miller, Department of Psychology & Neuroscience, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-3270, USA.
Keely A Muscatell, Department of Psychology & Neuroscience, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-3270, USA; Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27514, USA.
Acknowledgements
We are grateful to Samantha Brosso for her help with manuscript preparation and to members of the Carolina Social Neuroscience and Health Laboratory for feedback on the ideas presented in this paper. We also acknowledge members of the UNC Human Neuroimaging Group, particularly Gary Wilkins, for the creation of the data preprocessing pipeline and with his assistance troubleshooting analyses. We also wish to thank Stacey M. Schaefer, Barry Radler and the numerous staff members and investigators involved in the MIDUS data collection efforts.
Funding
Gabriella M. Alvarez is funded by the National Science Foundation Graduate Research Fellowship Program, the Ford Foundation Predoctoral Program and the Robert Wood Johnson Health Policy Research Scholars Program. Funding for the data was provided by the John D. and Catherine T. MacArthur Foundation Research Network, National Institute on Aging (P01-AG020166), National Institute on Aging (U19-AG051426) and NIH National Center for Advancing Translational Sciences (NCATS) Clinical and Translational Science Award (CTSA) (1UL1RR025011).
Conflict of interest
The authors declare no conflict of interest.
References
- Appleton A.A., Buka S.L., Loucks E.B., Gilman S.E., Kubzansky L.D. (2013). Divergent associations of adaptive and maladaptive emotion regulation strategies with inflammation. Health Psychology, 32(7), 748–56. doi: 10.1037/a0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage, 54(3), 2033–44. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J.M., Reeves G., Billman G.E., Sturmberg J.P. (2018). Inflammation-nature’s way to efficiently respond to all types of challenges: implications for understanding and managing “the epidemic” of chronic diseases. Frontiers in Medicine, 5, 316. doi: 10.3389/fmed.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L., Pagnoni G., Drake D.F., et al. (2012). Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Archives of General Psychiatry, 69(10), 1044–53. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti A., Guo Q., Giannetti P., et al. A. (2016). Hippocampal neuroinflammation, functional connectivity, and depressive symptoms in multiple sclerosis. Biological Psychiatry, 80(1), 62–72. doi: 10.1016/j.biopsych.2015.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., Konsman J.P., Bluthé R.M., Kelley K.W. (2000). Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Autonomic Neuroscience: Basic & Clinical, 85(1–3), 60–5. doi: 10.1016/S1566-0702(00)00220-4. [DOI] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience, 9(1), 46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dev S.I., Moore R.C., Soontornniyomkij B., Achim C.L., Jeste D.V., Eyler L.T. (2017). Peripheral inflammation related to lower fMRI activation during a working memory task and resting functional connectivity among older adults: a preliminary study. International Journal of Geriatric Psychiatry, 32(3), 341–9. doi: 10.1002/gps.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson S.S., Gable S.L., Irwin M.R., Aziz N., Kemeny M.E. (2009). Social-evaluative threat and proinflammatory cytokine regulation: an experimental laboratory investigation. Psychological Science, 20(10), 1237–44. doi: 10.1111/j.1467-9280.2009.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Inagaki T.K., Rameson L.T., Mashal N.M., Irwin M.R. (2009). An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. NeuroImage, 47(3), 881–90. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Berkman E.T., Inagaki T.K., Rameson L.T., Mashal N.M., Irwin M.R. (2010a). Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological Psychiatry, 68(8), 748–54. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Moieni M., Inagaki T.K., Muscatell K.A., Irwin M.R. (2017). Sickness and in health: the co-regulation of inflammation and social behavior. Neuropsychopharmacology, 42(1), 242–53. doi: 10.1038/npp.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Inagaki T.K., Mashal N.M., Irwin M.R. (2010b). Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, Behavior, and Immunity, 24(4), 558–63. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. (2016). Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–5. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Creswell J.D., Verstynen T.D., Gianaros P.J. (2014). Health neuroscience: defining a new field. Current Directions in Psychological Science: A Journal of the American Psychological Society, 23(6), 446–53. doi: 10.1177/0963721414549350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Miller A.H. (2012). Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Frontiers in Neuroendocrinology, 33(3), 315–27. doi: 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Treadway M.T. (2017). Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology, 42(1), 216–41. doi: 10.1038/npp.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Li Z., Haroon E., et al. (2016). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular Psychiatry, 21(10), 1358–65. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C., Campisi J. (2014). Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 69(Suppl 1), S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Furman D., Campisi J., Verdin E., et al. (2019). Chronic inflammation in the etiology of disease across the life span. Nature Medicine, 25(12), 1822–32. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C. (2006). Interleukin-6 and chronic inflammation. Arthritis Research & Therapy, 8(Suppl 2), S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulou A., Grigoriadis N., Bekiari C., et al. (2013). Acute inflammation alters adult hippocampal neurogenesis in a multiple sclerosis mouse model. Journal of Neuroscience Research, 91(7), 890–900. doi: 10.1002/jnr.23226. [DOI] [PubMed] [Google Scholar]
- Gianaros P.J., Marsland A.L., Kuan D.C.-H., et al. (2014). An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biological Psychiatry, 75(9), 738–45. doi: 10.1016/j.biopsych.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene A.S., Gao S., Scheinost D., Constable R.T. (2018). Task-induced brain state manipulation improves prediction of individual traits. Nature Communications, 9(1), 2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M.N., Hwang K., Luna B. (2013). The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage, 82, 208–25. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N.A., Brydon L., Walker C., Gray M.A., Steptoe A., Critchley H.D. (2009). Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry, 66(5), 407–14. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartanto A., Lee S.T.H., Yong J.C. (2019). Dispositional gratitude moderates the association between socioeconomic status and Interleukin-6. Scientific Reports, 9(1), 802. doi: 10.1038/s41598-018-37109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A.S., Reekum C.M., Schaefer S.M., et al. (2013). Sustained striatal activity predicts eudaimonic well-being and cortisol output. Psychological Science, 24(11), 2191–200. doi: 10.1177/0956797613490744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren M.B., Lamkin D.M., Suls J. (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic Medicine, 71(2), 171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Inagaki T.K., Muscatell K.A., Irwin M.R., Cole S.W., Eisenberger N.I. (2012). Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage, 59(4), 3222–6. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T.K., Muscatell K.A., Irwin M.R., et al. (2015). The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain, Behavior, and Immunity, 44, 247–52. doi: 10.1016/j.bbi.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M.R., Cole S.W. (2011). Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology, 11(9), 625–32. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaye D.L., Waites K.B. (1997). Clinical applications of C-reactive protein in pediatrics. The Pediatric Infectious Disease Journal, 16(8), 735–46 quiz 746. [DOI] [PubMed] [Google Scholar]
- Jiang R., Zuo N., Ford J.M., et al. (2020). Task-induced brain connectivity promotes the detection of individual differences in brain-behavior relationships. NeuroImage, 207, 116370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Maren S. (2015). Prefrontal-hippocampal interactions in memory and emotion. Frontiers in Systems Neuroscience, 9, 170. doi: 10.3389/fnsys.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. (2012). FSL. NeuroImage, 62(2), 782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kok B.E., Coffey K.A., Cohn M.A., et al. (2013). How positive emotions build physical health: perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychological Science, 24(7), 1123–32. doi: 10.1177/0956797612470827. [DOI] [PubMed] [Google Scholar]
- Kraynak T.E., Marsland A.L., Wager T.D., Gianaros P.J. (2018). Functional neuroanatomy of peripheral inflammatory physiology: a meta-analysis of human neuroimaging studies. Neuroscience and Biobehavioral Reviews, 94, 76–92. doi: 10.1016/j.neubiorev.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraynak T.E., Marsland A.L., Hanson J.L., Gianaros P.J. (2019). Retrospectively reported childhood physical abuse, systemic inflammation, and resting corticolimbic connectivity in midlife adults. Brain, Behavior, and Immunity, 82, 203–13. doi: 10.1016/j.bbi.2019.08.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann J.S., Grigoleit J.-S., Lichte P., et al. (2013). Neural response to emotional stimuli during experimental human endotoxemia. Human Brain Mapping, 34(9), 2217–27. doi: 10.1002/hbm.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8, Gainesville, FL: University of Florida. [Google Scholar]
- Lekander M., Karshikoff B., Johansson E., et al. (2016). Intrinsic functional connectivity of insular cortex and symptoms of sickness during acute experimental inflammation. Brain, Behavior, and Immunity, 56, 34–41. doi: 10.1016/j.bbi.2015.12.018. [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Straccia M.A., Meyer M.L., Du M., Tan K.M. (2019). Social, self, (situational), and affective processes in medial prefrontal cortex (MPFC): causal, multivariate, and reverse inference evidence. Neuroscience and Biobehavioral Reviews, 99, 311–28. doi: 10.1016/j.neubiorev.2018.12.021. [DOI] [PubMed] [Google Scholar]
- Lindquist K.A., Satpute A.B., Wager T.D., Weber J., Barrett L.F. (2016). The brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex, 26(5), 1910–22. doi: 10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-Z., Wang Y.-X., Jiang C.-L. (2017). Inflammation: the common pathway of stress-related diseases. Frontiers in Human Neuroscience, 11, 316. doi: 10.3389/fnhum.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland A.L., Cohen S., Rabin B.S., Manuck S.B. (2006). Trait positive affect and antibody response to hepatitis B vaccination. Brain, Behavior, and Immunity, 20(3), 261–9. doi: 10.1016/j.bbi.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Marsland A.L., Kuan D.C.-H., Sheu L.K., et al. (2017a). Systemic inflammation and resting state connectivity of the default mode network. Brain, Behavior, and Immunity, 62, 162–70. doi: 10.1016/j.bbi.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland A.L., Walsh C., Lockwood K., John-Henderson N.A. (2017b). The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain, Behavior, and Immunity, 64, 208–19. doi: 10.1016/j.bbi.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M. (2012). The emotion paradox in the aging brain. Annals of the New York Academy of Sciences, 1251, 33–49. doi: 10.1111/j.1749-6632.2012.06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga M., Isowa T., Kimura K., et al. (2008). Associations among central nervous, endocrine, and immune activities when positive emotions are elicited by looking at a favorite person. Brain, Behavior, and Immunity, 22(3), 408–17. doi: 10.1016/j.bbi.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Matsunaga M., Bai Y., Yamakawa K., et al. (2013). Brain-immune interaction accompanying odor-evoked autobiographic memory. PLoS One, 8(8), e72523. doi: 10.1371/journal.pone.0072523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage, 61(4), 1277–86. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N.D., Haroon E., Xu X., Woolwine B.J., Li Z., Felger J.C. (2018). Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: preliminary results. Brain, Behavior, and Immunity, 73, 725–30. doi: 10.1016/j.bbi.2018.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C., Pfau M.L., Hodes G.E., et al. (2017). Social stress induces neurovascular pathology promoting depression. Nature Neuroscience, 20(12), 1752–60. doi: 10.1038/s41593-017-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz C.N., Tracey K.J. (2005). It takes nerve to dampen inflammation. Nature Immunology, 6(8), 756–7. doi: 10.1038/ni0805-756. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V., Goodrick S., Rawesh A., et al. (1997). Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. The Journal of Clinical Endocrinology and Metabolism, 82(12), 4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Moieni M., Tan K.M., Inagaki T.K., et al. (2019). Sex differences in the relationship between inflammation and reward sensitivity: a randomized controlled trial of endotoxin. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(7), 619–26. doi: 10.1016/j.bpsc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Eisenberger N.I., Dutcher J.M., Cole S.W., Bower J.E. (2016a). Links between inflammation, amygdala reactivity, and social support in breast cancer survivors. Brain, Behavior, and Immunity, 53, 34–8. doi: 10.1016/j.bbi.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell K.A., Moieni M., Inagaki T.K., et al. (2016b). Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain, Behavior, and Immunity, 57, 21–9. doi: 10.1016/j.bbi.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisticò R., Mango D., Mandolesi G., et al. (2013). Inflammation subverts hippocampal synaptic plasticity in experimental multiple sclerosis. PLoS One, 8(1), e54666. doi: 10.1371/journal.pone.0054666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R., Miller G.E. (2016). Early-life adversity and physical and emotional health across the lifespan: a Neuroimmune network hypothesis. Biological Psychiatry, 80(1), 23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R., Brody G.H., Armstrong C.C., et al. (2019). Higher peripheral inflammatory signaling associated with lower resting-state functional brain connectivity in emotion regulation and central executive networks. Biological Psychiatry, 86(2), 153–62. doi: 10.1016/j.biopsych.2019.03.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M.-F., Bower J.E., Cho H.J., et al. (2009a). To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain, Behavior, and Immunity, 23(7), 887–97. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M.-F., Irwin M.R., Wellisch D.K. (2009b). When grief heats up: pro-inflammatory cytokines predict regional brain activation. NeuroImage, 47(3), 891–6. doi: 10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki G., Solanki N., Atrooz F., Allam F., Salim S. (2013). Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Research, 1539, 73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V.A., Tracey K.J. (2012). The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nature Reviews. Endocrinology, 8(12), 743–54. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche A. (2011). A four-dimensional registration algorithm with application to joint correction of motion and slice timing in fMRI. IEEE Transactions on Medical Imaging, 30(8), 1546–54. doi: 10.1109/TMI.2011.2131152. [DOI] [PubMed] [Google Scholar]
- Sallet J., Mars R.B., Noonan M.P., et al. (2013). The organization of dorsal frontal cortex in humans and macaques. The Journal of Neuroscience, 33(30), 12255–74. doi: 10.1523/JNEUROSCI.5108-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellar J.E., John-Henderson N., Anderson C.L., Gordon A.M., McNeil G.D., Keltner D. (2015). Positive affect and markers of inflammation: discrete positive emotions predict lower levels of inflammatory cytokines. Emotion, 15(2), 129–33. doi: 10.1037/emo0000033. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Wardle J., Marmot M. (2005). Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proceedings of the National Academy of Sciences of the United States of America, 102(18), 6508–12. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg E.M. (2006). Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nature Reviews. Immunology, 6(4), 318–28. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz J.R., Prather A.A., Hariri A.R. (2017). Threat-related amygdala activity is associated with peripheral CRP concentrations in men but not women. Psychoneuroendocrinology, 78, 93–6. doi: 10.1016/j.psyneuen.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi A.C., Haber S.N., Searle G.E., et al. (2014). Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cerebral Cortex, 24(5), 1165–77. doi: 10.1093/cercor/bhs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reekum C.M., Schaefer S.M., Lapate R.C., et al. (2018). Aging is associated with a prefrontal lateral-medial shift during picture-induced negative affect. Social Cognitive and Affective Neuroscience, 13(2), 156–63. doi: 10.1093/scan/nsx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson L.L., Bilbo S.D. (2013). Chemokines and the hippocampus: a new perspective on hippocampal plasticity and vulnerability. Brain, Behavior, and Immunity, 30, 186–94. doi: 10.1016/j.bbi.2013.01.077. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–41. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Behrens T.E.J., Beckmann C.F., Jenkinson M., Smith S.M. (2004). Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage, 21(4), 1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


