Abstract
Trauma exposure is associated with increased inflammatory biomarkers (e.g. C-reactive protein [CRP] and cytokines), and inflammation has been shown to impact corticostriatal reward circuitry and increase anhedonia-related symptoms. We examined resting-state functional MRI in a high-trauma inner-city population of African-American women (n = 56), who reported on average five different types of trauma exposures, to investigate whether inflammation correlated with functional connectivity (FC) in corticostriatal reward circuitry in association with symptoms of anhedonia and PTSD. Plasma CRP negatively correlated with bilateral ventral striatum (VS) to ventromedial prefrontal cortex (vmPFC) FC (P < 0.01). In participants where plasma was available to also measure cytokines and their soluble receptors, left (L)VS-vmPFC FC negatively correlated with an inflammatory composite score (previously shown to be increased in plasma and cerebrospinal fluid of depressed patients with high CRP) only in women with significant PTSD symptoms (n = 14; r = −0.582, P = 0.029) and those who experienced moderate–severe childhood trauma (r = −0.595, P = 0.009). Exploratory analyses indicated that LVS-vmPFC FC correlated with anhedonia-related subscales from the Beck Depression Inventory (r = −0.691, P = 0.004) and PTSD Symptom Scale (avoidance/numbness; r = −0.514, P = 0.042) in participants with an inflammatory score over the median (n = 16). Results suggest that inflammation contributes to compromised reward circuitry and symptoms of anhedonia and PTSD in trauma-exposed women.
Keywords: inflammation, resting-state functional connectivity, ventral striatum, PTSD, anhedonia, childhood trauma
Introduction
Trauma exposure, and particularly early-life trauma, is associated with both increased levels of inflammatory biomarkers and greater risk for medical and psychiatric illnesses such as posttraumatic stress disorder (PTSD) and depression (Danese et al., 2007; Danese et al., 2008; Michopoulos et al., 2015). Recent evidence has revealed biologic mechanisms by which psychological stress is converted into inflammatory signaling in the brain and periphery (Fleshner et al., 2017; Weber et al., 2017), and inflammation impacts neurotransmitters and neurocircuits that contribute to behavioral symptoms (Felger, 2018). Despite growing interest in the role of inflammation in trauma-related behavioral disturbance, little work has been done to link increased inflammation associated with trauma exposure to changes in neurocircuitry that may precipitate risk for symptoms of PTSD and depression.
Trauma exposure and PTSD can be as high as 88% and 46%, respectively, in low socioeconomic status (SES) communities living in urban settings with a high incidence of violence (Schwartz et al., 2005; Gillespie et al., 2009). Both low SES (Miller et al., 2009) and/or exposure to trauma (Pace et al., 2006; Danese et al., 2011; Baumeister et al., 2016) in childhood has been associated with an increased, chronic inflammatory state in adulthood, including elevated concentrations of inflammatory cytokines and the acute-phase reactant C-reactive protein (CRP). Increased inflammation in response to psychosocial stress occurs via neuroendocrine mechanisms that promote immune cell activation and trafficking into circulation and thus the brain, as well as direct ‘sterile’ inflammatory signaling in both the periphery and central nervous system (Wohleb et al., 2011; Iwata et al., 2013; Weber et al., 2017). Inflammatory cytokines then act in the brain to affect neurotransmitters and neurocircuits, serving as one biologic mechanism by which trauma exposure may contribute to symptoms of PTSD and depression (Nusslock and Miller, 2015; Felger and Treadway, 2017; Felger, 2018), particularly in persons experiencing chronic inflammation as the result of early-life adversity. Accordingly, a significant proportion of patients with PTSD and/or depression have been reported to exhibit increased systemic inflammation, as measured by inflammatory cytokines and CRP (Passos et al., 2015; Goldsmith et al., 2016; Michopoulos et al., 2017; O'Donovan et al., 2017). Furthermore, increased inflammation prior to or immediately following a traumatic event has been associated with increased risk for PTSD symptoms (Pervanidou et al., 2007; Eraly et al., 2014).
Although relationships between inflammation, neurocircuitry and severity of PTSD and depressive symptoms in high-trauma populations have not been established, a growing body of literature has described effects of inflammation on circuits and symptoms relevant to these disorders (Michopoulos et al., 2017; Felger, 2018; Savitz and Harrison, 2018). For example, administration of inflammatory cytokines or cytokine inducers such as typhoid vaccine or endotoxin (either as clinical therapy or in laboratory experiments in healthy controls) has consistently been shown to affect the basal ganglia and reward circuitry (for review see Felger and Treadway, 2017; Harrison, 2017), including reduced activation of the ventral striatum (VS) to hedonic reward (Eisenberger et al., 2010; Capuron et al., 2012; Harrison et al., 2016). Inflammation effects on reward-responsive brain regions and dopamine metabolism and release have been associated with symptoms of anhedonia and reduced motivation both in humans and laboratory animals (Capuron et al., 2012; Felger et al., 2013; Haroon et al., 2014; Yohn et al., 2016). Anhedonia is a core symptom of depression but is prevalent in other psychiatric disorders including PTSD (Kashdan et al., 2006). Indeed, approximately two-thirds of patients with PTSD exhibit symptoms of anhedonia, which has been associated with poor outcomes including suicidality and length of disease (Hassija et al., 2012; Nawijn et al., 2015). Additionally, patients with PTSD exhibited reduced activation of reward-related regions including both ventral and dorsal striatum, which correlated with reported symptoms of emotional numbing and motivational deficits (Sailer et al., 2008; Elman et al., 2009; Felmingham et al., 2014; Elman et al., 2018).
Our previous work has shown that endogenous elevations in peripheral inflammatory markers indicative of chronic low-grade inflammation may likewise impact corticostriatal reward circuitry to contribute to symptoms of anhedonia in patients with depression (Felger et al., 2016). Specifically, we found that higher levels of plasma CRP were associated with reduced functional connectivity (FC) between the left (L)VS and ventromedial prefrontal cortex (vmPFC) that correlated with the severity of anhedonia. Of note, we observed similar relationships between LVS-vmPFC FC and levels of inflammatory cytokines and their soluble receptors, which were found to be increased in both plasma and cerebrospinal fluid (CSF) of depressed patients with elevated CRP (Felger et al., in press). Additionally, we recently reported that LVS and the vmPFC were the two most significant hubs within a broadly distributed network of decreased FC identified in whole brain in association with CRP in patients with depression, which was highly predictive of anhedonia severity (Yin et al., 2019). Interestingly, we also found that a single nucleotide polymorphism within the CRP gene, rs1130864, was significantly associated with increased risk for PTSD and correlated with PTSD symptom severity including the ‘loss of interest’ item from the PTSD Symptom Scale (PSS) (Michopoulos et al., 2015). However, whether increased inflammation is associated with similar alterations in reward circuitry and symptoms of anhedonia in persons exposed to trauma who are at risk for PTSD and depression has not been examined.
Herein, we used a targeted, hypothesis-driven approach similar to that of our previous work (Felger et al., 2016) to first determine whether peripheral inflammation (as measured by plasma CRP and inflammatory cytokines and their soluble receptors) was associated with deficits in FC between the VS and vmPFC in a high-trauma sample of African-American (AA) women. Based on previous studies linking both inflammation and altered reward circuitry to PTSD and depression (see above), we examined whether relationships between inflammation and FC were stronger in women with PTSD and/or depression. We also explored whether associations between inflammation and FC depended on severity of childhood trauma, which has been associated with increased inflammation and risk for PTSD and depression in adulthood (Danese et al., 2009). Given the role of deficits in reward circuit function in anhedonia, e.g. Whitton et al. (2015), we explored whether FC in reward circuitry correlated with symptoms of anhedonia. Because inflammation and its effects on reward circuitry may be one pathway to symptoms of anhedonia, we also examined whether inflammation had an interaction effect on the relationship between FC in reward circuitry and anhedonia.
Methods
Participants
Fifty-six AA women (21–65 years) were recruited from the general medical clinics of Grady Memorial Hospital, a publicly funded hospital that serves economically disadvantaged individuals in Atlanta, Georgia through a larger ongoing study of risk factors for PTSD, the Grady Trauma Project (GTP). High trauma and posttraumatic symptoms have been observed in this population (Binder et al., 2008) that is >85% AA. Only subjects self-reporting AA race/ethnicity were studied to enhance homogeneity. Only women were studied because they represent >80% of the Grady patient population, show higher rates of PTSD and depression, and to limit variability in circuits involving sexually dimorphic brain regions (Kessler, 2003; Stevens and Hamann, 2012; Stevens et al., 2014). Furthermore, women have been found to be more sensitive to the effects of inflammation on the brain and behavior (Derry et al., 2015). Participants had no neurological disorder; bipolar disorder, schizophrenia or any other psychotic disorder as determined by Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV or Mini-International Neuropsychiatric Interview administered by trained clinical staff under the supervision of licensed clinicians; current psychotropic medication; and metal clips or implants. Due to interest in exposure to trauma and symptoms of anhedonia, participants with PTSD and depression were not excluded. Subjects were screened for alcohol abuse and drugs of abuse (past year) and pregnancy (see Supplement). All participants provided written informed consent prior to participating. The institutional review board of Emory University approved all procedures.
Study design and procedures
Blood was collected in the morning after participants had at least 30 minutes of rest. Blood was drawn an average of 56 days (s.d. = 135 days) prior to scans. On the day of scans, subjects completed self-report behavioral assessments followed by functional magnetic resonance imaging (fMRI) scans.
Inflammatory biomarkers
High-sensitivity plasma CRP was measured with a Beckman AU480 chemistry analyzer and Ultra WR CRP kit (Sekisui Diagnostics). Inflammatory cytokines, interleukin (IL)-6, IL-1beta and tumor necrosis factor (TNF) and their soluble receptors (IL-6sr, IL-1ra, sTNFR2) were assessed using multiplex bead-based assays (R&D Systems) as described (Felger et al., 2016; Mehta et al., 2018; Felger et al., in press). No immune variables were below the limits of assay detection (see Table S1), and coefficients of variation were reliably <10%. A composite score of inflammation was calculated as the sum of Z scores for concentrations of the inflammatory cytokines IL-6, TNF, IL-1beta and their soluble receptors (Felger et al., in press). See Supplement and Figure S1.
Trauma exposure and behavioral symptoms
Lifetime trauma history was assessed using the Traumatic Events Inventory (TEI) (Schwartz et al., 2005), and childhood trauma was assessed by Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 1994; Scher et al., 2001). The modified PSS was used to assess PTSD (Foa and Tolin, 2000) and the self-report Beck Depression Inventory (BDI) was used to assess depression symptom severity (Beck et al., 1988). A PSS score of ≥21 was defined symptom severity consistent with probable PTSD (Foa et al., 1993), and a BDI score ≥19 determined moderate depression (Beck et al., 1988), on the day of the fMRI scan. No scores for BDI or PSS were exclusionary. Symptoms of anhedonia were assessed using an anhedonia subscale from BDI (Pizzagalli et al., 2005; Treadway et al., 2009) and the avoidance/numbing scale from PSS (Foa et al., 1993). See Supplement.
Blood oxygenation level dependent (BOLD) fMRI data acquisition and pre-processing
Data were acquired on a Siemens 3.0 Tesla Magnetom Trio TIM whole-body MR scanner (Siemens, Malvern, PA) using a 12-channel head coil. Structural images were acquired using a gradient-echo, T1-weighted pulse sequence and wakeful resting-state fMRI images were acquired using a Z-saga pulse sequence to minimize signal loss due to inhomogeneous susceptibility (Heberlein and Hu, 2004). Analysis of rfMRI data was conducted with AFNI (http://afni.nimh.nih.gov/). Pre-processing steps included outlier detection (~5.5 times median absolute deviation), despiking, slice timing correction, motion correction, anatomy-to-functional image co-registration, nuisance signal (head motion parameters and derivatives, cerebral spinal fluid and white matter) regression, band-pass filtering (0.009 < f < 0.08 Hz) and 5 mm full-width half-maximum spatial smoothing. After the pre-processing pipeline, individual’s 4D fMRI data were transformed into the standard Montreal Neurological Institute (MNI) space. See Supplement.
BOLD fMRI data analysis
Resting-state VS-vmPFC FC was examined between 3 mm radius spherical seeds centered on bilateral inferior VS (including nucleus accumbens; MNI coordinates: x = ±14, y = 8, z = −9) (Capuron et al., 2012; Felger et al., 2016) and a ROI in vmPFC (MNI coordinates: x = 0, y = 44, z = −8 and cluster size = 1408 mm3) previously reported to be associated with neural activation in response to receipt of reward vs loss in a meta-analysis of neuroimaging studies (Diekhof et al., 2012) and with decreased FC with VS in our previous work (Felger et al., 2016). The FC between LVS and right (R)VS and vmPFC was assessed separately, consistent with previous studies using similar methods and because inflammation may have greater effects on left striatum (Capuron et al., 2007; Di Martino et al., 2008; Kelly et al., 2009; Eisenberger et al., 2010; Haroon et al., 2014; Felger et al., 2016). Subject-level FC correlations were extracted and Fisher’s Z-transformed for correlation with inflammatory markers and behavior (Felger et al., 2016).
Statistical analysis
Patient characteristics and inflammatory markers were summarized using mean and SD. Subject-level connectivity Z-scores for targeted relationships between bilateral VS ROIs and the vmPFC were entered into bivariate correlation models to assess relationships between inflammatory markers (as independent variables) and symptoms from PTSD and depression scales that are related to anhedonia (as dependent variables). Partial correlations were performed to control for clinical covariates including age and body mass index (BMI), which may influence or confound relationships between inflammation and neural circuitry. To explore the potential influence of probable PTSD and/or depression, or that of early-life trauma (in separate analyses), on relationships between inflammation and FC, and to examine whether inflammation and FC interact to influence symptoms of anhedonia, multiple linear regression including interaction terms and covariates were employed. Significant interactions were subsequently explored by examining separate correlations in women with vs without probable PTSD/depression or significant childhood trauma, and in those above and below the median for the inflammatory composite score (−0.27, n = 16 per group), respectively. To determine which marker from the inflammatory composite score was most significantly associated with FC in patients with probable PTSD/depression, linear regression models were conducted with backward and forward selection including clinical covariates (age and BMI) and using the same selection criteria (Felger et al., 2016; Mehta et al., 2018). Significant correlations were inspected visually, and potential outliers were examined by Grubb’s test for each analysis; results were reported with and without identified outliers. Significance was two-tailed, α < 0.05, and statistics were conducted in IBM SPSS Statistics 25.0.
Results
Patient characteristics
See Table 1 for sample characteristics. Consistent with previous GTP studies, subjects reported experiencing on average five types of potentially traumatic events on the TEI. Only three women did not report experiencing any childhood or lifetime trauma. On the scan day, 18 participants were considered to have probable PTSD (PSS severity score ≥21), and 6 of these 18 participants also exhibited symptom severity consistent with moderate depression (BDI score ≥19). Patients with probable PTSD and/or depression (referred to as PTSD+) exhibited significantly higher severity of PTSD and depressive symptoms, including the symptoms related to anhedonia (from BDI) and avoidance/numbing (from PSS), and experienced significantly greater childhood trauma as measured by CTQ scores (P < 0.01). Age, BMI and inflammatory markers did not significantly differ between groups (all P > 0.05). Of note, there were no significant correlations between CRP or inflammatory cytokines or their receptors and PSS or BDI scores (all P > 0.05).
Relationships between inflammatory markers and VS to vmPFC FC in the group as a whole and in women with PTSD
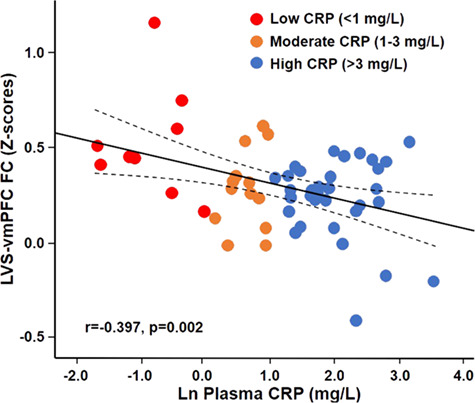
Based on previous findings of a relationship between circulating inflammatory markers and decreased FC in reward circuits in depression (Felger et al., 2016), associations between inflammation and bilateral VS-vmPFC FC were first examined using plasma CRP (in the group as a whole, n = 56), then cytokines and their soluble receptors using a composite score of inflammation (in subjects for which additional plasma was available, n = 32). Increasing concentrations of log transformed plasma CRP (lnCRP) were associated with reduced FC between both LVS (r = −0.397, P = 0.002) and RVS (r = −0.382, P = 0.004) to vmPFC in the group as a whole. Figure 1 depicts the relationship between lnCRP and LVS-vmPFC where colored dots reflect the respective unlogged CRP concentrations across levels of inflammation from low (<1 mg/L, blue) to medium (1–3 mg/L, orange) and high (>3 mg/L, red), as per American Heart Association and Center for Disease Control guidelines for increased risk of medical illness (Ridker, 2003). The data from one subject for both LVS and RVS to vmPFC FC was determined to be an outlier, but its removal did not affect these relationships with CRP (both P < 0.05). Moreover, relationships between CRP and LVS (r = −0.382, P = 0.005) and RVS (r = −0.362, P = 0.008) to vmPFC FC remained significant when controlling for demographic variables including age and BMI. Negative associations were also observed between CRP and both LVS (r−0.390, P = 0.025) and RVS (r = −0.353, P = 0.044) to vmPFC FC in patients where plasma was available for measurement of other inflammatory markers (n = 32, see below), and there was no interaction for CRP and probable PTSD on the relationship with FC (r = −0.118, P = 0.387).
Fig. 1. Peripheral inflammation, as measured by natural lnCRP, was negatively associated with FC between the LVS and the vmPFC (LVS-vmPFC) in a sample of trauma-exposed, inner-city AA women (n = 56) who underwent resting-state functional MRI. Blue dots = CRP <1 mg/L; Orange dots = CRP >1 and <3 mg/L; Red dots = CRP >3 mg/L.
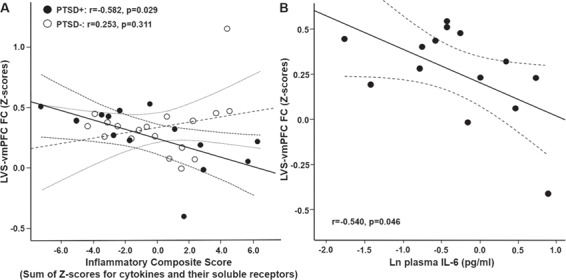
In participants where plasma was available to measure cytokines and their soluble receptors (n = 32), a composite score of inflammation (which we previously found to be increased in both the plasma and CSF of depressed patients with high plasma CRP, Felger et al., in press, and which correlated with CRP in the current sample, r = 0.377, P = 0.034) was calculated (see Supplement). Although no significant relationship was observed between this composite score and LVS (r = −0.169, P = 0.354) or RVS (r = −0.113, P = 0.539)-vmPFC FC in the participant group as a whole, the relationship between this composite score and LVS-vmPFC (r = −0.428, P = 0.016) (but not RVS-vmPFC FC, r = −0.315, P = 0.084) was significant after removal of the FC outlier, and remained significant when controlling for covariates (r = −0.378, P = 0.043). Furthermore, a significant interaction effect of probable PTSD and the inflammatory composite score on LVS-vmPFC (r = −0.398, P = 0.029) was observed and remained significant when controlling for clinical covariates (P < 0.05). To further interpret this interaction, women were divided based on whether or not they had probable PTSD. A negative relationship between the inflammatory composite score and LVS-vmPFC FC was observed in participants with (n = 14; r = −0.582, P = 0.029) but not without (n = 18; r = 0.253, P = 0.311) probable PTSD (Figure 2A), and these relationships persisted when removing an FC outlier from each group (PTSD+: r = −0.729, P = 0.005; PTSD−: r−0.119, P = 0.648). Of the six markers included in the inflammatory composite score, IL-6 (shown as lnIL6; Figure 2B) was revealed to be the strongest predictor of LVS-vmPFC FC in women with PTSD in backward and forward linear regression models containing clinical covariates (age and BMI) and using the same selection criteria (r = −0.683, P = 0.007). Of note, the negative relationships between both the inflammatory composite score (r = −0.577, P = 0.039) and IL-6 (r = −0.703, P = 0.007) and LVS-vmPFC FC in participants with probable PTSD persisted after controlling for depressive symptoms as measured by the BDI. There were no significant relationships between the inflammatory composite score and RVS-vmPFC FC in subjects with or without PTSD (both P > 0.36).
Fig. 2. A significant interaction between PTSD and inflammation (as measured by a composite factor for cytokines and their soluble receptors) with respect to their relationship with FC was observed. Specifically, inflammation was negatively associated with LVS-vmPFC (LVS-vmPFC) FC in women with (PTSD+: solid circles, n = 14) but not without (PTSD−: open circles, n = 18) probable PTSD (A). This relationship between inflammation and FC in PTSD+ patients was driven primarily by plasma interleukin (IL)-6 (B), as determined by linear regression models with selection including clinical covariates.
The role of childhood trauma in the relationship between inflammation and VS to vmPFC FC: exploratory results
To determine whether severity of childhood trauma had an independent or interactive effect on the relationships between inflammation and LVS-vmPFC FC, regression models were conducted including CTQ scores as well as interaction terms for CTQ scores and inflammatory markers. The CTQ scores did not have an effect on FC (P > 0.169) or the relationship between CRP (r = −0.458, P = 0.019) or the inflammatory composite score (r = −0.436, P = 0.016) and FC. Conversely, there was a significant interaction effect of total CTQ scores on the relationship between the inflammatory composite score (r = −0.564, P = 0.001), but not CRP (r = −0.137, P = 0.232), and LVS-vmPFC. This interaction remained significant when controlling for clinical covariates (P < 0.01). To further interpret this interaction, women were divided based on whether or not they experienced ≥1 moderate to severe form of childhood neglect or abuse (n = 18/32, see Supplement). The inflammatory composite score significantly correlated with LVS-vmPFC FC in women with (r = −0.595, P = 0.009) but not without (r = 0.290, P = 0.315) childhood trauma.
Relationships between LVS-vmPFC FC and symptoms of anhedonia and a potential role for increased inflammation: exploratory results
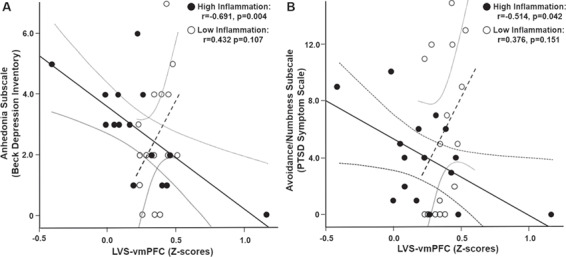
In our previous work in patients with depression, LVS-vmPFC FC was negatively associated with symptoms of anhedonia (Felger et al., 2016). Therefore, relationships between LVS-vmPFC FC and anhedonia-related symptoms were explored using subscales from the BDI and PSS (Foa et al., 1993; Pizzagalli et al., 2005; Treadway et al., 2009). In the group as a whole, LVS-vmPFC FC did not correlate with symptoms related to anhedonia derived from the BDI (anhedonia subscale: r = −0.027, P = 0.843) or the PSS (avoidance/numbing subscale: r = 0.004, P = 0.975). To determine whether inflammation influenced the relationship between FC and anhedonia, linear regression models including interaction terms for LVS-vmPFC FC and either the inflammatory composite score or CRP were employed in exploratory analyses. Although neither CRP nor the inflammatory composite score alone significantly predicted anhedonia or affected the relationship between FC and anhedonia in these models (all P > 0.23), inflammation by LVS-vmPFC FC interactions were observed when considering the inflammatory composite score but not CRP (P > 0.208). Specifically, a significant interaction was observed for anhedonia measured by the BDI subscale (r = −0.372, P = 0.047) and a trend was observed for the avoidance/numbing scale of PSS (r = −0.305, P = 0.069). Further examination of this relationship separately in women with high and low inflammation, defined as having an inflammatory composite score below or above the median (−0.27, n = 16/32, Figure S1), revealed a negative relationship between LVS-vmPFC FC and symptoms related to anhedonia as defined by both the BDI anhedonia subscale (r = −0.691, P = 0.004, Figure 3A) and PSS avoidance/numbing subscale (r = −0.514, P = 0.042, Figure 3B) in women with high inflammation. Both relationships remained significant after outlier removal from FC data and when controlling for covariates (all P < 0.05). In subjects with low inflammation, a significant positive relationship was observed between LVS-vmPFC FC and BDI anhedonia (r = 0.593, P = 0.015) and not PSS avoidance/numbing (r = 0.376, P = 0.151) (Figure 3), but this relationship was not significant after removal of an anhedonia outlier that was >4 SDs above the mean (r = 0.432, P = 0.107) (Figure 3A).
Fig. 3. A significant interaction between inflammation (as measured by a composite factor for cytokines and their soluble receptors) and FC with respect to their relationship with symptoms of anhedonia was observed. Specifically, VS-vmPFC (LVS-vmPFC) FC was negatively associated with anhedonia as measured by the BDI anhedonia subscale (A) and the PSS avoidance/numbing subscale (B), with lower LVS-vmPFC FC predicting increased symptom severity in women with (solid circles) but not without (open circles) an inflammatory composite score above the median. Of note, one subject did not complete one item on the BDI anhedonia subscale (see Supplement) and one subject had an anhedonia score >4 s.d. above the mean, thus n = 15/group in A and 16/group in B.
Discussion
A highlight of the current study is that the primary results, an association between higher concentrations of plasma CRP and low LVS-vmPFC FC, replicated our previous findings in medically stable, medication-free male and female adult patients with depression (Felger et al., 2016; Yin et al., 2019). This replication was striking considering the substantial methodological differences in recruitment between our previous sample and the AA women with high trauma exposure recruited from the GTP herein (see Limitations below). Interestingly, however, correlations between FC in reward circuitry and other inflammatory markers (including cytokines and their soluble receptors) in this sample were only observed in patients with significant PTSD symptoms, and relationships between FC and symptoms related to anhedonia were specific to subjects with high inflammation. The collective findings from this and our prior study suggest a strong association between peripheral inflammation and functioning of reward circuitry that is generalizable across participant samples with varying demographic backgrounds and clinical presentations. However, it should be noted that of the patients with depression from our previous cohort that underwent fMRI, ~63% were AA and ~71% women, indicating that this population may be particularly vulnerable to the impact of inflammation on reward circuitry. Although the significance is yet unknown, it is also worthy to mention that as with our previous data (Felger et al., 2016; Yin et al., 2019), relationships between endogenous inflammation and FC were stronger for LVS compared to RVS, consistent with previous studies reporting stronger effects of exogenously administered inflammatory stimuli on the left side of basal ganglia, e.g. (Capuron et al., 2007; Eisenberger et al., 2010; Haroon et al., 2014).
Specifically, a relationship between LVS-vmPFC FC and plasma CRP was observed in the group as a whole (n = 56) and in the subset of women where additional plasma was available to measure other inflammatory markers (n = 32); however, the association between FC and the other inflammatory markers (cytokines and their soluble receptors) was observed only in women with significant PTSD symptoms. These differences in findings between inflammatory markers may be due to design considerations (see Study Limitations below, e.g. lack of repeat CRP testing to establish stability), thus elevated CRP in some women may be due to acute infections that were not identified or reported, or from other acute sources not related to low-grade chronic inflammation resulting from psychological stress or trauma. Considering that CRP is produced primarily as an acute phase reactant by the liver, it may reflect inflammation not only from stress-related cytokine activity, but also from metabolic and/or medical and health-related mechanisms that may be present in all subjects (Aronson et al., 2004a; Aronson et al., 2004b; Park et al., 2005). Cytokines can be released from adipose tissue, and also by circulating or tissue-specific immune cells that may be more relevant to the effects of stress reactivity on inflammation (Hodes et al., 2014; Wohleb et al., 2014; Fleshner et al., 2017; Weber et al., 2017). Therefore, patients with significant PTSD symptoms may experience a higher burden of chronic stress that contributes to increased peripheral cytokine production (Michopoulos et al., 2017), which may subsequently impact brain dopamine and reward circuitry (Felger and Treadway, 2017), as evidenced by our finding of a relationship between inflammatory cytokines and their soluble receptors and FC only in women with probable PTSD.
Accordingly, regression models in women with PTSD revealed that relationships between LVS-vmPFC FC and inflammatory markers were driven by IL-6, a cytokine reliably elevated in patients with depression and PTSD (Musselman et al., 2001; Lindqvist et al., 2009; Lindqvist et al., 2014; Passos et al., 2015). In animal models of chronic social defeat, levels of IL-6 in particular have been identified to predict susceptibility vs resilience to the behavioral effects of this social stress (Hodes et al., 2014). Moreover, stress-susceptible mice exposed to chronic social defeat showed reduced integrity of the blood brain barrier in the nucleus accumbens, causing infiltration of IL-6 into the brain parenchyma and increased depressive-like behavior (Menard et al., 2017). Accordingly, IL-6 is a likely inflammatory moderator between chronic stress and trauma exposure and anhedonia-related symptoms in depression and PTSD, and may serve as a potential therapeutic target. In the current study, women with significant PTSD symptoms were those most likely to exhibit variability in reward circuit function that was associated with inflammation and its effects on the brain, and that may subserve relevant symptoms of depression or PTSD. Indeed, relationships between VS-vmPFC FC and symptoms related to anhedonia from both the depression (BDI) and PTSD (PSS) scale was only observed in women with high inflammation (as determined by cytokines and their soluble receptors), a group where cytokine activity may affect reward circuitry to contribute to symptom severity. It should be noted that in addition to general symptoms related to anhedonia (i.e. loss of interest), the PSS avoidance/numbing subscale probes anhedonia-related symptoms that may be specific to PTSD (e.g. avoiding activities or people that remind you of the traumatic event) as well as lack of memory of a traumatic event and hopelessness. The association between FC and this subscale remained significant even when excluding lack of interest (item #9), suggesting that this circuit may be related to a broader spectrum of symptoms. Indeed, relationships between reduced neural activation of VS to rewarding stimuli (happy faces) have been previously associated with severity of emotional numbing in PTSD (Felmingham et al., 2014).
Exploratory analysis indicated that childhood trauma interacts with inflammation in association with deficits in FC in reward circuitry. These findings contribute to a proposed causal pathway by which stress exposure in early life increases inflammatory load in children and adolescents (Baldwin et al., 2018), which may affect developing neurocircuitry, contributing to symptom burden that persists into adulthood (Danese et al., 2007). Indeed, our results support a model by which childhood abuse can lead to increased systemic inflammation, which subsequently impacts reward circuits to cause symptoms related to anhedonia in adulthood. As noted above, fMRI studies in PTSD found reduced neural responsiveness to reward in association with emotional numbing and motivational deficits similar to that of depression (Sailer et al., 2008; Elman et al., 2009; Felmingham et al., 2014). Anhedonia as a symptom of PTSD has also been shown to correlate with comorbid depression, leading to greater symptom severity related to mental function, quality of life and suicidal ideation (Armour et al., 2015). Thus, it is important to understand mechanisms by which inflammation in trauma-exposed individuals may drive circuit changes to contribute to symptoms of anhedonia and emotional numbing.
Study limitations and future directions
One important limitation is the small sample size, particularly considering that inflammatory cytokines and receptors were not measured in the entire sample due to lack of plasma availability. Another potential concern is that whereas lifetime history of depression and PTSD was assessed by DSM-IV criteria during screening, probable PTSD and depression was based on symptom severity scales collected on the day of scanning. This method was preferred in this study and our previous work in order to more accurately reflect behavior of the participants in the days leading up to the study visit and improve correlations with neuroimaging (Stevens et al., 2013; Stevens et al., 2014). We also did not have a high SES group for comparison, or severity measure for adult trauma to teases out specificity of childhood vs adult trauma. Laboratory screening and clinical examination to exclude/control for medical illnesses or acute infections that could confound relationships between inflammation, neurocircuitry and behavior and repeat CRP tests to establish stability were not conducted. Additionally, MRI scans were not conducted at the same time of day (to control for diurnal variations), and drug screens were conducted prior to MRI but not blood sampling. Although blood sampling and neuroimaging were significantly spaced (~56 days), this delay between biological and neuroimaging sampling further solidifies the validity and stability of the relationship between peripheral inflammation and FC in reward circuitry. Additionally, measurement of inflammatory cytokines and receptors allowed for more specificity in understanding relationships between inflammatory molecules and changes in FC in reward circuitry. Our report, although relatively preliminary, uncovered interesting relationships between inflammation, deficits in reward circuitry and anhedonia in women with trauma exposure that can be examined more thoroughly in future work. Future GTP studies will assess inflammatory markers in larger cross-sectional and longitudinal studies to further examine relationships between trauma, inflammation, neurocircuitry and behavior.
Translational health implications
Our hypothesis-generating study uncovered preliminary findings indicating that inflammation is associated with reduced FC within reward circuitry in relation to depressive and PTSD symptoms of anhedonia and emotional numbing in a trauma-exposed sample of inner-city women. These results paralleled our previous findings in patients with depression, suggesting that inflammation may contribute to symptoms related to anhedonia that are prevalent in both PTSD and depression. Increased inflammation in PTSD and depression are often comorbid with high BMI and/or evidence of metabolic dysfunction, which together contribute not only to changes in the brain and behavior, but also to risk for medical illnesses, e.g. diabetes, heart disease and cancer (Couzin-Frankel, 2010; Osborn and Olefsky, 2012; Delgado et al., 2018). Additionally, the presence of high inflammation and its associated metabolic disturbance in psychiatric illnesses like PTSD and depression has been associated with resistance to standard antidepressant therapies, increased morbidity and mortality (Rush, 2007; Uher et al., 2009; Cattaneo et al., 2013; Capuron et al., 2017; Haroon et al., 2018). Better understanding the impact of inflammation on reward circuitry may provide avenues for novel therapies to improve anhedonia and motivation and confer general health benefits in these patients (Raison et al., 2013; Bekhbat et al., 2018).
Contributor Information
Neeti D Mehta, Neuroscience Graduate Program, Graduate Division of Biological and Biomedical Sciences, Emory University, Atlanta, GA 30322, USA; Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, GA 30322, USA.
Jennifer S Stevens, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, GA 30322, USA.
Zhihao Li, School of Psychology and Sociology, Shenzhen University, Shenzhen, Guangdong Sheng 518060, China; Shenzhen Key Laboratory of Affective and Social Cognitive Science, Shenzhen University, Shenzhen, Guangdong Sheng 518060, China.
Charles F Gillespie, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, GA 30322, USA.
Negar Fani, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, GA 30322, USA.
Vasiliki Michopoulos, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, GA 30322, USA; Yerkes National Primate Research Center, Atlanta, GA 30322, USA.
Jennifer C Felger, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, GA 30322, USA; Winship Cancer Institute, Emory University, Atlanta, GA 30322, USA.
Conflict of interest
All authors declare no conflicts of interest. In the past 12 months, Dr Felger has consulted for Otsuka on a topic unrelated to this research.
Funding
The current work was supported by funds from the National Institute of Mental Health (grants R01MH109637, R01MH096764, R01MH099211 and R01MH071537), the Dana Foundation (grant number CADF49143), the National Natural Science Foundation of China (grant number 31671169, 31530031 to Z.L.), and the Shenzhen high-level overseas talents award (grant number 000099 to Z.L.). In addition, the study was supported in part by Public Health Service Grants (grant number UL1TR000454, KL2TR000455 and K12 HD085850) from the Clinical and Translational Science Award program, and by the National Institute of Health/National Cancer Institute under award number P30CA138292.
References
- Armour C., Tsai J., Durham T.A., et al. (2015). Dimensional structure of DSM-5 posttraumatic stress symptoms: support for a hybrid Anhedonia and Externalizing Behaviors model. Journal of Psychiatric Research, 61, 106–13. [DOI] [PubMed] [Google Scholar]
- Aronson D., Bartha P., Zinder O., et al. (2004a). Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. International Journal of Obesity and Related Metabolic Disorders, 28(5), 674–9. [DOI] [PubMed] [Google Scholar]
- Aronson D., Bartha P., Zinder O., et al. (2004b). Association between fasting glucose and C-reactive protein in middle-aged subjects. Diabetic Medicine, 21(1), 39–44. [DOI] [PubMed] [Google Scholar]
- Baldwin J.R., Arseneault L., Caspi A., et al. (2018). Childhood victimization and inflammation in young adulthood: a genetically sensitive cohort study. Brain, Behavior, and Immunity, 67, 211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D., Akhtar R., Ciufolini S., Pariante C.M., Mondelli V. (2016). Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Molecular Psychiatry, 21(5), 642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Carbin M.G. (1988). Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review, 8(1), 77–100. [Google Scholar]
- Bekhbat M., Chu K., Le N.A., et al. (2018). Glucose and lipid-related biomarkers and the antidepressant response to infliximab in patients with treatment-resistant depression. Psychoneuroendocrinology, 98, 222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D.P., Fink L., Handelsman L., et al. (1994). Initial reliability and validity of a new retrospective measure of child abuse and neglect. The American Journal of Psychiatry, 151(8), 1132–6. [DOI] [PubMed] [Google Scholar]
- Binder E.B., Bradley R.G., Liu W., et al. (2008). Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA, 299(11), 1291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L., Lasselin J., Castanon N. (2017). Role of adiposity-driven inflammation in depressive morbidity. Neuropsychopharmacology, 42(1), 115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L., Pagnoni G., Demetrashvili M.F., et al. (2007). Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology, 32(11), 2384–92. [DOI] [PubMed] [Google Scholar]
- Capuron L., Pagnoni G., Drake D.F., et al. (2012). Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Archives of General Psychiatry, 69(10), 1044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A., Gennarelli M., Uher R., et al. (2013). Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology, 38(3), 377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin-Frankel J. (2010). Inflammation bares a dark side. Science, 330(6011), 1621. [DOI] [PubMed] [Google Scholar]
- Danese A., Caspi A., Williams B., et al. (2011). Biological embedding of stress through inflammation processes in childhood. Molecular Psychiatry, 16(3), 244–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., Moffitt T.E., Harrington H., et al. (2009). Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatrics & Adolescent Medicine, 163(12), 1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., Moffitt T.E., Pariante C.M., Ambler A., Poulton R., Caspi A. (2008). Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of General Psychiatry, 65(4), 409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A., Pariante C.M., Caspi A., Taylor A., Poulton R. (2007). Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America, 104(4), 1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado I., Huet L., Dexpert S., et al. (2018). Depressive symptoms in obesity: relative contribution of low-grade inflammation and metabolic health. Psychoneuroendocrinology, 91, 55–61. [DOI] [PubMed] [Google Scholar]
- Derry H.M., Padin A.C., Kuo J.L., Hughes S., Kiecolt-Glaser J.K. (2015). Sex differences in depression: does inflammation play a role. Current Psychiatry Reports, 17(10), 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Scheres A., Margulies D.S., et al. (2008). Functional connectivity of human striatum: a resting state FMRI study. Cerebral Cortex, 18(12), 2735–47. [DOI] [PubMed] [Google Scholar]
- Diekhof E.K., Kaps L., Falkai P., Gruber O. (2012). The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude—an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia, 50(7), 1252–66. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Berkman E.T., Inagaki T.K., Rameson L.T., Mashal N.M., Irwin M.R. (2010). Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological Psychiatry, 68(8), 748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I., Lowen S., Frederick B.B., Chi W., Becerra L., Pitman R.K. (2009). Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biological Psychiatry, 66(12), 1083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I., Upadhyay J., Langleben D.D., Albanese M., Becerra L., Borsook D. (2018). Reward and aversion processing in patients with post-traumatic stress disorder: functional neuroimaging with visual and thermal stimuli. Translational Psychiatry, 8(1), 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraly S.A., Nievergelt C.M., Maihofer A.X., et al. (2014). Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry, 71(4), 423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C. (2018). Imaging the role of inflammation in mood and anxiety-related disorders. Current Neuropharmacology, 16(5), 533–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Haroon E., Wommack E.C., et al. (in press). What does plasma CRP tell us about peripheral and central inflammation in depression. Molecular Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Li Z., Haroon E., et al. (2016). Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular Psychiatry, 21(10), 1358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Mun J., Kimmel H.L., et al. (2013). Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology, 38(11), 2179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Treadway M.T. (2017). Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology, 42(1), 216–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K.L., Falconer E.M., Williams L., et al. (2014). Reduced amygdala and ventral striatal activity to happy faces in PTSD is associated with emotional numbing. PLoS One, 9(9), e103653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M., Frank M., Maier S.F. (2017). Danger signals and inflammasomes: stress-evoked sterile inflammation in mood disorders. Neuropsychopharmacology, 42(1), 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E.B., Riggs D.S., Dancu C.V., Rothbaum B.O. (1993). Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress, 6(4), 459–73. [Google Scholar]
- Foa E.B., Tolin D.F. (2000). Comparison of the PTSD symptom scale-interview version and the clinician-administered PTSD scale. Journal of Traumatic Stress, 13(2), 181–91. [DOI] [PubMed] [Google Scholar]
- Gillespie C.F., Bradley B., Mercer K., et al. (2009). Trauma exposure and stress-related disorders in inner city primary care patients. General Hospital Psychiatry, 31(6), 505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith D.R., Rapaport M.H., Miller B.J. (2016). A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Molecular Psychiatry, 21(12), 1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E., Daguanno A.W., Woolwine B.J., et al. (2018). Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology, 95, 43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E., Woolwine B.J., Chen X., et al. (2014). IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology, 39(7), 1777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison N.A. (2017). Brain structures implicated in inflammation-associated depression. Current Topics in Behavioral Neurosciences, 31, 221–48. [DOI] [PubMed] [Google Scholar]
- Harrison N.A., Voon V., Cercignani M., Cooper E.A., Pessiglione M., Critchley H.D. (2016). A neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biological Psychiatry, 80(1), 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassija C.M., Jakupcak M., Gray M.J. (2012). Numbing and dysphoria symptoms of posttraumatic stress disorder among Iraq and Afghanistan war veterans: a review of findings and implications for treatment. Behavior Modification, 36(6), 834–56. [DOI] [PubMed] [Google Scholar]
- Heberlein K.A., Hu X. (2004). Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-shot z-shim: Z-SAGA. Magnetic Resonance in Medicine, 51(1), 212–6. [DOI] [PubMed] [Google Scholar]
- Hodes G.E., Pfau M.L., Leboeuf M., et al. (2014). Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proceedings of the National Academy of Sciences, 111(45), 16136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M., Ota K.T., Duman R.S. (2013). The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain, Behavior, and Immunity, 31, 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan T.B., Elhai J.D., Frueh B.C. (2006). Anhedonia and emotional numbing in combat veterans with PTSD. Behaviour Research and Therapy, 44(3), 457–67. [DOI] [PubMed] [Google Scholar]
- Kelly C., de Zubicaray G., Di Martino A., et al. (2009). L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. The Journal of Neuroscience, 29(22), 7364–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C. (2003). Epidemiology of women and depression. Journal of Affective Disorders, 74(1), 5–13. [DOI] [PubMed] [Google Scholar]
- Lindqvist D., Janelidze S., Hagell P., et al. (2009). Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biological Psychiatry, 66(3), 287–92. [DOI] [PubMed] [Google Scholar]
- Lindqvist D., Wolkowitz O.M., Mellon S., et al. (2014). Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain, Behavior, and Immunity, 42, 81–8. [DOI] [PubMed] [Google Scholar]
- Mehta N.D., Haroon E., Xu X., Woolwine B.J., Li Z., Felger J.C. (2018). Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: preliminary results. Brain, Behavior, and Immunity, 73, 725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C., Pfau M.L., Hodes G.E., et al. (2017). Social stress induces neurovascular pathology promoting depression. Nature Neuroscience, 20(12), 1752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V., Powers A., Gillespie C.F., Ressler K.J., Jovanovic T. (2017). Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology, 42(1), 254–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V., Rothbaum A.O., Jovanovic T., et al. (2015). Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. The American Journal of Psychiatry, 172(4), 353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.E., Chen E., Fok A.K., et al. (2009). Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America, 106(34), 14716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman D.L., Miller A.H., Porter M.R., et al. (2001). Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. The American Journal of Psychiatry, 158(8), 1252–7. [DOI] [PubMed] [Google Scholar]
- Nawijn L., van Zuiden M., Frijling J.L., Koch S.B.J., Veltman D.J., Olff M. (2015). Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neuroscience & Biobehavioral Reviews, 51, 189–204. [DOI] [PubMed] [Google Scholar]
- Nusslock R., Miller G.E. (2015). Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biological Psychiatry, 80(1), 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A., Ahmadian A.J., Neylan T.C., Pacult M.A., Edmondson D., Cohen B.E. (2017). Current posttraumatic stress disorder and exaggerated threat sensitivity associated with elevated inflammation in the Mind Your Heart Study. Brain, Behavior, and Immunity, 60, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn O., Olefsky J.M. (2012). The cellular and signaling networks linking the immune system and metabolism in disease. Nature Medicine, 18(3), 363–74. [DOI] [PubMed] [Google Scholar]
- Pace T.W., Mletzko T.C., Alagbe O., et al. (2006). Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. The American Journal of Psychiatry, 163(9), 1630–3. [DOI] [PubMed] [Google Scholar]
- Park H.S., Park J.Y., Yu R. (2005). Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Research and Clinical Practice, 69(1), 29–35. [DOI] [PubMed] [Google Scholar]
- Passos I.C., Vasconcelos-Moreno M.P., Costa L.G., et al. (2015). Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry, 2(11), 1002–12. [DOI] [PubMed] [Google Scholar]
- Pervanidou P., Kolaitis G., Charitaki S., et al. (2007). Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology, 32(8–10), 991–9. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A., Jahn A.L., O'Shea J.P. (2005). Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biological Psychiatry, 57(4), 319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Rutherford R.E., Woolwine B.J., et al. (2013). A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry, 70(1), 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker P.M. (2003). Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation, 107(3), 363–9. [DOI] [PubMed] [Google Scholar]
- Rush A.J. (2007). STAR*D: what have we learned. The American Journal of Psychiatry, 164(2), 201–4. [DOI] [PubMed] [Google Scholar]
- Sailer U., Robinson S., Fischmeister F.P., et al. (2008). Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia, 46(11), 2836–44. [DOI] [PubMed] [Google Scholar]
- Savitz J., Harrison N.A. (2018). Interoception and inflammation in psychiatric disorders. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(6), 514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C.D., Stein M.B., Asmundson G.J., McCreary D.R., Forde D.R. (2001). The childhood trauma questionnaire in a community sample: psychometric properties and normative data. Journal of Traumatic Stress, 14(4), 843–57. [DOI] [PubMed] [Google Scholar]
- Schwartz A.C., Bradley R.L., Sexton M., Sherry A., Ressler K.J. (2005). Posttraumatic stress disorder among African Americans in an inner city mental health clinic. Psychiatric Services, 56(2), 212–5. [DOI] [PubMed] [Google Scholar]
- Stevens J.S., Almli L.M., Fani N., et al. (2014). PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 111(8), 3158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J.S., Hamann S. (2012). Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia, 50(7), 1578–93. [DOI] [PubMed] [Google Scholar]
- Stevens J.S., Jovanovic T., Fani N., et al. (2013). Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. Journal of Psychiatric Research, 47(10), 1469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway M.T., Buckholtz J.W., Schwartzman A.N., Lambert W.E., Zald D.H. (2009). Worth the 'EEfRT'? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One, 4(8), e6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R., Mors O., Hauser J., et al. (2009). Body weight as a predictor of antidepressant efficacy in the GENDEP project. Journal of Affective Disorders, 118(1–3), 147–54. [DOI] [PubMed] [Google Scholar]
- Weber M.D., Godbout J.P., Sheridan J.F. (2017). Repeated social defeat, neuroinflammation, and behavior: monocytes carry the signal. Neuropsychopharmacology, 42(1), 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton A.E., Treadway M.T., Pizzagalli D.A. (2015). Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current Opinion in Psychiatry, 28(1), 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E.S., Hanke M.L., Corona A.W., et al. (2011). Beta-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. The Journal of Neuroscience, 31(17), 6277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E.S., McKim D.B., Shea D.T., et al. (2014). Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biological Psychiatry, 75(12), 970–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Xu X., Chen G., et al. (2019). Inflammation and decreased functional connectivity in a widely-distributed network in depression: centralized effects in the ventral medial prefrontal cortex. Brain, Behavior, and Immunity, 80, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn S.E., Arif Y., Haley A., et al. (2016). Effort-related motivational effects of the pro-inflammatory cytokine interleukin-6: pharmacological and neurochemical characterization. Psychopharmacology, 233(19–20), 3575–86. [DOI] [PubMed] [Google Scholar]


