Abstract
Interpersonal touch and social support can influence physical health, mental well-being and pain. However, the mechanisms by which supportive touch promotes analgesia are not well understood. In Study 1, we tested how three kinds of social support from a romantic partner (passive presence, gentle stroking and handholding) affect pain ratings and skin conductance responses (SCRs). Overall, support reduced pain ratings in women, but not men, relative to baseline. Support decreased pain-related SCRs in both women and men. Though there were no significant differences across the three support conditions, effects were largest during handholding. Handholding also reduced SCRs in the supportive partner. Additionally, synchronicity in couples’ SCR was correlated with reductions in self-reported pain, and individual differences in synchrony were correlated with the partner’s trait empathy. In Study 2, we re-analyzed an existing dataset to explore fMRI activity related to individual differences in handholding analgesia effects in women. Increased activity in a distributed set of brain regions, including valuation-encoding frontostriatal areas, was correlated with lower pain ratings. These results may suggest that social support can reduce pain by changing the value of nociceptive signals. This reduction may be moderated by interpersonal synchrony and relationship dynamics.
Keywords: touch, pain, synchrony, social support
Introduction
Social support can have significant psychological and physiological benefits including reduced disease risk, increased self-determination and a longer life span (Coleman and Iso-Ahola, 1993; Brown et al., 2003; Uchino, 2006; Yang et al., 2016). Experimentally, social support has been shown to mitigate self-reported pain intensity and unpleasantness (Brown et al., 2003; Master et al., 2009) and to reduce stress-related physiology in response to pain and threat (Coan et al., 2006; Sambo et al., 2010). These effects are likely potentiated in part by touch. Though pain relief by touch is a well-known phenomenon among caregivers and medical providers, it has only recently come under rigorous scientific scrutiny (e.g. Mancini et al., 2014).
Three common types of touch are associated with pain relief—gentle stroking, massage and handholding. These different types of touch have unique peripheral signaling mechanisms yet yield potentially similar effects on pain. Gentle stroking activates C-tactile (CT) afferents, low-threshold mechanoreceptors found under the hairy skin (Olausson et al., 2010) that signal positive affective touch (Pawling et al., 2017). In rodent models, gentle stroking increases pain tolerance and plasma levels of oxytocin, a neuropeptide with analgesic and social bonding properties (Agren et al., 1995). Recent evidence suggests these findings translate to humans: gentle stroking delivered via a mechanical device designed to optimally simulate CT afferents reduces self-reported pain ratings (Liljencrantz et al., 2017). Massage activates mechanoreceptors sensitive to deep pressure and may enact pain relief by stimulating nerve fibers competing with nociceptors (for a review see Wall et al., 1996). Handholding involves medium to light pressure and primarily stimulates the glabrous skin of the palm, where there are no CT fibers. Handholding can increase physiological and neural synchrony within dyads (Goldstein et al., 2017, 2018) and reduce pain expression in the brain and in self-reports (López-Solà et al., 2019). Evidence suggests adolescents undergoing cancer treatment identify handholding as the most effective coping strategy for their pain and prefer to hold the hand of a family member or friend (Weekes et al., 1993). In experimental contexts with adults, pain relief associated with handholding is more effective when the support giver is a romantic partner rather than a stranger (Coan et al., 2006; Master et al., 2009; Younger et al., 2010; Eisenberger et al., 2011) or a mechanical device (López-Solà et al., 2019).
Some of the effects of touch are likely due to one’s perception of the support. Social factors such as one’s familiarity with the support giver (Gazzola et al., 2012), gender norms (Kirschbaum et al., 1995), relationship quality (Hennessy et al., 2009) and attachment styles (Sambo et al., 2010) can moderate the effects of support on pain and stress (for a review see Krahé et al., 2013). Though it may potentiate support, touch may not be required at all. In some cases, the mere presence of a supportive other can reduce pain ratings relative to pain experienced alone (Montoya et al., 2004; Master et al., 2009). These various pieces of evidence converge on the idea that one’s evaluation of the social context may be the primary determinant of social support’s influence on health outcomes.
There is a long history of sometimes contradictory reports of sex and gender effects on pain (for reviews see Chesterton et al., 2003; Greenspan et al., 2007). Prior to 2007, 79% of animal pain research was performed in male animals alone (Greenspan et al., 2007); since then, the field has recognized a critical need to examine sex and gender effects in pain studies. However, many extant experiments related to social support and pain recruit only women as the pain receivers (e.g. López-Solà et al., 2019). Here, we included men and women in order to assess gender effects.
Brain data support the notion that affective evaluation of the social context mediates the effects of social support on pain. The dopaminergic system in the nucleus accumbens (NAc)/striatum is critical for stimulus valuation and reward prediction errors (for a meta-analysis, see Garrison et al., 2013) and is activated during the offset of pain (Navratilova et al., 2015), opioid analgesia (Borsook et al., 2010) and placebo analgesia (Scott et al., 2008; for review, see Wager and Atlas, 2015). Positive social stimuli, and other elicitors of positive expectations, can increase activity and opioidergic activity in the NAc/striatum. For example, Nummenmaa et al. (2016) found that a romantic partner’s gentle caress increases the availability of μ-opioid receptors in the NAc and frontal cortices of men. Similarly, López-Solà et al., (2019) found that activity in several brain regions including the left ventral striatum mediated the expression of the neural pain signature (Wager et al., 2013) when women receiving thermal pain held their partner’s hand. Conversely, negative expectations about pain may reduce μ-opioid receptor and dopamine receptor signaling in the NAc (Carlino et al., 2014).
When we better understand how social support impacts the brain and the body, we will be better able to predict if, when and how it can be effective for any one person. This study aimed to improve our understanding of how social support influences nociceptive processing by investigating (i) how three kinds of social support between romantic couples (partner presence, gentle stroking and handholding) affect experiences of pain relative to pain experienced in isolation and (ii) what brain networks are related to individual differences in the effectiveness of handholding analgesia.
Materials and method
Study 1
Participants
Fifty-one romantic couples (N = 102) in a relationship lasting over 6 months participated together in this study. Participants were recruited from the campus of the University of Colorado Boulder as well as the wider Boulder community. Participants had no history of psychiatric, neurological or pain disorders and no current pain. The member of the couple who responded to our participation advertisements was pre-assigned to be the ‘main participant’ (22 women, age = 27.44 ± 0.84 SE years). Their romantic partner (30 women, age = 26.41 ± 0.96 SE years) was pre-assigned to be the provider of interpersonal support. Fifty couples were straight cisgendered pairs, and one couple was a lesbian couple (both cis women). Eighty-nine participants were White, 7 Latino, 3 Native American, 3 Asian American and 2 African American. Couples had been together for 4.84 ± 0.74 SE years on average and self-reported high relationship satisfaction (mean = 92.45 ± 1.03 SE) on a scale from 0 to 100. The majority of couples were sexually active (N = 47), monogamous but unmarried (N = 32), living together (N = 33) and with no children (N = 48). See Table 1 for more information about participant demographics as well as Supplementary Figure S1 for more information about the couples. All participants gave written informed consent and were compensated $12 an hour for their participation. The timeframe of data collection for this study was Fall 2015–2016.
Table 1.
Participant demographics from Study 1
| Age (years) | Gender identity | Sexual orientation | Ethnicity | Average relationship length (years) | Average relationship satisfaction (scale 1–100) | |
|---|---|---|---|---|---|---|
| Main participant | 27.44 ± 0.84 SE | 22 Women 29 Men | 1 Lesbian 4 Bisexual 44 Heterosexual 2 No response | 44 White 2 Latina 1 Asian American 2 Native American 2 African American | 4.84 ± 0.74 SE | 92.45 ± 1.03 SE |
| Romantic partner | 26.41 ± 0.96 SE | 30 Women 21 Men | 1 Lesbian 5 Bisexual 44 Heterosexual 1 No response | 45 White 5 Latino 2 Asian American 1 Native American |
Study design
After both members of the couple gave their informed consent, the romantic partner was shown how to gently stroke a 9-cm area marked on the main participant’s left forearm so that the velocity and force of the stroking would match what is thought to be the optimal velocity and force for CT afferent stimulation; 3 cm/s at 0.20–0.40 N, respectively (Löken et al., 2009). The pleasantness of this stroking was verified with a questionnaire called the Touch Perception Task (Supplementary Figure S2). See the Supplementary Material for a script of what participants were told before participation.
The experiment involved five task conditions. Each condition consisted of 10 trials of painful thermal stimulation delivered in a pseudorandom order to three different sites on the main participant’s left leg (Figure 1). Moment-by-moment pain intensity ratings were collected from the main participant each trial. Overall pain intensity and unpleasantness ratings were collected from the main participants at the end of each condition (for details of the rating scales, see Supplementary Methods). The first condition was a ‘Pre-manipulation’ condition where the main participant experienced the pain stimulations alone, without their partner present. The presentation order of the next three conditions was pseudorandomized so that there were six total orders. These conditions were (a) gentle ‘Stroking’ condition; (b) a ‘Handholding’ condition, where the partner held the main participant’s left hand, specifically holding mostly the glabrous skin of the palm; and (c) a ‘Present’ condition, where the partner was present but did not touch or significantly interact with the main participant. Lastly, the main participant underwent a ‘Post-manipulation’ condition, where they again experienced pain without their partner present.
Fig. 1.
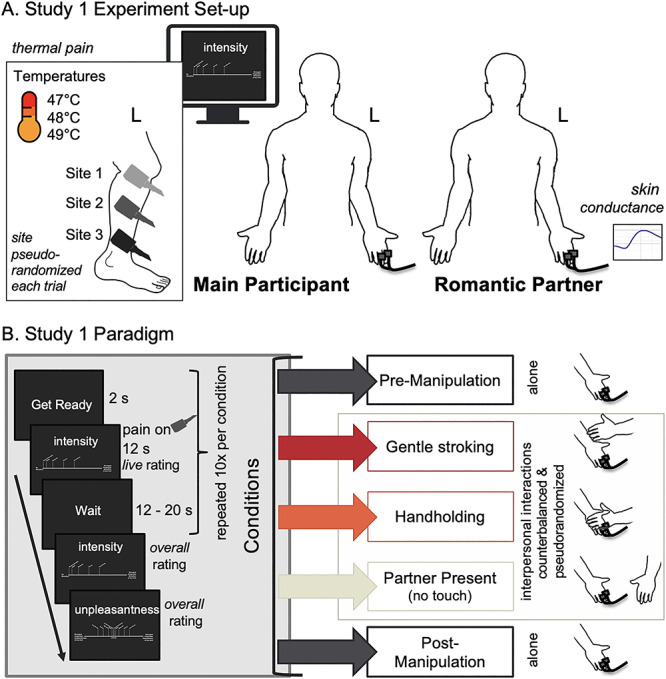
Method detail. (A) Study 1 Experiment setup. The main participant was positioned in front of the rating computer. EDA electrodes were attached to the fingers of their left hand. Ratings were given with their right hand using a mouse, regardless of subject’s handedness. Three sites of the main participant’s left leg were marked for stimulation. When their romantic partner was in the room, they sat to the main participant’s right and interacted with the main participant’s left hand using their right hand during the touch conditions. EDA electrodes were attached to the left hand of the romantic partner. (B) Study 1 Paradigm. The experiment involved five task conditions. Each condition consisted of 10 trials of painful thermal stimulation delivered in a pseudorandom order to three different sites on the main participant’s left leg. Moment-by-moment pain intensity ratings were collected from the main participant each trial. Overall pain ratings were collected from the main participants at the end of each condition. The first condition was a ‘Pre-manipulation’ condition where the main participant experienced the pain stimulations alone, without their partner present. The presentation order of the next three conditions was stratified so that there were six total orders. These conditions were (a) gentle ‘Stroking,’ (b) ‘Handholding’ and (c) a ‘Present’ condition where the partner was present but did not touch or significantly interact with the main participant. Lastly, the main participant underwent a ‘Post-manipulation’ condition alone.
Experimenters
There were three experimenters trained to run this study, and at any given session two were present. All experimenters were White women between the ages of 18 and 26.
Thermal stimulation
Thermal stimulation was delivered to the volar surface of the left inner forearm applied using an ATS Pathway System (Medoc Ltd) with a 16-mm Peltier thermode end-plate. Heat stimulations were delivered to three sites located on the participants’ left leg. Skin site selection was fixed a priori before the experiment and was the same for each person (on the outer left leg, right below the knee, in the center of the leg and right above the ankle; see Figure 1A). Each stimulation lasted 12 seconds, with 3.5-second ramp-up and 1-second ramp-down periods and 7.5 seconds at target temperature. Three levels of temperature were administered to the participants (level 1, 47°C; level 2, 48°C; level 3, 49°C).
Physiological recording
Electrodermal activity (EDA) was recorded from the main participants across all conditions in order to analyze participant skin conductance responses (SCRs). EDA was also recorded from the romantic partners during the three support conditions using 11-mm Ag/AgCl electrodes (Biopac systems, Goleta, CA) attached to the medial phalanges of the middle and ring fingers of the left hand at a sampling rate of 1000 Hz. One couple’s EDA data were lost due to a technical error. The data from 5 additional romantic partners were also lost due to a technical error, so there are only 45 participants in the partner skin SCR analyses, while there are 50 participants in the pain-receiver analysis. Additionally, three pain receivers were missing data during the ‘Pre’ condition due to technical errors.
Skin conductance preprocessing and signal decomposition
Skin conductance analysis was performed using the MATLAB package, Ledalab (Benedek and Kaernbach, 2010). Raw traces were downsampled to 100 Hz and then preprocessed with the software’s adaptive smoothing function, which applies a Gaussian low-pass filter to the data. Finally, the signal was deconvolved using a continuous decomposition analysis (CDA). This analysis separates the traces into continuous signals of tonic and phasic activity. The ‘phasic’ driving component of the signal can then be analyzed while controlling for slow, tonic changes. Event-related responses to stimuli were assessed by extracting the average phasic activity of SCRs via area under the curve, occurring within a window 3.5 s after stimulus onset until 11 s for each trial because there was a 3.5-sec ramp-up and 1 s ramp-down period for the pain stimulation. Event-related phasic SCRs were range normalized within each subject and log transformed. This procedure was done so that individual variability in the signal magnitude would not bias further analyses. SCR trial responses were then averaged within conditions.
Post-experiment surveys
After the experimental session, participants were asked to complete surveys related to their perception of touch and interpersonal support in their daily lives. The main participant completed the Social Touch Questionnaire (STQ; Wilhelm et al., 2001) where lower scores indicate more liking of social touch and the Touch Perception Task (Guest et al., 2011), which was used to characterize how they perceived the gentle stroking. Both participants completed the Interpersonal Reactivity Index (IRI; Davis, 1983) which describes one’s dispositional empathy, the Interpersonal Support Evaluation List (ISEL; Cohen et al., 1985), which describes one’s perceptions of social support and belonging in one’s life and how that impacts one’s stress response, and the TACTYPE (Deethardt and Hines, 1983) which describes one’s attitudes towards touch in their daily lives, where higher scores indicate a more welcoming attitude towards social touch.
Model construction and gender effects
A linear mixed effects (LME) analysis of the relationship between interpersonal support and outcomes in dyads (N = 51) was performed using the lme4 (Bates et al., 2014) package in R (R Core Team, 2018). Pain reports and painful stimulus-evoked SCRs were tested as outcomes in separate models. In the pain intensity model Condition, Gender and their interaction were entered as fixed effects. Subject intercepts and slopes were modeled as random effects. The model was fit by REML, and Satterthwaite’s method for estimating degrees of freedom was used for inference (t-tests).
LME Model 1 treated condition as a binary variable, averaging the Pre and Post sessions into a single ‘Alone’ condition and contrasting it against ‘Support’ which is the average of the Stroking, Handholding and Present conditions (Figure 2A). This provides a planned within-person comparison of Alone vs Support effects on pain.
Fig. 2.
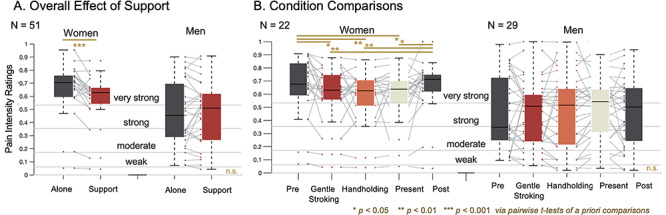
Interpersonal support decreases self-reported pain intensity in women but not men. (A) Overall effect of support. Social support reduced self-reported pain ratings in women receiving pain, but not men. (B) Condition comparisons. When conditions were analyzed separately, we find that each support condition significantly decreases pain reports relative to the Pre and Post conditions in women but not men.
In LME Model 2, we contrast coded all five conditions (Pre, Stroking, Handholding, Present, Post) to compare the effects of the different types of interpersonal support. Planned pairwise comparison were performed between each condition (Figure 2B). Subject intercepts were modeled as random effects; Condition and Gender were modeled as fixed effects.
Model 3 was developed to test for effects of support condition Order, to check whether effects of interest could be explained by manipulation order, habituation or sensitization effects. We tested for order effects by regressing stimulus Order on overall ratings in a Type III ANOVA with Satterthwaite’s method. Subject intercepts were modeled as random effects.
To quantify support effects on physiological synchrony, the main participant and partner SCR traces were extracted from each trial, smoothed (100 point moving average) and then correlated with one another by calculating the Pearson’s r on a trial-by-trial basis. These r values were then averaged within condition for each couple, and the average r values or ‘SCR synchrony’ were compared across conditions. Because SCR was recorded from the partner only when the partner was in the experiment room, we could not assess a ‘baseline’ level of synchrony. Therefore, we used one-sample t-tests to test for the existence of synchrony (r value greater than 0). To compare conditions, we used an LME model treating the three support conditions (Stroking, Handholding, Present) as fixed effects and subject intercepts (support effect magnitude) as random.
To assess Gender effects, we first report results across Gender on average, including terms for Support by Gender interactions in the model. Gender was treated as binary in this model because all participants self-identified as gender binary. If there was a significant interaction, we disaggregated results by Gender. If there was no interaction, we did not divide the sample by gender and instead interpreted effects across men and women.
Study 2
Study 2 comprised a novel re-analysis of a previous paper (López-Solà et al., 2019); that is, this analysis fit a new model to different independent and dependent variables from that same data set. This new analysis was conducted in light of findings from Study 1 of this manuscript in order to link the behavioral findings with possible brain mechanisms. Please see the Supplementary Methods for the complete description of all procedures performed in the full investigation from which we derived Study 2.
Participants
The study included 30 healthy cis women (mean age of 24.50 ± 6.65 years) with no history of psychiatric, neurological or pain disorders and no current pain symptoms, who were in a committed and monogamous romantic relationship for at least 3 months. All participants (cis women) and their romantic partners (cis men) gave written informed consent that was approved by the institutional review board of the University of Colorado Boulder and were paid for their participation. All participants were able to complete the fMRI task and were considered appropriate for inclusion in the final analysis. The timeframe of data collection for this study was October 2012 to June 2013.
Study design
A within-subjects a-b-b-a design was used where each condition consisted of 8 trials divided into 2 runs per condition (4 trials each). During condition ‘a’ the participant received thermal pain (47°C, 11-second stimuli, 7.5-second plateau temperature) while holding an inert rubber device (squeeze ball); during condition ‘b’ the participant was holding their partners’ hand (left hand of participant holding the left hand of the partner). After each pain stimulus (trial), participants rated, using a computerized visual analogue scale (VAS), pain intensity (‘how intense was the painful stimulus?’, ranging from 0, ‘not intense at all’ to 100, ‘the most intense imaginable’).
Analysis
We conducted an analysis to identify brain regions where pain-related activity was predictive of individual differences in pain report in the Handholding vs Squeeze ball control conditions. We ran two robust regressions. In one, brain activity during Handholding was predicted by individual differences in pain intensity ratings during Handholding. In the second, brain activity during the Squeeze ball control was predicted by individual differences in pain intensity ratings during the control. We compared the slopes of these two regressions to test where brain activity was more predictive of pain during Handholding. To conduct a statistical test on the difference of the slopes, we used bootstrap resampling to draw 1000 samples (with replacement), selecting all data from a participant as a unit to accommodate the error covariate structure in the within-person design. We fit both regression models and compared the slopes of the two regression lines and used the bootstrap distribution to estimate P values for the difference between Handholding and Squeeze ball slopes. This allowed us to test whether the brain/pain correlation was stronger during Handholding than control at a FDR-correction threshold of q < 0.05.
Note that this is different from, and we believe preferable to, the more common procedure of correlating the brain differences for pain-related activity in [Handholding–Squeeze ball] with pain differences in [Handholding–Squeeze ball]. This type of correlation has several shortcomings. First, it does not directly test the effect of greatest a priori interest—the relationship between brain and pain during Handholding. It tests the correlation between incremental differences in [Handholding–Squeeze ball]. Correlations between behavioral variables and difference scores ([Handholding–Squeeze ball]) are harder to interpret; they can be driven by correlations in the control condition (Squeeze ball) as well as the active condition (Handholding). In addition, correlating difference scores is substantially noisier, as the variance of the difference is the sum of the variances of individual conditions. Instead, we perform a more direct test of whether brain responses to pain predict pain ratings during Handholding and whether this effect is stronger during Handholding than control.
Results
Study 1
Interpersonal support decreases perceived pain intensity and unpleasantness overall
In Model 1, there was a significant effect of Support (the within-person Alone vs Support contrast; t(49.03) = −2.40, P = 0.02); participants reported less pain intensity when receiving Support than when Alone overall. Regardless of condition, men reported less pain intensity than women overall (main effect of Gender; t(49.00) = −2.404, P = 0.02). The interaction between Gender and Support was significant (t(49.03) = 3.22, P < 0.01); women reported a greater decrease in pain intensity when receiving Support. We therefore disaggregated by Gender. Planned pairwise comparisons revealed that pain intensity ratings reported by women were greater when Alone (Mean = 0.66, SD = 0.20) vs receiving Support (Mean = 0.58 SD = 0.19; t(21) = 4.00, P < 0.001; Cohen’s d = 0.36). There was no difference between Alone and Support conditions for men (t(28) = −0.60, P = 0.56).
There was also a significant effect of Support when Model 1 was fit to pain unpleasantness ratings (within-person Alone vs Support contrast; t(49.00) = 2.16, P = 0.04); participants reported less pain unpleasantness when receiving Support than when Alone overall. There was also a main effect of Gender (t(49.00) = 2.70, P = 0.01), but no Gender and Support interaction (t(49.00) = −1.15, P = 0.26). See Supplementary Figure S3A for more details.
In Model 2, there was no main effect of Support (F(4,196) = 1.60, P = 0.18), but there was a main effect of Gender (F(1,49) = 5.20, P = 0.03), with men reporting lower pain overall. As before, there was a significant interaction between Gender and Support (F(4,196) = 2.98, P = 0.02), and we thus disaggregated by Gender. In women, each of the three Support manipulations reduced pain relative to the Pre and Post conditions. Gentle stroking decreased pain relative to the Pre (Mean = 0.66, SD = 0.23; t(21) = −2.15, P = 0.04, Cohen’s d = 0.31) and Post conditions (Mean = 0.65, SD = 0.20; t(21) = −2.83, P = 0.01, Cohen’s d = 0.30). Handholding decreased pain relative to Pre (t(21) = −2.84, P = 0.01, Cohen’s d = 0.37) and Post (t(21) = −3.00, P = 0.01, Cohen’s d = 0.37). Partner presence decreased pain relative to Pre (t(21) = 2.30, P = 0.03, Cohen’s d = 0.34) and Post (t(21) = −2.36, P = 0.03, Cohen’s d = 0.33) conditions. There were no significant pairwise differences between the Support conditions. However, the largest effect was for Handholding (Cohen’s d = 0.37 in both Pre and Post contrasts). There were no significant pairwise effects in men (see Supplementary Table S1 for details).
There was no main effect of Support condition in the unpleasantness ratings (F(4,196) = 1.78, P = 0.13), only a main effect of Gender (F(1,49) = 7.05, P = 0.01) and no interaction (F(4,196) = 0.88, P = 0.47; see Supplementary Figure S3B).
Model 3 confirmed that there was no effect of condition Order on pain intensity (F(5,45) = 1.04, P = 0.41) nor pain unpleasantness (F(5,45) = 0.25, P = 0.94), nor was there an interaction between Condition and Order for intensity (F(20,180) = 1.44, P = 0.11) or unpleasantness (F(20,180) = 0.92, P = 0.56) indicating that the sequential order of the three interpersonal Support conditions did not influence ratings.
Interpersonal support decreases perceived pain-related physiological responses in women and men
When fit to pain-related SCRs, as with pain, Model 1 revealed a main effect of Support (F(1,48.1) = 7.19/equivalent t(49) = 2.61, P = 0.01, d = 0.37; Figure 3A). Support (Mean = 0.31, SD = 0.11) reduced pain-related physiological responses compared with Alone (Mean = 0.35, SD = 0.95). Here, there was no effect of Gender (F(1,47.9) = 1.15, P = 0.29) and no significant interaction (F(1,48.1) = 0.07, P = 0.79).
Fig. 3.
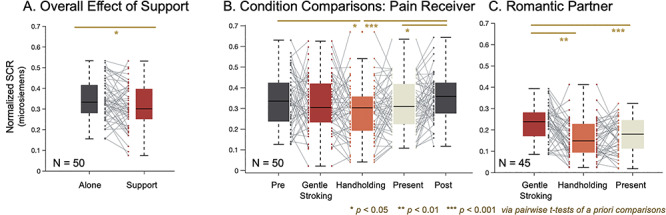
Interpersonal support decreases pain-related physiological responses in women and men. (A) Overall effect of support. Social support reduced pain-related physiological responses across all main participants. (B) Condition comparisons. When conditions were analyzed separately in the pain receiver, we find that this effect is driven by the Handholding condition, where only the Handholding condition is significantly less than the Pre and Post conditions. (C) Romantic partner. Partner SCRs are higher during the Stroking condition than during the passive Present and Handholding conditions.
Model 2 revealed a main effect of Support (F(4,188.2) = 3.11, P = 0.02; Figure 3B), indicating that SCRs varied across Support conditions. This effect was driven by the Handholding condition. There was no significant effect of Gender (F(1,47.8) = 1.01, P = 0.32) or Support x Gender interaction (F(1,188.2) = 0.56, P = 0.70). Pairwise comparisons revealed that Handholding (Mean = 0.29, SD = 0.13) reduced pain-related SCR compared to Pre (Mean = 0.34, SD = 0.13; t(45) = −2.11, P = 0.04, SD = 0.15, CI = [0.00 0.09], Cohen’s d = −0.38) and Post (Mean = 0.35, SD = 0.11; t(48) = −4.02, P < 0.01, SD = 0.12, CI = [−0.10–0.03], Cohen’s d = −0.55). Presence without touch (Mean = 0.31, SD = 0.12) reduced pain-related SCR relative to only Post (Mean = 0.35, SD = 0.11; t(49) = −2.16, P = 0.04, SD = 0.13, CI = [−0.08–0.00], Cohen’s d = −0.35). There were no significant differences among the Support conditions (Handholding vs Stroking: t(48) = −1.55, P = 0.13, Cohen’s d = −0.25; Handholding vs Present: t(48) = −1.39, P = 0.17, Cohen’s d = −0.25; Stroking vs Present: t(49) = 0.39, P = 0.70, Cohen’s d = 0.06), though effect sizes are largest for Handholding.
Type of interpersonal touch influences physiological responses in the supportive partner
Applying Model 2 to SCR responses in the supportive caregivers revealed a main effect of condition (F(2,83.59) = 11.71, P < 0.01). Pairwise comparisons revealed that partner SCR was higher during Stroking (Mean = 0.23, SD = 0.09) than Handholding (Mean = 0.16, SD = 0.09; t(41) = 4.16, P < 0.01, SD = 0.11, CI = [0.04 0.11], Cohen’s d = 0.85) and Present (Mean = 0.18, SD = 0.08; t(42) = 3.81, P < 0.01, SD = 0.10, CI = [0.03 0.09], Cohen’s d = 0.68) conditions. There was no significant difference between the Handholding and Present conditions (t(41) = −0.98, P = 0.34, SD = 0.10, CI = [−0.04 0.02], Cohen’s d = −0.20).
Interpersonal support induced physiological synchrony
Partner support induced physiological synchrony in SCR traces within each condition (Stroking: t(44) = 2.86, P < 0.01, SD = 0.21, CI = [0.03 0.15]; Handholding: t(43) = 5.71, P < 0.001, SD = 0.31, CI = [0.17 0.36]; Present: t(44) = 5.15, P < 0.001, SD = 0.24, CI = [0.11 0.26]; Figure 4A).
Fig. 4.
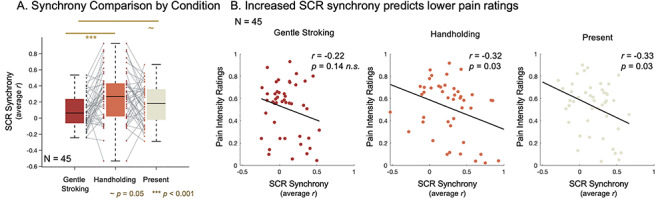
Interpersonal support induces physiological synchrony related to pain reduction. (A) Synchrony comparison by condition. Three one-sample t-tests revealed that partner support induced physiological synchrony, on average, within each condition. Pairwise comparisons revealed that synchrony was higher during Handholding than Stroking. Synchrony during Handholding was nonsignificantly higher than during the no-touch Present condition. Synchrony during the Present condition was a borderline significantly higher than Stroking. (B) Increased SCR synchrony predicts lower pain ratings. Pearson correlations between pain intensity ratings and average SCR synchrony within each condition.
Next, we compared synchronicity across support conditions. The synchrony LME model revealed a main effect of Condition (F(2,87.45) = 6.77, P < 0.01). Pairwise comparisons revealed that synchrony was higher during Handholding (Mean = 0.27, SD = 0.31) than Stroking (Mean = 0.09, SD = 0.21; t(43) = −3.74, P < 0.001, SD = 0.32, CI = [−0.28–0.08], Cohen’s d = −0.67). Synchrony during Handholding was nonsignificantly higher than during the Present condition (Mean = 0.18, SD = 0.24; t(43) = 1.68, P = 0.10, SD = 0.33, CI = [−0.02 0.18], Cohen’s d = 0.29). Synchrony during the Present condition was the borderline significantly higher than Stroking (t(44) = −2.01, P = 0.05, SD = 0.32, CI = [−0.19 0.00], Cohen’s d = −0.43; Figure 4A).
Increased synchrony during Handholding was positively correlated with the main participant’s self-reported degree of comfort with interpersonal touch in their daily life as measured by the TACTYPE questionnaire (r = 0.31, P = 0.04, Figure 5). This relationship between comfort with touch and synchrony was not found in the Stroking (r = −0.01, P = 0.94) or Present conditions (r = −0.09, P = 0.53).
Fig. 5.
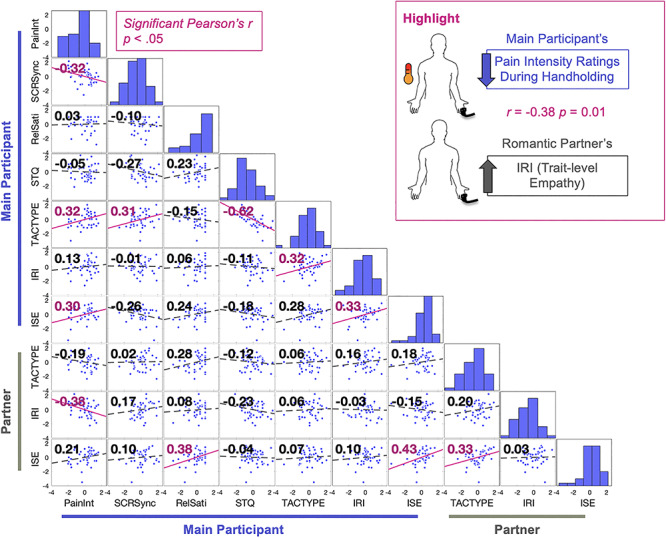
Individual differences in interpersonal tendencies and beliefs are related to handholding analgesia. To explore relationships among the effect of Handholding on pain and synchrony with an individual’s empathic traits, enjoyment of touch and perceptions of support in their lives, we computed a pairwise Pearson’s correlation matrix using participant survey data as well as their average pain reports and SCR synchrony during Handholding. This plot focuses on Handholding because Handholding had the largest effect on pain reports and physiology. Pearson’s r values are on the top left corner of each plot. Significant correlations in pink. Significance threshold set at P < 0.05 uncorrected. Frequency histograms are plotted on the diagonal. For the purpose of this plot, the data were z-scored to standardize the range of data for easier viewing. Legend: PainInt, average pain intensity ratings during Handholding; SCRsync, average couple SCR synchrony during Handholding; RelSatis, Relationship Satisfaction; STQ, Social Touch Questionnaire; TACTYPE, attitudes about touch; IRI, Interpersonal reactivity index (empathy); ISE, Interpersonal Support Evaluation.
Increased interpersonal synchrony was negatively correlated with pain intensity ratings
Increased SCR synchrony was significantly negatively correlated with self-reported overall pain intensity ratings during Handholding (r = −0.32, P = 0.03) and during the passive Present condition (r = −0.33, P = 0.03). There was a nonsignificant negative correlation during the Stroking condition (r = −0.22, P = 0.14; Figure 4).
Individual differences in interpersonal tendencies and beliefs are related to the effects of handholding on pain reports
To explore relationships among the effect of Handholding on pain intensity self-reports and SCR synchrony with empathic traits, enjoyment of touch and appraisals of support, we computed a pairwise Pearson’s correlation matrix using participant survey data, average pain reports and SCR synchrony during Handholding. Surveys with subscales were collapsed so that one average score was reported per survey (Figure 5). This analysis centered on Handholding because Handholding had the largest effect on pain reports and physiology. Because this analysis was exploratory, the significance threshold was set at P < 0.05 and not corrected for multiple comparisons and therefore may be at risk of increased Type I error. More definitive and adequately powered analyses of individual differences require substantially larger sample sizes (e.g. N > ~300).
This analysis revealed the following exploratory correlations, which provide preliminary support for testing individual differences in larger samples:
Average scores on the main participant’s TACTYPE questionnaire, which reflects positive attitudes towards interpersonal touch in their daily life, were (i) positively correlated with their pain ratings (r = 0.32, P = 0.03), (ii) positively correlated with SCR synchrony with their partner (r = 0.31, P = 0.04), (iii) negatively correlated with average scores on their STQ (where negative scores indicate greater liking of social touch; r = −0.62, P < 0.001) and (iv) positively correlated with average IRI score, which reflects empathic tendencies (r = 0.32, P = 0.03). To summarize, people who rate high on the TACTYPE, and therefore may have a more welcoming attitude towards interpersonal touch, reported more pain during Handholding, had greater SCR synchrony with their partners during Handholding and had greater empathic tendencies.
Average scores on the main participant’s ISE, which reflects one’s sense of social support in their daily life, were positively correlated with average pain intensity ratings during Handholding (r = 0.30, P = 0.05) and with their average IRI scores (r = 0.33, P = 0.03). Thus, people who rate high on ISE, and therefore may perceive their social support network as strong, have greater empathic tendencies and report more pain during Handholding. While exploratory, this may indicate that those who feel more supported by their partner may also be more pain-sensitive or feel more comfortable expressing pain.
Average scores on the partner’s ISE were positively correlated with the couple’s self-reported relationship satisfaction (r = 0.38, P = 0.01), their partner’s ISE (r = 0.43, P < 0.01) and their own TACTYPE (r = 0.33, P = 0.03). Note, the main participant’s ISE was also positively correlated with relationship satisfaction, but this relationship was not significant (r = 0.30, P = 0.11). This may indicate that feeling socially supported is related to greater relationship satisfaction and a more positive attitude towards interpersonal touch.
Finally, average scores on the partner’s IRI were negatively correlated with the main participant’s average pain intensity reports during Handholding (r = −0.38, P = 0.01). Furthermore, we found that across all support conditions, increased empathic tendency (measured via the IRI) within the supportive partner was significantly correlated with reductions in the main participant’s pain intensity reports (Stroking: r = −0.40, P < 0.01; Handholding: r = −0.39, P = 0.01; Present: r = −0.44; P < 0.01; Supplementary Figure S4). In addition, the partner’s average IRI scores were positively correlated with SCR synchrony in only the Present condition (Present: r = −0.44; P < 0.01; see Supplementary Figure S4 for comparison with other conditions). Thus, receiving social support from a partner with greater empathic tendency may be related to greater physiological synchrony and larger effects of support on pain.
Study 2: brain activity relevant to somatic sensation and valuation predicts decreases in self-reported pain intensity during handholding
To better understand how these effects relate to brain activity, we analyzed the difference in slopes between two robust regression models (Wager et al., 2005) of data from Study 2. Model 1 predicts pain ratings from brain data during Handholding trails and Model 2 predicts pain ratings from brain data during Squeeze ball trials. That is, for each voxel, Model 1 regressed average participant pain intensity ratings (X1) during Handholding onto average brain activity during Handholding (y1). Model 2 regressed average participant pain intensity ratings (X2) during the holding of a Squeeze ball onto average brain activity during the holding of the Squeeze ball (y2). We then took the difference in the slopes (Model 1–Model 2). We bootstrapped the slope difference (1000 samples; participants sampled with replacement) to assess significance. The final maps were corrected for multiple comparisons at q < 0.05 False Discovery Rate (FDR; Figure 6). Increased activity during Handholding (relative to holding a Squeeze ball) predicted reduced pain self-reports (i.e. negative differences in slope) in the right NAc/ventral striatum, bilateral primary somatosensory cortex (S1), right inferior parietal sulcus (IPS) and left medial prefrontal cortex (PFC; see Supplementary Table S2 for a complete list).
Fig. 6.
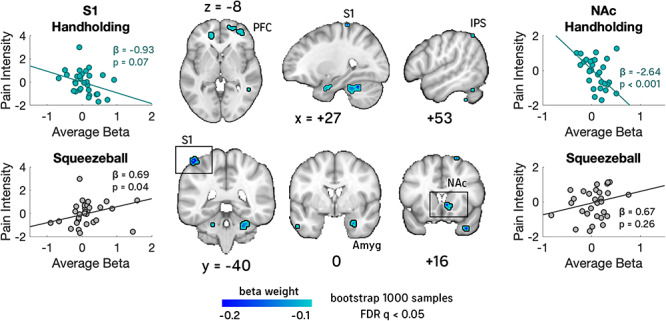
Brain regions that predict decreased pain self-reports during handholding. Two robust regressions were fit on data from Study 2. In one, brain activity during Handholding was predicted by individual differences in pain intensity ratings during Handholding. In the second, brain activity during the Squeeze ball control was predicted by individual differences in pain intensity ratings during the control. We compared the slopes of these two regressions to test where brain activity was significantly more predictive of pain during Handholding. This analysis revealed negative correlations with pain during Handholding—indicative of pain-regulation or other competing processes—in the right nucleus accumbens (NAc)/ventral striatum, bilateral primary somatosensory cortex (S1), amygdala (Amyg), right inferior parietal sulcus (IPS) and medial PFC. NAc and medial PFC have all been associated with pain valuation and meaning, amygdala with pain affect and somatosensory cortex with social touch-related pain reduction in previous studies. Breakout scatter plots of select ROIs are pictured for descriptive purposes only.
To provide a better description of the effects found in the NAc and S1, we plotted the subject-wise data from significant clusters within the NAc and S1 for Models 1 and 2 and included the slopes and statistics for a biased robust regression performed within those clusters. This is done for descriptive purposes only to expand on the effects found from the whole-brain regression. Statistics yielded from this descriptive analysis are necessarily biased as we selected significant regions of interested after creating the whole-brain results map, and therefore these statistics are presented only to descriptively expand upon the results of the whole-brain analysis.
Discussion
The results of our two-part investigation indicate that receiving social support impacts pain perception and physiology and that the strength of this effect is influenced by several social variables related to the conception of support, including gender, empathic traits in the support giver and preferences for social touch. Receiving social support from a partner reduced pain in women, but not men. In contrast, pain-related SCRs, which are less subject to demand characteristics and evaluative biases, were reduced by social support in both women and men. Though there were no significant differences across the different types of support, effect sizes were largest during handholding in both pain ratings and SCR. Relative to the other touch condition, handholding may have an anxiolytic effect in the partner: the supportive partner’s SCR decreased during handholding relative to stroking, and SCR synchrony was higher during handholding relative to stroking.
It is possible that the absence of an effect of social support in the pain ratings of men is related to gender biases in self-reporting and self-expression (Fillingim et al., 2009; Louie and Ward, 2010; Paller et al., 2009). Men reported lower pain than women, but did not show lower pain-related SCRs. They may be unwilling to report high levels of pain in the presence of their partners or the experimenters. There is evidence that men who more strongly identify as ‘masculine’ more often volunteer for pain experiments and report less pain (Mattos Feijó et al., 2018). Conversely, women may feel more comfortable reporting pain or may more often use pain reporting as a social support-garnering strategy (Chambers et al., 2002). The dissociation in this study between pain reports and SCRs provides evidence of an interesting separation between presumably lower-level nocifensive processes (here, sympathetic autonomic activation) and higher-level pain reports.
Greater physiological synchrony during handholding vs stroking may reflect benefits of handholding in participants’ perceptions of support. It may also occur because gentle stroking is a repetitive motion that stimulates the skin of the receiver, which may influence SCRs independent of shared physiological processes induced by the context. However, the association between increased interpersonal SCR synchrony and participants’ pain reports suggests that shared physiology is functionally important for pain. To our knowledge this is the first experiment to find this effect in SCR to date; however, other studies have found that synchrony in heart rate, respiration and electroencephalographic (EEG) measurements in romantic couples are related to analgesia (Goldstein et al., 2017, 2018).
One interpretation of the synchrony effect is that when two people attend to one another, they simulate each other’s affective state. It is possible that as a romantic partner responds to the pain-receiving participant’s distress and tries to soothe them, they reduce their own stress-related physiology because they are taking an action to help their partner. Their personal reduction in distress may be simulated by their partner who then may begin to reappraise the pain as less dangerous. This cycle of support may underlie pain modification by altering the threat value associated with the painful stimulus in the pain receiver. More direct tests of these complex dynamics await further study. Here, the positive correlation between synchrony and one’s reported comfort with interpersonal touch in their daily life may indicate that the benefits of interpersonal touch are related to how one conceptualizes touch: people who desire social touch and evaluate it as pleasing and safe benefit more from it.
In the brain, individual differences in the effectiveness of handholding analgesia were related to activity within frontostriatal valuation pathways and brain regions involved in somatic sensation and action. Participants with greater striatum activation during handholding reported less pain on average. The striatum is associated with reinforcement learning and is a critical brain region for stimulus evaluation. Activation of the striatum during handholding might reflect a positive appraisal of the social context, and this appraisal may interact with the pain representation in a way that mitigates the danger associated with the painful stimulus. This finding is consistent with evidence that NAc/striatum-dependent reward processing is related to pain reductions when one is reminded of a supportive context (Younger et al., 2010) and during placebo analgesia (Scott et al., 2008). In this way, social support may influence one’s pain experience by altering one’s ‘top-down’ expectations about pain.
Some of the activity in brain regions related to somatic sensation and action (right S1 and right IPS) was contralateral both to the hand being held and to the arm being stimulated by the painful thermode. Activity in S1 is commonly implicated in pain experiencing (Wager et al., 2013), typically in relation to the site of painful stimulation. Activity in the IPS has been shown to be related to proprioceptive space with respect to the hands and activates with hand-specific tactile input (Binkofski et al., 1998; Avillac et al., 2005; Culham et al., 2006). Makin et al. (2007) propose that the anterior IPS integrates multisensory information to represent immediate space around the hand. It is possible that evaluative processing interacts with somatic representations of handholding or of the pain site (left forearm) to alter the ‘embodied’ value of painful stimulus (Niedenthal, 2007; Niedenthal and Barsalou, 2009; Seth, 2013) or that attention to the space around the hand gives higher weighting to the supportive context and in this way diminishes the danger associated with the painful stimulus.
The pain experience is constructed in the brain through the integration of nociceptive input with cognitive and affective information (for a review, see Reddan and Wager, 2018, 2019). Social support may influence pain by signaling concepts of safety that integrate with the pain experience and alter its valuation. Supportive touch may have dual ‘bottom-up’ and ‘top-down’ paths by which it influences pain signaling, where both the peripheral mechanisms of touch as well as conceptualizations of safety interact with the nociceptive input. Handholding is one such clear social signal of safety and care.
Supportive touch provides an important window into both the sensory and motivational–affective dimensions of pain perception that are likely to be influenced by psychological processes. Advancements in our basic scientific understanding of these interactions will elucidate how social context impacts motivational brain systems and health and will broaden our understanding of the biology of cooperation, affection and compassion.
Conflict of interest statement
The all of the authors of this manuscript—Touch and social support influence interpersonal synchrony and pain—certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Funding
The neural bases of placebo effects and their relation to regulatory processes Agency National Institute of Health (NIH). Institute National Institute of Mental Health (NIMH). Type Research Project (R01) Project # 7R01MH076136-12. Application # 9967395. Study Section Biobehavioral Mechanisms of Emotion, Stress and Health Study Section (MESH).
Supplementary Material
Contributor Information
Marianne C Reddan, Department of Psychology, Stanford University, Stanford, CA 94305, USA; Department of Psychology and Neuroscience, Institute of Cognitive Science, University of Colorado, Boulder, CO 80309-0344, USA.
Hannah Young, Department of Psychology and Neuroscience, Institute of Cognitive Science, University of Colorado, Boulder, CO 80309-0344, USA.
Julia Falkner, Department of Psychology and Neuroscience, Institute of Cognitive Science, University of Colorado, Boulder, CO 80309-0344, USA.
Marina López-Solà, Department of Psychology and Neuroscience, Institute of Cognitive Science, University of Colorado, Boulder, CO 80309-0344, USA; Department of Medicine, School of Medicine and Health Sciences, University of Barcelona, Barcelona 08036, Spain.
Tor D Wager, Department of Psychology and Neuroscience, Institute of Cognitive Science, University of Colorado, Boulder, CO 80309-0344, USA; Department of Psychological Brain Sciences, Dartmouth College, Hanover, NH 03755, USA.
References
- Agren G., Lundeberg T., Uvnäs-Moberg K., Sato A. (1995). The oxytocin antagonist 1-deamino-2-d-Tyr-(Oet)-4-Thr-8-Orn-oxytocin reverses the increase in the withdrawal response latency to thermal, but not mechanical nociceptive stimuli following oxytocin administration or massage-like stroking in rats. Neuroscience Letters. doi: 10.1016/0304-3940(95)11335-T. [DOI] [PubMed] [Google Scholar]
- Avillac M., Denève S., Olivier E., Pouget A., Duhamel J.R. (2005). Reference frames for representing visual and tactile locations in parietal cortex. Nature Neuroscience. 8(7), 941–949. doi: 10.1038/nn1480. [DOI] [PubMed] [Google Scholar]
- Bartoshuk L.M., Duffy V.B., Green B.G., Hoffman H.J., Ko C.W., Lucchina L.A., et al. (2004). Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 82, 109–14. [DOI] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., Walker S. (2014). lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-7 . Available: http://CRAN.R-project.org/package=lme4. R Package Version.
- Benedek M., Kaernbach C. (2010). A continuous measure of phasic electrodermal activity. Journal of Neuroscience Methods. 190(1), 80–91. doi: 10.1016/j.jneumeth.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F., Dohle C., Posse S., et al. (1998). Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology. 50(5), 1253–1259. doi: 10.1212/WNL.50.5.1253. [DOI] [PubMed] [Google Scholar]
- Borsook D., Upadhyay J., Chudler E.H., Becerra L. (2010). A key role of the basal ganglia in pain and analgesia - insights gained through human functional imaging. Molecular Pain. 6, 1744-8069-6-27. doi: 10.1186/1744-8069-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.L., Sheffield D., Leary M.R., Robinson M.E. (2003). Social support and experimental pain. Psychosomatic Medicine. 65(2), 276–283. doi: 10.1097/01.PSY.0000030388.62434.46. [DOI] [PubMed] [Google Scholar]
- Carlino E., Frisaldi E., Benedetti F. (2014). Pain and the context. Nature Reviews Rheumatology. 10(6), 348–355. doi: 10.1038/nrrheum.2014.17. [DOI] [PubMed] [Google Scholar]
- Chambers C.T., Craig K.D., Bennett S.M. (2002). The impact of maternal behavior on children’s pain experiences: an experimental analysis. Journal of Pediatric Psychology. 27(3), 293–301. doi: 10.1093/jpepsy/27.3.293. [DOI] [PubMed] [Google Scholar]
- Chesterton L.S., Barlas P., Foster N.E., Baxter G.D., Wright C.C. (2003). Gender differences in pressure pain threshold in healthy humans. Pain. 101(3), 259–266. doi: 10.1016/S0304-3959(02)00330-5. [DOI] [PubMed] [Google Scholar]
- Coan J.A., Schaefer H.S., Davidson R.J. (2006). Lending a hand: social regulation of the neural response to threat. Psychological Science. 17(12), 1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- Cohen S., Mermelstein R., Kamarck T., Hoberman H. M. (1985). Measuring the functional components of social support In Social Support: Theory, Research and Applications. 10.1007/978-94-009-5115-0_5 [DOI] [Google Scholar]
- Coleman D., Iso-Ahola S.E. (1993). Leisure and health: the role of social support and self-determination. Journal of Leisure Research. doi: 10.1080/00222216.1993.11969913. [DOI] [Google Scholar]
- Culham J.C., Cavina-Pratesi C., Singhal A. (2006). The role of parietal cortex in visuomotor control: what have we learned from neuroimaging? Neuropsychologia. doi: 10.1016/j.neuropsychologia.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Davis M.H. (1983). Measuring individual differences in empathy: evidence for a multidimensional approach. Journal of Personality and Social Psychology. 44(1), 113–126. doi: 10.1037/0022-3514.44.1.113. [DOI] [Google Scholar]
- Deethardt J.F., Hines D.G. (1983). Tactile communication and personality differences. Journal of Nonverbal Behavior. 8(2), 143–156. doi: 10.1007/BF00987000. [DOI] [Google Scholar]
- Eisenberger N.I., Master S.L., Inagaki T.K., et al. (2011). Attachment figures activate a safety signal-related neural region and reduce pain experience. Proceedings of the National Academy of Sciences of the United States of America. 108(28), 11721–11726. doi: 10.1073/pnas.1108239108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim R.B., King C.D., Ribeiro-Dasilva M.C., Rahim-Williams B., Riley J.L. 3rd (2009). Sex, gender, and pain: a review of recent clinical and experimental findings. The Journal of Pain: Official Journal of the American Pain Society, 10(5), 447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison J., Erdeniz B., Done J. (2013). Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neuroscience and Biobehavioral Reviews. 37(7), 1297–1310. doi: 10.1016/j.neubiorev.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Gazzola V., Spezio M.L., Etzel J.A., Castelli F., Adolphs R., Keysers C. (2012). Primary somatosensory cortex discriminates affective significance in social touch. Proceedings of the National Academy of Sciences of the United States of America. 109(25), E1657–E1666. doi: 10.1073/pnas.1113211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein P., Weissman-Fogel I., Shamay-Tsoory S.G. (2017). The role of touch in regulating inter-partner physiological coupling during empathy for pain. Scientific Reports. 115(11), E2528–E2537. doi: 10.1038/s41598-017-03627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein P., Weissman-Fogel I., Dumas G., Shamay-Tsoory S.G. (2018). Brain-to-brain coupling during handholding is associated with pain reduction. Proceedings of the National Academy of Sciences of the United States of America. doi: 10.1073/pnas.1703643115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan J.D., Craft R.M., LeResche L., et al. (2007). Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 132, S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest S., Dessirier J.M., Mehrabyan A., et al. (2011). The development and validation of sensory and emotional scales of touch perception. Attention, Perception, & Psychophysics. 73(2), 531–550. doi: 10.3758/s13414-010-0037-y. [DOI] [PubMed] [Google Scholar]
- Hennessy M.B., Kaiser S., Sachser N. (2009). Social buffering of the stress response: diversity, mechanisms, and functions. Frontiers in Neuroendocrinology. 30(4), 470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Klauer T., Filipp S.H., Hellhammer D.H. (1995). Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine. 57(1), 23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Krahé C., Springer A., Weinman J.A., Fotopoulou A. (2013). The social modulation of pain: others as predictive signals of salience - a systematic review. Frontiers in Human Neuroscience. 7, 386. doi: 10.3389/fnhum.2013.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljencrantz J., Strigo I., Ellingsen D.M., et al. (2017). Slow brushing reduces heat pain in humans. European Journal of Pain (United Kingdom). 21(7), 1173–1185. doi: 10.1002/ejp.1018. [DOI] [PubMed] [Google Scholar]
- Löken L.S., Wessberg J., Morrison I., McGlone F., Olausson H. (2009). Coding of pleasant touch by unmyelinated afferents in humans. Nature Neuroscience. 12(5), 547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- López-Solà M., Geuter S., Koban L., Coan J.A., Wager T.D. (2019). Brain mechanisms of social touch-induced analgesia. Pain. 160(9), 2072–2085. [DOI] [PubMed] [Google Scholar]
- Louie G.H., Ward M.M. (2010). Sex disparities in self-reported physical functioning: true differences, reporting bias, or incomplete adjustment for confounding? Journal of the American Geriatrics Society, 58(6), 1117–22. doi: 10.1111/j.1532-5415.2010.02858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin T.R., Holmes N.P., Zohary E. (2007). Is that near my hand? Multisensory representation of peripersonal space in human intraparietal sulcus. Journal of Neuroscience. 27(4), 731–740. doi: 10.1523/JNEUROSCI.3653-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini F., Nash T., Iannetti G.D., Haggard P. (2014). Pain relief by touch: a quantitative approach. Pain. 155(3), 635–642. doi: 10.1016/j.pain.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master S.L., Eisenberger N.I., Taylor S.E., Naliboff B.D., Shirinyan D., Lieberman M.D. (2009). A picture’s worth: partner photographs reduce experimentally induced pain. Psychological Science. 20(11), 1316–1318. doi: 10.1111/j.1467-9280.2009.02444.x. [DOI] [PubMed] [Google Scholar]
- Mattos Feijó L., Tarman G.Z., Fontaine C., Harrison R., Johnstone T., Salomons T. (2018). Sex-specific effects of gender identification on pain study recruitment. The Journal of Pain. 19(2), 178–185. doi: 10.1016/j.jpain.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Montoya P., Larbig W., Braun C., Preissl H., Birbaumer N. (2004). Influence of social support and emotional context on pain processing and magnetic brain responses in fibromyalgia. Arthritis and Rheumatism. 50(12), 4035–4044. doi: 10.1002/art.20660. [DOI] [PubMed] [Google Scholar]
- Navratilova E., Atcherley C.W., Porreca F. (2015). Brain circuits encoding reward from pain relief. Trends in Neurosciences. 38(11), 741–750. doi: 10.1016/j.tins.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedenthal P.M. (2007). Embodying emotion. Science. 316(5827), 1002–1005. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- Niedenthal P.M., Barsalou L.W. (2009). Embodiment In: Sander D., Scherer K., editors. Oxford Companion to the Handbook of Affective Sciences, London: Oxford University Press; https://psycnet.apa.org/record/2009-16563-000 [Google Scholar]
- Nummenmaa L., Tuominen L., Dunbar R., et al. (2016). Social touch modulates endogenous μ-opioid system activity in humans. NeuroImage. 138, 242–247. doi: 10.1016/j.neuroimage.2016.05.063. [DOI] [PubMed] [Google Scholar]
- Olausson H., Wessberg J., Morrison I., McGlone F., Vallbo Å. (2010). The neurophysiology of unmyelinated tactile afferents. Neuroscience and Biobehavioral Reviews. 34(2), 185–191. doi: 10.1016/j.neubiorev.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Paller C.J., Campbell C.M., Edwards R.R., Dobs A.S. (2009). Sex-based differences in pain perception and treatment. Pain Medicine (Malden, Mass.), 10(2), 289–299. doi: 10.1111/j.1526-4637.2008.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawling R., Cannon P.R., McGlone F.P., Walker S.C. (2017). C-tactile afferent stimulating touch carries a positive affective value. PLoS One. 12(3), e0173457. doi: 10.1371/journal.pone.0173457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at https://www.R-project.org/. [Google Scholar]
- Reddan M.C., Wager T.D. (2018). Modeling pain using fMRI: from regions to biomarkers. Neuroscience Bulletin. 34(1), 208–215. doi: 10.1007/s12264-017-0150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddan M.C., Wager T.D. (2019). Brain systems at the intersection of chronic pain and self-regulation. Neuroscience Letters. 702, 24–33. doi: 10.1016/j.neulet.2018.11.047. [DOI] [PubMed] [Google Scholar]
- Sambo C.F., Howard M., Kopelman M., Williams S., Fotopoulou A. (2010). Knowing you care: effects of perceived empathy and attachment style on pain perception. Pain. 151(3), 687–693. doi: 10.1016/j.pain.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Scott D.J., Stohler C.S., Egnatuk C.M., Wang H., Koeppe R.A., Zubieta J.K. (2008). Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Archives of General Psychiatry. 65(2), 220. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Seth A.K. (2013). Interoceptive inference, emotion, and the embodied self. Trends in Cognitive Sciences. 17(11), 565–573. doi: 10.1016/j.tics.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Uchino B.N. (2006). Social support and health: a review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine. 29(4), 377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Atlas L.Y. (2015). The neuroscience of placebo effects: connecting context, learning and health. Nature Reviews. Neuroscience. 16(7), 403–418. doi: 10.1038/nrn3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Keller M.C., Lacey S.C., Jonides J. (2005). Increased sensitivity in neuroimaging analyses using robust regression. NeuroImage. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Atlas L.Y., Lindquist M.A., Roy M., Woo C.W., Kross E. (2013). An fMRI-based neurologic signature of physical pain. The New England Journal of Medicine. 368(15), 1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall P.D., Morgan M.M., Sorkin L.S., et al. (1996). Comments after 30 years of the gate control theory. Pain Forum. 5(1), 12–22. [Google Scholar]
- Weekes D.P., Kagan S.H., James K., et al. (1993). The phenomenon of hand holding as a coping strategy in adolescents experiencing treatment-related pain. Journal of Pediatric Oncology Nursing. 10(1), 19–25. doi: 10.1177/104345429301000105. [DOI] [PubMed] [Google Scholar]
- Wilhelm F.H., Kochar A.S., Roth W.T., Gross J.J. (2001). Social anxiety and response to touch: incongruence between self-evaluative and physiological reactions. Biological Psychology. 58(3), 181–202. doi: 10.1016/S0301-0511(01)00113-2. [DOI] [PubMed] [Google Scholar]
- Yang Y.C., Boen C., Gerken K., Li T., Schorpp K., Harris K.M. (2016). Social relationships and physiological determinants of longevity across the human life span. Proceedings of the National Academy of Sciences of the United States of America. 113(3), 578–583. doi: 10.1073/pnas.1511085112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger J., Aron A., Parke S., Chatterjee N., Mackey S. (2010). Viewing pictures of a romantic partner reduces experimental pain: involvement of neural reward systems. PLoS One. 5(10), 1932–6203. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


