Abstract
Pregnancy and the transition to parenthood is an important period marked by dramatic neurobiological and psychosocial changes that may have implications for the health of women and offspring. Although human and non-human animal research suggests that the brain undergoes alterations during the peripartum period, these changes are poorly understood. Here, we review existing research, particularly human neuroimaging and psychophysiological research, to examine changes in brain structure and function during the peripartum period and discuss potential implications for the health of women and offspring. First, we discuss the potential causes of these changes across pregnancy, including physiological and psychosocial factors. Next, we discuss the evidence for structural and functional changes in the brain during pregnancy and into the postpartum period, noting the need for research conducted prospectively across human pregnancy. Finally, we propose potential models of individual differences in peripartum neurobiological changes (i.e. hypo-response, typical response, hyper-response) and emphasize the need to consider trajectories of change in addition to pre-existing factors that may predict maternal adjustment to parenthood. We suggest that the consideration of individual differences in neurobiological trajectories across pregnancy may contribute to a better understanding of risk for negative health and behavior outcomes for women and offspring.
Keywords: peripartum mental health, brain function, brain structure, postpartum depression, neurobiology
Each year, ~4 million women in USA experience pregnancy and childbirth (Martin et al., 2018). The transition to motherhood is a dynamic period in life marked by a combination of dramatic neurobiological and psychosocial changes. These changes may have significant implications for the physical and mental health of women and offspring. Depressive symptoms affect more than 25% of women in the peripartum period (Gavin et al., 2005), and the prevalence rates of anxiety and related disorders are estimated at 10–20% (Fairbrother et al., 2016). There is also evidence that maternal depression and anxiety symptoms can have profound negative effects on infant development (Anniverno et al., 2013; Pearlstein et al., 2013; Righetti-Veltema et al., 2003).
Risk for peripartum depression and anxiety may be attributed to some of the physiological changes (e.g. shifts in hormones, neurobiological changes) that women undergo during this period (Yim et al., 2015; Barba-Müller et al., 2018). This risk may also be in part to the host of new challenges (e.g. ‘around the clock’ child-rearing demands) that take place as women transition to motherhood (Saxbe et al., 2018). In addition, it may be that interactions between psychosocial and physiological factors shape new mothers’ ability to respond sensitively to the needs of their infant (Kim et al., 2010; Ostlund et al., 2017; Young et al., 2017). Such interactions have cascading effects on child outcomes, as early maternal sensitivity has been found to predict children’s social and academic functioning from childhood into adulthood (Leerkes, 2010; Raby et al., 2015).
Both human and non-human animal research suggests that the brain undergoes substantial structural and functional changes during pregnancy and the postpartum period (Oatridge et al., 2002; Brunton & Russell, 2008; Barba-Müller et al., 2018). Yet, the purpose of these changes and the ways in which they may increase—or buffer against—risk for maternal psychopathology and transition to parenthood remain unclear. Some researchers theorize that at least some of these changes may be adaptive and prepare women for the emotional and cognitive demands necessary for caring for a new child (Barba-Müller et al., 2018). However, the peripartum period is also a unique time when neurobiological changes in women have the potential to affect the health of both women and the fetus. As such, it is possible that some peripartum brain changes are a result of processes necessary for promoting healthy offspring development (e.g. hormonal changes), but may have deleterious effects on women (e.g. increased psychopathology risk, cognitive deficits) (De et al., 2006; Anderson & Rutherford, 2012; Yim et al., 2015). Given the potential impact of structural and functional change across gestation on health and well-being of women and offspring, it is critical to identify both typical and atypical changes across this period. Understanding neurobiological changes in the peripartum period, as well as how individuals differ in these trajectories, may help us identify risk factors and targets for early interventions for high-risk women in order to promote mental health and sensitive caregiving in the peripartum period.
To this end, we review current knowledge of the structural and functional brain changes across the peripartum period and implications for the health of women and offspring. We first discuss the potential causes of change in brain structure and function across pregnancy, including both physiological and psychosocial factors. Next, we review the limited evidence for structural and functional changes in the brain during pregnancy and into the postpartum period, with a focus on human research. We focus predominantly on cross-sectional and longitudinal neuroimaging and psychophysiological studies in pregnant women in order to identify changes that may be unique to the experience of pregnancy, as opposed to the experience of parenting more broadly. To complement these findings, we also integrate findings from the larger literature on postpartum women as well as non-human animals. Finally, we conclude by proposing potential models of individual differences in peripartum neurobiological changes. Specifically, we highlight three possible trajectories of neurobiological change during pregnancy (i.e. hypo-response, typical response, hyper-response) to characterize differences across individuals and potential health and behavior implications for women and offspring.
Potential contributors to peripartum brain changes
There are several reasons the brain may change during pregnancy and postpartum. The peripartum period is marked by significant alterations in hormones, immune function, sleep, psychosocial stress and caregiving responsibilities. There is evidence that these changes contribute to structural and functional brain alterations outside of the context of pregnancy. Drawing from this literature may provide insight into factors driving neurobiological change during the peripartum period, as well as the possible consequences of these changes for the health of women and their offspring.
Among the most dramatic biological changes experienced by women during pregnancy are large increases and then decreases in hormones thought to support pregnancy, fetal growth and labor. Although a comprehensive review is outside the scope of the current review, levels of progesterone, estrogen, cortisol, oxytocin, dopamine and testosterone, as well as other hormones, change dramatically across this period (for reviews, Magon & Kumar, 2013; Yim et al., 2015). Progesterone and estrogen levels are critical for preparing women’s bodies to support fetal development. Levels of both hormones rise exponentially during pregnancy and drop considerably post-birth (Magon & Kumar, 2013). Cortisol levels in pregnancy gradually increase, peak at delivery and then quickly decline to baseline level within 3 days postpartum (Thompson & Trevathan, 2008). Gestational increases in cortisol ensure the fetal lungs, central nervous system and other organs are fully developed and also activates pathways associated with labor (Murphy & Clifton, 2003). Oxytocin levels generally increase during the first to third trimesters and are thought to help facilitate the onset of labor, as well as shape mother–infant attachment and bonding (Feldman et al., 2007; Galbally et al., 2011; Prevost et al., 2014). Further, oxytocin is released after birth and is thought to facilitate the mother–infant bond (Douglas, 2010). Maternal testosterone levels rise during the course of pregnancy, and the mean concentrations decrease after delivery. Relatively elevated testosterone levels during pregnancy are associated with growth restrictions in utero as well as lower birth weight and length (Carlsen et al., 2006). High levels of prenatal testosterone exposure also impact infant cognitive, motor and language development (Cho & Holditch-Davis, 2014). In summary, hormone changes appear to be critical for preparing women’s bodies for the maintenance of the pregnancy (e.g. progesterone, estrogen), the development of fetal organs (e.g. testosterone, cortisol) and labor and the production of breast milk (e.g. oxytocin) (Nissen et al., 1998; Thompson & Trevathan, 2008; Douglas, 2010; Galbally et al., 2011; Magon & Kumar, 2013). Hormonal changes are also likely to influence maternal brain function (Carmona et al., 2019). Different patterns of hormone fluctuations across pregnancy may correspond with distinct trajectories of structural and functional brain changes.
Extensive research in non-human animals, specifically rodents, suggests that hormonal changes shape the maternal brain (Brunton & Russell, 2008). Consistent with this, outside of the context of pregnancy, fluctuations in hormones have been linked to structural and functional changes in the human brain. For instance, changes in sex steroid availability during puberty seem to trigger alterations in gray and white matter volume (Peper et al., 2011). During menopause, estrogen levels decline rapidly, and this reduction is associated with a reduction in gray matter volume (Kim et al., 2018a). Typical fluctuations in estrogen across the human menstrual cycle have also been found to modulate brain activation in some regions (e.g. dorsal lateral prefrontal cortex [PFC]) (Amin et al., 2006). Testosterone and estrogen therapies have been associated with alterations in brain cortical thickness (Zubiaurre-Elorza et al., 2014). Further, administration of hormones (e.g. testosterone, oxytocin administration in randomized control trials) has also been associated with changes in brain function, including activation of the amygdala, inferior frontal gyrus and insula to the sound of crying infants (Bos et al., 2010; Riem et al., 2011). Finally, endogenous hormones (e.g. testosterone, progesterone, estradiol) have been shown to modulate neural circuitry important for emotion regulation (e.g. amygdala, medial PFC [mPFC], orbitofrontal cortex [OFC]) (Van Wingen et al., 2011). A recent longitudinal study compared changes in cortical thickness across pubertal development in female adolescents and changes in cortical thickness before and after pregnancy in first-time mothers (Carmona et al., 2019). Adolescence and pregnancy are both periods associated with dramatic hormone changes, such as increased estrogen secretion (Blakemore et al., 2010; Magon & Kumar, 2013), and the adolescent and pregnant samples had similar neuroanatomical changes (e.g. reductions in volume and cortical thickness) (Carmona et al., 2019). The similar structural changes in both samples provide support for the hypothesis that hormonal changes drive changes in brain structure.
In addition, the maternal immune system undergoes changes during pregnancy as it works to balance protecting the mother and the fetus, while also tolerating the fetus, a foreign body (Munoz-Suano et al., 2011). In particular, levels of pro-inflammatory cytokines—mediators of immune responses that promote inflammation—increase during pregnancy (Bränn et al., 2017). Although inflammatory responses are adaptive for preventing infection, elevated inflammation levels are associated with a range of physical and psychological health conditions (Kendall-Tackett, 2007; Dooley et al., 2018). Inflammation is also associated with alteration in brain structure and function (Jefferson et al., 2007; Miller et al., 2013; Marsland et al., 2015). For example, one study found that an experimental inflammatory challenge, compared to a placebo, led to selectively increased amygdala activity to socially threatening images in a sample of non-pregnant adults (Inagaki et al., 2012).
Perhaps in part related to hormonal and inflammatory changes in pregnancy, as well as physical changes in the body that may influence typical sleep positions, 78% of women report having more disturbed sleep during pregnancy than before pregnancy (Hashmi et al., 2016). Women experience disruption to their sleep, inadequate sleep and high rates of symptoms of sleep disorder throughout pregnancy (Mindell et al., 2015). Following birth, it may be months until the infant sleeps through the night (Lillis et al., 2018). Mothers, compared to fathers, tend to intervene more often when infants wake and report more sleep disturbances (Gay et al., 2004; Saxbe et al., 2018). Indeed, it has been estimated that mothers lose an average of 80 h of sleep a year when caring for young children due to frequent infant waking and nighttime infant care (e.g. need for frequent feeding and diaper changes) (Acebo et al., 2005). Several studies have found that reduced sleep duration and sleep quality have an impact on gray matter volume and functional connectivity at rest (Liu et al., 2014; Khalsa et al., 2017; Krause et al., 2017). Even just one night of sleep deprivation has been linked to changes in brain function, including a 60% increase in amygdala response to emotionally negative pictures (Goldstein & Walker, 2014). As such, structural and functional changes in brain across the peripartum period may be driven in part by fragmented or insufficient sleep (Lillis et al., 2018).
In parallel with physiological changes women experience during this period, the peripartum period is also accompanied with a host of new challenges and potential stressors. Many women experience pregnancy-specific stressors during this period, such as fears and anxiety about childbirth (Alehagen et al., 2006; Hall et al., 2009). Women also experience new stressors after delivery (e.g. sleep loss) (Gay et al., 2004). Caring for an infant has significant emotional (e.g. providing affection to the infant), cognitive (e.g. attentional demands of caregiving) and financial demands (e.g. additional child care costs and/or unpaid maternity leave, hospital bills) (Marshall & Tracy, 2009; Saxbe et al., 2018). The financial strain associated with caring for a child may be particularly difficult for women that are already disadvantaged socioeconomically; compared to middle-income mothers, low-income mothers demonstrate altered neural responses to emotional infant cues (e.g. negative infant faces, positive infant faces, infant cry sounds) (Kim et al., 2016, 2017). The demands of caring for a new child also contribute to occupational stress, as well as strain on romantic relationships (Rosand et al., 2011; Saxbe et al., 2018). In addition, many women report having decreases in self-esteem after the birth of their first child, in part a result of the physical changes that accompany pregnancy (e.g. fat stores, stretch marks, breast changes) (Gjerdingen et al., 2009; van et al., 2018). Outside of the context of pregnancy, experimental evidence finds that even time-limited psychosocial stress is linked with changes in brain functioning, including amygdala–hippocampal connectivity (Liston et al., 2009; Fan et al., 2015). The psychosocial stressors women experience during pregnancy and postpartum may also moderate the degree of cognitive changes (e.g. deficits in memory) and brain-related alterations women experience (Brown & Schaffir, 2018).
A final possibility is that the experiences of caregiving alter the brain. Consistent with this finding, one cross-sectional neuroimaging study assessed how the duration of caregiving in new mothers (infants between 1 and 14 months) impacted neural activity (Parsons et al., 2017). Results indicated that mothers with older infants, and more time caregiving, had greater neural activation in the OFC and the amygdala to infant-specific cues. An event-related potential (ERP) study in women 3 months postpartum found that maternal experience, or how many children a mother had, modulated late neural responses (e.g. P300) to infant stimuli (Maupin et al., 2015). Specifically, first-time mothers had greater neural responses to infant cues (e.g. infant faces, cry stimuli) than women with more than one child. These studies may reflect the gradual changes in the brain as it adjusts to the role of motherhood. Further evidence that neurobiological changes result from experiences of caregiving originates from a study of three groups of first-time parents: heterosexual primary-caregiving mothers that gave birth, heterosexual secondary-caregiving fathers and primary-caregiving homosexual fathers (Abraham et al., 2014). The study found that primary caregiving fathers exhibited elevated amygdala activation similar to primary caregiving mothers. Among all fathers, time spent caregiving (average weekly hours alone with the infant) was positively associated with the degree of functional connectivity between the amygdala and the superior temporal sulcus while watching a video of themselves interacting with their infant. Another study found that foster mothers demonstrated an association between oxytocin, brain activity and caregiving behavior that paralleled patterns in biologically related mothers (Bick et al., 2013). A recent neuroimaging study in older adults found a positive association between cortical thickness and number of offspring, regardless of parent sex/gender (Orchard et al., 2019). Taken together, these findings suggest that structural and functional changes in the brain are partly a function of experience-dependent processes from caregiving, rather than exclusively physiological consequences of childbirth and postpartum recovery.
In summary, there is evidence that hormonal changes, inflammation, lack of sleep and stress all have effects on brain structure and function outside of pregnancy. As such, it is likely that the combination of these physiological and psychosocial factors—along with the experience of caregiving itself—contribute to changes in the brain across the peripartum period. The next section will detail research on brain structure and function in women across pregnancy and the postpartum period in order to evaluate the evidence for specific brain changes, as well as the timing of such changes.
Brain changes in the peripartum period
Emerging neuroimaging research has provided insight into changes in the maternal brain during gestation and in the postpartum period. In this section, we first review methods used to assess structural and functional brain changes in humans across the peripartum period, as well as strengths and weaknesses of each method. Second, we discuss research on structural changes in the maternal brain across the peripartum period. Finally, we review the literature on functional alterations in networks underlying reward and motivation, salience and fear, executive function, and social cognition and attachment.
Methods used to assess brain structure and function in pregnancy and postpartum
In pregnancy and the postpartum period, brain structure and function are studied using non-invasive imaging methods, including magnetic resonance imaging (MRI), functional MRI (fMRI), diffusion weighted imaging (DWI), electroencephalogram (EEG), ERP and functional near-infrared spectroscopy (fNIRS). MRI generates high-resolution images of the brain, while DWI detects white matter tracts in the brain (Brammer, 2009; Silver et al., 2018). fMRI measures blood flow in the brain to detect areas of activity. Imaging in pregnancy is considered acceptable for diagnostic purposes when there are clear benefits for the woman (Obstetrics Gynecology, 2017). The concerns about imaging during pregnancy include the potential effects of tissue heating and the acoustic noise level on the fetus (Ray et al., 2016). The interaction between the radiofrequency pulses of the MRI and the magnetic variations in tissues generates energy or heat that can be passed onto individuals in the scanner (Victoria et al., 2014). In order to reduce this likelihood, some researchers have selected scanning parameters that are low in heat (e.g. decreasing the number of slices, using parallel imaging techniques) (van den & Thomason, 2016). Studies have not found evidence of risk of hearing loss among neonates whom as fetuses were exposed to MRI (Reeves et al., 2010; Jaimes et al., 2018). Additional precautions (e.g. adding padding for sound insulation) can also be taken to lower the noise during the scan (van den & Thomason, 2016). In the postpartum period, several research groups have used fMRI to assess functional differences between new mothers and nulliparous women. Perhaps due to concern about the potential risks of using fMRI and MRI during pregnancy, there is only one study that has followed mothers longitudinally from pregnancy to the early postpartum (Oatridge et al., 2002). However, a second longitudinal study assessed women before and after, but not during, pregnancy (Hoekzema et al., 2017).
Other psychophysiological and imaging methods (e.g. EEG, ERP, fNIRS) can be implemented as alternative procedures. These tools examine neural activity related to sensory, cognitive and affective processes that may be particularly relevant for longitudinal research across pregnancy (Hajcak et al., 2010; Luck et al., 2000). Compared to neuroimaging approaches like MRI/fMRI, ERP has more limited spatial resolution, but has excellent temporal resolution and has been used in studies across pregnancy (Itthipuripat & Sprague, 2019). There are few studies (one fNIRS and a handful of EEG/ERP studies) that have utilized these methods to assess functional changes across pregnancy through the postpartum period. A review of grant applications funded and searchable through NIH RePORTER indicates that a number of new studies on this important period are forthcoming.
In the next sections, we present a review of the literature using neuroimaging and psychophysiological methods to examine brain structure and function in the peripartum period. We focus on studies examining neural measures in pregnancy and the postpartum. With longitudinal or cross-sectional designs. We also reference behavioral studies that do not assess brain activity to help ground our interpretation of neural findings. To further complement these findings, we briefly review studies comparing mothers and comparison women in the postpartum period, and studies of related processes in the non-human animal literature. It is critical to note that there have been significant advances in imaging techniques since its advent (e.g. changes in magnetic field strength). Further, many early imaging studies had small sample sizes (e.g. Lorberbaum et al., 2002: n = 10; Ranote et al., 2004: n = 10). We acknowledge the limitations of many of these early studies and direct the reader to Table S1 (supplementary file), which outlines details of the key studies discussed.
Structural brain changes
Extensive research on non-human animals has provided a foundation for our understanding of the changes in brain structure across pregnancy, from which we can infer changes that are likely in humans (Brunton & Russell, 2008; Kim et al., 2016). This work is complemented by a relatively small literature examining peripartum structural alterations in humans. In this section, we will first discuss global brain alterations in gray and white matter volume across pregnancy. Next, we will review changes in anatomical structures that are most commonly studied in this period (i.e. pituitary gland, hippocampus, amygdala).
Both rodent and human research has found evidence of reductions in brain volume (both overall and in specific regions) and increases in ventricular size across the peripartum period. A study in a sample of lactating and nulliparous rodents found that lactating animals had both decreased absolute and relative brain weight compared with nulliparous animals (Hillerer et al., 2014). The first human study on this topic assessed ventricular and brain volume changes in a small sample of healthy pregnant women and patients with preeclampsia across late pregnancy and the early postpartum period (Oatridge et al., 2002). Both women with and without preeclampsia had a reduction in brain size during pregnancy, with the greatest observed reduction in brain size at term (37th–42nd week of pregnancy). Ventricular size also increases during pregnancy. Interestingly, at an assessment conducted at 24 weeks post-delivery, the participants’ overall brain volume had returned to pre-pregnancy size; ventricular size also decreased in size after delivery. This postpartum readjustment in brain volume suggests that most pregnancy-related anatomical alterations may be temporary.
The second, and more recent, longitudinal MRI study assessed gray matter alterations before and after pregnancy in a larger sample of first-time mothers compared to a sample of nulliparous women (Hoekzema et al., 2017). Compared to the nulliparous women, pregnant women showed greater reductions in gray matter from pre-conception to post-birth sessions, specifically in the anterior and posterior cortical midline and sections of the bilateral prefrontal and temporal cortex. Hoekzema et al. (2017) argue that this change in gray matter, if reflecting neurobiological pruning, may actually be beneficial. In fact, changes in gray matter volume across pregnancy were associated with mothers’ self-reported connection to their infant in the postpartum period. However, replication is necessary to bolster these findings. Further, in contrast to Oatridge et al.’s (2002) findings on overall brain volume, which used a 1.0 T scanner, this more recent study using a 3 T scanner found that the pregnancy-related alterations were maintained from the post-pregnancy session (~10 weeks after delivery) to another follow-up session 2 years post-pregnancy, with the exception of partial volume recovery in the left hippocampal cluster. There is also emerging evidence of enduring structural changes associated with the number of children birthed (e.g. positive associations between parity and brain structures) in a sample well beyond the peripartum period (de Lange et al., 2019). Together, these studies suggest that pregnancy and child rearing are associated with changes in some human brain structure. Some of these changes may be enduring, while others may return to pre-pregnancy size.
The pituitary gland also undergoes structural alterations during pregnancy. The pituitary gland has a critical role in hormone production during the peripartum period. The anterior pituitary undergoes 2-to-3-fold enlargement during pregnancy (Laway & Mir, 2013), which is attributed to an increase in hyperplasia of prolactin-secreting (PRL) cell size (Asa et al., 1982). The PRL cells return to pre-pregnancy size ~8 months after delivery in humans or 7 days after delivery in rodents (Haggi et al., 1986; Miki et al., 2007). This profound anatomical change facilitates the hormone production necessary for the body to support pregnancy (e.g. adequate nutritional support for the developing fetus, preparation for labor and lactation) (Laway & Mir, 2013; Reshef et al., 2015).
There is also evidence of structural changes in subcortical regions of the brain, including the hippocampus and amygdala, across pregnancy. There is evidence of volume decreases in the hippocampus—a region involved in memory consolidation among other processes (Vann & Albasser, 2011)—from pre-conception to after delivery (Hoekzema et al., 2017). A portion of the hippocampus (left hippocampal cluster) returned to pre-pregnancy baseline volume by 2 years postpartum (Hoekzema et al., 2017). Similarly, the hippocampus has been characterized by a reduction in both cell proliferation and volume during pregnancy and the peripartum period in rodents (Galea et al., 2000; Rolls et al., 2008). Research in rodents has indicated alterations in the neurogenesis of hippocampal neurons in both mothers and fathers during the postpartum period (Glasper et al., 2011). This finding suggests that hippocampal plasticity may not be solely a result of hormonal fluctuations due to pregnancy, but instead reflects experience-dependent alterations that are a consequence of the demands of parenthood.
Similar to the hippocampus, the amygdala, which plays a key role in emotion and salience detection, is also thought to undergo structural alterations in the peripartum period (Rasia-Filho et al., 2004). Specifically, rodents have a decrease in dendritic spine density in the medial nucleus of the amygdala following pregnancy (Rasia-Filho et al., 2004). Although there is evidence from non-human animal research of changes in amygdala structure (e.g. dendritic spine density), this finding has yet to be demonstrated in humans.
Functional brain changes
Reward and motivation networks
Both non-human animal and human research has linked dopamine and oxytocin pathways with the development of caregiving behaviors. Findings from human research suggest that the dopaminergic system plays a crucial role in driving behavior related to pleasurable stimuli and rewards, including food, drugs, sex or the face of a loved one (Sell et al., 1999; Bartels & Zeki, 2004; Alonso-Alonso et al., 2015). Oxytocin and dopamine interact in brain regions associated with reward and motivation (e.g. ventral tegmental area, ventral striatum) and have been found to be critical in the establishment and maintenance of social bonds (Shahrokh et al., 2010). In mothers, these structures associated with reward are sensitive to infant cues and may play a critical role in motivating caregiving behaviors. Functional imaging studies (Lorberbaum et al., 2002; Strathearn et al., 2009) in humans support changes in reward circuitry sensitivity to infant cues during the transition to motherhood. However, to our knowledge, all of these studies were conducted during the postpartum period and it is unclear whether these are pre-existing differences, changes that emerge across gestation or alterations driven by the experience of caregiving.
In the postpartum period, new mothers have been found to display more activity in regions of the striatum, mPFC, midbrain and thalamus when listening to the sounds of an infant crying relative to both a white noise sound and a rest condition (Lorberbaum et al., 2002). Similar patterns of activation in the striatum and thalamus have been observed when mothers view images of their own infant versus an unknown child (Strathearn et al., 2008). Further, activation was greatest for happy relative to neutral or sad infant face stimuli. Similar patterns of increased activation of reward-related brain regions to mothers’ own vs. unknown child cues have been observed in other studies (Leibenluft et al., 2004; Ranote et al., 2004; Noriuchi et al., 2008). A recent study utilizing resting-state functional connectivity found that connectivity between the left amygdala and the left nucleus accumbens was positively associated with positive maternal behavior (i.e. the ability of the mother to scaffold appropriate interactions with her infant) (Dufford et al., 2019). Mothers that were further along in the postpartum period also exhibited greater resting-state functional connectivity between the right amygdala and the bilateral caudate and right putamen. This finding suggests that connectivity in structures associated with reward and motivation (e.g. striatum) may contribute to positive caregiving behaviors and that experiences of caregiving may shape this function.
Non-human animal studies also demonstrate increased activation in areas of the reward system when mothers are presented with stimuli associated with their offspring. For instance, rodent mothers have been found to have more activation in the dopamine reward system to suckling stimulation than cocaine (Ferris, 2005). Further, findings suggest that lactating dams prefer spending time with their pups than receiving food (Lee et al., 1998). Along with facilitating bond formation and caregiving, the reward system has also been implicated in protective maternal behavior. Several regions, including the ventral striatum and periaqueductal gray, are activated during maternal aggression when a rodent mother is protecting her pups from an intruder (Nephew et al., 2009). Taken together, existing research suggests that, on average, new mothers exhibit increased activation of reward-related brain regions particularly to their own infant cues, although it remains unclear when in the peripartum period this emerges.
Salience and fear networks
In addition to positive valence systems, the peripartum period appears to be a time of alterations in salience and fear networks that work together to process threats in the environment. Neural structures in the salience network, including the dorsal anterior cingulate cortex (dACC), orbital fronto-insular cortex and anterior insula, integrate sensory information relayed from subcortical and brainstem structures and overlap with brain structures that are involved in processing threat and emotional cues (e.g. amygdala, insula, bed nucleus of the stria terminalis) (Naaz et al., 2018).
There is evidence that women exhibit heightened neural reactivity to threat and distress stimuli across gestation. Some of these changes appear to emerge during pregnancy, whereas others seem linked to the experience of parenting. ERP and fNIRS studies of women during pregnancy have found support for heightened reactivity to threat in pregnancy. That is, pregnant women in their third trimester exhibited greater neural reactivity to emotional stimuli compared to non-pregnant women (Raz, 2014). Another study using fNIRS assessed neural activation and attention bias to fear-relevant stimuli (e.g. fearful faces) across all three trimesters (Roos et al., 2011). Specifically, women exhibited greater activation of the PFC and attention bias towards threatening images in their second trimester compared to the first or third trimester. PFC activity during response to threat was also associated with some neuroendocrine changes, such as changes in cortisol and testosterone. This finding suggests that hormonal changes in pregnancy may be contributing to this increased responsiveness. Behavioral studies have also indicated that women have an enhanced ability to encode emotional faces of threat, such as fearful and angry faces, from early to late pregnancy (Pearson et al., 2009). This alteration in emotional processing could be linked with an increase in estrogen across pregnancy (Magon & Kumar, 2013). For instance, estrogen receptors within the amygdala have been linked with alterations in fear expression in both human and non-human animals (Jasnow et al., 2006).
A larger literature has examined salience and fear networks in the postpartum period, a time in which increased activation of these networks may facilitate protective and sensitive caregiving. Studies in postpartum women have consistently found that infant distress stimuli, including videos, photos and sounds, activate networks including the amygdala (Seifritz et al., 2003; Atzil et al., 2012; Barrett et al., 2012; Rocchetti et al., 2014). These patterns may not be specific to negatively valenced stimuli, as there is also evidence that happy infant stimuli and images of one’s child compared to a familiar child increases amygdala activation in mothers (Leibenluft et al., 2004; Barrett et al., 2012). Interestingly, a recent cross-sectional study with new mothers (1–14 months postpartum) found that a longer duration of motherhood was associated with greater activation in the amygdala and OFC in response to infant vs. adult cues (Parsons et al., 2017). This result suggests that maternal experience may continue to shape these networks beyond the changes observed in pregnancy.
Executive network
In addition to emotionally relevant circuits, there is evidence of changes in neural systems involved in cognitive functioning. Consistent with this evidence, women in pregnancy and postpartum exhibit deficits in working memory performance and information processing compared to non-pregnant women (Brett & Baxendale, 2001; De et al., 2006). One fMRI study sought to assess response inhibition in postpartum women using a Go/No-Go task, in which participants have to inhibit responses to no-go stimuli (Bannbers et al., 2013). Women in the postpartum period exhibited reduced activity in the ventrolateral PFC and dACC during response inhibition relative to non-postpartum women. However, no differences between groups were found in their behavioral performance (i.e. correct Go/NoGo trials). This suggests that differences in brain function are not solely driven by differences in performance, ability or effort between the groups but rather a distinction in activation of neural circuitry (i.e. decreased activity in prefrontal areas in postpartum women). Further, results from a recent fMRI study revealed that women in the postpartum period (had a child in the preceding 3 months) had decreased neural activity at rest in the posterior cingulate cortex and prefrontal cortex compared to age and education matched nulliparous women (Zheng et al., 2018). These activation patterns were correlated with specific impairments in cognitive functioning (e.g. memory, attention) in the peripartum period. These findings suggest that mothers in the peripartum period may experience alterations in neural systems supporting cognitive processes, which drive impairments observed at the behavioral and self-report level (Henry & Rendell, 2007). There is evidence of selected impairments in executive functioning (e.g. memory impairments, slower processing speed) during pregnancy and early motherhood, which may indicate that these neurobiological alterations emerge earlier than the postpartum period (Buckwalter et al., 2001; De et al., 2006; Henry & Sherwin, 2012). For example, one behavioral study found evidence of impairments in memory encoding and retrieval in pregnancy and the postpartum period (De et al., 2006). There are also strong associations between fluctuations in hormone levels during late pregnancy and the early postpartum period and cognitive abilities, including verbal recall and processing speed (Henry & Sherwin, 2012). Additional imaging studies are necessary to assess if changes in neural systems involved in cognitive functioning emerge during pregnancy.
Social cognition and attachment
Neural systems that support the ability to understand and empathize with the mental states of others are fundamental in caregiving (Oppenheim et al., 2001; Hein & Singer, 2008). Regions such as the dorsal mPFC, lateral mPFC, precuneus/posterior cingulate, posterior superior temporal sulcus and temporoparietal junction have been found to activate during mentalizing tasks (Frith & Frith, 2006; Mitchell, 2009).
To our knowledge, there are no imaging studies that have assessed changes in social cognition across pregnancy. However, there is some evidence of change in behavioral measures. For example, women exhibit improvements in ability to encode emotional faces (e.g. angry faces, sad faces, fearful faces) during late pregnancy (Pearson et al., 2009). There is also evidence that women display enhanced facial recognition as pregnancy progresses (Anderson & Rutherford, 2011). This finding has been interpreted as a socially adaptive mechanism to allow a mother to more quickly identify their child’s facial expression and discern their needs (Anderson & Rutherford, 2012).
In the postpartum period, fMRI studies have found that mothers have greater activation in areas associated with theory of mind (e.g. paracingulate cortex, posterior cingulate, insula) when viewing images of their own child compared to a familiar child (Leibenluft et al., 2004). Neural regions associated with social cognition were also activated in a novel fMRI study in which mothers both observed and imitated the facial expressions of their child and unknown child (Lenzi et al., 2008). In particular, the mirror neuron system (ventral premotor cortex, inferior frontal gyrus and posterior parietal cortex) was more activated in response to one’s own child compared to unknown children. Given the behavioral changes (e.g. enhanced facial recognition, improvements in emotional face encoding) that women experience as pregnancy progresses (Pearson et al., 2009; Anderson & Rutherford, 2011), these neural alterations appear to emerge before the postpartum period.
Summary
Although there is compelling and growing evidence that women undergo complex structural and functional changes in the brain across the peripartum period, there are few longitudinal studies in humans mapping the sequence of these changes across gestation. Studies assessing structural alterations suggest that there are reductions in overall gray matter volume across gestation. One study assessed structural changes from pregnancy to early postpartum period with two to three MRI scans over that period (Oatridge et al., 2002) and the other compared gray matter volume in women before conception and again after delivery (Hoekzema et al., 2017). However, there is no longitudinal research that has charted the trajectory of this reduction across gestation. There is also evidence supporting anatomical alterations in specific structures (i.e. amygdala, hippocampus, pituitary gland). Additional research is necessary to map the specific timing of these neuroanatomical adjustments and the potential factors driving these alterations across the peripartum period.
Further, findings support the possibility of functional changes in the brain in regions related to reward and motivation, salience and fear, executive functioning and social cognition and attachment. There is relatively consistent evidence from research on the postpartum period, where new mothers exhibit increased activation in reward-related brain regions when presented with stimuli associated with their infant (Ranote et al., 2004). Findings from both an ERP study and a fNIRS study suggest that women exhibit heightened reactivity to threat during pregnancy (Roos et al., 2011; Raz, 2014), and these patterns of heightened activation of salience and fear networks appear to persist into the postpartum period (Seifritz et al., 2003). Although limited, research in the postpartum period suggests that systems that support cognitive processes may be impaired, and these changes in brain function may contribute to memory impairments and slower processing speed during pregnancy and early motherhood (Buckwalter et al., 2001; De et al., 2006). Longitudinal research suggests that across pregnancy, women exhibit enhanced emotional coding that may prepare women for caring for their offspring (Pearson et al., 2009). In the postpartum period, fMRI studies have found that women have greater activation in regions associated with theory of mind and empathy when viewing images of their own child compared to a familiar child (Leibenluft et al., 2004).
Overall, there is a paucity of studies capturing brain alterations across the peripartum period. There is also limited research assessing how these trajectories of change can contribute to a woman’s health and behavior in the peripartum period. In the next section, we will propose potential models of change in brain function across the peripartum period that may promote or inhibit healthy adjustment to parenting.
Potential trajectories of changes across the peripartum period and health implications
Taken together, there is evidence that the human brain undergoes a range of structural and functional alterations during pregnancy and into the postpartum period—from widespread decreases in gray matter volume (Oatridge et al., 2002) to changes in reactivity of motivation and salience networks (Raz, 2014). A range of variables, including hormonal fluctuations and changes in the social environment and psychosocial stress, likely drive these neural changes (Yim et al., 2015; Saxbe et al., 2018). Some researchers posit that at least some alterations in brain function and structure could be adaptive for new mothers and play a preparative role for the challenges of caregiving and behavioral synchrony with the infant (Abraham & Feldman, 2018; Barba-Müller et al., 2018). We argue that neurobiological alterations in the peripartum period, while set into motion by processes necessary for fetal development (e.g. hormone alterations maintain pregnancy and trigger fetal maturation) (Kaludjerovic & Ward, 2012), may be an evolutionary trade-off, discussed further below. These changes (e.g. fluctuations in estrogen and progesterone) may confer risk to the mother by increasing the likelihood for the development of psychopathology, such as depression and anxiety (Schiller et al., 2015; Barba-Müller et al., 2018).
Although many new mothers demonstrate healthy adjustments to parenting and recovery from the physical and psychological effects of pregnancy, the peripartum period is also a high-risk time for depression and anxiety in mothers, as well as an important time for mother–infant bonding. Pre-existing risk factors including prior depressive history and exposure to life stress are established predictors of postpartum psychopathology (Beck, 2001; O’Hara, 2011). Similarly, women’s own childhood experiences, poverty and chronic stress before the peripartum period are associated with increased levels of harsh or insensitive caregiving (Belsky et al., 2009).
We argue that in addition to these established risk factors, considering trajectories of neurobiological changes may be critical for predicting psychopathology risk (e.g. particularly if the neurobiological changes match those that outside of the context of pregnancy are associated with increased psychopathology risk, such as dysfunctions in reward processing) (Luking et al., 2016). That is, integrating pre-existing psychosocial risk factors with measurement of neurobiological changes during pregnancy could improve the ability to detect women and families at greatest risk and in need of early intervention.
In combination with established risk factors, specific trajectories of brain changes across pregnancy may increase a woman’s likelihood of developing psychopathology or engaging in insensitive infant care. At the same time, there is also potential that certain trajectories of neurobiological changes buffer against negative outcomes and aid in adaptation for women during this transition. Here, we propose three illustrative trajectories of neurobiological change during pregnancy (i.e. hypo-response, typical response, hyper-response; see Figure 1). Next, we highlight possible health implications and future research for identifying the consequences of each trajectory pattern for mothers and offspring. Importantly, we recognize that trajectories leading to physical and psychological health of mothers and offspring may differ across systems and levels of analysis, and as such, future longitudinal research integrating multiple measures of brain function and structure is critically needed.
Figure 1. Distinct trajectories of mothers’ neurobiological change during pregnancy (hypo-response, typical response, hyper-response).
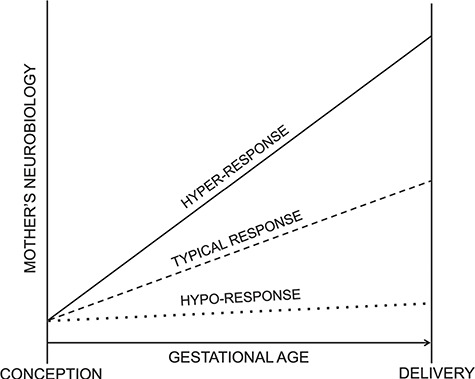
In considering the implications of typical and atypical neurobiological changes across the peripartum period, we argue that it is essential to consider the potential mechanisms of the changes and the trade-offs in terms of health implications for both mothers and infants. For example, pregnant women have limited immune function to support themselves and the fetus, and the body may redirect more resources to support fetal versus maternal immunological maintenance (Abrams & Miller, 2011). In this way, changes occurring during pregnancy may come at a cost for women. The shifts in hormones likely driving neurobiological and cognitive changes in women across the peripartum period may be critical for offspring development (Brunton & Russell, 2008), but may result in suboptimal functioning in women. The findings for overall reductions in brain volume across the peripartum period may have detrimental consequences for a woman’s cognitive functioning considering evidence that gray matter volume reductions have been associated with declines in cognitive performance in studies of adult aging (Ramanoël et al., 2018). Typical peripartum changes may, therefore, confer increased risk for mental health problems. For instance, almost immediately after delivery of the placenta, progesterone levels decrease to pre-pregnancy levels and estrogen levels remain elevated (Schiller et al., 2015). An important line of work, with obvious intervention implications already recognized in the form of treatments for postpartum depression prevention (Meltzer-Brody et al., 2018), indicates that changes in reproductive hormone concentrations post-delivery may drive symptoms of depression in some women (Moses-Kolko et al., 2014; O’Hara & Wisner, 2014). In addition, some brain changes, such as gray matter volume reductions (Oatridge et al., 2002; Ramanoël et al., 2018) and changes in resting-state functional connectivity (Zheng et al., 2018), may result in cognitive changes in women including memory disturbances, trouble concentrating and absentmindedness (colloquially called ‘pregnancy brain’) during pregnancy and the postpartum period (Henry & Rendell, 2007).
Consistent with this possibility, studies assessing cognitive functioning during the peripartum period indicate that pregnant women are impaired on some measures of memory, specifically those that place a high demand on executive cognitive control (Henry & Rendell, 2007). Finally, heightened reactivity to threatening stimuli appears to emerge at some point in pregnancy or postpartum (Roos et al., 2011; Raz, 2014). This alteration may promote protective behavior towards infants but, at the same time, could increase risk for internalizing symptoms (Pearson et al., 2009).
There are likely individual differences in the trajectory of neurobiological changes across this period, with some women exhibiting hypo-responsiveness, characterized by reduced change or a slower pace of change, and others exhibiting hyper-responsiveness, characterized by more dramatic or faster pace of change. If typical peripartum neurobiological change confers some moderate risk for mothers, hypo-responsiveness in these domains, such as attenuated gray matter volume decreases and blunted threat responding, may be relatively promotive of mothers’ mental health during this period (Young et al., 2017; Barba-Müller et al., 2018; Bjertrup et al., 2019), but potentially problematic for the developing fetus (i.e. if a lack of change is due to insufficient biological support for the fetus). On the other hand, hyper-responsiveness in these domains may confer increased risk for mothers (i.e. due to greater neurobiological changes from a preconception baseline), but potentially lead to greater resources directed towards the developing fetus. That is, greater hormone-driven remodeling in the maternal brain may be associated with better outcomes for the fetus at the expense of the mother’s cognitive and affective functioning. Consistent with this possibility, reduced ACC, left parahippocampal gyrus and left superior temporal gyrus volume in postpartum women have been linked to increased risk for postpartum psychosis (Fusté et al., 2017). Although cross-sectional, this suggests that, at the extreme end, greater change in brain structure across this period can increase risk for peripartum psychopathology.
Several studies have been conducted in the postpartum period exclusively, including both cross-sectional and longitudinal studies occurring shortly after birth (Kim et al., 2018b, 2010; Lorberbaum et al., 2002; Noriuchi et al., 2008; Parsons et al., 2017; Zheng et al., 2018), and find individual differences in brain structure and function related to caregiving. One fMRI study found gray matter volume increases from 2–4 weeks postpartum to 2–4 months postpartum (Kim et al., 2010), providing further evidence of a recovery of brain volume during the months following birth. From an individual difference perspective, the degree of volume change varied, with this study finding that positive perceptions of one’s infant shortly after birth predicted greater volume change in a cluster including the hypothalamus, amygdala and substantia nigra. A recent neuroimaging study in a sample of first-time mothers identified a positive association between postpartum months and cortical thickness (Kim et al., 2018). Further, cortical thickness in the prefrontal cortex was positively associated with self-reported parental self-efficacy. These findings hint at the possibility that those individuals who had greater reduction in gray matter across pregnancy, assuming those were the individuals with the most opportunity for recovery, were the mothers with the most positive perceptions of their newborn infants. Such investigations would require longitudinal imaging in pregnancy and would indicate the possibility that more dramatic brain changes could be beneficial for both the mother and the infant.
Clearly, other patterns are possible and perhaps even probable (e.g. an inverted-U shape relationship between changes in the peripartum period and healthy outcomes) (Henry & Sherwin, 2012). The peripartum period involves alterations in systems that may impact the physical and psychological health of both women and their offspring, and we know little about the individual factors and experiences that may contribute to variation in these neurobiological changes and the implications of those differences. For instance, substance use is likely to disrupt the typical trajectories of brain change across the peripartum period (Swain et al., 2019). Consistent with this, substance use in the early postpartum period has been associated with disruptions in neural response to infant cues (e.g. cry and faces) (Landi et al., 2011). However, more research is needed to better understand how substance use alters trajectories of change across the peripartum period.
Future directions
Research examining neurobiological changes during the peripartum has at least two important implications. First, from a basic science perspective, critical questions centered on brain plasticity, including the potential causes, timing and specificity of effects of hormones on brain structure and function, can be discovered through the use of pregnancy as a special case in which we have increased understanding that events of interest will occur (e.g. increases in specific hormones at set gestational ages). This type of research program will enhance understanding of the brain both within and outside of pregnancy. Second, focusing on pregnant women and their offspring as a population of interest, women have an increased vulnerability for psychopathology during the peripartum period. Detailing the trajectories of neurobiological change across pregnancy may provide insight into why women are at greater risk during this period and the specific factors associated with vulnerability. This empirical work may guide the development of interventions to mitigate poor outcomes during this period. Further, several biomarkers have been identified as early predictors of depression (e.g. blunted activation in the striatum to reward) (Kujawa & Burkhouse, 2017). There may be neural predictors of psychopathology and insensitive caregiving that emerge during pregnancy and have yet to be identified. With improvements in technology and development of norms and standardized procedures, it is possible that assessment of certain biomarkers (e.g. EEG/ERP) could be integrated into clinics serving high-risk women in the future, with the potential to aid in identifying women would benefit from early intervention and the ideal timing and targets for treatment.
There is also a need to integrate human and non-human animal research. Research in non-human animal models can provide important parallels to understand neurobiological change in humans (Brett et al., 2015). For instance, non-human animal research has provided valuable insight into the effect of neuroendocrine and experiential factors on the brain during the perinatal period (Brunton & Russell, 2008). Although this research is useful, both neurobiological distinctions and psychosocial differences in human and non-human animals limit comparisons about the influence of hormonal and neural changes on emotion and behavior (Brett et al., 2015; Spinelli et al., 2009). Complementary non-human animal research focusing on pregnancy and the postpartum period may provide a more comprehensive understanding of the mechanisms driving neurobiological changes. They also may inform early biomarkers of risk in the peripartum period. The conceptual framework gleaned from this transdisciplinary lens can be used to further our understanding of the neurobiological changes we see in human samples.
Longitudinal imaging (e.g. fMRI, MRI, fNIRS) and psychophysiology (e.g. EEG, ERP) studies of brain structure and function starting pre-conception through the postpartum period are needed to allow researchers to chart trajectories of neurobiological change. Prospective studies of neural predictors of maternal psychopathology and insensitive caregiving provision are critical to informing our understanding of baseline risk factors during this transition. Further, future research will need to compare baseline neurobiological predictors and trajectories of change as predictors of outcomes. Pre-existing factors that may moderate the course of neurobiological change and risk (e.g. chronic stress, trauma, psychopathology history, physical health conditions, maternal caregiving experience, maternal substance use) will also need to be incorporated into analyses. Distinct trajectories of change across the peripartum period (e.g. hyper-response, typical response, hypo-response) may be adaptive for some processes, but not others. Future longitudinal studies will enable greater understanding of these changes in the brain during this time, whether and to what degree neurobiological changes represent a trade-off between mothers’ mental health risk and healthy offspring development and may facilitate early identification and clinical interventions for high-risk women and dyads.
Conclusions
In this review, we have considered evidence for neurobiological changes across the peripartum period and addressed the reasons we expect drive these alterations to brain structure (both in overall and specific anatomical regions) and functional networks. Although longitudinal research across pregnancy is limited, the existing literature supports the possibility of a brain decrease in overall gray matter volume across gestation, with specific alterations in the pituitary gland, hippocampus and amygdala (Bergland et al., 1968; Hoekzema et al., 2017). Additional longitudinal research mapping the trajectories and timing of these alterations across pregnancy and the postpartum period is necessary. Further, there is compelling evidence that the brain undergoes functional changes in regions involved in reward and motivation, salience and fear, executive function, and social cognition and attachment across the peripartum period. The majority of these studies have been conducted in the postpartum period, which makes it challenging to discern if these functional alterations are a consequence of changes in pregnancy or the experience of caregiving.
Finally, we have argued that charting trajectories of brain changes in the peripartum period and considering individual differences in these trajectories may be essential for understanding psychopathology risk and the emergence of caregiving behavior across this period. The neurobiological changes that most women undergo across the peripartum period may not necessarily be adaptive for women, but rather by-products of the physiological alterations necessary to support offspring development. It may be that some deviations from this typical neurobiological response, such as a hyper-response, confer risk for the mother while providing great support for the infant’s development. In contrast, hypo-response or less neurobiological alterations may buffer women from the cognitive and affective risks associated with pregnancy at the expense of supporting fetal and infant development. Understanding these potential reproductive trade-offs and their relationship with neurobiological trajectories across pregnancy may provide a better understanding of risks for negative health outcomes for women and offspring.
Funding
This work was supported by the Jacobs Foundation Early Career Research Fellowship to K.L.H., a Brain and Behavior Foundation (NARSAD) Award to A.K. and a Klingenstein Third Generation Foundation fellowship to A.K.
Conflict of interest
None declared.
Supplementary Material
Contributor Information
Emilia F Cárdenas, Department of Psychology and Human Development, Vanderbilt University, 37203, Nashville, USA.
Autumn Kujawa, Department of Psychology and Human Development, Vanderbilt University, 37203, Nashville, USA.
Kathryn L Humphreys, Department of Psychology and Human Development, Vanderbilt University, 37203, Nashville, USA.
References
- Abraham E., Feldman R. (2018). The many faces of human faregiving: perspective on flexibility of the parental brain, hormonal systems, and parenting behaviors and their long-term implications for child development. International Handbook of Social Neuroendocrinology, 298–317. [Google Scholar]
- Abraham E., Hendler T., Shapira-Lichter I., Kanat-Maymon Y., Zagoory-Sharon O., Feldman R. (2014). Father’s brain is sensitive to childcare experiences. Proceedings of the National Academy of Sciences, 111(27), 9792–7. 10.1073/pnas.1402569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams E.T., Miller E.M. (2011). The roles of the immune system in Women’s reproduction: evolutionary constraints and life history trade-offs. American Journal of Physical Anthropology, 146(SUPPL. 53), 134–54. 10.1002/ajpa.21621. [DOI] [PubMed] [Google Scholar]
- Acebo C., Sadeh A., Seifer R., Tzischinsky O., Hafer A., Carskadon M.A. (2005). Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep, 28(12), 1568–77. 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]
- Alehagen S., Wijma B., Wijma K. (2006). Fear of childbirth before, during, and after childbirth. Acta Obstetricia et Gynecologica Scandinavica, 85(1), 56–62. 10.1080/00016340500334844. [DOI] [PubMed] [Google Scholar]
- Alonso-Alonso M., Woods S.C., Pelchat M., Grigson P.S., Stice E., Beauchamp G.K. (2015). Food reward system: current perspectives and future research needs. Nutrition Reviews, 73(5), 296–307. 10.1093/nutrit/nuv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin Z., Epperson C.N., Constable R.T., Canli T. (2006). Effects of estrogen variation on neural correlates of emotional response inhibition. NeuroImage, 32(1), 457–64. 10.1016/j.neuroimage.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Anderson M.V., Rutherford M.D. (2011). Recognition of novel faces after single exposure is enhanced during pregnancy. Evolutionary Psychology, 9(1), 47–60. 10.1177/147470491100900107. [DOI] [PubMed] [Google Scholar]
- Anderson M.V., Rutherford M.D. (2012). Cognitive reorganization during pregnancy and the postpartum period: an evolutionary perspective. Evolutionary Psychology, 10(4), 659–87. 10.1177/147470491201000402. [DOI] [PubMed] [Google Scholar]
- Anniverno R., Bramante A., Mencacci C., Durbano F. (2013). Anxiety disorders in pregnancy and the postpartum period. New Insights into Anxiety Disorders.. doi: 10.5772/52786. [DOI] [Google Scholar]
- Asa S.L., Penz G., Kovacs K., Ezrin C. (1982). Prolactin cells in the human pituitary. A quantitative immunocytochemical analysis. Archives of Pathology and Laboratory Medicine, 106(7), 360–3. [PubMed] [Google Scholar]
- Atzil S., Hendler T., Zagoory-Sharon O., Winetraub Y., Feldman R. (2012). Synchrony and specificity in the maternal and the paternal brain: relations to oxytocin and vasopressin. Journal of the American Academy of Child and Adolescent Psychiatry, 51(8), 798–811. 10.1016/j.jaac.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Bannbers E., Gingnell M., Engman J., Morell A., Sylvén S., et al. (2013). Prefrontal activity during response inhibition decreases over time in the postpartum period. Behavioural Brain Research, 241(1), 132–8. 10.1016/j.bbr.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Barba-Müller E., Craddock S., Carmona S., Hoekzema E. (2018). Brain plasticity in pregnancy and the postpartum period: links to maternal caregiving and mental health. Archives of Women’s Mental Health, 1–11. 10.1007/s00737-018-0889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J., Wonch K.E., Gonzalez A., Ali N., Steiner M., Fleming A.S. (2012). Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Social Neuroscience, 7(3), 252–68. 10.1080/17470919.2011.609907. [DOI] [PubMed] [Google Scholar]
- Bartels A., Zeki S. (2004). The neural correlates of maternal and romantic love. NeuroImage, 21(3), 1155–66. 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Beck C.T. (2001). Predictors of postpartum depression: an update. Nursing Research, 50(5), 275–85. 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Belsky J., Conger R., Capaldi D.M. (2009). The intergenerational transmission of parenting: introduction to the special section. Developmental Psychology, 45(5), 1201–4. 10.1037/a0016245. [DOI] [PubMed] [Google Scholar]
- Bergland R.M., Ray B.S., Torack R.M. (1968). Anatomical variations in the pituitary gland and adjacent structures in 225 human autopsy cases. Journal of Neurosurgery, 28(2), 93–9. 10.3171/jns.1968.28.2.0093. [DOI] [PubMed] [Google Scholar]
- Bick J., Dozier M., Bernard K., Grasso D., Simons R. (2013). Foster mother-infant bonding: associations between Foster mothers’ oxytocin production, electrophysiological brain activity, feelings of commitment, and caregiving quality. Child Development, 84(3), 826–40. 10.1111/cdev.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjertrup A.J., Friis N.K., Miskowiak K.W. (2019). The maternal brain: neural responses to infants in mothers with and without mood disorder. Neuroscience and Biobehavioral Reviews, 107, 196–307. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Burnett S., Dahl R.E. (2010). The role of puberty in the developing adolescent brain. Human Brain Mapping, 31(6), 926–33. 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos P.A., Hermans E.J., Montoya E.R., Ramsey N.F., van Honk J. (2010). Testosterone administration modulates neural responses to crying infants in young females. Psychoneuroendocrinology, 35(1), 114–21. 10.1016/j.psyneuen.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Brammer M. (2009). The role of neuroimaging in diagnosis and personalized medicine--current position and likely future directions. Dialogues in Clinical Neuroscience, 11(4), 389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bränn E., Papadopoulos F., Fransson E., White R., Edvinsson Å., et al. (2017). Inflammatory markers in late pregnancy in association with postpartum depression—a nested case-control study. Psychoneuroendocrinology, 79, 146–59. 10.1016/j.psyneuen.2017.02.029. [DOI] [PubMed] [Google Scholar]
- Brett M., Baxendale S. (2001). Motherhood and memory: a review. Psychoneuroendocrinology, 26(4), 339–62. 10.1016/S0306-4530(01)00003-8. [DOI] [PubMed] [Google Scholar]
- Brett Z.H., Humphreys K.L., Fleming A.S., Kraemer G.W., Drury S.S. (2015). Using cross-species comparisons and a neurobiological framework to understand early social deprivation effects on behavioral development. Development and Psychopathology, 27(2), 347–67. 10.1017/S0954579415000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E., Schaffir J. (2018). CME review article. Pediatric Emergency Care, 34(1), 59–60. 10.1097/01.pec.0000530052.69853.4a. [DOI] [Google Scholar]
- Brunton P.J., Russell J.A. (2008). The expectant brain: adapting for motherhood. Nature Reviews Neuroscience, 25, 11–25. 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- Buckwalter J.G., Buckwalter D.K., Bluestein B.W., Stanczyk F.Z. (2001). Pregnancy and post partum: changes in cognition and mood. Progress in Brain Research, 133(3), 303–19. 10.1016/S0079-6123(01)33023-6. [DOI] [PubMed] [Google Scholar]
- Carlsen S.M., Jacobsen G., Romundstad P. (2006). Maternal testosterone levels during pregnancy are associated with offspring size at birth. European Journal of Endocrinology, 155(2), 365–70. 10.1530/eje.1.02200. [DOI] [PubMed] [Google Scholar]
- Carmona S., Martínez-García M., Paternina-Die M., Barba-Müller E., Wierenga L.M., Hoekzema E. (2019). Pregnancy and adolescence entail similar neuroanatomical adaptations: a comparative analysis of cerebral morphometric changes. Human Brain Mapping, 40(7), 2143–52. 10.1002/hbm.24513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J., Holditch-Davis D. (2014). Effects of perinatal testosterone on infant health, mother-infant interactions, and infant development. Biological Research for Nursing, 16(2), 228–36. 10.1177/1099800413486340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot R.H.M., Vuurman E.F.P.M., Hornstra G., Jolles J. (2006). Differences in cognitive performance during pregnancy and early motherhood. Psychological Medicine, 36(7), 1023–32. 10.1017/S0033291706007380. [DOI] [PubMed] [Google Scholar]
- Dooley L.N., Kuhlman K.R., Robles T.F., Eisenberger N.I., Craske M.G., Bower J.E. (2018). The role of inflammation in core features of depression: insights from paradigms using exogenously-induced inflammation. Neuroscience and Biobehavioral Reviews, 94(July), 219–37. 10.1016/j.neubiorev.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas A.J. (2010). Baby love? Oxytocin-dopamine interactions in mother-infant bonding. Endocrinology, 151(5), 1978–80. 10.1210/en.2010-0259. [DOI] [PubMed] [Google Scholar]
- Dufford A.J., Erhart A., Kim P. (2019). Maternal brain resting-state connectivity in the postpartum period. Journal of Neuroendocrinology, (October 2018), e12737 10.1111/jne.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbrother N., Janssen P., Antony M.M., Tucker E., Young A.H. (2016). Perinatal anxiety disorder prevalence and incidence. Journal of Affective Disorders, 200, 148–55. 10.1016/j.jad.2015.12.082. [DOI] [PubMed] [Google Scholar]
- Fan Y., Pestke K., Feeser M., Aust S., Pruessner J.C., Grimm S. (2015). Amygdala-hippocampal connectivity changes during acute psychosocial stress: joint effect of early life stress and oxytocin. Neuropsychopharmacology, 40(12), 2736–44. 10.1038/npp.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R., Weller A., Zagoory-Sharon O., Levine A. (2007). Evidence for a Neuroendocrinological Foundation of Human Affiliation. Psychological Science, 18(11), 965–70. 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Ferris C.F. (2005). Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. Journal of Neuroscience, 25(1), 149–56. 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C.D., Frith U. (2006). The neural basis of mentalizing. Neuron, 50(4), 531–4. 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Fusté M., Pauls A., Worker A., Reinders A.A.T.S., Simmons A., Dazzan P. (2017). Brain structure in women at risk of postpartum psychosis: an MRI study. Translational Psychiatry, 7(12). 10.1038/s41398-017-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbally M., Lewis A.J., Ijzendoorn M., V., Permezel M. (2011). The role of oxytocin in mother-infant relations: a systematic review of human studies. Harvard Review of Psychiatry, 19(1), 1–14. 10.3109/10673229.2011.549771. [DOI] [PubMed] [Google Scholar]
- Galea L.A.M., Ormerod B.K., Sampath S., Kostaras X., Wilkie D.M., Phelps M.T. (2000). Spatial working memory and hippocampal size across pregnancy in rats. Hormones and Behavior, 37(1), 86–95. 10.1006/hbeh.1999.1560 10.1006/hbeh.1999.1560. [DOI] [PubMed] [Google Scholar]
- Gavin N.I., Gaynes B.N., Lohr K.N., Meltzer-Brody S., Gartlehner G., Swinson T. (2005). Perinatal depression: a systematic review of prevalence and incidence. Obstetrics and Gynecology, 106, 1071–83. 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gay C.L., Lee K.A., Lee S.-Y. (2004). Sleep patterns and fatigue in new mothers and fathers. Biological Research for Nursing, 5(4), 311–8. 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerdingen D., Fontaine P., Crow S., McGovern P., Center B., Miner M. (2009). Predictors of mothers’ postpartum body dissatisfaction. Women and Health, 49(6–7), 491–504. 10.1080/03630240903423998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasper E.R., Kozorovitskiy Y., Pavlic A., Gould E. (2011). Paternal experience suppresses adult neurogenesis without altering hippocampal function in Peromyscus californicus. Journal of Comparative Neurology, 519(11), 2271–81. 10.1002/cne.22628. [DOI] [PubMed] [Google Scholar]
- Goldstein A., Walker M. (2014). The role of sleep in emotional brain function. Ssrn.. doi: 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S.H., Garber J. (2017). Evidence-based interventions for depressed mothers and their young children. Child Development, 88(2), 368–77. 10.1111/cdev.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gynecol O. (2017). ACOG committee opinion contrast in pregnancy. In Obstetrics & Gynecology, 130. [Google Scholar]
- Haggi E.S., Torres A.I., Maldonado C.A., Aoki A. (1986). Regression of redundant lactotrophs in rat pituitary gland after cessation of lactation. Journal of Endocrinology, 111(3), 367–73. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Macnamara A., Olvet D.M. (2010). Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology, 35(2), 129–55. 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hall W.A., Hauck Y.L., Carty E.M., Hutton E.K., Fenwick J., Stoll K. (2009). Childbirth fear, anxiety, fatigue, and sleep deprivation in pregnant women. JOGNN - Journal of Obstetric, Gynecologic, and Neonatal Nursing, 38(5), 567–76. 10.1111/j.1552-6909.2009.01054.x. [DOI] [PubMed] [Google Scholar]
- Hashmi A.M., Bhatia S.K., Bhatia S.K., Khawaja I.S. (2016). Insomnia during pregnancy: diagnosis and rational interventions. Pakistan Journal of Medical Sciences, 32(4), 1030–7. 10.12669/pjms.324.10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G., Singer T. (2008). I feel how you feel but not always: the empathic brain and its modulation. Current Opinion in Neurobiology, 18(2), 153–8. 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Henry J.D., Rendell P.G. (2007). A review of the impact of pregnancy on memory function. Journal of Clinical and Experimental Neuropsychology, 29(8), 793–803. 10.1080/13803390701612209. [DOI] [PubMed] [Google Scholar]
- Henry J.F., Sherwin B.B. (2012). Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behavioral Neuroscience, 126(1), 73–85. 10.1037/a0025540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillerer K.M., Neumann I.D., Couillard-Despres S., Aigner L., Slattery D.A. (2014). Lactation-induced reduction in hippocampal neurogenesis is reversed by repeated stress exposure. Hippocampus, 24(6), 673–83. 10.1002/hipo.22258. [DOI] [PubMed] [Google Scholar]
- Hoekzema E., Barba-Müller E., Pozzobon C., Picado M., Lucco F., et al. (2017). Pregnancy leads to long-lasting changes in human brain structure. Nature Neuroscience, 20(2), 287–96. 10.1038/nn.4458. [DOI] [PubMed] [Google Scholar]
- Inagaki T.K., Muscatell K.A., Irwin M.R., Cole S.W., Eisenberger N.I. (2012). Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage, 59(4), 3222–6. 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itthipuripat S., Sprague T.C. (2019). Functional MRI and EEG index complementary attentional modulations. The Journal of Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes C., Delgado J., Cunnane M.B., Hedrick H.L., Adzick N.S., Victoria T. (2018). P MRS at 1. 5 and 3 T. NMR. Biomedicine, (June), 793–8. 10.1002/nbm. [DOI] [Google Scholar]
- Jasnow A.M., Schulkin J., Pfaff D.W. (2006). Estrogen facilitates fear conditioning and increases corticotropin- releasing hormone mRNA expression in the central amygdala in female mice. Hormones and Behavior, 49(2), 197–205. 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Jefferson A.L., Massaro J.M., Wolf P.A., Seshadri S., Au R., Decarli C. (2007). Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology, 68(13), 1032–8. 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaludjerovic J., Ward W.E. (2012). The interplay between estrogen and fetal adrenal cortex. Journal of Nutrition and Metabolism, 2012, 1–12. 10.1155/2012/837901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall-Tackett K. (2007). A new paradigm for depression in new mothers: the central role of inflammation and how breastfeeding and anti-inflammatory treatments protect maternal mental health. International Breastfeeding Journal, 2, 1–14. 10.1186/1746-4358-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa S., Hale J.R., Goldstone A., Wilson R.S., Mayhew S.D., et al. (2017). Habitual sleep durations and subjective sleep quality predict white matter differences in the human brain. Neurobiology of Sleep and Circadian Rhythms, 3, 17–25. 10.1016/j.nbscr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Leckman J.F., Mayes L.C., Feldman R., Wang X., Swain J.E. (2010). The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behavioral Neuroscience, 124(5), 695–700. 10.1037/a0020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Capistrano C., Congleton C. (2016). Socioeconomic disadvantages and neural sensitivity to infant cry: role of maternal distress. Social Cognitive and Affective Neuroscience, 11(10), 1597–607. 10.1093/scan/nsw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Capistrano C.G., Erhart A., Gray-Schiff R., Xu N. (2017). Socioeconomic disadvantage, neural responses to infant emotions, and emotional availability among first-time new mothers. Behavioural Brain Research, 325, 188–96. 10.1016/j.bbr.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.W., Park K., Jeong G.W. (2018a). Effects of sex hormones and age on brain volume in post-menopausal women. Journal of Sexual Medicine, 15(5), 662–70. 10.1016/j.jsxm.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Kim P., Dufford A.J., Tribble R.C. (2018b). Cortical thickness variation of the maternal brain in the first 6 months postpartum: associations with parental self-efficacy. Brain Structure and Function, 223(7), 3267–77. 10.1007/s00429-018-1688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause A.J., Simon E.B., Mander B.A., Greer S.M., Saletin J.M., Walker M.P. (2017). The sleep-deprived human brain HHS public access. Nature Reviews. Neuroscience, 18(7), 404–18. 10.1038/nrn.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A., Burkhouse K.L. (2017). Vulnerability to depression in youth: advances from affective neuroscience. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(1), 28–37. 10.1016/j.bpsc.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi N., Montoya J., Kober H., Rutherford H.J.V., Mencl W.E., Mayes L.C. (2011). Maternal neural responses to infant cries and faces: relationships with substance use. Frontiers in Psychiatry, 2 10.3389/fpsyt.2011.00032 10.3389/fpsyt.2011.00032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange A.-M.G., de T., Meer D., van der L., Alnæs D., Westlye L.T. (2019). Population-based neuroimaging reveals traces of childbirth in the maternal brain. BioRxiv, 650952 10.1101/650952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laway B., Mir S. (2013). Pregnancy and pituitary disorders: challenges in diagnosis and management. Indian Journal of Endocrinology and Metabolism, 17(6), 996 10.4103/2230-8210.122608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Clancy S., Fleming A.S. (1998). Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behavioural Brain Research, 114(2), 175–81. 10.1002/hc. [DOI] [PubMed] [Google Scholar]
- Leerkes E.M. (2010). Predictors of maternal sensitivity to infant distress. Parenting, 10(3), 219–39. 10.1080/15295190903290840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E., Gobbini M.I., Harrison T., Haxby J.V. (2004). Mothers’ neural activation in response to pictures of their children and other children. Biological Psychiatry, 56(4), 225–32. 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lenzi D., Lenzi G.L., Pantano P., Trentini C., Macaluso E. (2008). Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cerebral Cortex, 19(5), 1124–33. 10.1093/cercor/bhn153. [DOI] [PubMed] [Google Scholar]
- Lillis T.A., Hamilton N.A., Pressman S.D., Ziadni M.S., Khou C.S., et al. (2018). Sleep quality buffers the effects of negative social interactions on maternal mood in the 3–6 month postpartum period: a daily diary study. Journal of Behavioral Medicine, 41(5), 733–46. 10.1007/s10865-018-9967-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C., McEwen B.S., Casey B.J. (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences, 106(3), 912–7. 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Kong X.Z., Liu X., Zhou R., Wu B. (2014). Long-term total sleep deprivation reduces thalamic gray matter volume in healthy men. Neuroreport, 25(5), 320–3. 10.1097/WNR.0000000000000091. [DOI] [PubMed] [Google Scholar]
- Lorberbaum J.P., Newman J.D., Horwitz A.R., Dubno J.R., Lydiard R.B., et al. (2002). A potential role for thalamocingulate circuitry in human maternal behavior. Biological Psychiatry, 51(6), 431–45. [DOI] [PubMed] [Google Scholar]
- Luck S.J., Woodman G.F., Vogel E.K. (2000). Event-related potential studies of attention. Trends in Cognitive Sciences, 4(11), 432–40. 10.1016/S1364-6613(00)01545-X. [DOI] [PubMed] [Google Scholar]
- Luking K.R., Pagliaccio D., Luby J.L., Barch D.M. (2016). Reward processing and risk for depression across development. Trends in Cognitive Sciences, 20(6), 456–68. 10.1016/j.tics.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magon N., Kumar P. (2013). Hormones in pregnancy. Nigerian Medical Journal, 53(4), 179 10.4103/0300-1652.107549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N.L., Tracy A.J. (2009). After the baby: work-family conflict and working mothers’ psychological health. Family Relations, 58(4), 380–91. 10.1111/j.1741-3729.2009.00560.x. [DOI] [Google Scholar]
- Marsland A.L., Gianaros P.J., Kuan D.C.H., Sheu L.K., Krajina K., Manuck S.B. (2015). Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain, Behavior, and Immunity, 48, 195–204. 10.1016/j.bbi.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J.A., Hamilton B.E., Osterman M.J.K., Driscoll A.K., Drake P. (2018). Births: final for 2017. National Vital Statistics Reports, 67(8), 1–49. [PubMed] [Google Scholar]
- Maupin A.N., Hayes N.J., Mayes L.C., Rutherford H.J.V. (2015). The application of electroencephalography to investigate the neural bases of parenting: a review. Parenting, 15(1), 9–23. 10.1080/15295192.2015.992735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S., Colquhoun H., Riesenberg R., Epperson C.N., Deligiannidis K.M., Kanes S. (2018). Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. The Lancet, 392(10152), 1058–70. 10.1016/S0140-6736(18)31551-4. [DOI] [PubMed] [Google Scholar]
- Miki Y., Kataoka M.L., Shibata T., Haque T.L., Kanagaki M., Konishi J. (2007). The pituitary gland: changes on MR images during the 1st year after delivery. Radiology, 235(3), 999–1004. 10.1148/radiol.2353040243. [DOI] [PubMed] [Google Scholar]
- Miller A.H., Haroon E., Raison C.L., Felger J.C. (2013). Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depression and Anxiety, 30(4), 297–306. 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell J.A., Cook R.A., Nikolovski J. (2015). Sleep patterns and sleep disturbances across pregnancy. Sleep Medicine, 16(4), 483–8. 10.1016/j.sleep.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Mitchell J.P. (2009). Inferences about mental states. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1521), 1309–16. 10.1098/rstb.2008.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko E.L., Horner M.S., Phillips M.L., Hipwell A.E., Swain J.E. (2014). In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. Journal of Neuroendocrinology, 26(10), 665–84. 10.1111/jne.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Suano A., Hamilton A.B., Betz A.G. (2011). Gimme shelter - the immune system during pregnancy (Munoz-Suano 2011) (impressão ok). pdf. Immunological Reviews, 241, 20–38. [DOI] [PubMed] [Google Scholar]
- Murphy V.E., Clifton V.L. (2003). Alterations in human placental 11β=hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta, 24(7), 739–44. 10.1016/S0143-4004(03)00103-6. [DOI] [PubMed] [Google Scholar]
- Naaz F., Knight L.K., Depue B.E. (2018). Explicit and ambiguous threat processing: functionally dissociable roles of the amygdala and bed nucleus of the stria terminalis. Journal of Cognitive Neuroscience, 31(4), 543–59. 10.1162/jocn_a_01369. [DOI] [PubMed] [Google Scholar]
- Nephew B.C., Bridges R.S., Lovelock D.F., Byrnes E.M. (2009). Enhanced maternal aggression and associated changes in neuropeptide gene expression in multiparous rats. Behavioral Neuroscience, 123(5), 949–57. 10.1037/a0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen E., Gustavsson P., Widström A.M., Uvnäs-Moberg K. (1998). Oxytocin, prolactin, milk production and their relationship with personality traits in women after vaginal delivery or cesarean section. Journal of Psychosomatic Obstetrics and Gynaecology, 19(1), 49–58. 10.3109/01674829809044221. [DOI] [PubMed] [Google Scholar]
- Noriuchi M., Kikuchi Y., Senoo A. (2008). The functional neuroanatomy of maternal love: mother’s response to infant’s attachment behaviors. Biological Psychiatry, 63(4), 415–23. 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- O’Hara M.W. (2011). Prospective study of postpartum blues. Archives of General Psychiatry, 48(9), 801 10.1001/archpsyc.1991.01810330025004. [DOI] [PubMed] [Google Scholar]
- O’Hara M.W., Wisner K.L. (2014). Perinatal mental illness: definition, description and aetiology. Best Practice and Research: Clinical Obstetrics and Gynaecology, 28(1), 3–12. 10.1016/j.bpobgyn.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatridge A., Holdcroft A., Saeed N., Hajnal J.V., Puri B.K., Bydder G.M. (2002). Change in brain size during and after pregnancy: study in healthy women and women with preeclampsia. American Journal of Neuroradiology, 23(1), 19–26. 10.1002/etc.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim D., Koren-Karie N., Sagi A. (2001). Mothers empathic understanding of their preschoolers’ internal experience: relations with early attachment. International Journal of Behavioral Development, 25(1), 16–26. 10.1080/01650250042000096. [DOI] [Google Scholar]
- Orchard E.R., Ward P.G.D., Sforazzini F., Storey E., Egan G.F., Jamadar S.D. (2019). Cortical changes associated with parenthood are present in late life. BioRxiv, 0–3. [Google Scholar]
- Ostlund B.D., Measelle J.R., Laurent H.K., Conradt E., Ablow J.C. (2017). Shaping emotion regulation: attunement, symptomatology, and stress recovery within mother–infant dyads. Developmental Psychobiology, 59(1), 15–25. 10.1002/dev.21448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C.E., Young K.S., Petersen M.V., Jegindoe Elmholdt E.M., Vuust P., et al. (2017). Duration of motherhood has incremental effects on mothers’ neural processing of infant vocal cues: a neuroimaging study of women. Scientific Reports, 7(1), 1–9. 10.1038/s41598-017-01776-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein T., Howard M., Salisbury A., Zlotnick C. (2013). Postpartum depression. Journal of Nursing, 60(6), 22–6. 10.6224/JN.60.6.22. [DOI] [PubMed] [Google Scholar]
- Pearson R.M., Lightman S.L., Evans J. (2009). Emotional sensitivity for motherhood: late pregnancy is associated with enhanced accuracy to encode emotional faces. Hormones and Behavior, 56(5), 557–63. 10.1016/j.yhbeh.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Peper J.S., Hulshoff Pol H.E., Crone E.A., van Honk J. (2011). Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience, 191, 28–37. 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Prevost M., Zelkowitz P., Tulandi T., Hayton B., Feeley N., Gold I. (2014). Oxytocin in pregnancy and the postpartum: relations to labor and its management. Frontiers in Public Health, 2 10.3389/fpubh.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raby K.L., Roisman G.I., Fraley R.C., Simpson J.A. (2015). The enduring predictive significance of early maternal sensitivity: social and academic competence through age 32 years. Child Development, 86(3), 695–708. 10.1111/cdev.12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanoël S., Hoyau E., Kauffmann L., Renard F., Pichat C., Baciu (2018). M, Gray matter volume and cognitive performance during normal aging. A voxel-based morphometry study. Frontiers in Aging Neuroscience, 10(AUG), 1–10. 10.3389/fnagi.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranote S., Elliott R., Abel K.M., Mitchell R., Deakin J.F.W., Appleby L. (2004). The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport, 15(11), 1825–9. 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- Rasia-Filho A.A., Fabian C., Rigoti K.M., Achaval M. (2004). Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience, 126(4), 839–47. 10.1016/j.neuroscience.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Ray J.G., Vermeulen M.J., Bharatha A., Montanera W.J., Park A.L. (2016). Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA - Journal of the American Medical Association, 316(9), 952–61. 10.1001/jama.2016.12126. [DOI] [PubMed] [Google Scholar]
- Raz S. (2014). Behavioral and neural correlates of cognitive-affective function during late pregnancy: an Event-Related Potentials study. Behavioural Brain Research, 267, 17–25. 10.1016/j.bbr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- Reeves M.J., Brandreth M., Whitby E.H., Hart A.R., Paley M.N.J., Stevens J.C. (2010). Neonatal cochlear function: measurement after exposure to acoustic noise during in utero MR imaging. Radiology, 257(3), 802–9. 10.1148/radiol.10092366. [DOI] [PubMed] [Google Scholar]
- Reshef T., Hugh T., Burney R., Mooney S., Giudice L. (2015). Endocrinology of pregnancy. Recurrent Pregnancy Loss, 128–39 10.1201/b17855-16. [DOI] [Google Scholar]
- Riem M.M.E., Bakermans-Kranenburg M.J., Pieper S., Tops M., Boksem M.A.S., Rombouts S.A.R.B. (2011). Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biological Psychiatry, 70(3), 291–7. 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Righetti-Veltema M., Bousquet A., Manzano J. (2003). Impact of postpartum depressive symptoms on mother and her 18-month-old infant. European Child and Adolescent Psychiatry, 12(2), 75–83. 10.1007/s00787-003-0311-9. [DOI] [PubMed] [Google Scholar]
- Rocchetti M., Radua J., Paloyelis Y., Xenaki L.A., Frascarelli M., Fusar-Poli P. (2014). Neurofunctional maps of the “maternal brain” and the effects of oxytocin: a multimodal voxel-based meta-analysis. Psychiatry and Clinical Neurosciences, 68(10), 733–51. 10.1111/pcn.12185. [DOI] [PubMed] [Google Scholar]
- Rolls A., Schori H., London A., Schwartz M. (2008). Decrease in hippocampal neurogenesis during pregnancy: a link to immunity. Molecular Psychiatry, 13(5), 468–9. 10.1038/sj.mp.4002126. [DOI] [PubMed] [Google Scholar]
- Roos A., Robertson F., Lochner C., Vythilingum B., Stein D.J. (2011). Altered prefrontal cortical function during processing of fear-relevant stimuli in pregnancy. Behavioural Brain Research, 222(1), 200–5. 10.1016/j.bbr.2011.03.055. [DOI] [PubMed] [Google Scholar]
- Rosand G.M., Slinning K., Eberhard-Gran M., Roysamb E., Tambs K. (2011). Partner relationship satisfaction and maternal emotional distress in early pregnancy. BMC Public Health. 10.1186/1471-2458-11-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe D., Rossin-Slater M., Goldenberg D. (2018). The transition to parenthood as a critical window for adult health. American Psychologist, 73(9), 1190–200. 10.1037/amp0000376. [DOI] [PubMed] [Google Scholar]
- Schiller C.E., Meltzer-Brody S., Rubinow D.R. (2015). The role of reproductive hormones in postpartum depression. CNS Spectrums, 20(01), 48–59. 10.1017/S1092852914000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifritz E., Esposito F., Neuhoff J.G., Lüthi A., Mustovic H., Di Salle F. (2003). Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biological Psychiatry, 54(12), 1367–75. 10.1016/S0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Sell L.A., Morris J., Bearn J., Frackowiak R.S., Friston K.J., Dolan R.J. (1999). Activation of reward circuitry in human opiate addicts [in process citation]. The European Journal of Neuroscience, 11(3), 1042–8. [DOI] [PubMed] [Google Scholar]
- Shahrokh D.K., Zhang T.Y., Diorio J., Gratton A., Meaney M.J. (2010). Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology, 151(5), 2276–86. 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M., Moore C.M., Villamarin V., Jaitly N., Hall J.E., Deligiannidis K.M. (2018). White matter integrity in medication-free women with peripartum depression: a tract-based spatial statistics study. Neuropsychopharmacology, 43(7), 1573–80. 10.1038/s41386-018-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S., Chefer S., Suomi S.J., Higley J.D., Barr C.S., Stein E. (2009). Early-life stress induces long-term morphologic changes in primate brain. Archives of General Psychiatry, 66(6), 658–65. 10.1001/archgenpsychiatry.2009.52 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L., Li J., Fonagy P., Montague R.P. (2008). What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics, 122(1), 40–51. 10.1542/peds.2007-1566 10.1542/peds.2007-1566 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L., Fonagy P., Amico J., Montague P.R. (2009). Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology, 34(13), 2655–66. 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain J.E., Ho S.S., Fox H., Garry D., Brummelte S. (2019). Effects of opioids on the parental brain in health and disease. Frontiers in Neuroendocrinology, 54(May), 100766 10.1016/j.yfrne.2019.100766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den, Heuvel M.I., Thomason M.E. (2016). Functional connectivity of the human brain in utero. Trends in Cognitive Sciences, 20(12), 931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L.A., Trevathan W.R. (2008). Cortisol reactivity, maternal sensitivity, and learning in 3-month-old infants. Infant Behavior and Development, 31(1), 92–106. 10.1016/j.infbeh.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wingen G.A., Ossewaarde L., Bäckström T., Hermans E.J., Fernández G. (2011). Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience, 191, 38–45. 10.1016/j.neuroscience.2011.04.042. [DOI] [PubMed] [Google Scholar]
- Vann S.D., Albasser M.M. (2011). Hippocampus and neocortex: recognition and spatial memory. Current Opinion in Neurobiology, 21(3), 440–5. 10.1016/j.conb.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Victoria T., Jaramillo D., Roberts T.P.L., Zarnow D., Johnson A.M., et al. (2014). Fetal magnetic resonance imaging: jumping from 1.5 to 3 tesla (preliminary experience). Pediatric Radiology, 44(4), 376–86. 10.1007/s00247-013-2857-0. [DOI] [PubMed] [Google Scholar]
- Yim I., Tanner Stapleton L., Guardino C., Hahn-Holbrook J., Dunkel Schetter C. (2015). Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Ssrn. doi: 10.1146/annurev-clinpsy-101414-020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K.S., Parsons C.E., Stein A., Vuust P., Craske M.G., Kringelbach M.L. (2017). The neural basis of responsive caregiving behaviour: investigating temporal dynamics within the parental brain. Behavioural Brain Research, 325, 105–16. 10.1016/j.bbr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Zheng J.X., Chen Y.C., Chen H., Jiang L., Bo F., Gu J.P. (2018). Disrupted spontaneous neural activity related to cognitive impairment in postpartum women. Frontiers in Psychology, 9, 1–10. 10.3389/fpsyg.2018.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou R., Tiemeier H., van der Ende J., Verhulst F.C., Muetzel R.L., El Marroun H. (2019). Exposure to maternal depressive symptoms in fetal life or childhood and offspring brain development: a population-based imaging study. American Journal of Psychiatry, appi.ajp.2019.1 10.1176/appi.ajp.2019.18080970. [DOI] [PubMed] [Google Scholar]
- Zubiaurre-Elorza L., Junque C., Gómez-Gil E., Guillamon A. (2014). Effects of cross-sex hormone treatment on cortical thickness in transsexual individuals. Journal of Sexual Medicine, 11(5), 1248–61. 10.1111/jsm.12491. [DOI] [PubMed] [Google Scholar]
- van Scheppingen M.A., Denissen J.J.A., Chung J.M., Tambs K., Bleidorn W. (2018). Self-esteem and relationship satisfaction during the transition to motherhood. Journal of Personality and Social Psychology, 114(6), 973–91. 10.1037/pspp0000156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


