Abstract
Background:
Despite pulmonary arterial hypertension (PAH) directly affects the right ventricle (RV), important structural, functional, and molecular changes also occur in left ventricle (LV). The objective of our study was to analyze the hypothetical cardioprotective effects of exercise preconditioning on LV in rats with monocrotaline (MCT)-induced PAH.
Methods:
Forty male Wistar rats were randomly separated in sedentary (SED) and trained group (EX; running sessions of 60 min/day, 5 days/wk, at 25 m/min, for 4 weeks). After 4 weeks, animals were injected with MCT (60 mg/kg; SED + MCT; EX + MCT) or vehicle (SED + V). Following an additional period of 4 weeks where all animals remained sedentary, we completed LV hemodynamic evaluation in baseline and isovolumic conditions and collected LV samples for histological and molecular analysis.
Results:
Preconditioning with exercise was capable to restore LV systolic and diastolic dysfunction in both baseline and isovolumic conditions (P < .05). This improved was paralleled with prevention of LV cardiomyocytes atrophy, fibrosis, and endothelin 1 mRNA levels (P < .05).
Conclusions:
Our findings suggest that exercise preconditioning can prevent LV dysfunction secondary to MCT-induced PAH, which is of particular interest for the familial form of the disease that is manifested by greater severity or earlier onset.
Keywords: cardioprotection, exercise training, pulmonary arterial hypertension
Introduction
Pulmonary arterial hypertension (PAH) is a serious disease often accompanied by right ventricular (RV) dysfunction and failure (RVF). Besides contributing to exercise intolerance, RVF is associated with reduced quality of life and poor survival in patients with PAH.1–3 Accumulating evidence suggests that left ventricle (LV) performance may also be impaired since the early stages of this condition, further contributing to the limited prognosis.4 LV dysfunction in patients with PAH is characterized by reduced ejection fraction (EF) and impaired diastolic function, with some studies reporting a reduction in LV-free wall mass due to cardiomyocyte atrophy.5 Mechanisms contributing to LV dysfunction in PAH include ventricular interdependence and impaired LV filling, but there are evidences from both human and animal studies that intrinsic LV myocardial abnormalities also contribute.6,7
The role of exercise training (ExT) in PAH is gaining interest as several studies demonstrate its safety and efficacy in improving important clinical outcomes such as exercise tolerance, quality of life, and pulmonary arterial pressure.8,9 These benefits have been attributed mainly to noncardiac changes, but both clinical and preclinical studies are suggesting that ExT may also enhance RV function and prevent maladaptive remodeling.10–12 Because both ventricles are coupled in series and their performance is interrelated, it might be predicted that ExT will also improve LV function, but this remains poorly characterized. A recent small pre-post study of women with PAH addressed this issue for the first time, suggesting that vigorous aerobic ExT could lead to a reduction in systemic vascular resistance and preserve LV diastolic function.13 To further advance in the comprehension of the effects of exercise training against LV dysfunction secondary to PAH, we performed the effects of exercise preconditioning on LV function and remodeling in a study using a rat model of PAH induced by monocrotaline (MCT). Our aim was to analyze the hypothetical cardioprotective effects of exercise preconditioning on LV function and remodeling.
Methods
Animal models and experimental design
All experimental procedures involving animal care and sacrifice were performed according to the Portuguese law on animal welfare and specifications of the National Institute of Health Guide for Care and Use of Laboratory Animals and approved by the ethical committee of the University of Porto, Portugal.
Following 1 week of quarantine after arrival, 40 male Wistar rats (age = 4 weeks; Charles River Laboratories, Barcelona), were housed in groups of 5 rats per cage, maintained in an inverted 12 h light/dark cycle, in an environment with controlled temperature of 22°C, and had free access to food. After that, they were randomly separated into 2 experimental groups: sedentary (SED; n = 25; remained with movement confined to the cage's space for 4 weeks) and exercise (EX; n = 15; exercised on a treadmill 5 days/wk for 4 weeks). After ending exercise protocol some animals from each group received 1 subcutaneous injection of MCT (60 mg/kg, Sigma, Barcelona, Spain) or an equal volume of vehicle (V; 1 mL/kg of saline), originating the following groups: (i) SED + MCT (n = 15), (ii) SED + V (n = 10), and (iii) EX + MCT (n = 15). At this point, all animals remained sedentary for an additional 4-week period, until the time of hemodynamic evaluation.
Exercise training protocol
Animals were acclimated to the treadmill for 1 week. Running speed and exercise duration were progressively increased until animals reached a maximum of 25 m/min (estimated work rate of 70% maximum oxygen consumption14 during 60 minutes, at the end of the week. Then, animals exercised for 4 weeks, 5 days per week, 60 min/day, at 25 m/min, with no grade. Sedentary animals were placed on the treadmill 3 times per week, during 10 minutes, without movement, in order to expose them to the stress of being in the device.
Hemodynamic evaluation
Procedure
At day 28 and 29 after MCT or vehicle administration, animals were prepared for left ventricular hemodynamic evaluation with a pressure-volume catheter as previously described in detail.15 In brief, rats were anaesthetized by inhalation with a mixture 4% sevoflurane with oxygen, intubated for mechanical ventilation (TOPO, Kent Scientific, Torrington, CT, USA). The right jugular vein was cannulated for fluid administration (prewarmed 0.9% NaCl solution) and the heart was exposed by a median sternotomy and the pericardium was widely opened. LV hemodynamic function was measured with conductance catheter (model-FTM-1912B-8018, 1.9F; Scisense, London, ON, Canada) inserted by apical puncture on the LV cavity, along the ventricular long axis. The catheter was connected to MVP-300 conductance system through interface cable (PCU-2000 MPVS, FC-MR-4, Scisense, USA), coupled to PowerLab16/30 converter (AD Instruments, Dunedin, New Zealand) and a personal computer for data acquisitions. After complete instrumentation, the animal preparation was allowed to stabilize for 15 minutes. Hemodynamic recordings were made with respiration suspended at the end of expiration under steady-state conditions and in response to isovolumetric contractions induced by sudden and acute ascending aortic occlusion.15 Parameters from conductance catheter were recorded at a sampling rate of 1000 Hz and analyzed with Millar conductance data acquisition and analysis software (PVAN3.5). The following parameters were recorded: heart rate (HR), peak systolic pressure (Pmax), end-diastolic pressure, end-systolic pressure, peak rate of pressure rise (dP/dtmax), peak rate of pressure fall (dP/dtmin), constant time of isovolumetric pressure decay (Tau), cardiac output (CO), end-diastolic volume (EDV), end-systolic volume, stroke volume, EF, and maximal elastance (Emax).
Animal sacrifice and tissue harvesting
After collecting all hemodynamic data, the animals were sacrificed by exsanguination, the heart was excised and weighed, and samples from LV + septum (LV + S) were dissected, washed in cold phosphate-buffered saline (pH 7.2) and weighted together. Heart weight and LV + S weight were normalized to tibia length. Then, the LV was separated from the septum and divided in samples that were prepared for light microscopy analysis [cardiomyocyte cross-sectional area (CSA) and collagen deposition] and endothelin (ET)-1 mRNA quantification by reverse transcription polymerase chain reaction (RT-PCR).
Histological analysis
Cubic pieces coming from intermediate cardiac region from LV were fixed (4% paraformaldehyde) by diffusion for 24 hours and subsequently dehydrated through graded ethanol solutions, cleared in xylene and mounted in paraffin. Transverse 6 μm thick sections were cut and used for assessing cardiomyocyte CSA and fibrous tissue accumulation as previously described by us in detail.10 In brief, LV sections were stained with hematoxylin and eosin and images were analyzed with ImageJ software (National Institute of Health, Bethesda, MD). CSA was determined in 7 animals per group, 10 pictures per animal, totalizing around 1000 cardiomyocytes per group. In order to determine the amount of cardiac fibrosis, LV sections were stained with Picrosirius red and quantified. Paraffin sections were dewaxed and hydrated, stained 4-μm-slide with Picrosirius red for 1 hour and 30 minutes, followed by two washes with acidified water. Finally, they were dehydrated in 80, 90 and 100% of ethanol, cleared in xylene and mounted in a resinous medium. Images were analyzed with Image-Pro Plus 6.0 software (Media Cybernetics, Inc.) for quantification of the percentage area covered by collagen and muscle tissue. For quantitative comparisons, random microscopic fields (magnification of ×400) were considered and 10 representative images per animal were obtained, from 7 animals per group.
Relative quantification of mRNA
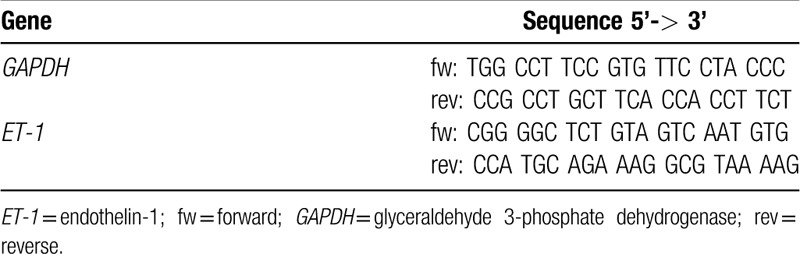
Two-step real-time RT-PCR was performed as previously described.16 Briefly, after total mRNA extraction (no. 74124; Qiagen), standard curves were obtained for each gene correlating (R ≥ 0.98) the mRNA quantities in graded dilutions of a rat cardiac tissue sample with the respective threshold cycles (second derivative maximum method). Equal amounts of mRNA from every sample underwent 3 separate 2-step real-time RT-PCR experiments for each gene, using SYBR green as marker (no. 204143; Qiagen). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as internal control and results are relative to the mean obtained for the SED + V group and normalized for GAPDH (fold increase). All the analysis was performed in duplicates. Specific PCR primer pairs for the studied genes are presented in Table 1.
Table 1.
Primers used in mRNA quantification by real-time reverse transcription polymerase chain reaction
Statistical analysis
The Kolmogorov-Smirnov test was used to investigate within-group normality for a given variable. One-way analysis with Tukey's posthoc test to compare all groups was used for normally distributed data. Kruskal-Wallis test followed by Dunns test was used for non-normal data. All results are presented as mean ± standard deviation. Differences were considered significantly when P < .05. Statistical analysis was performed with Graph Pad Prism software (version 6.0).
Results
Survival and general morphometric characteristics
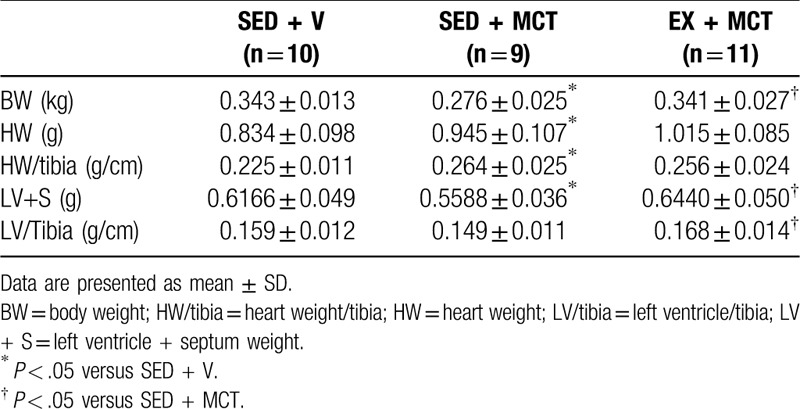
During the protocol 6 rats from SED + MCT (40%) and 4 from EX + MCT (27%) groups died. Survival curves were not statistically different (P = .07; Fig. 1). Table 2 summarizes the analyzed morphometric parameters. SED + MCT group presented lower body weight (P < .05 vs SED + V), whereas this was prevented in EX + MCT (P < .05 vs SED + MCT). Cardiac hypertrophy (heart weight and heart/tibia) was present in both MCT groups (P < .05 vs SED + V). Left ventricular + septum weight was decreased in SED + MCT (P < .05 vs SED + V), but not in EX + MCT group (P < .05 vs SED + MCT). Exercise preconditioning also prevented the decline in LV weight after normalization for tibia length in EX + MCT (P < .05 vs SED + MCT).
Figure 1.
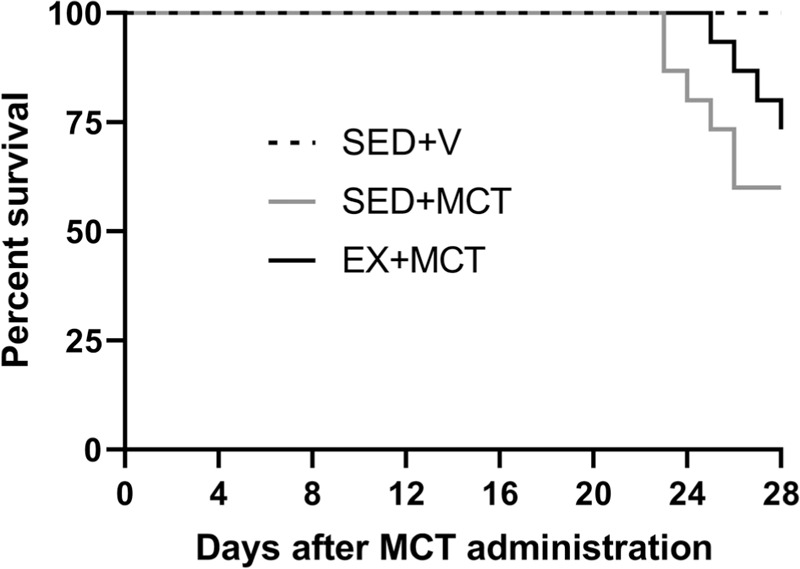
Effects of exercise training on survival. MCT = monocrotaline, SED = separated in sedentary.
Table 2.
General morphometric characteristics
Histological analysis
The reduced LV mass observed in SED + MCT was accompanied by LV cardiomyocytes atrophy (Fig. 2). In addition, animals from SED + MCT group also showed increased tissue fibrosis (P < .05 vs SED + V). All these features were prevented in EX + MCT (P < .05 vs SED + MCT).
Figure 2.
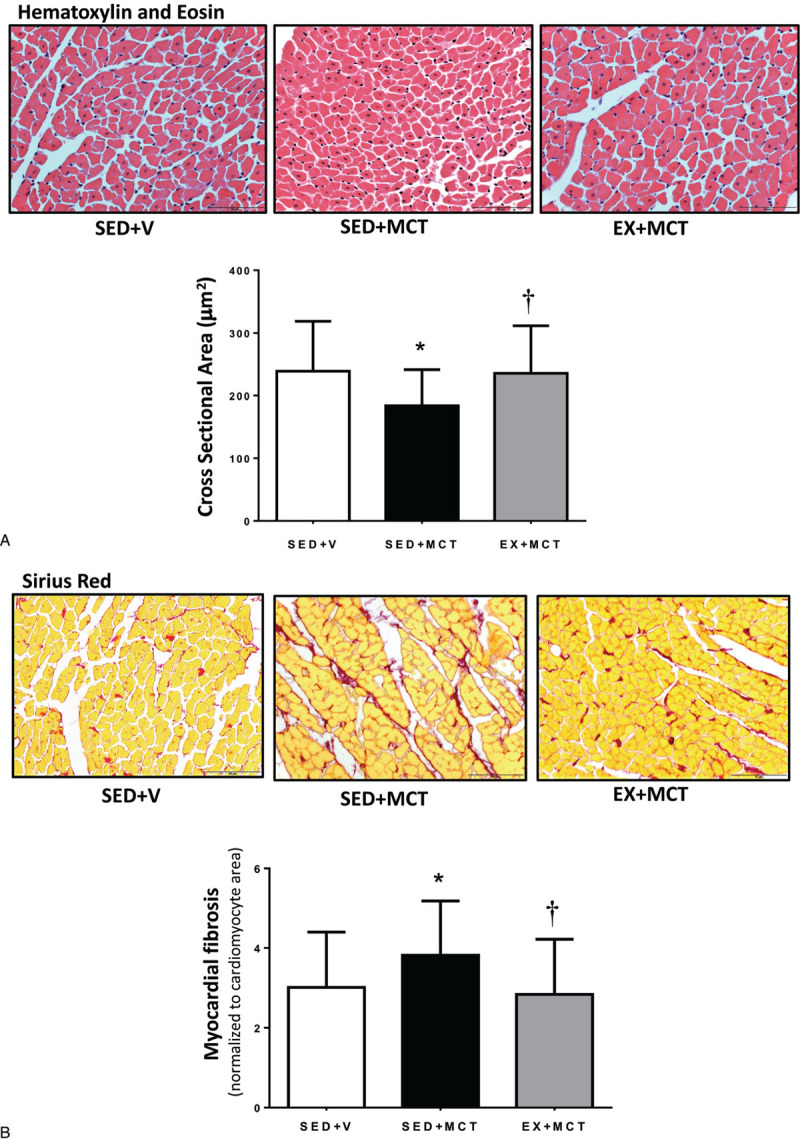
Effects of exercise training on left ventricle cross-section area (A) and myocardial fibrosis (B). Values are presented as mean ± SD (n = 7 animals per group). *P < .05 versus SED + V and †P < .05 versus SED + MCT. MCT = monocrotaline, SED = separated in sedentary.
Characterization of cardiac function: baseline and isovolumetric conditions
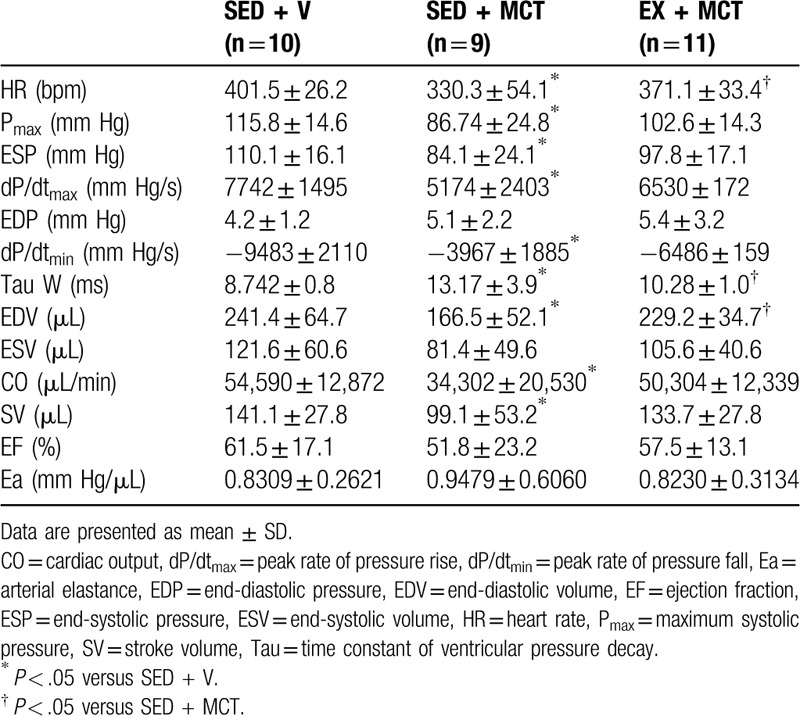
The results from hemodynamic evaluation are summarized in Table 3. HR was significantly reduced in SED + MCT and normalized in EX + MCT group (P < .05 vs SED + MCT). In opposition to EX + MCT, LV systolic dysfunction was present in SED + MCT as shown by the lower Pmax, end-systolic pressure, dP/dtmax, stroke volume, and CO. Regarding to LV diastolic function, it was significantly compromised in SED + MCT, with animals from this group exhibiting a reduced dP/dtmin and an increased constant time Tau and EDV (P < .05 vs SED + V). Preconditioning with exercise training prevented the changes in Tau and EDV (P < .05 vs SED + MCT), but not in dP/dtmin (P < .05 vs SED + V) in animals from EX + MCT.
Table 3.
Baseline hemodynamic evaluation parameters
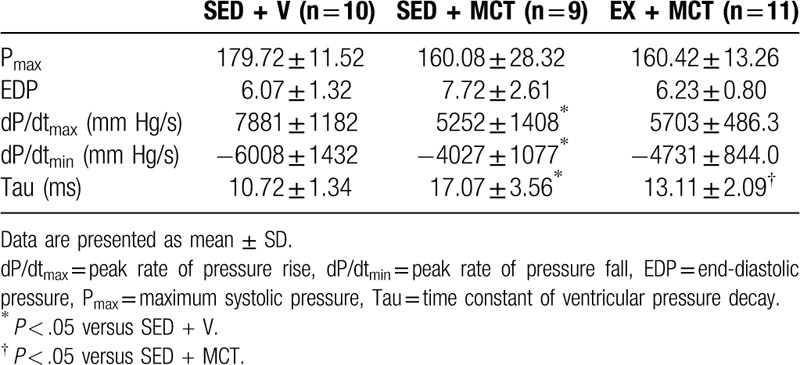
A sudden and acute increase in pressure overload induced by total occlusion of the ascending aorta was performed in all animals to stress the heart (Table 4). Under similar loading conditions, systolic dysfunction was present in SED + MCT as shown by the decrease in dP/dtmax. Diastolic dysfunction was also present in SED + MCT as illustrated by the reduction in dP/dtmin, increased end-diastolic pressure and Tau (P < .05 vs SED + V). Preconditioning with exercise prevented all these alterations (P < .05 vs SED + MCT).
Table 4.
Hemodynamic evaluation under isovolumetric conditions
Neurohumoral activation
Due to the important role of ET-1 activation in PAH pathophysiology, the ET-1 gene expression was quantified on LV. ET-1 mRNA levels were increased in LV of SED + MCT group and normalized in EX + MCT group (P < .05) (Fig. 3).
Figure 3.
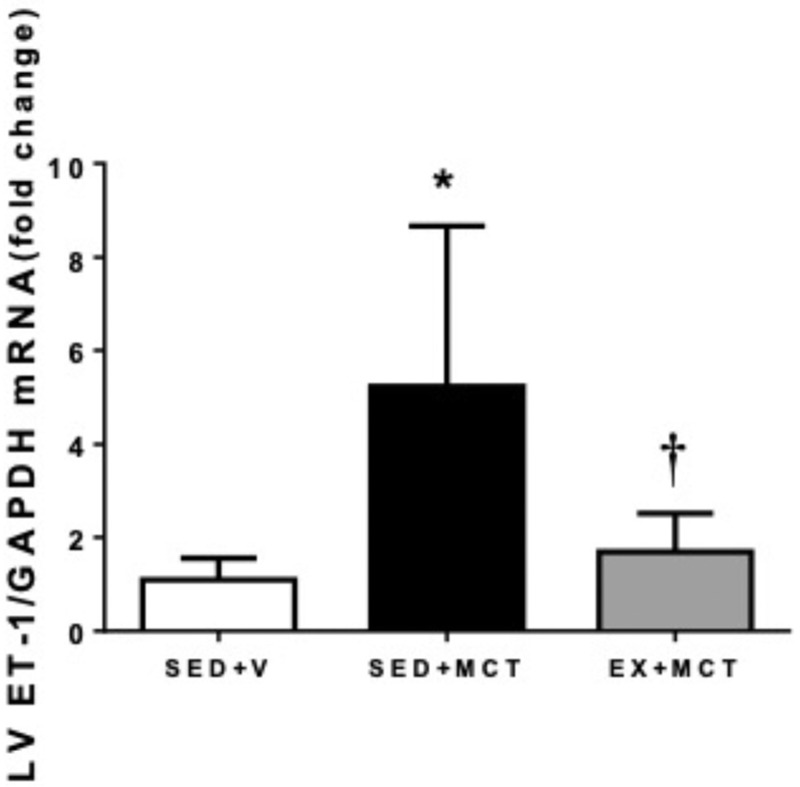
Effects of exercise training in left ventricle endothelin-1 mRNA. Values are mean ± SD (n = 7 animals per group). *P < .05 versus SED + V and †P < .05 versus SED + MCT. MCT = monocrotaline, SED = separated in sedentary.
Discussion
The present study shows that preconditioning the heart with aerobic exercise can rescue LV dysfunction secondary to MCT-induced PAH. These benefits were paralleled with prevention of cardiomyocytes atrophy and fibrosis, and with normalization of ET-1 mRNA levels. Of note, these improvements were observed 4 weeks after the cessation of exercise training, highlighting that the protective phenotype promoted by exercise training is maintained for several days.
In order to study the cardioprotective phenotype afforded by exercise training, we submitted previously exercised animals to experimental MCT-induced PAH. This experimental model is widely used to study therapeutic targets to PAH as well as a model of RVF.7,17–22 In the current work, we show that preconditioning the heart with exercise training can prevent LV CO decline and preserve several indexes of LV contractility and relaxation. Our data corroborate a previous observation that vigorous aerobic ExT could preserve LV diastolic function in women with PAH.13 Exercise training also prevented the fall of LV Pmax and HR, which have been previously related with the severity of the disease and reduced responsiveness to sympathetic stimulation, respectively.7 Of note, exercise-induced cardiac protection was observed even when the LV was submitted to an additional acute stressful stimulus. Previous data from our group showed that exercise training, performed at different stages of PAH, prevents RV dysfunction, and maladaptive remodeling.10,11 Specifically regarding exercise preconditioning, we showed that exercise prevented right ventricular cardiac hypertrophy, diastolic dysfunction, inflammation, and proteolysis.11 We now extend the benefits of exercise preconditioning to the LV, which was the main focus of the present work. This is of main importance since although PAH selectively overloads the RV, who ultimately fails, LV function is also affected.7,21,22
The improved LV hemodynamic response in EX + MCT group was accompanied by the prevention of LV mass loss, cardiomyocyte atrophy, fibrosis, and ET-1 mRNA. LV mass and cardiomyocyte atrophy can be attributed to decreases in both diastolic and systolic loading of the LV through a mechanism of ventricular interdependence.23 Contrarily to SED + MCT, EX + MCT showed preserved loading conditions (EDV, CO, and Pmax), probably due to the improved right ventricular function,11 thus contributing to prevent LV atrophy. Although cardiomyocyte death may also be responsible for the loss of some myocardial mass in the unloaded LV,21 atrophy is thought to be mainly responsible.22 Extracellular remodeling is another hallmark in cardiac remodeling and has also been described in this experimental model.21 Accumulation of fibrosis has a negative impact on cardiac function, affecting cardiac stiffness, promoting arrhythmias, and impairing the diffusion of oxygen to cardiomyocytes.24 Exercise preconditioning was able to prevent its increase. This could be due to the anti-inflammatory and antineurohumoral proprieties of exercise training.10,11 Indeed, we found lower levels of ET-1 mRNA in LV from exercised animals. ET-1 is known to favor the accumulation of fibrosis by activating myofibroblasts.25–27 Moreover, ET-1 is implicated in the modulation of LV function since its blockade with chronic administration of Bosentan prevented LV functional deterioration in PAH.5
Previous studies using exercise at different stages of PAH have shown worsening28 or improvement of survival.10,29 Despite the trends for greater survival rate in EX + MCT, this outcome was not significantly improved in exercised animals. However, it must be noted that exercise was interrupted after the induction of the disease. To conserve the benefits, exercise must be maintained.30 Because these animals presented better cardiac function and remodeling, we hypothesize that they would be in better clinical status to continue exercising if exercise was maintained throughout the disease course and, ultimately, improve their survival.
In conclusion, our findings suggest that exercise preconditioning can prevent LV dysfunction secondary to MCT-induced PAH. Our data also highlight that cardioprotection can be afforded for several weeks after the end of the last training session. Mechanisms underlying exercise-induced protection can be related to the prevention of LV atrophy, fibrosis, and neurohumoral activation.
Acknowledgments
None.
Assistance with the study
None.
Financial support
This work was supported by UnIC (UID/IC/00051/2019) and CIAFEL (UID/DTP/00617/2019). C.S. was funded by CAPES (BEX 0554/14-6).
Conflicts of interest
None.
Presentation
Some of these results were previously presented in scientific meetings.
References
- [1].Babu AS, Arena R, Myers J, et al. Exercise intolerance in pulmonary hypertension: mechanism, evaluation and clinical implications. Expert Rev Respir Med. 2016;10:979–990. [DOI] [PubMed] [Google Scholar]
- [2].Van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–2519. [DOI] [PubMed] [Google Scholar]
- [3].Van de Veerdonk MC, Bogaard HJ, Voelkel NF. The right ventricle and pulmonary hypertension. Heart Fail Rev. 2016;21:259–271. [DOI] [PubMed] [Google Scholar]
- [4].Gan CLJW, Marcus JT, Westerhof N, et al. Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. Am J Physiol-Heart C. 2006;290:H1528–H1533. [DOI] [PubMed] [Google Scholar]
- [5].Hardziyenka M, Campian ME, Verkerk AO, et al. Electrophysiologic remodeling of the left ventricle in pressure overload-induced right ventricular failure. J Am Coll Cardiol. 2012;59:2193–2202. [DOI] [PubMed] [Google Scholar]
- [6].Manders E, Bogaard HJ, Handoko ML, et al. Contractile dysfunction of left ventricular cardiomyocytes in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2014;64:28–37. [DOI] [PubMed] [Google Scholar]
- [7].Lourenco AP, Roncon-Albuquerque R, Jr, Bras-Silva C, et al. Myocardial dysfunction and neurohumoral activation without remodeling in left ventricle of monocrotaline-induced pulmonary hypertensive rats. Am J Physiol Heart Circ Physiol. 2006;291:H1587–H1594. [DOI] [PubMed] [Google Scholar]
- [8].Ehlken N, Lichtblau M, Klose H, et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: a prospective, randomized, controlled trial. Eur Heart J. 2016;37:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pandey A, Garg S, Khunger M, et al. Efficacy and safety of exercise training in chronic pulmonary hypertension: systematic review and meta-analysis. Circ Heart Fail. 2015;8:1032–1043. [DOI] [PubMed] [Google Scholar]
- [10].Moreira-Goncalves D, Ferreira R, Fonseca H, et al. Cardioprotective effects of early and late aerobic exercise training in experimental pulmonary arterial hypertension. Basic Res Cardiol. 2015;110:57. [DOI] [PubMed] [Google Scholar]
- [11].Nogueira-Ferreira R, Moreira-Gonçalves D, Silva AF, et al. Exercise preconditioning prevents MCT-induced right ventricle remodeling through the regulation of TNF superfamily cytokines. Int J Cardiol. 2016;203:858–866. [DOI] [PubMed] [Google Scholar]
- [12].Moreira-Goncalves D, Ferreira-Nogueira R, Santos M, et al. Exercise training in pulmonary hypertension and right heart failure: insights from pre-clinical studies. Adv Exp Med Biol. 2017;999:307–324. [DOI] [PubMed] [Google Scholar]
- [13].Woolstenhulme JG, Guccione AA, Herrick JE, et al. Left ventricular function before and after aerobic exercise training in women with pulmonary arterial hypertension. J Cardiopulm Rehabil Prev. 2019;39:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lawler JM, Powers SK, Hammeren J, et al. Oxygen cost of treadmill running in 24-month-old Fischer-344 rats. Med Sci Sports Exerc. 1993;25:1259–1264. [PubMed] [Google Scholar]
- [15].Moreira-Goncalves D, Henriques-Coelho T, Fonseca H, et al. Intermittent cardiac overload results in adaptive hypertrophy and provides protection against left ventricular acute pressure overload insult. J Physiol. 2015;593:3885–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Henriques-Coelho T, Correia-Pinto J, Roncon-Albuquerque R, Jr, et al. Endogenous production of ghrelin and beneficial effects of its exogenous administration in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2004;287:H2885–H2890. [DOI] [PubMed] [Google Scholar]
- [17].Kasahara Y, Kiyatake K, Tatsumi K, et al. Bioactivation of monocrotaline by P-450 3A in rat liver. J Cardiovasc Pharmacol. 1997;30:124–129. [DOI] [PubMed] [Google Scholar]
- [18].Schultze AE, Roth RA. Chronic pulmonary hypertension-the monocrotaline model and involvement of the hemostatic system. J Toxicol Environ Health B Crit Rev. 1998;1:271–346. [DOI] [PubMed] [Google Scholar]
- [19].Henriques-Coelho T, Roncon-Albuquerque Junior R, Lourenco AP, et al. Ghrelin reverses molecular, structural and hemodynamic alterations of the right ventricle in pulmonary hypertension. Rev Port Cardiol. 2006;25:55–63. [PubMed] [Google Scholar]
- [20].Henriques-Coelho T, Oliveira SM, Moura RS, et al. Thymulin inhibits monocrotaline-induced pulmonary hypertension modulating interleukin-6 expression and suppressing p38 pathway. Endocrinology. 2008;149:4367–4373. [DOI] [PubMed] [Google Scholar]
- [21].Correia-Pinto J, Henriques-Coelho T, Roncon-Albuquerque R, et al. Time course and mechanisms of left ventricular systolic and diastolic dysfunction in monocrotaline-induced pulmonary hypertension. Basic Res Cardiol. 2009;104:535–545. [DOI] [PubMed] [Google Scholar]
- [22].Hardziyenka M, Campian ME, Reesink HJ, et al. Right ventricular failure following chronic pressure overload is associated with reduction in left ventricular mass evidence for atrophic remodeling. J Am Coll Cardiol. 2011;57:921–928. [DOI] [PubMed] [Google Scholar]
- [23].Dell’Italia LJ. The forgotten left ventricle in right ventricular pressure overload. J Am Coll Cardiol. 2011;57:929–930. [DOI] [PubMed] [Google Scholar]
- [24].Harvey PA, Leinwand LA. The cell biology of disease: cellular mechanisms of cardiomyopathy. J Cell Biol. 2011;194:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rizvi MA, Katwa L, Spadone DP, et al. The effects of endothelin-1 on collagen type I and type III synthesis in cultured porcine coronary artery vascular smooth muscle cells. J Mol Cell Cardiol. 1996;28:243–252. [DOI] [PubMed] [Google Scholar]
- [26].Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. [DOI] [PubMed] [Google Scholar]
- [27].Gonzalez A, Ravassa S, Beaumont J, et al. New targets to treat the structural remodeling of the myocardium. J Am Coll Cardiol. 2011;58:1833–1843. [DOI] [PubMed] [Google Scholar]
- [28].Handoko ML, De Man FS, Happe CM, et al. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation. 2009;120:42–49. [DOI] [PubMed] [Google Scholar]
- [29].Souza-Rabbo MP, Silva LF, Auzani JA, et al. Effects of a chronic exercise training protocol on oxidative stress and right ventricular hypertrophy in monocrotaline-treated rats. Clin Exp Pharmacol Physiol. 2008;35:944–948. [DOI] [PubMed] [Google Scholar]
- [30].Lennon SL, Quindry J, Hamilton KL, et al. Loss of exercise-induced cardioprotection after cessation of exercise. J Appl Physiol (1985). 2004;96:1299–1305. [DOI] [PubMed] [Google Scholar]


