Abstract
Background:
The commonly available platelet indices are platelet distribution width (PDW), plateletcrit (PCT), and mean platelet volume (MPV). They have been used in diagnosis and prognosis of various abdominal disorders. They have never been used to predict severity of alcoholic hepatitis.
Methods:
A retrospective analysis of chronic alcohol consumers presenting with jaundice and deranged liver function tests was performed. Maddrey discriminant function (MDF) and modified end-stage liver disease (MELD) scores were calculated and patients compared between severe and nonsevere alcoholic hepatitis (MDF ≥32 vs MDF <32 and MELD >20 vs MELD ≤20). Logistic regression analysis was performed to find significant predictors. Receiver operating characteristic was used to find the area under the curve. Spearman correlation was performed to discover association between platelet indices and severity scores.
Results:
There were 119 patients in the study. Coexisting illnesses included pancreatitis, cirrhosis, infections, and alcohol withdrawal syndrome. The mean age (years), duration of alcohol consumption (years), and ethanol (g/day) were 45.13 ± 11.53, 18.84 ± 11.40, and 65.61 ± 45.42, respectively. The average MELD and Maddrey scores were 14.13 ± 5.17 and 36.45 ± 29.63, respectively. The mean platelet counts, PDW, MPV, and PCT were 194.01 ± 178.82 × 109/L, 17.10 ± 1.21, 5.99 ± 0.96, and 0.14 ± 0.04, respectively. PDW >18 and MPV had a significant positive correlation with MELD scores. Only bilirubin and prothrombin prolongation were significant predictors of severe alcoholic hepatitis. The area under the curve was highest for PCT at 0.622 (P = .07; confidence interval = 0.500–0.743).
Conclusions:
Platelet indices appear to be significantly altered in alcoholic hepatitis, but they do not predict severe disease. Whether this inability to predict severe alcoholic hepatitis is due to coexisting illnesses such as pancreatitis, cirrhosis, and infection needs to be studied further.
Keywords: alcoholic hepatitis, ethanol, platelet indices, platelets
Introduction
The platelet indices include platelet distribution width (PDW) in percentage, plateletcrit (PCT) in percentage, mean platelet volume (MPV) in femtoliters, mean platelet component in grams/deciliter, mean platelet mass in picograms, platelet larger cell ratio in percentage, and immature platelet fraction in percentage.1 The first 3 indices are available in automated hematology analyzers.1 Alcoholic hepatitis is an inflammatory liver disorder occurring in chronic alcohol consumers (>6 months and abstinence <60 days) manifesting with fatigue, anorexia, and jaundice (within prior 8 weeks).2 It is characterized by elevation of transaminases <400 and aspartate transaminase/alanine transaminase (AST/ALT) ratio >1.5 with total serum bilirubin >3 mg/dL.2 Prognostic models for alcoholic hepatitis include both static [model for end-stage liver disease (MELD), Maddrey discriminant function (MDF)] and dynamic (Lillie) models.3 Platelet indices have been used in chronic viral hepatitis, hepatic fibrosis, nonalcoholic steatohepatitis, chronic alcohol consumption, alcohol-related fatty liver, and cirrhosis.4–7 But they have not been used to prognosticate alcoholic hepatitis. Therefore, we attempted to utilize the platelet indices to predict severity of alcoholic hepatitis based on MELD and MDF scoring systems.
Materials and methods
This retrospective cross-sectional study was performed at Indira Gandhi Medical College and Research Institute, Pondicherry, a government-funded teaching hospital catering to patients from Pondicherry and the neighboring districts of Tamil Nadu. The aims of the study were to determine whether platelet indices could predict severe alcoholic hepatitis and to compare the platelet indices in patients with and without severe alcoholic hepatitis. Following the approval of the Institute Ethics Committee (IEC/PP/2015/42 dated July 2, 2015), patients were recruited between April 1, 2017 and October 31, 2019.
Alcoholic hepatitis was diagnosed in patients presenting with jaundice who had consumed at least 40 g/day of alcohol for ≥6 months, and their AST/ALT ratio was at least >1.5 (Consensus Definitions for Alcoholic Hepatitis-Minimum criteria for clinical diagnosis).2 Grams of ethanol were calculated as follows: 1.5 oz of 80 proof liquor and 12 oz of beer contain 14 g of ethanol.8 There was no equivalent for country-made liquor such as arrack (made from fermented coconut sap) and hence the calculation for 80 proof liquor was used. Drug-related liver injury was ruled out by history at the time of admission. HIV testing was negative in all individuals. Three patients positive for hepatitis B and 2 of hepatitis C were excluded from the study. Ceruloplasmin and screening for Kayser-Fleischer ring was performed for patients <40 years and they were noncontributory. Testing for autoimmune hepatitis could not be done, since the facility was not available in the hospital. The complete blood count sent at the time of admission was perused for obtaining the values of platelet indices. The complete blood count was performed using a Celltac Alpha MEK-6400 automated hematology analyzer within 2 hours of obtaining a 2-mL venepuncture sample into an ethylenediamine tetraacetic acid tube. MDF and MELD scores were calculated for each patient using an app Qx Calculate v.7.1.12.0. An MDF score of ≥32 and MELD score of >20 was considered as severe alcoholic hepatitis. Patients with and without severe alcoholic hepatitis were compared using both scoring systems.
Demographics, clinical, and laboratory data were noted in an Excel spreadsheet and analyzed using IBM SPSS v22 for Windows. Frequencies of historical and clinical variables were calculated, and Chi-square analysis was used to compare these frequencies with severe alcoholic hepatitis. Means ± standard deviations were calculated for continuous variables and independent-samples t test was used to compare the means in patients with and without severe alcoholic hepatitis. Logistic regression analysis was performed to find significant predictors of severe alcoholic hepatitis. Spearman correlation was used to find out significant association between platelet indices and scoring systems. Statistical significance was considered if the P value <.05.
Results
The study had 119 patients of whom the overwhelming majority were men (115/119). Seventy-two patients (60.5%) presented with abdominal pain, whereas 36 complained of fever. Hepatomegaly (n = 56) and disorientation (n = 41) were the most common systemic findings. Coexisting illnesses included pancreatitis (n = 14), cirrhosis (n = 19), infections (n = 23), alcohol withdrawal syndrome (n = 103), and anemia (n = 61). The mean age (years), duration of alcohol consumption (years), ethanol (g/day), and duration of smoking (years) was 45.13 ± 11.53, 18.84 ± 11.40, 65.61 ± 45.42, and 7.29 ± 12.05, respectively. The average MELD and Maddrey scores were 14.13 ± 5.17 and 36.45 ± 29.63, respectively. The mean platelet counts, PDW, MPV, and PCT were 194.01 ± 178.82 × 109/L, 17.10 ± 1.21, 5.99 ± 0.96, and 0.14 ± 0.04, respectively. Mean AST and ALT were 147.49 ± 87.21 and 69.28 ± 49.74, respectively. PDW >18 (r = 0.214; P = .03) and MPV (r = 0.225; P = .02) had a significant positive correlation with MELD scores.
Comparison between patients with and without severe alcoholic hepatitis based upon the 2 scoring systems are given in Tables 1 and 2. Bilirubin and prothrombin (PT) prolongation were significant in univariate analysis using both scoring systems (Table 3). Multivariate analysis using the significant variables obtained from univariate analysis showed that only bilirubin and PT prolongation were significant predictors of severe alcoholic hepatitis. The area under the curve was highest for PCT at 0.622 (P = .07; confidence interval = 0.500–0.743; Fig. 1).
Table 1.
Demographics, comorbid illnesses, clinical features, and laboratory parameters based on modified end-stage liver disease score
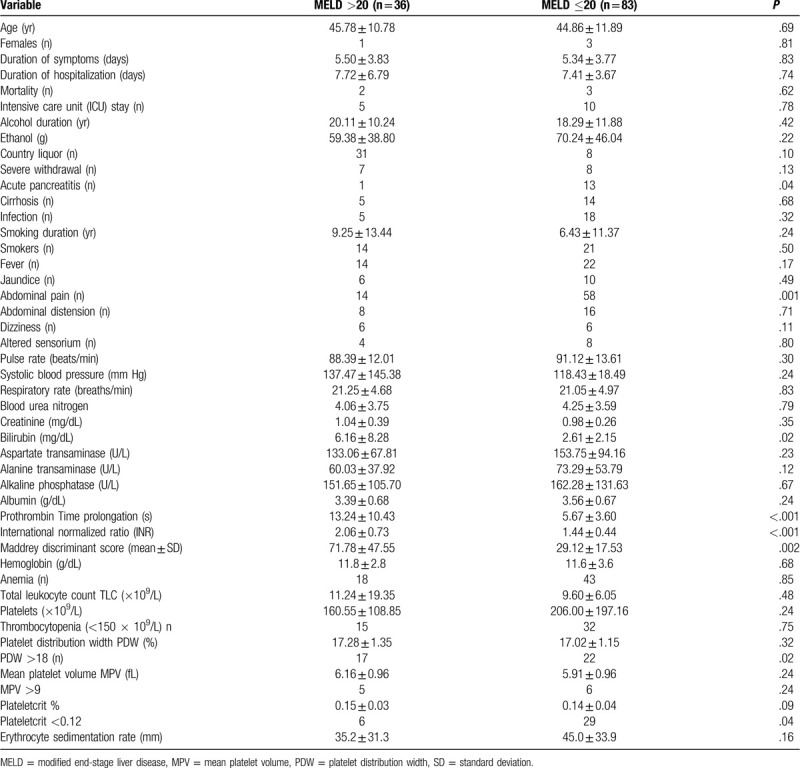
Table 2.
Demographics, comorbid illnesses, clinical features, and laboratory parameters based on Maddrey discriminant function score
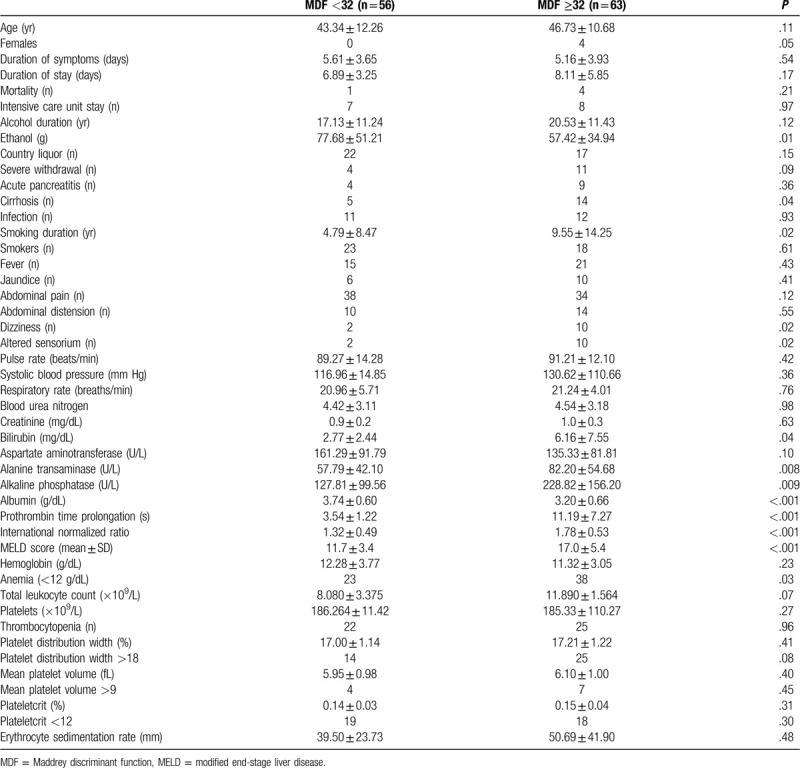
Table 3.
Univariate and multivariate analysis of variables in relation to prognostic scoring systems
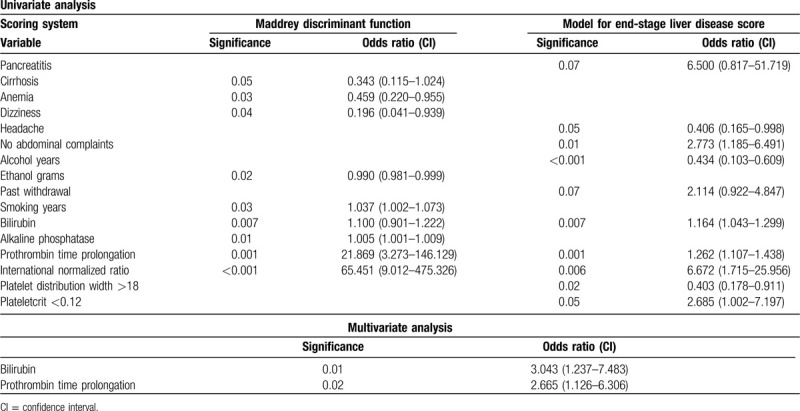
Figure 1.
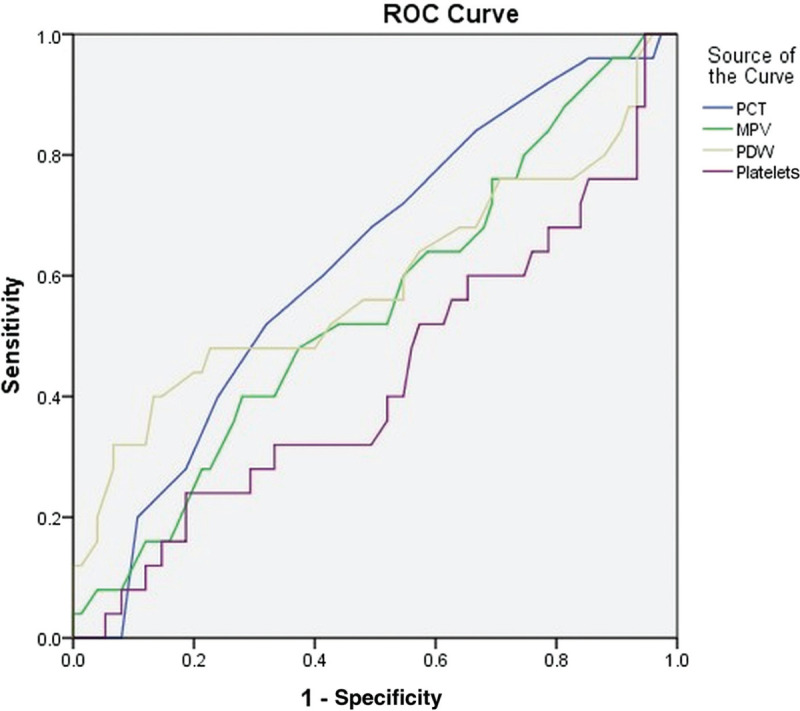
Receiver operating characteristic curve shows highest area under the curve for plateletcrit. MPV = mean platelet volume, PCT = plateletcrit, PDW = platelet distribution width, ROC = receiver operating characteristic.
Discussion
Platelets are cytoplasmic fragments of megakaryocytes that contain 3 types of granules, and play a role in hemostasis, inflammation, defense against microbes, angiogenesis, and wound healing.1 The amount of cytoplasm is directly proportional to the ploidy of the nucleus.9 Thrombopoietin activates megakaryocytes, and thrombopoietin release is stimulated by interleukin-6 in the liver. Similarly, interleukin-6 and tumor necrosis factor-α can cause platelet activation in inflammatory states. The progenitor maturity, megakaryocyte ploidy, and platelet activation affect the platelet counts, its mass, and its morphology.9 Platelets remain in blood for 8 to 12 days and 30% of the total platelets are in the spleen. Platelets assist leukocytes in combating bacterial infection and subsequent healing by means of platelet microparticles, reactive oxygen species, platelet-derived growth factor, and transforming growth factor-β.9
MPV is a measurement of volume dimension and varies from 7.5 to 12 fL, whereas our MPV values ranged from 3.6 to 8.3 fL.9 PDW is calculated from the platelet-size distribution curve at the level of 20%, and platelet anisocytosis increases PDW.1 It ranges from 8.3% to 56.6%, compared to 13.9% to 19.4% in our study.10 The volume that platelets occupy in blood is the PCT. The normal range of PCT is 0.22% to 0.24%.1 The ranges of platelet parameters for the South Indian population like ours was obtained from a study in blood donors: platelets 160 to 478 × 109/L, MPV 6 to 9.2 fL, PCT 0.12 to 0.29, and PDW 15.2% to 18.5%, with the corresponding means being 251 × 109/L, 7.35 fL, 0.19% and 16.38%, respectively.11 The cut-offs used in our study for PDW (>18), PCT (<0.12), and MPV (>9) were obtained from these ranges.
Megakaryocytes determine the MPV—cytokines, age of platelets, rate of production, and platelet number influence the MPV and, increased MPV denotes increased platelet activation and increased rate of production. Thus MPV is a marker of platelet activity.9 More cell granules and adhesion molecules are found in large platelets and hence platelet aggregation also correlates with elevated MPV.9 Age, sex, and ethnicity may influence MPV.9 Larger platelet size with pseudopodia formation can lead to larger PDW.10 Increased PDW and MPV suggests increased marrow release of platelets, whereas thrombocytopenia and reduced PCT suggest excessive platelet consumption.1 Usually, MPV and PDW change in the same direction.1 Under physiological conditions the platelet count is inversely correlated with the MPV.9
Many studies have found an association of platelet indices with thrombotic disorders, infections, malignancies, inflammatory disorders, and trauma.1 They have been studied in abdominal disorders such as acute pancreatitis, acute cholecystitis, and acute mesenteric ischemia.1,10 MPV can act like an acute phase reactant and denotes disease activity in acute pancreatitis.1 In a study by Wang et al,10 higher PDW (>16.5) was associated with higher incidence of organ failure and mortality, and PDW at admission was an independent predictor of persistent organ failure. Reduced MPV was seen in patients with acute appendicitis when compared with controls.1 MPV was also lower in patients with acute cholecystitis compared to controls or patients with chronic cholecystitis.1 MPV has also been evaluated in mesenteric ischemia and was found to be higher in patients than in controls, and in nonsurvivors compared to survivors.1
Among the liver disorders unrelated to alcohol, nonalcoholic fatty liver disease, chronic hepatitis B–related fibrosis, and ascitic fluid infection have been studied using platelet indices. In a study of 130 patients by Saremi et al,5 mean MPV was significantly higher in patients with nonalcoholic fatty liver disease than those without. In a Turkish study of patients with chronic hepatitis B, MPV was significantly higher in patients with higher grade of fibrosis and was described as an independent predictor of hepatic fibrosis.4 PDW and platelet had a significant negative relationship with the degree of inflammation and degree of fibrosis in chronic hepatitis B–related infection. MPV was not useful for inflammation or fibrosis in this study.12 Increased MPV and PDW was observed in patients with ascitic fluid infection.13 MPV had high sensitivity and specificity for predicting spontaneous bacterial peritonitis with a cut-off of 8.7 fL at 95.9% and 91.7%, respectively.13
Many studies have shown that alcohol has an inhibitory effect on platelet aggregation in response to collagen or adenosine diphosphate14; ethanol can inhibit as well as enhance platelet activation.14 In a study of 178 patients, the mean MPV was increased in patients with chronic alcohol abuse compared to that of controls.7 MPV/platelet ratio differed significantly in patients with chronic alcohol abuse.7 There was no significant difference between our patient groups (MELD >20 and MELD ≤20) with respect to MPV/platelet ratio (0.058 vs 0.066).
Another study from Japan compared healthy controls with patients with fatty liver, alcohol-related cirrhosis, and hepatitis C virus-related cirrhosis.6 Monoclonal antibodies (anti-PAC-1) to fibrinogen receptor on GPIIb/IIIa molecule, anti-CD62P antibody to P-selectin (from α granules), and anti-CD61 antibodies to GPIIIa (present in all platelets) were used in the study.6 Platelet counts correlated inversely with anti-PAC-1 and anti-CD62P antibodies. CD62P, a marker of platelet activation was increased in all groups, with the highest in alcohol-related fatty liver. PAC-1 has affinity to the GPIIb/IIIa only in the activated state, and increased positivity was seen in fatty liver and hepatitis C–related cirrhosis. CD62P and platelet microparticle (platelet-derived microparticles, based on anti-CD61) positivity were higher in patients with alcoholic liver disease compared to controls indicating increased platelet activation. Alcoholic hepatitis was not studied.6 In a Portuguese study of platelet indices in alcohol-related chronic liver disease (n = 65), it was found that PCT and PDW varied significantly in patients compared with controls, the PCT being lower and PDW being higher.15 Using a cut-off of PCT <0.12 and PDW >18, our study echoed similar findings. Using a receiver operating characteristic curve, the area under the curve was highest for PCT (0.622; confidence interval: 0.500–0.743).
The 5 most important scores used in alcoholic hepatitis are MDF, MELD, age bilirubin INR creatinine, Glasgow Alcoholic Hepatitis Score and Lillie. The first 2 scoring systems are used routinely in clinical practice.16 An MDF ≥32 has been shown to markedly reduce survival and is hence termed severe hepatitis. Its static nature is its biggest shortcoming and often, combination with other prognostic scores predicts outcomes more accurately.17 The absolute value of the prothrombin time used in MDF varies with the assay used in different laboratories and is the other limitation.17 The highest sensitivity and specificity in predicting mortality is with an MELD score of >20.18 Thirty-six patients had severe disease according to MELD compared to 63 in MDF. MELD identifies patients at low risk for short-term mortality.17 MELD, age bilirubin INR creatinine, and Glasgow Alcoholic Hepatitis Score were superior to MDF in predicting mortality.3 Nevertheless MDF is the commonest criteria used for entry into clinical trials. Bilirubin and PT prolongation were the only significant predictors of severe alcoholic hepatitis in our study. Since this finding was previously expected, and these variables are already included in the above prognostic models used to predict severity, the findings in this study further support the use of these variables in the models. Other markers studied in alcoholic hepatitis include keratin-18, an intermediate filament protein in the epithelial tissue that is released into blood during hepatic necrosis along with its cleaved form.19 Keratin-18 and the ratio of keratin-18 to its caspase-cleaved form were higher in nonsurviving patients of alcoholic hepatitis compared to survivors and correlated well with mortality in alcoholic hepatitis. K-18 did not correlate well with transaminases, bilirubin, and prothrombin time.19 A need for noninvasive tests to assess disease severity has had progress made in the form of breath tests. A combination of trimethylamine and pentane (TAP model) breath levels could predict diagnosis of alcoholic hepatitis with high sensitivity and specificity.18 We have also attempted a simple, inexpensive and noninvasive test that is routinely available in most hospitals to predict severe alcoholic hepatitis.
Most patients with severe alcoholic hepatitis may have cirrhosis.2 In our study, only 14/63 with severe alcoholic hepatitis had cirrhosis. The prevalence of infections in alcoholic hepatitis varies from 12% to 26% and was in line with the findings of our study (19%).2 Liver biopsy is helpful for confirming diagnosis and prognostication. None of our patients underwent the procedure.
Limitations
This was a retrospective study, and some data regarding biochemical parameters were missing. Alcohol quantity was calculated based on patient's own admission regarding his/her daily consumption and the actual quantity could be higher. It is probable that the interviewer's understanding of patient's terms such as 1 drink, 1 peg, 1 “quarter”(=180 mL of 80 proof liquor), 1 bottle (60, 90, 180, 375, 750, and 1000 mL for 80 proof liquor; 330, 500, and 650 mL for beer available in India) could have underestimated the actual quantity of ethanol, since alcoholic hepatitis occurs with alcohol consumption of ∼100 g/day.20 There was no equivalent for country liquor (arrack) and the same calculation for 80-proof liquor was used; hence, the dose of ethanol may be higher.8 Autoimmune hepatitis could not be ruled out in our patients; considering the overwhelmingly male predominance and heavy alcohol use, the possibility of autoimmune hepatitis appeared to be remote. Bilirubin and transaminase values used were taken at the time of admission and did not necessarily reflect the highest values in the individual patient. There were some comorbid illnesses such as infection, pancreatitis, and cirrhosis that could have affected the platelet indices.
Conclusion
Well-validated severity scoring systems are available for alcoholic hepatitis. Platelet indices have been found to be a marker for several inflammatory disorders, and hence the intent to study them in alcoholic hepatitis. MPV, PDW > 18, and PCT < 0.12 were associated only with MELD scores. Platelet indices appear to be significantly altered in alcoholic hepatitis, but they do not predict severe disease. Whether this inability to predict severe alcoholic hepatitis is due to coexisting illnesses such as pancreatitis, cirrhosis, and infection needs to be studied further.
Acknowledgements
The data that support the findings of this study are openly available in Figshare at http://doi.org/10.6084/m9.figshare.11559591.
Assistance with the study: none.
Conflicts of interest
None.
Presentation
None.
Author contributions
S.V.: Concept, design, and manuscript preparation. S.V.: Statistical analysis, tables, literature search, and manuscript editing. D.J.: Data acquisition and manuscript review.
Conflicts of interest
None.
References
- [1].Budak YU, Polat M, Huysal K. The use of platelet indices, plateletcrit, mean platelet volume and platelet distribution width in emergency non-traumatic abdominal surgery: a systematic review. Biochem Med (Zagreb). 2016;26:178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Im GY. Acute alcoholic hepatitis. Clin Liver Dis. 2019;23:81–98. [DOI] [PubMed] [Google Scholar]
- [3].Forrest EH, Atkinson SR, Richardson P, et al. Application of prognostic scores in the STOPAH trial: discriminant function is no longer the optimal scoring system in alcoholic hepatitis. J Hepatol. 2018;68:511–518. [DOI] [PubMed] [Google Scholar]
- [4].Karagoz E, Ulcay A, Tanoglu A, et al. Clinical usefulness of mean platelet volume and red blood cell distribution width to platelet ratio for predicting the severity of hepatic fibrosis in chronic hepatitis B virus patients. Eur J Gastroenterol Hepatol. 2014;26:1320–1324. [DOI] [PubMed] [Google Scholar]
- [5].Saremi Z, Rastgoo M, Mohammadifard M, Bijari B, Akbari E. Comparison of platelet number and function between nonalcoholic fatty liver disease and normal individuals. J Res Med Sci. 2017;22:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ogasawara F, Fusegawa H, Haruki Y, Shiraishi K, Watanabe N, Matsuzaki S. Platelet activation in patients with alcoholic liver disease. Tokai J Exp Clin Med. 2005;30:41–48. [PubMed] [Google Scholar]
- [7].Cho SY, Lee HJ, Kim JW, Park TS. Mean platelet volume and mean platelet volume/platelet count ratio in patients with chronic alcohol consumption. Platelets. 2015;26:371–372. [DOI] [PubMed] [Google Scholar]
- [8].Brick J. Standardization of alcohol calculations in research. Alcohol Clin Exp Res. 2006;30:1276–1287. [DOI] [PubMed] [Google Scholar]
- [9].Korniluk A, Koper-Lenkiewicz OM, Kamińska J, Kemona H, Dymicka-Piekarska V. Mean platelet volume (MPV): new perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediators Inflamm. 2019;2019:9213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang F, Meng Z, Li S, Zhang Y, Wu H. Platelet distribution width levels can be a predictor in the diagnosis of persistent organ failure in acute pancreatitis. platelet distribution width levels can be a predictor in the diagnosis of persistent organ failure in acute pancreatitis. Gastroenterol Res Pract. 2017;2017:8374215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Naina HVK, Harris S. Platelet and red blood cell indices in Harris platelet syndrome. Blood. 2010;21:303–306. [DOI] [PubMed] [Google Scholar]
- [12].Pan Y, Muheremu A, Wu X, Liu J. Relationship between platelet parameters and hepatic pathology in patients with chronic hepatitis B infection – a retrospective cohort study of 677 patients. J Int Med Res. 2016;44:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Abdel-Razik A, Eldars W, Rizk E. Platelet indices and inflammatory markers as diagnostic predictors for ascitic fluid infection. Eur J Gastroenterol Hepatol. 2014;26:1342–1347. [DOI] [PubMed] [Google Scholar]
- [14].Salem RO, Laposata M. Effects of alcohol on hemostasis. Am J Clin Pathol. 2005;123 (suppl):S96–S105. [DOI] [PubMed] [Google Scholar]
- [15].Costa AC, Ribeiro B, Costa E. Índices plaquetários em indivíduos com doença hepática alcoólica crónica. Arq Gastroenterol. 2007;44:201–204. [DOI] [PubMed] [Google Scholar]
- [16].Singal AK, Kodali S, Vucovich LA, Darley-Usmar V, Schiano TD. Diagnosis and treatment of alcoholic hepatitis: a systematic review. Alcohol Clin Exp Res. 2016;40:1390–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gholam PM. Prognosis and prognostic scoring models for alcoholic liver disease and acute alcoholic hepatitis. Clin Liver Dis. 2016;20:491–497. [DOI] [PubMed] [Google Scholar]
- [18].Dugum MF, McCullough AJ. Acute alcoholic hepatitis, the clinical aspects. Clin Liver Dis. 2016;20:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Woolbright BL, Bridges BW, Dunn W, Olson JC, Weinman SA, Jaeschke H. Cell death and prognosis of mortality in alcoholic hepatitis patients using plasma keratin-18. Gene Expr. 2017;17:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Indian liquor [Internet] 2014. https://delhiexcise.gov.in/pdf/IL_PriceList.pdf.


