Abstract
Introduction
The novel coronavirus disease 2019 (COVID-19) has rapidly spread across the globe. Pre-existing comorbidities have been found to have a dramatic effect on the disease course. We sought to analyze the effect of asthma on the disease progression and outcomes of COVID-19 patients.
Methods
We conducted a multi-center retrospective study of positively confirmed COVID-19 patients. The primary outcome of interest was in-hospital mortality. Secondary outcomes were the Intensive Care Unit (ICU) admission, intubation, mechanical ventilation, and length of hospital stay.
Results
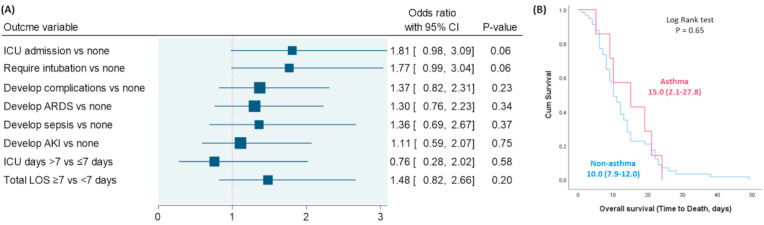
A total of 502 COVID-19 adult patients (72 asthma and 430 non-asthma cohorts) with mean age of 60.7 years were included in the study. The frequency of asthma in hospitalized cohorts was 14.3%. Univariate analysis revealed that asthma patients were more likely to be obese (75% versus 54.2%, p = 0.001), with a higher frequency of intubation (40.3% versus 27.8%, p = 0.036), and required a longer duration of hospitalization (15.1 ± 12.5 versus 11.5 ± 10.6, p = 0.015). After adjustment, multivariable analysis showed that asthmatic patients were not associated with higher risk of ICU admission (OR = 1.81, 95%CI = 0.98–3.09, p = 0.06), endotracheal intubation (OR = 1.77, 95%CI = 0.99–3.04, p = 0.06) or complications (OR = 1.37, 95%CI = 0.82–2.31, p = 0.23). Asthmatic patients were not associated with higher odds of prolonged hospital length of stay (OR = 1.48, 95%CI = 0.82–2.66, p = 0.20) or with ICU stay (OR = 0.76, 95%CI = 0.28–2.02, p = 0.58). Kaplan-Meier curve showed no significant difference in the overall survival of the two groups (p = 0.65).
Conclusion
Despite the increased prevalence of hospitalization in elder asthmatic COVID-19 patients, after adjustment for other variables, it was neither associated with increased severity nor worse outcomes.
Keywords: Chronic lung disease, SARS-CoV-2, Prognosis
Graphical abstract
Introduction
Since its initial description in late 2019, the novel coronavirus (COVID-19) has become a global pandemic, overwhelming our healthcare systems and depleting resource stockpiles [1]. In some pandemic hotspots, physicians have had to make difficult decisions regarding lifesaving interventions due to a lack of supplies such as ventilators [2]. Further complicating management of this disease, COVID-19 has a broad clinical presentation. Patients can be asymptomatic carriers or have mild non-respiratory and respiratory symptoms. On the other hand, the virus can also present with severe pneumonia or lethal respiratory failure. Because of these severe outcomes, gaining an understanding of COVID-19's disease course is essential to guiding treatment approaches and optimizing individual patient outcomes.
Preexisting comorbidities can have a dramatic impact on the course of a COVID-19 infection. It is, therefore, crucial to identify which comorbidities most contribute to severe infections, which may influence individual patient care plans [3]. To better understand these relationships, the Centers for Disease Control and Prevention (CDC) is actively incorporating new and emerging data to update their treatment recommendations for patients with varying comorbid conditions. Currently, the CDC reports that asthma is present in about 17% of hospitalized COVID-19 patients, making it the fourth most prevalent comorbidity behind hypertension, obesity, and diabetes [4]. Obese and diabetic patients have been categorized as high-risk, but there is still limited data regarding the impact of bronchial asthma on COVID-19 outcomes [5].
Because asthma is a chronic lung disease, it follows that asthmatics are at a greater risk for negative outcomes with a respiratory virus. However, there is a debate over the expected outcomes in COVID-19 infected patients with asthma. Some members of the coronaviridiae family that are associated with the common cold have been linked to asthma exacerbations [6]. In contrast, Jackson et al. reported an observed protective relationship for asthma and atopic conditions in COVID-19 patients [7]. In efforts to explain this phenomenon, it was proposed that patients with asthma and ectopic diseases may exhibit a lower concentration of respiratory tract angiotensin-converting enzyme (ACE) receptors in patients. As has been widely reported, ACE receptors and specifically, ACE2, are known to be the host receptor site for SARS-CoV-2 [7,8].
Considering that more than 19 million adults in the United States have asthma [9], it is crucial to characterize asthma in the setting of COVID-19. Therefore, we retrospectively evaluated hospitalized patients with laboratory-confirmed SARS-CoV-2, focusing on the differing outcomes between asthmatic and non-asthmatic patients. This study aimed to help further understand asthma's impact on COVID-19 outcomes, hopefully enabling physicians to tailor management and resource allocation in response to the ongoing pandemic.
Methodology
Study population
Following Institutional Review Board (IRB) approval, data of hospitalized patients with laboratory-confirmed SARS-CoV-2 infection was gathered and entered into the web-based data collection platform, RedCap. Patients included in the study presented to either University Medical Center New Orleans (UMCNO), Tulane Medical Center (TMC), Saint Francis Medical Center Monroe (SFMC), or Our Lady of the Lake Regional Medical Center (OLOL) during the period between March 15 to June 9, 2020.
Variables
A broad range of data was recorded, including but not limited to, the following: demographics, medical history, comorbidities, clinical presentation, daily laboratory values, complications, and outcomes. Patients were excluded if they were below 18 years of age or did not have recorded outcome data. Obesity was defined as body mass index (BMI) ≥30 kg/m2 [10]. For severity assessment, the CURB-65 score was estimated based on confusion status, respiratory rate≥30, blood pressure (systolic <90 mmHg or diastolic ≤60 mmHg), age ≥65 years, and blood urea nitrogen level >19 mg/dL (>7 mmol/L), and ranged from 0 to 5 [11]. Quick Sequential Organ Failure Assessment (qSOFA) score was calculated using Glasgow coma score <15, respiratory rate ≥22, and systolic blood pressure ≤100, and was set to be a positive indicator for the poor outcome if ≥ 2 [12]. Horowitz index (PaO2/FIO2 ratio) was calculated as the ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen to determine a patient's respiratory efficiency [13]. The neutrophil-lymphocyte ratio (NLR), an indicator for poor outcome, was also estimated [14].
Outcomes
A comparison between the asthmatic and non-asthmatic cohorts was performed. The primary outcome of interest was in-hospital mortality. Secondary outcomes were risk of Intensive Care Unit (ICU) admission, risk of endotracheal intubation, duration of mechanical ventilation, and length of hospital stay. Clinical diagnosis of complications was made using standard definitions such as the Risk, Injury, Failure, Loss of Kidney Function, and End-stage kidney disease (RIFLE) score for renal injury [15]. Berlin criteria were also utilized to identify cases of acute respiratory distress syndrome (ARDS) [16].
Statistical analysis
Data management was performed in SAS 9.4 (SAS Institute Inc., SAS 9.4, Cary, NC: SAS Institute Inc., 2013), and statistical analysis was carried out using SPSS 26.0 (SPSS, Inc., Chicago, IL, USA) and STATA 16.0 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). Continuous variables were described as medians and interquartile ranges, while categorical data were presented as frequencies and percentages. Chi-square or Fisher's Exact tests were used for categorical variables. Student's t and Mann-Whitney U tests were applied for quantitative variables to examine the difference between asthmatic and non-asthmatics groups. Shapiro-Wilk test was used to test the normality of the continuous variables. Kaplan-Meier survival analysis and the Log Rank test was used to compare the in-hospital mortality in the two groups. Binary logistic regression analysis was used to assess the role of asthma comorbidity in the outcomes of COVID-19 disease. Age, gender, and obesity were adjusted in the model. Hosmer-Lemeshow test was used to assess goodness-of-fit. Results were reported as odds ratio (OR), and 95% confidence interval (CI) and two-sided p values below 0.05 were set as significant.
Results
Characteristics of the study population
A total of 502 confirmed positive COVID-19 patients were identified from multiple hospitals in Louisiana, including 72 bronchial asthma patients and 430 non-asthmatic controls. Their mean age was 60.6 ± 13.9 years and 60.8 ± 15.7 years, respectively (p = 0.89). There was no observed age or gender difference. Females accounted for 54.2% (39 patients) in the asthma group versus 52.0% (222 patients) in the non-asthma group (p = 0.79). The frequency of obesity among asthmatic patients (75%) was significantly higher than those without current asthma (54.2%), p = 0.001. On admission, asthma cohorts presented with greater respiratory rate (24.14 ± 7.03 vs 21.77 ± 7.20 breaths per minute, p = 0.015) and lower bicarbonate level (21.73 ± 5.41 vs 24.67 ± 3.57 mEq/L, p = 0.022). No other significant differences were found regarding clinical and laboratory features (Table 1 ).
Table 1.
Baseline characteristics of COVID-19 patients at admission.
| Characteristics | Non-asthma (n = 430) | Asthma (n = 72) | P-value | |
|---|---|---|---|---|
| Age | Mean ± SD | 60.8 ± 15.7 | 60.6 ± 13.9 | 0.89 |
| 18–49 years | 91 (21.3) | 20 (27.8) | 0.20 | |
| 50–64 years | 155 (36.2) | 29 (40.3) | ||
| ≥65 years | 182 (42.5) | 23 (31.9) | ||
| Sex | Female | 222 (52.0) | 39 (54.2) | 0.79 |
| Male | 205 (48.0) | 33 (45.8) | ||
| Race | African American | 325 (75.6) | 50 (69.4) | 0.09 |
| White | 66 (15.3) | 18 (25.0) | ||
| Not Reported | 39 (9.1) | 4 (5.6) | ||
| BMI, kg/m2 | Mean ± SD | 32.90 ± 8.32 | 35.79 ± 9.43 | 0.034 |
| Smoking | None | 299 (69.5) | 47 (65.3) | 0.71 |
| Past smoker | 94 (21.9) | 17 (23.6) | ||
| Current smoker | 37 (8.6) | 8 (11.1) | ||
| Chief complaint | Shortness of breath | 231 (53.7) | 44 (61.1) | 0.25 |
| Fever | 94 (21.9) | 14 (19.4) | 0.76 | |
| Cough | 93 (21.6) | 21 (29.2) | 0.17 | |
| Flu-like symptoms | 26 (6) | 6 (8.3) | 0.44 | |
| Fatigue | 28 (6.5) | 4 (5.6) | 0.71 | |
| Chest pain | 10 (2.3) | 3 (4.2) | 0.41 | |
| Altered mental status | 64 (14.9) | 6 (8.3) | 0.20 | |
| Headache | 3 (0.7) | 2 (2.8) | 0.15 | |
| Nausea, vomiting, diarrhea | 27 (6.3) | 4 (5.6) | 0.81 | |
| Comorbidities | Hypertension | 306 (71.2) | 51 (70.8) | 0.95 |
| Diabetes | 183 (42.6) | 28 (38.9) | 0.60 | |
| Chronic heart failure | 46 (10.7) | 4 (5.6) | 0.20 | |
| Arrhythmia | 43 (10) | 5 (6.9) | 0.52 | |
| COPD | 31 (7.2) | 5 (6.9) | 0.93 | |
| Chronic kidney disease | 68 (15.8) | 8 (11.1) | 0.37 | |
| Cancer | 47 (10.9) | 8 (11.1) | 0.96 | |
| Coronary artery disease | 46 (10.7) | 3 (4.2) | 0.08 | |
| Cerebrovascular disease | 34 (7.9) | 4 (5.6) | 0.63 | |
| Obesity | 233 (54.2) | 54 (75) | 0.001 | |
| Severity | qSOFA score | 0.69 ± 0.70 | 0.89 ± 0.72 | 0.06 |
| CURB65 score | 1.47 ± 1.13 | 1.33 ± 1.20 | 0.41 | |
| Orientation | Glasgow coma score | 13.59 ± 3.11 | 13.57 ± 3.57 | 0.98 |
| Vital signs | Temperature | 99.47 ± 1.67 | 99.75 ± 2.09 | 0.24 |
| Pulse rate | 89.31 ± 19.41 | 93.78 ± 20.38 | 0.09 | |
| Systolic blood pressure | 125.52 ± 21.06 | 127.41 ± 20.20 | 0.51 | |
| Diastolic blood pressure | 73.66 ± 15.30 | 73.73 ± 13.44 | 0.97 | |
| Mean arterial pressure | 100.89 ± 18.52 | 101.75 ± 18.93 | 0.73 | |
| Respiratory rate | 21.77 ± 7.20 | 24.14 ± 7.03 | 0.015 | |
| ABG findings | SaO2 | 92.99 ± 7.80 | 93.59 ± 7.94 | 0.57 |
| pH respiratory | 7.26 ± 1.01 | 7.39 ± 0.08 | 0.51 | |
| PaCO2 | 39.24 ± 13.75 | 38.08 ± 10.53 | 0.68 | |
| PaO2 | 89.67 ± 67.34 | 82.39 ± 78.09 | 0.62 | |
| HCO3 | 24.67 ± 3.57 | 21.73 ± 5.41 | 0.022 | |
| FiO2 (%) | 40.52 ± 29.58 | 43.18 ± 30.99 | 0.78 | |
| PaO2/FiO2 ratio | 244.62 ± 111.31 | 236.00 ± 115.17 | 0.82 | |
| Laboratory findings | White blood cells | 8.18 ± 5.70 | 7.95 ± 3.46 | 0.77 |
| Hemoglobin | 12.11 ± 2.03 | 11.97 ± 2.31 | 0.64 | |
| Hematocrit | 36.16 ± 5.91 | 36.70 ± 5.93 | 0.62 | |
| Platelet count | 236.56 ± 104.9 | 246.15 ± 103. | 0.51 | |
| Neutrophil count | 7.08 ± 9.91 | 6.20 ± 3.25 | 0.50 | |
| Lymphocyte count | 1.33 ± 1.95 | 1.09 ± 0.43 | 0.34 | |
| Neutrophil lymphocyte ratio | 7.97 ± 10.42 | 6.55 ± 4.51 | 0.30 | |
| Serum sodium | 204.48 ± 902.78 | 137.24 ± 24.9 | 0.65 | |
| Serum potassium | 4.09 ± 1.11 | 4.08 ± 0.86 | 0.98 | |
| Serum chloride | 101.47 ± 8.85 | 101.82 ± 4.03 | 0.82 | |
| Calcium corrected | 9.01 ± 0.66 | 8.99 ± 0.86 | 0.88 | |
| Random blood sugar | 145.04 ± 86.29 | 155.97 ± 88.81 | 0.36 | |
| Blood urea nitrogen | 26.50 ± 20.20 | 22.95 ± 20.89 | 0.20 | |
| Serum creatinine | 1.82 ± 2.05 | 1.57 ± 1.88 | 0.37 | |
| Albumin | 3.24 ± 0.56 | 3.36 ± 0.59 | 0.18 | |
| Bilirubin | 0.64 ± 0.49 | 0.52 ± 0.19 | 0.23 | |
| Alkaline phosphatase | 77.07 ± 45.08 | 65.54 ± 19.23 | 0.22 | |
| AST | 48.59 ± 35.23 | 53.63 ± 38.84 | 0.53 | |
| ALT | 33.01 ± 27.20 | 45.67 ± 35.96 | 0.05 | |
| Anion gap | 12.22 ± 10.08 | 10.61 ± 2.68 | 0.50 | |
| Lactic acid | 52.89 ± 104.58 | 1.75 ± 0.78 | 0.51 | |
| Troponin | 3.04 ± 14.72 | 0.60 ± 0.94 | 0.78 | |
| HbA1c | 7.51 ± 2.81 | 9.80 ± 4.81 | 0.33 | |
| C-reactive protein | 48.21 ± 63.08 | 38.47 ± 58.98 | 0.64 | |
| Procalcitonin | 11.49 ± 66.12 | 0.24 ± 0.22 | 0.51 | |
| Ferritin | 987.87 ± 1881.3 | 1632.3 ± 3293.5 | 0.26 | |
Data are presented as mean and standard deviation or frequency and percentage. BMI: body mass index. SaO2: oxygen saturation, PaO2: partial pressure of oxygen, PaCO2: partial pressure of carbon dioxide, HCO3: bicarbonate, FiO2: Fraction of inspired oxygen, AST: Aspartate transaminase, ALT: alanine transaminase, HbA1c: glycosylated hemoglobin. Chi-square, Fisher's Exact, Student's t, or Mann-Whitney U tests were used. P-value at <0.05 was considered significant.
Outcomes of asthma patients
Univariate analysis of COVID-19 outcomes revealed that asthma was significantly associated with higher rate of endotracheal intubation (40.3% vs 27.8%, p = 0.036), mechanical ventilation (both invasive and non-invasive) (70.7% vs 52.2%, p = 0.039), and longer hospital length of stay (15.14 ± 12.48 days vs 11.51 ± 10.58 days, p = 0.015). Asthma was not associated with a higher rate of Intensive Care Unit (ICU) admission (22.2% vs 14.9%, p = 0.12), acute respiratory distress syndrome (37.5% vs 30.9%, p = 0.27), or death (9.7% vs 13.5%, p = 0.45) among COVID-19 patients. No other clinical and laboratory findings were different between patients with and without asthma (Table 2 ).
Table 2.
Comparison of the outcomes between asthmatic and non-asthmatic groups.
| Characteristics | Non-asthma (n = 423) | Asthma (n = 72) | P value | |
|---|---|---|---|---|
| Procedures | Intubation | 118 (27.8) | 29 (40.3) | 0.036 |
| Extubation | 76 (64.4) | 25 (86.2) | 0.026 | |
| Re-intubation | 10 (13.2) | 4 (16.0) | 0.74 | |
| Mechanical ventilation | 109 (52.2) | 29 (70.7) | 0.039 | |
| ICU admission | 63 (14.9) | 16 (22.2) | 0.12 | |
| Days of events | Intubation days | 1.69 ± 2.53 | 2.62 ± 3.53 | 0.13 |
| Ventilation days | 2.05 ± 4.48 | 1.77 ± 2.70 | 0.72 | |
| ICU LOS | 9.14 ± 7.22 | 9.00 ± 7.65 | 0.93 | |
| ICU-free days | 9.77 ± 10.16 | 12.17 ± 10.68 | 0.10 | |
| Total LOS | 11.51 ± 10.58 | 15.14 ± 12.48 | 0.015 | |
| Time to death | 12.89 ± 8.79 | 14.71 ± 6.99 | 0.60 | |
| Complications | None | 216 (50.2) | 30 (41.7) | 0.20 |
| One or more | 214 (49.8) | 42 (58.3) | ||
| Types of complications | ARDS | 133 (30.9) | 27 (37.5) | 0.27 |
| RF/AKI | 100 (23.3) | 16 (22.2) | 0.84 | |
| Sepsis | 61 (14.2) | 14 (19.4) | 0.28 | |
| Bacteremia | 28 (6.5) | 6 (8.3) | 0.61 | |
| Current state | Still hospitalized | 7 (1.6) | 0 (0) | 0.60 |
| Closed cases | 423 (98.4) | 72 (100) | ||
| Mortality | Discharged alive | 366 (86.5) | 65 (90.3) | 0.45 |
| Died | 57 (13.5) | 7 (9.7) | ||
| Death location | ICU | 5 (8.8) | 1 (14.3) | 0.51 |
| Floor | 52 (91.2) | 6 (85.7) | ||
Seven non-asthmatic cases were still hospitalized thus was excluded from the analysis. ICU: intensive care unit, LOS: length of stay, ARDS: acute respiratory distress syndrome, RF/AKI: renal failure/acute kidney injury. Chi-square, Fisher's Exact, or Student's t were used. P-value at <0.05 was considered significant.
Comparison stratified by obesity showed both asthmatic and non-asthmatic patients had overall similar demographic features, presenting manifestations and comorbidities in each subgroup. However, clinical courses differed significantly between some groups (Table 3 ). On comparison to non-asthmatic obese patients, obese asthmatic patients were more likely to develop sepsis (25.9% vs 14.2%, p = 0.042), had higher risk of ICU admission (48.1% vs 33.2%, p = 0.042), and required prolonged intubation (2.73 ± 3.63 days vs 1.38 ± 2.07, p = 0.032).
Table 3.
Demographic and clinical outcomes of asthma and non-asthma patients stratified by obesity.
| Characteristics | Non-obese |
Obese |
|||||
|---|---|---|---|---|---|---|---|
| Non-asthma (n = 197) | Asthma (n = 18) | P value | Non-asthma (n = 233) | Asthma (n = 54) | P-value | ||
| Age | Mean ± SD | 65.02 ± 16.32 | 59.50 ± 18.30 | 0.18 | 58.28 ± 14.38 | 57.09 ± 13.03 | 0.58 |
| Sex | Female | 80 (41) | 8 (44.4) | 0.81 | 135 (57.9) | 38 (71.7) | 0.09 |
| Male | 115 (59) | 10 (55.6) | 98 (42.1) | 15 (28.3) | |||
| Race | African American | 136 (69) | 15 (83.3) | 0.07 | 177 (76) | 47 (87) | 0.12 |
| White | 43 (21.8) | 0 (0) | 38 (16.3) | 3 (5.6) | |||
| Not Reported | 18 (9.1) | 3 (16.7) | 18 (7.7) | 4 (7.4) | |||
| Smoking | Active smokers | 20 (10.2) | 2 (11.1) | 0.98 | 17 (7.3) | 6 (11.1) | 0.48 |
| Severity | qSOFA | 0.65 ± 0.71 | 0.67 ± 0.65 | 0.95 | 0.71 ± 0.69 | 0.95 ± 0.74 | 0.05 |
| CURB65 | 1.63 ± 1.16 | 1.58 ± 1.31 | 0.90 | 1.33 ± 1.08 | 1.25 ± 1.17 | 0.69 | |
| PF ratio | 269.47 ± 118.89 | 207.67 ± 67.00 | 0.39 | 229.33 ± 104.62 | 248.14 ± 133.52 | 0.67 | |
| Respiratory rate | 20.96 ± 6.98 | 20.94 ± 5.04 | 0.99 | 22.48 ± 7.33 | 25.21 ± 7.32 | 0.022 | |
| Lab testing | Ferritin | 802.44 ± 771.40 | 3192.06 ± 5897.4 | 0.012 | 1121.2 ± 2379.5 | 923.37 ± 767.81 | 0.79 |
| NLR | 8.37 ± 8.81 | 6.10 ± 4.33 | 0.34 | 7.61 ± 11.68 | 6.69 ± 4.60 | 0.61 | |
| Procedures | Intubation | 37 (19.2) | 6 (33.3) | 0.22 | 81 (34.9) | 23 (42.6) | 0.29 |
| Extubation | 22 (59.5) | 5 (83.3) | 0.39 | 54 (66.7) | 20 (87) | 0.06 | |
| Re-intubation | 3 (13.6) | 2 (40) | 0.22 | 7 (13) | 2 (10) | 0.73 | |
| Mechanical ventilation | 35 (39.8) | 5 (62.5) | 0.27 | 74 (61.2) | 24 (72.7) | 0.22 | |
| ICU admission | 42 (21.4) | 4 (22.2) | 0.93 | 77 (33.2) | 26 (48.1) | 0.042 | |
| Days of events | Intubation days | 2.45 ± 3.30 | 2.00 ± 3.37 | 0.80 | 1.38 ± 2.07 | 2.73 ± 3.63 | 0.032 |
| Ventilation days | 0.91 ± 2.85 | 0.63 ± 1.77 | 0.78 | 2.88 ± 5.23 | 2.11 ± 2.86 | 0.46 | |
| ICU LOS | 8.11 ± 7.17 | 7.50 ± 4.95 | 0.91 | 9.00 ± 6.84 | 7.50 ± 5.02 | 0.54 | |
| Total LOS | 11.72 ± 11.22 | 17.79 ± 10.82 | 0.05 | 11.34 ± 10.06 | 14.39 ± 12.91 | 0.08 | |
| Complications | None | 107 (54.3) | 9 (50) | 0.81 | 109 (46.8) | 21 (38.9) | 0.36 |
| One or more | 90 (45.7) | 9 (50) | 124 (53.2) | 33 (61.1) | |||
| Types of complications | ARDS | 45 (22.8) | 5 (27.8) | 0.57 | 88 (37.8) | 22 (40.7) | 0.69 |
| RF/AKI | 44 (22.3) | 3 (16.7) | 0.77 | 56 (24) | 13 (24.1) | 1.00 | |
| Sepsis | 28 (14.2) | 0 (0) | 0.14 | 33 (14.2) | 14 (25.9) | 0.042 | |
| Bacteremia | 17 (8.6) | 0 (0) | 0.37 | 11 (4.7) | 6 (11.1) | 0.07 | |
| Mortality | Discharged alive | 171 (89.1) | 17 (94.4) | 0.70 | 195 (84.4) | 48 (88.9) | 0.52 |
| Died | 21 (10.9) | 1 (5.6) | 36 (15.6) | 6 (11.1) | |||
PF ratio: PaO2: FiO2 ratio, ICU: intensive care unit, LOS: length of stay, ARDS: acute respiratory distress syndrome, RF/AKI: renal failure/acute kidney injury.
Impact of asthma comorbidity on COVID-19 outcomes
Using multivariate regression analysis, bronchial asthma was not associated with higher risk of ICU admission (OR = 1.81, 95%CI = 0.98–3.09, p = 0.06) and endotracheal intubation (OR = 1.77, 95%CI = 0.99–3.04, p = 0.06). In addition, the presence of asthma was not associated with greater odds of complications (OR = 1.37, 95%CI = 0.82–2.31, p = 0.23), prolonged hospital length of stay (OR = 1.48, 95%CI = 0.82–2.66, p = 0.20) nor with the duration of ICU stay (OR = 0.76, 95%CI = 0.28–2.02, p = 0.58) (Fig. 1 A). Kaplan-Meier curve showed no significant difference in overall survival of the two groups (p = 0.65) (Fig. 1B).
Fig. 1.
Impact of bronchial asthma on COVID-19 outcomes. (A) Binary regression analysis was performed. Odds ratio (OR) with a 95% confidence interval (CI) was reported. The vertical reference line was set at 1. P-value at <0.05 was considered significant. Data were adjusted by age, gender, and obesity. ARDS: acute respiratory distress syndrome, AKI: acute kidney injury, ICU: intensive care unit, LOS: length of stay. (B) Kaplan-Meier survival curve comparing survival duration of asthma and non-asthma groups.
Discussion
As the COVID-19 pandemic evolves, countries consider policies to protect those at increased risk of severe disease. To the best of our knowledge, this is the first study evaluating the relationship between asthma and COVID-19 outcomes in hospitalized patients in Louisiana. In the 502 patients included, the prevalence of asthma was 14.3%. This rate is higher than the national prevalence of asthma reported by the CDC (7.7%), but in line with CDC reports on the prevalence of asthma in COVID-19 patients [17]. This increased prevalence of asthma in COVID-19 patients may be attributable to increased disease awareness and concern to increased severity of the disease course among asthmatics.
Our initial univariate analysis of asthmatic patients showed significantly higher rates of endotracheal intubation and mechanical ventilation, and increased duration of hospital stay. Patients with asthma were also more likely to be obese, a finding consistent with prior studies [18,19]. As has been well-documented and widely reported, obesity alone has been associated with poor clinical outcomes in hospitalized COVID-19 patients [4]. Fittingly, in initial univariate analysis of obese asthmatic, there were significantly elevated rates of sepsis, ICU admission, and prolonged intubation. However, after multivariate adjustment, asthma comorbidity did not drive poor outcomes for our primary and secondary endpoints of interest - ICU admission, intubation, mechanical ventilation, ARDS, and case fatality rate. Although these results may not be expected, there is a plausible mechanism, the downregulation of ACE2 receptors, that could hypothetically account for these observations [[20], [21]].
Early data published by Jackson et al. suggested that patients with allergic asthma have decreased ACE2 expression in nasal and bronchial epithelial cells [7]. The possible explanation for this observation is two-fold. First, it has been postulated that type II immune modulation decreases the expression of the receptor for cellular entry of COVID-19 – ACE2 [22]. Second, individuals with chronic respiratory conditions often use inhaled corticosteroids (ICS). The Severe Asthma Research Program (SARP) demonstrated that ICS use in asthmatic patients leads to decreased expression of ACE2 and TMPRSS2 - a host serine protease which primes host cells for entry via the spike protein [23]. Additional in vitro studies have demonstrated that treatment with ciclesonide, an ICS used for suppressing asthma attacks, demonstrated viral suppression of SARS-CoV-2 [24]. It must be remembered that these experimental studies occurred in non-COVID-19 patients; nonetheless, the results suggest further investigation is necessary to understand the possible protective role of type 2 inflammation in asthma and COVID-19. Based on these studies, it may be possible that ICS treatment is protective via viral suppression or ACE2/TMPRSS2 reduction. These protective effects may explain the lack of significant difference in outcomes among asthmatic and non-asthmatic patients.
The potential limitations of this study include self-reporting of asthma history and the inability to identify the severity of asthma. To prevent false reporting, each patient's home medications were reviewed to confirm the diagnosis. Asthma severity may be assessed retrospectively based on symptoms or via risk assessment requiring measurement of airflow limitation using spirometry or Peak flow meter. However, these Aerosol Generating Procedures (AGPs) may put healthcare workers at an increased risk of coronavirus exposure.
The results of the current study show that while pre-existing asthma is more prevalent in our COVID-19 cohort than in the general population, after adjustment for other covariates, it was neither associated with disease severity nor negative outcomes. Therefore, although a seemingly poor prognosis, asthma does not imply a worse outcome than non-asthmatics. This information can help physicians and researchers identify people with other underlying medical conditions at risk for more severe COVID-19 disease.
Declaration of competing interest
The authors declare the absence of conflict of interest.
Funding source
None of the authors have any funding regarding this publication.
Authors contribution
MHH, EAT, EK designed the study. ASA, NB, AZ, JR, AH, NA collected patients' data. MHH performed data management. MHH, EAT performed statistical analyses. MH, EAT, EK contributed to data interpretation. MHH, EAT, NB, AZ, JR wrote the first draft. MHH, EAT drafted the final manuscript. All authors critically reviewed and approved the final version of the manuscript.
CRediT authorship contribution statement
Mohammad H. Hussein: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Visualization. Eman A. Toraih: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Writing - original draft, Visualization. Abdallah S. Attia: Methodology, Resources, Writing - original draft. Nicholas Burley: Methodology, Resources, Writing - original draft. Allen D. Zhang: Methodology, Resources, Writing - original draft. Jackson Roos: Methodology, Resources, Writing - original draft. August Houghton: Methodology, Resources, Writing - original draft. Nedum Aniemeka: Methodology, Resources, Writing - original draft. Mahmoud Omar: Writing - review & editing. Mohamed Aboueisha: Writing - review & editing. Mohamed A. Shama: Writing - review & editing. Juan Duchesne: Resources, Writing - review & editing. Emad Kandil: Conceptualization, Investigation, Resources, Writing - review & editing, Visualization, Supervision, Project administration.
Acknowledgment
We thank numerous doctors, nurses, government, and civilians working together to fight against the SARS-CoV-2.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmed.2020.106205.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hamid S., Mir M.Y., Rohela G.K. Novel coronavirus disease (COVID-19): a pandemic (epidemiology, pathogenesis and potential therapeutics) New Microbes New Infect. 2020;35:100679. doi: 10.1016/j.nmni.2020.100679. Published 2020 April 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hick J.L., Hanfling D., Wynia M.K., Pavia A.T. National Academy of Medicine; Washington, DC: 2020. Duty to Plan: Health Care, Crisis Standards of Care, and Novel Coronavirus SARS-CoV-2. NAM Perspectives. Discussion paper. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Liang W.H., Zhao Y., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. Published 2020 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg S., Kim L., Whitaker M., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and prevention . US Department of Health and Human Services, CDC; Atlanta, GA: 2020. Coronavirus Disease 2019 (COVID-19): Are You at Higher Risk for Severe Illness?https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/high-risk-complications.html [Google Scholar]
- 6.Zheng X.Y., Xu Y.J., Guan W.J., Lin L.F. Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review. Arch. Virol. 2018;163:845–853. doi: 10.1007/s00705-017-3700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson D.J., Busse W.W., Bacharier L.B., et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J. Allergy Clin. Immunol. 2020;146(1):203–206. doi: 10.1016/j.jaci.2020.04.009. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Summary Health Statistics Tables for U.S . 2018. Adults: National Health Interview Survey.https://www.cdc.gov/nchs/fastats/asthma.htm Tables A-2b, A-2c. (Last accessed July first) [Google Scholar]
- 10.Weir C.B., Jan A. BMI classification percentile and cut off points. StatPearls. 2019 https://pubmed.ncbi.nlm.nih.gov/31082114/ [PubMed] [Google Scholar]
- 11.George N., Elie-Turenne M.C., Seethala R.R., et al. External validation of the qSOFA score in emergency department patients with pneumonia. J. Emerg. Med. 2019;57(6):755–764. doi: 10.1016/j.jemermed.2019.08.043. [DOI] [PubMed] [Google Scholar]
- 12.Su Y., Tu G.W., Ju M.J., et al. Comparison of CRB-65 and quick sepsis-related organ failure assessment for predicting the need for intensive respiratory or vasopressor support in patients with COVID-19 [published online ahead of print, 2020 May 7] J. Infect. 2020;S0163–4453(20):30281–30284. doi: 10.1016/j.jinf.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao H.C., Lai T.Y., Hung H.L., et al. Sequential oxygenation index and organ dysfunction assessment within the first 3 days of mechanical ventilation predict the outcome of adult patients with severe acute respiratory failure. ScientificWorldJournal. 2013;2013:413216. doi: 10.1155/2013/413216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang A.P., Liu J.P., Tao W.Q., Li H.M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int. Immunopharm. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kara I., Yildirim F., Kayacan E., Bilaloğlu B., Turkoglu M., Aygencel G. Importance of RIFLE (risk, injury, failure, Loss, and end-stage renal failure) and AKIN (acute kidney injury network) in hemodialysis initiation and intensive care unit mortality. Iran. J. Med. Sci. 2017;42(4):397–403. [PMC free article] [PubMed] [Google Scholar]
- 16.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: the Berlin Definition. J. Am. Med. Assoc. 2012 June 20;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and prevention Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm Source: 2018 National Health Interview Survey (NHIS) Data, Table 3-1 and Table 4-1.
- 18.Khalid F., Holguin F. A review of obesity and asthma across the life spac. J. Asthma. 2018;55:1286–1300. doi: 10.1080/02770903.2018.1424187. [DOI] [PubMed] [Google Scholar]
- 19.Peters U., Dixon A.E., Forno E. Obesity and asthma. J. Allergy Clin. Immunol. 2018;141:1169–1179. doi: 10.1016/j.jaci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finney L.J., et al. Inhaled corticosteroids downregulate the SARS-Cov-2 receptor ACE2 in COPD through suppression of type I interferon. bioRxiv preprint. 2020 doi: 10.1101/2020.06.13.149039. http://biorxiv.org/cgi/content/short/2020.06.13.149039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chhiba K.D., Patel G.B., Vu T.H.T., et al. Prevalence and characterization of asthma in hospitalized and non-hospitalized patients with COVID-19 [published online ahead of print, 2020 June 15] J. Allergy Clin. Immunol. 2020;S0091–6749(20):30840–X. doi: 10.1016/j.jaci.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters M.C., Sajuthi S., Deford P., et al. COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am. J. Respir. Crit. Care Med. 2020;202(1):83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani W., et al. The inhaled corticosterois ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. Bio. 2020 doi: 10.1101/2020.03.11.987016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.