Lipoic acid is an essential cofactor for GCS and 2-oxoacid dehydrogenases, and α-lipoic acid has been utilized as a medicine and attracted attention as a supplement due to its antioxidant activity. The biosynthesis pathways of lipoic acid have been established in Bacteria and Eucarya but not in Archaea. Although some archaeal species, including Sulfolobus, possess a classical lipoyl synthase (LipA) gene homolog, many archaeal species, including T. kodakarensis, do not. In addition, the biosynthesis mechanism of the octanoyl moiety, a precursor for lipoyl group biosynthesis, is also unknown for many archaea. As the enzyme identified in T. kodakarensis most likely represents a new group of lipoyl synthases in Archaea, the results obtained in this study provide an important step in understanding how lipoic acid is synthesized in this domain and how the two structurally distinct lipoyl synthases evolved in nature.
KEYWORDS: lipoic acid, lipoyl synthase, biosynthesis, Archaea, hyperthermophile, Thermococcus, cofactor, metabolism
ABSTRACT
Lipoic acid is a sulfur-containing cofactor and a component of the glycine cleavage system (GCS) involved in C1 compound metabolism and the 2-oxoacid dehydrogenases that catalyze the oxidative decarboxylation of 2-oxoacids. Lipoic acid is found in all domains of life and is generally synthesized as a lipoyl group on the H-protein of the GCS or the E2 subunit of 2-oxoacid dehydrogenases. Lipoyl synthase catalyzes the insertion of two sulfur atoms to the C-6 and C-8 carbon atoms of the octanoyl moiety on the octanoyl-H-protein or octanoyl-E2 subunit. Although the hyperthermophilic archaeon Thermococcus kodakarensis seemed able to synthesize lipoic acid, a classical lipoyl synthase (LipA) gene homolog cannot be found on the genome. In this study, we aimed to identify the lipoyl synthase in this organism. Genome information analysis suggested that the TK2109 and TK2248 genes, which had been annotated as biotin synthase (BioB), are both involved in lipoic acid metabolism. Based on the chemical reaction catalyzed by BioB, we predicted that the genes encode proteins that catalyze the lipoyl synthase reaction. Genetic analysis of TK2109 and TK2248 provided evidence that these genes are involved in lipoic acid biosynthesis. The purified TK2109 and TK2248 recombinant proteins exhibited lipoyl synthase activity toward a chemically synthesized octanoyl-octapeptide. These in vivo and in vitro analyses indicated that the TK2109 and TK2248 genes encode a structurally novel lipoyl synthase. TK2109 and TK2248 homologs are widely distributed among the archaeal genomes, suggesting that in addition to the LipA homologs, the two proteins represent a new group of lipoyl synthases in archaea.
IMPORTANCE Lipoic acid is an essential cofactor for GCS and 2-oxoacid dehydrogenases, and α-lipoic acid has been utilized as a medicine and attracted attention as a supplement due to its antioxidant activity. The biosynthesis pathways of lipoic acid have been established in Bacteria and Eucarya but not in Archaea. Although some archaeal species, including Sulfolobus, possess a classical lipoyl synthase (LipA) gene homolog, many archaeal species, including T. kodakarensis, do not. In addition, the biosynthesis mechanism of the octanoyl moiety, a precursor for lipoyl group biosynthesis, is also unknown for many archaea. As the enzyme identified in T. kodakarensis most likely represents a new group of lipoyl synthases in Archaea, the results obtained in this study provide an important step in understanding how lipoic acid is synthesized in this domain and how the two structurally distinct lipoyl synthases evolved in nature.
INTRODUCTION
Lipoic acid is an organosulfur compound and an essential cofactor for the glycine cleavage system (GCS) and 2-oxoacid dehydrogenases such as pyruvate dehydrogenase, 2-oxoglutarate dehydrogenase, and branched-chain 2-oxoacid dehydrogenase (1–4). The GCS plays an important role in one-carbon (C1) compound metabolism and is composed of four proteins (H-, L-, P-, and T-proteins), while the latter dehydrogenases produce acyl coenzyme A (acyl-CoA) from 2-oxoacids and consist of three subunits (E1, E2, and E3) (1, 4, 5). Lipoic acid is covalently bound to lysine residues in the H-protein of the GCS and E2 subunits of 2-oxoacid dehydrogenases through an amide bond. From the aspect of application, free α-lipoic acid, also known as thioctic acid, is used in treatment for inner ear hearing loss and subacute necrotizing encephalomyelitis and is anticipated as a medicine to improve insulin sensitivity in type 2 diabetic patients (6). In addition, it is an attractive supplement due to its antioxidant activity (7, 8).
In the GCS, a C1 donor is generated with glycine and tetrahydrofolate (THF) (3, 5). The P-protein (glycine dehydrogenase) catalyzes decarboxylation of glycine and transfers the remaining aminomethyl moiety onto one of the sulfur atoms in the lipoyl group on the H-protein. The T-protein (aminomethyltransferase) then transfers the methylene group of the aminomethyl moiety to THF, which is coupled with deamination. The resulting N5,N10-methylenetetrahydrofolate can be utilized as a C1 donor in the metabolism of various compounds. In the case of l-serine (Ser) biosynthesis, glycine/serine hydroxymethyltransferase catalyzes the hydroxymethylation of glycine with N5,N10-methylenetetrahydrofolate and H2O to generate Ser. Finally, L-protein (dihydrolipoamide dehydrogenase) oxidizes the two intramolecular thiol groups and regenerates the disulfide bond of the lipoyl group using NAD+ as a cofactor (9, 10).
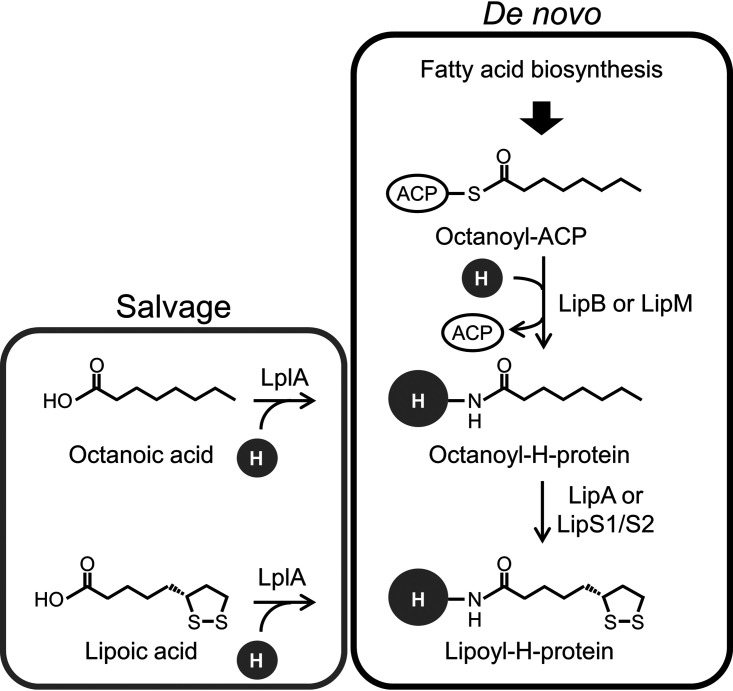
The final steps of lipoic acid biosynthesis are carried out on the H-protein (Fig. 1) or the E2 subunit of 2-oxoacid dehydrogenase (2–4). In a general scheme for generating lipoyl-H-protein, an octanoyl group is first synthesized on acyl carrier protein (ACP) through fatty acid biosynthesis. From the octanoyl-ACP, the octanoyl group is transferred onto the lysine residue of the H-protein, which is catalyzed by octanoyl transferase (e.g., LipB from Escherichia coli and LipM from Bacillus subtilis). On the other hand, lipoate-protein ligase (LplA) can also attach octanoic acid to the H-protein, which is dependent on ATP. Lipoyl synthase (e.g., LipA from E. coli) then catalyzes the covalent binding of sulfur atoms to the C-6 and C-8 carbons of the octanoyl group (designated sulfur insertion), generating lipoic acid, or, to be precise, the lipoyl group on the H-protein. Lipoyl synthase is classified into the radical S-adenosylmethionine (SAM) superfamily, which possesses the conserved sequence motif CX3CX2C (11, 12). Radical SAM family proteins catalyze reactions requiring radical chemistry. Lipoyl synthase contains two [4Fe-4S] clusters, and one of them (the auxiliary cluster) has been proposed to provide the sulfur atoms, while the other (the basic cluster) is used to generate the deoxyadenosyl radical, which initiates the reaction (12, 13). Deoxyadenosyl radical is synthesized by monoelectronic reduction of SAM via the basic [4Fe-4S] cluster and cleaves the C-H bond on the C-6 carbon of the octanoyl group. The produced carbon radical reacts with a sulfur atom in the auxiliary [4Fe-4S] cluster, generating a C-S bond (12–14). This reaction occurs at both the C-6 and C-8 positions, followed by the addition of two protons to generate the reduced form of the lipoyl group. In addition to this de novo pathway, the generation of lipoyl H-protein can also occur through the salvage and attachment of free lipoic acid onto the H-protein, which is also catalyzed by LplA (4).
FIG 1.
The classical and proposed lipoic acid biosynthesis pathways. In the proposed pathway, LipA is replaced by LipS1/S2 identified in this study. An enzyme corresponding to LipB or LipM is still unclear in a number of archaeal species. “H” refers to H-protein in the glycine cleavage system. Lipoic acid-activating enzyme and lipoyl transferase are utilized in mammalian cells instead of LplA. Abbreviations: LipA, classical lipoyl synthase; LipB and LipM, octanoyl transferase; LplA, lipoate-protein ligase; LipS1/S2, archaeal lipoyl synthase.
In archaea, the mechanisms for biosynthesis of several coenzymes, such as NAD (NAD+) (15–18), CoA (19–23), thiamine (24–26), heme (27, 28), FAD (29, 30, 60), and methyl coenzyme M (31–33), have been studied in recent years. The biosynthesis pathways of many coenzymes in archaea have been demonstrated to utilize atypical routes or enzymes which are different from those in bacteria and eukaryotes (23, 25, 28). On the other hand, the knowledge of the archaeal de novo biosynthesis pathway for lipoic acid is limited. Saccharolobus solfataricus P2 (formerly Sulfolobus solfataricus P2) harbors a classical LipA gene homolog, and the recombinant protein displayed lipoyl synthase activity toward octanoyl-tetrapeptide, whose peptide moiety was designed based on the E2 subunit of the pyruvate dehydrogenase complex (34, 35), implying that this archaeon is able to synthesize lipoic acid. However, the distribution of the classical LipA gene homologs is restricted to certain archaea, which include the halophiles, Aeropyrum, Sulfolobus, and Pyrobaculum. Intriguingly, although a number of archaeal species, including the hyperthermophilic archaeon Thermococcus kodakarensis, harbor a gene presumed to encode H-protein, classical LipA gene homologs are not found on their genomes.
In this study, we identified a structurally novel lipoyl synthase in T. kodakarensis, whose amino acid sequence differs from those of the classical LipA proteins. Activity and function were confirmed by in vivo genetic analysis and in vitro enzymatic analysis of the recombinant proteins. Most archaeal species, with a predicted H-protein gene but without the classical LipA gene homolog, possess the homologs of the lipoyl synthase genes identified in this study, suggesting that this structurally novel lipoyl synthase is widely distributed in archaea.
RESULTS
Candidates for previously unidentified lipoyl synthase in T. kodakarensis.
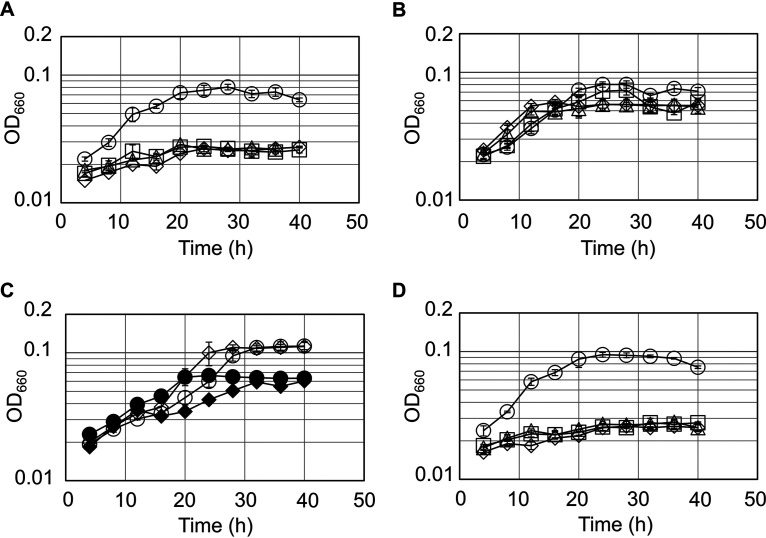
We presume that the GCS is present in T. kodakarensis, as homologs encoding classical H-, T-, and P-proteins are present on its genome (36). In addition, a disruption strain of the glycine/serine hydroxymethyltransferase gene (glyA; TK0528) displayed Ser auxotrophy in a synthetic medium with amino acids, indicating that Ser is synthesized exclusively from glycine (Gly) with GlyA in this medium (37). As the C1 group is most likely provided by the GCS, this further supports the notion that Ser biosynthesis in this organism is dependent on the GCS and, consequently, lipoic acid. We found that T. kodakarensis KU216 can grow in a synthetic amino acid medium, in which Ser was omitted, regardless of the presence or absence of lipoic acid (Fig. 2A and B). This result implies that this organism possesses the ability to synthesize lipoic acid. However, a gene encoding a classical LipA is absent on the T. kodakarensis genome. This raised the possibility that an unidentified protein(s) with lipoyl synthase activity is present in this archaeon.
FIG 2.
Growth properties of the host strain KU216 and the gene disruption strains. Growth of the host KU216 strain (circles) and the ΔTK2109 (squares), ΔTK2248 (triangles), and ΔTK2109 ΔTK2248 (diamonds) mutants was examined in a synthetic amino acid medium without serine and lipoic acid (mASW-AA-Ser[−]-S0-Ura-Lip[−]) (A), in a synthetic medium supplemented with lipoic acid (mASW-AA-Ser[−]-S0-Ura-Lip[+]) (B), and in a medium supplemented with octanoic acid (mASW-AA-Ser[−]-S0-Ura-Oct[+]) (D). (C) Growth of the host KU216 strain (circles) and ΔTK2109 ΔTK2248 mutant (diamonds) in a synthetic medium (mASW-AA-S0-Ura) with (open symbols) or without (closed symbols) biotin. Error bars indicate the standard deviations of three independent culture experiments. The vertical axis is represented in logarithmic scale.
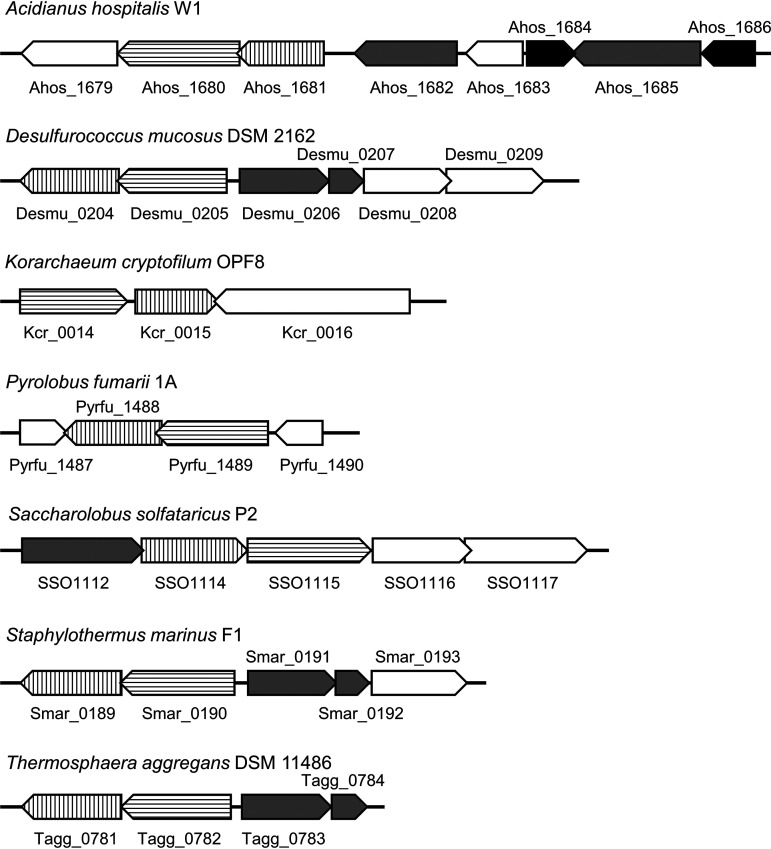
As candidates for lipoyl synthase in this organism, we focused on the TK2109 and TK2248 genes, which are annotated as biotin synthase (BioB). The protein products were 17 and 22% (TK2109) and 19 and 21% (TK2248) identical to BioB synthases from E. coli and the thaumarchaeon Nitrososphaera gargensis, respectively. T. kodakarensis also possesses three other proteins predicted to be involved in biotin biosynthesis (TK2217, TK1211, and TK2101). However, the TK2217 and TK2101 genes have recently been demonstrated to encode 2-amino-3-ketobutyric acid CoA ligase (37) and ornithine ω-aminotransferase (38), respectively, suggesting that these genes are not related to biotin biosynthesis. In addition, a gene encoding dethiobiotin synthetase (BioD) is not found on the genome. As the majority of genes related to biotin biosynthesis are absent in T. kodakarensis, we considered the possibility that TK2109 and TK2248 are involved in functions other than biotin biosynthesis. We searched for proteins related to the TK2109 and TK2248 proteins using STRING (https://string-db.org/) (39) and found that TK2109 and TK2248 homologs are located near one another on 32 genomes, among which in 20 the genes were next to each other. The adjacent location of the two genes was found in at least seven archaeal species (Fig. 3). Using the Archaeal Genome Browser (http://archaea.ucsc.edu/, University of California Santa Cruz), we found that one or two genes annotated as biotin or lipoate-protein ligase are present near the TK2109 and TK2248 homologs on the genomes of five organisms (Fig. 3). What we found most striking was that in Acidianus hospitalis, genes annotated as the GCS H-protein were also present near the TK2109 and TK2248 homologs. As the H-protein is subject to lipoylation, we considered the possibility that the TK2109 and TK2248 proteins are involved in lipoic acid biosynthesis. The reaction catalyzed by biotin synthase is a sulfur atom insertion to carbons in dethiobiotin, which is similar to that catalyzed by lipoyl synthase. Based on these facts, although their primary structures were not similar to those of classical LipA proteins (19% and 18% identical to the classical LipA [SSO3158] from S. solfataricus, respectively), we thought there was a possibility that the TK2109 and/or TK2248 genes encode lipoyl synthase.
FIG 3.
Gene arrangements of TK2109 and TK2248 homologs in seven archaea. Some archaeal organisms which possess TK2109 homolog, TK2248 homolog, and biotin or lipoate-protein ligase are shown. Vertical stripe, horizontal stripe, gray, and black arrowed boxes represent TK2109 homolog, TK2248 homolog, biotin or lipoate-protein ligase, and H-protein genes, respectively. Genes overlapping any of the above four genes, along with some neighboring genes, are shown with white arrowed boxes.
Construction and genotypic analysis of TK2109 and TK2248 gene disruption strains.
In order to examine the involvement of the TK2109 and TK2248 genes in lipoic acid and/or biotin biosynthesis, we took a genetic approach (40–44). We constructed a TK2109 gene disruption strain (ΔTK2109), a TK2248 gene disruption strain (ΔTK2248), and a double gene disruption strain (ΔTK2109 ΔTK2248) (see Fig. S1A in the supplemental material). The genotypes of the selected transformants were examined by PCR (Fig. S1B to S1I), which indicated that the target genes were successfully deleted. In all three transformants, the absence of unintended mutations in the homologous regions was confirmed by DNA sequencing analysis.
Growth properties of TK2109 and TK2248 gene disruption strains.
The growth properties of the host strain and gene disruption strains (ΔTK2109, ΔTK2248, and ΔTK2109 ΔTK2248) were first examined in a synthetic medium without Ser and lipoic acid (Fig. 2A). If any of the two genes were involved in lipoic acid biosynthesis, their disruption should result in Ser or lipoic acid auxotrophy, as proper function of the GCS is necessary for conversion of Gly to Ser. In contrast to the host strain KU216, none of the disruption strains displayed growth in this medium. Addition of 1 mM lipoic acid to this medium significantly complemented the growth defects of all the disruption strains (Fig. 2B). It is unlikely that the growth of the disruption strains was due to their utilizing lipoic acid as a substrate for growth, as the addition of lipoic acid at this concentration did not have any effect on the growth of the host strain. The results indicate that the ΔTK2109, ΔTK2248, and ΔTK2109 ΔTK2248 strains display lipoic acid auxotrophy and support our presumption that the genes are involved in lipoic acid biosynthesis. This also suggests that T. kodakarensis can salvage free lipoic acid and transfer it onto the H-protein. This may owe to the function of lipoate-protein ligase (LplA), whose gene homologs are present on the genome.
We next examined the involvement of TK2109 and TK2248 in biotin biosynthesis. The T. kodakarensis genome harbors several genes that suggest the presence of biotin-dependent enzymes in this organism. TK1624 and TK0990 are annotated as biotin carboxyl carrier protein subunit and pyruvate carboxylase subunit B, respectively, and do include the MKM motif for biotin ligation. However, irrespective of the presence or absence of biotin, the ΔTK2109 ΔTK2248 strain displayed growth through six serial cultures as in the case of the host strain (Fig. 2C). Although we cannot rule out the possibility that biotin is not necessary under these growth conditions, the results at least do not suggest that the genes are involved in biotin biosynthesis.
Based on these results, we further examined the involvement of the two genes in lipoic acid biosynthesis. We inoculated the disruption strains in medium supplemented with 1 mM octanoic acid instead of lipoic acid (Fig. 2D). The disruption strains did not display growth, suggesting that the TK2109 and TK2248 proteins catalyze a reaction downstream of octanoic acid biosynthesis, i.e., the conversion of either octanoic acid to lipoic acid or octanoyl-H-protein to lipoyl-H-protein. As described above, the TK2109 and TK2248 proteins display similarity to BioB, which catalyzes carbon-sulfur bond formation. In addition, both proteins harbor the CX3CX2C motif conserved in members of the radical SAM superfamily. Together with these structural characteristics, the results of our growth experiments provide strong support that these genes encode proteins catalyzing the lipoyl synthase reaction (Fig. 1).
Purification and reconstitution of the recombinant TK2109 and TK2248 proteins.
In order to examine whether the TK2109 protein and/or TK2248 protein exhibits lipoyl synthase activity, we produced the TK2109 and TK2248 recombinant proteins in E. coli. Judging from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis, the recombinant proteins were purified to apparent homogeneity by heat treatment, anion-exchange chromatography, and gel filtration chromatography (Fig. S2).
Knowledge on lipoyl synthase reactions and the sequences of TK2109 and TK2248 proteins suggest the presence of iron-sulfur clusters in these proteins. In order to generate, or reconstitute, the iron-sulfur clusters in the recombinant proteins, the proteins were incubated in the presence of dithiothreitol (DTT), ferric iron chloride, and sodium sulfide under anaerobic conditions. After precipitates were removed and proteins were concentrated, the protein solution displayed a dark brown color, and protein concentrations were determined. The UV-visible spectra of proteins with or without reconstitution (20 μM) were measured (Fig. S3). In addition to the absorbance at 280 nm derived from proteins, the reconstituted proteins exhibited absorbance at 420 nm, consistent with the UV-visible spectrum characteristic of [4Fe-4S] clusters (45–47).
Lipoyl synthase activity measurement of the TK2109 and TK2248 recombinant proteins using HPLC.
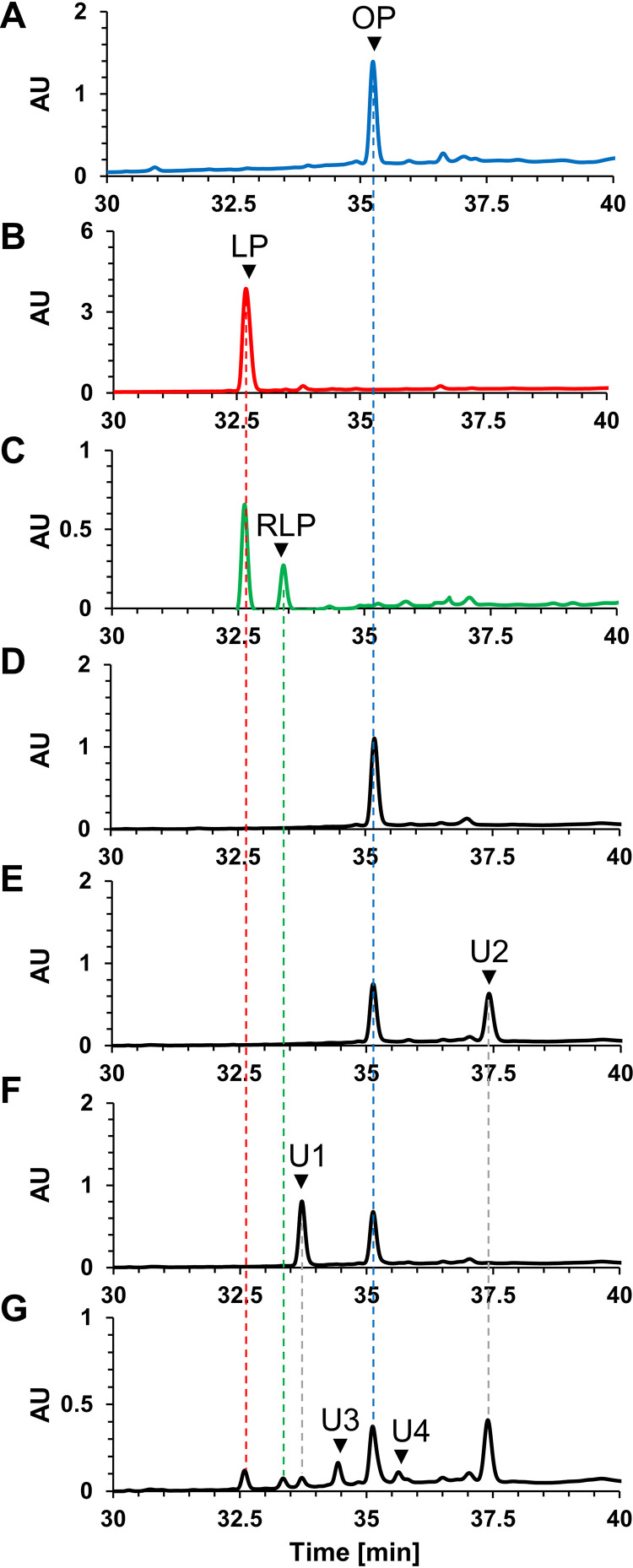
A chemically synthesized octanoyl-octapeptide, whose sequence corresponds to that of the lipoyl domain in the H-protein of T. kodakarensis (TK0150 protein), was used as a substrate for measuring lipoyl synthase activity of the reconstituted TK2109 and TK2248 proteins. Chemically synthesized octanoyl-peptide and lipoyl-peptide standards, the substrate and oxidized form of the product, respectively, and reaction products were analyzed by high-performance liquid chromatography (HPLC) (Fig. 4). The standard octanoyl-peptide and lipoyl-peptide were detected at 35.2 and 32.6 min, respectively (Fig. 4A and B). When only TK2109 protein or TK2248 protein was individually included in the reaction mixture, the production of lipoyl-peptide was not detected (Fig. 4E and F). However, in a reaction mixture with both proteins, we observed a peak whose retention time corresponded to that of the standard lipoyl-peptide (Fig. 4B and G). In addition, many other peaks, including those eluting at 33.4, 33.7, 34.4, 35.6, and 37.4 min, were observed. When the standard lipoyl-peptide was treated with DTT, a peak eluting at 33.4 min was observed (Fig. 4C), implying that the peak at 33.4 min corresponds to the octapeptide with a reduced lipoyl moiety, the true product of the lipoyl synthase reaction. The results suggest that the TK2109 and TK2248 proteins together display lipoyl synthase activity. Although we could not identify the compounds corresponding to the other peaks (at 33.7, 34.4, 35.6, and 37.4 min, indicated with U1, U3, U4, and U2, respectively) based on retention time, we note that the peaks with retention times of 33.7 min and 37.4 min (U1 and U2, respectively) were also observed in the reaction mixtures including only TK2248 protein or TK2109 protein, respectively.
FIG 4.
Lipoyl synthase activity measurement by HPLC analysis. HPLC analyses were performed with standard octanoyl-peptide (A), standard lipoyl-peptide (B), reduced lipoyl-peptide (C), reaction product without proteins (D), reaction product with reconstituted TK2109 protein (E), reaction product with reconstituted TK2248 protein (F), and reaction product with reconstituted TK2109 and TK2248 proteins (G). Abbreviations: OP, octanoyl-peptide; LP, lipoyl-peptide; RLP, reduced lipoyl-peptide; U1 to U4, unidentified compounds 1 to 4; AU, arbitrary units.
Identification of reaction products by LC-MS analysis.
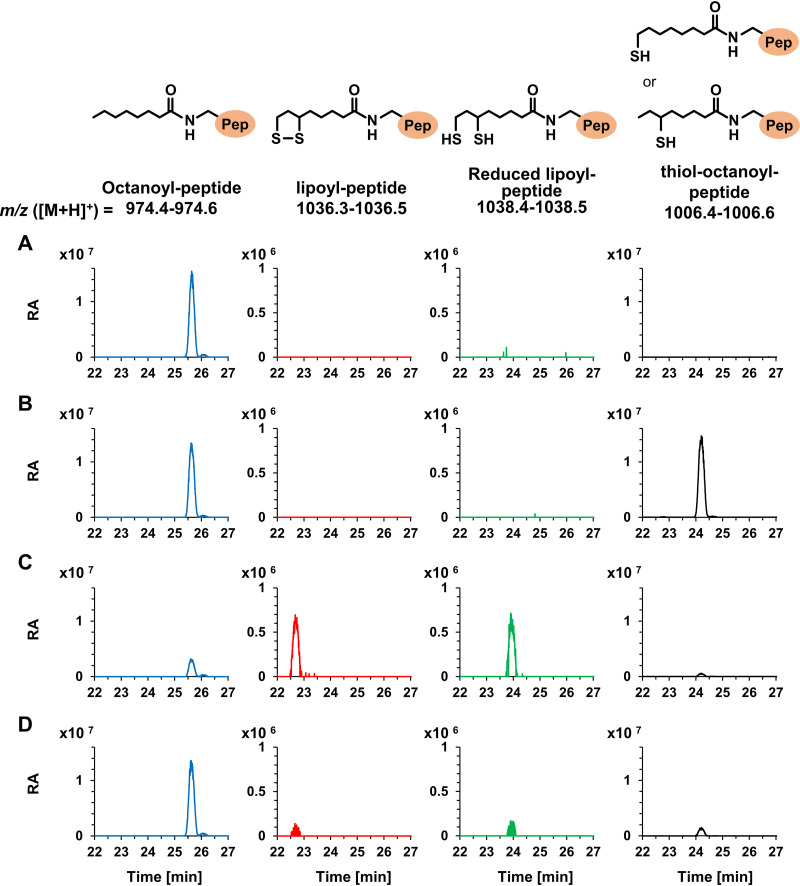
To identify the reaction products, liquid chromatography-mass spectrometry (LC-MS) analysis was carried out (Fig. 5). As the conditions (mobile phase and elution program) for LC differed from those in the above-described HPLC analysis, the retention times of the standard lipoyl-peptide (22.7 min), standard octanoyl-peptide (25.6 min), and reduced lipoyl-peptide (23.9 min) changed. Among the reaction products with TK2109 protein, TK2248 protein, and both proteins, compounds with exact masses corresponding to lipoyl-peptide ([M+H]+ = 1,036.47) and reduced lipoyl-peptide ([M+H]+ = 1,038.48) were detected only in the products from reactions using both proteins (Fig. 5A to C). LC-MS analysis also revealed that the reaction with TK2248 protein alone generated significant levels of a compound whose m/z value corresponded to that of the intermediate thiol-octanoyl-peptide ([M+H]+ = 1,006.51). Low levels of this compound were also detected after the reaction with both proteins. This raised the possibility that the unidentified peak U1 in the HPLC analysis described above corresponds to the intermediate thiol-octanoyl-peptide. On the other hand, we could not predict the compounds corresponding to peaks U2, U3, and U4. Concerning peak U2, the compound was detected in the reactions with TK2109 alone or with both proteins and displayed an m/z value of 845.49. Identification of this compound, as well as those corresponding to peaks U3 and U4, will have to await further understanding of the reaction mechanism. Furthermore, we performed the same experiments with TK2109 and TK2248 proteins, which were not subjected to reconstitution (Fig. 5D). As a result, very low levels of lipoyl-peptide, the reduced lipoyl-peptide, and the intermediate thiol-octanoyl-peptide were detected, implying that the procedure to reconstitute [4Fe-4S] clusters was important in generating an active lipoyl synthase enzyme. The results indicate that the TK2109 and TK2248 proteins function together as a lipoyl synthase and that both proteins are necessary for this activity. We designate the TK2109 and TK2248 proteins LipS1 and LipS2, respectively (Fig. 1).
FIG 5.
Identification of reaction products by LC-MS analysis. LC-MS analyses were carried out using reaction products with reconstituted TK2109 protein (A), reconstituted TK2248 protein (B), reconstituted TK2109 and TK2248 proteins (C), and nonreconstituted TK2109 and TK2248 proteins (D). Blue, red, green, and black lines represent the compounds whose m/z values corresponded to exact masses of octanoyl-peptide, lipoyl-peptide, reduced lipoyl-peptide, and intermediate thiol-octanoyl-peptide, respectively. Pep and RA, peptide and relative abundance, respectively.
DISCUSSION
In this study, we identified two genes whose products constitute a new type of lipoyl synthase in T. kodakarensis. The two proteins, designated LipS1 (TK2109) and LipS2 (TK2248), display primary structures with low identities to those of classical LipA proteins from E. coli (17% and 13% identical, respectively) and S. solfataricus (19% and 18% identical, respectively).
We examined the distributions of homologs of genes encoding the classical LipA, LipS1, LipS2, the E2 subunit of pyruvate dehydrogenase, the H-protein of the GCS, and the enzymes involved in the salvage of lipoic acid (LplA) in archaea (Table 1 and expanded in Table S1). Homologs of genes encoding LipS1 and/or LipS2, displaying E values lower than or equal to 2e−17, are distributed in a wide range of archaeal genera, including Palaeococcus, Pyrococcus, Thermococcus, Aciduliprofundum, Desulfurococcus, Staphylothermus, Thermosphaera, Hyperthermus, Pyrodictium, Pyrolobus, Acidianus, Metallosphaera, Saccharolobus, Stygiolobus, Sulfolobus, Sulfuracidifex, Sulfurisphaera, “Candidatus Korarchaeum,” and Lokiarchaeum. Almost all of these genera are (hyper)thermophiles, although we cannot estimate the precise growth temperature of the uncultured Lokiarchaeum sp. strain GC14_75. The members of these genera, with the exception of members of Saccharolobus and Sulfolobus, do not possess the classical LipA. It is noted that all the archaeal members with LipS1 and LipS2 gene homologs, except for a single species, Thermococcus piezophilus, possess a gene encoding H-protein in the GCS, a potential substrate for lipoylation. On the other hand, Methanocella, Pyrobaculum, and Thermoproteus harbor only a classical LipA homolog and an E2 subunit of pyruvate dehydrogenase. These tendencies in distribution suggest that classical LipA proteins in these specific organisms are lipoyl synthases for the E2 subunit, while the organisms harboring only the newly identified archaeal lipoyl synthases utilize LipS1/LipS2 for lipoylation of the H-protein. However, this distinction in roles does not seem to apply throughout the archaea. Many haloarchaea, as well as members of Cuniculiplasma, Ferroplasma, Picrophilus, Thermoplasma, Acidilobus, Caldisphaera, Aeropyrum, Fervidicoccus, Sulfodiicoccus, and “Candidatus Caldiarchaeum,” possess an H-protein gene homolog but do not harbor homologs of genes encoding LipS1 and LipS2. Among these organisms, those that possess a LipA gene homolog (many haloarchaea, Cuniculiplasma, Ferroplasma, Picrophilus, Aeropyrum, Sulfodiicoccus, and “Candidatus Caldiarchaeum”) may use LipA for H-protein lipoylation. On the other hand, there are genomes, including those of Halanaeroarchaeum, Thermoplasma, Acidilobus, Caldisphaera, and Fervidicoccus, that harbor neither LipS1, LipS2, nor classical LipA homologs. Whether these organisms utilize only salvage pathways involving LplA or unidentified biosynthesis pathways remains to be determined. The LplA gene homologs are widely distributed among archaea that possess the E2 subunit and/or the H-protein. An interesting point is that we found LipS1 and LipS2 gene homologs even in some (hyper)thermophilic bacteria, including Aquifex and Thermotoga (25 to 40% identical). Although in vitro examination of these proteins will be necessary to determine whether they exhibit lipoyl synthase activity, LipS1/LipS2-dependent lipoylation might occur in thermophilic archaea and bacteria.
TABLE 1.
Distribution of genes involved in lipoic acid metabolism in representatives of Archaea
| Organism or E value parameter | Gene returned for indicated querya |
|||||
|---|---|---|---|---|---|---|
| Lipoyl synthase |
Lipoylation substrate |
|||||
| LipA, SSO3158 | LipS1, TK2109 | LipS2, TK2248 | PDC E2 N, SSO1529 | PDC E2 C, SSO1530 | GCS H-protein, TK0150 | |
| Archaeoglobus fulgidus DSM 4304 | ||||||
| Halorhabdus utahensis | ||||||
| Halanaeroarchaeum sulfurireducens | HLASF_0883 | HLASF_0883 | HLASF_1709 | |||
| Halobacterium salinarum NRC-1 | VNG_2216G | VNG_2219G | VNG_2219G | VNG_1605G | ||
| Haloferax volcanii | HVO_2957 | HVO_0666 HVO_2960 | HVO_2960 HVO_0666 | HVO_2403 | ||
| Haloplanus sp. CBA1112 | DU484_02100 | DU484_02085 | DU484_12585 DU484_02085 | DU484_16075 | ||
| Halopiger xanaduensis | Halxa_1123 | Halxa_1119 | Halxa_1119 | Halxa_0801 | ||
| Methanothermobacter thermautotrophicus | ||||||
| Methanocaldococcus jannaschii | ||||||
| Methanocella paludicola | MCP_1716 | MCP_1719 | MCP_1719 | |||
| Methanospirillum hungatei | ||||||
| Methanosarcina barkeri Fusaro | ||||||
| Methanopyrus kandleri | ||||||
| Palaeococcus pacificus | PAP_09175 | PAP_08440 | PAP_07990 | PAP_00220 | ||
| Pyrococcus furiosus DSM 3638 | PF1158 | PF0144 | PF1492 | |||
| Thermococcus kodakarensis | TK2109 | TK2248 | TK0150 | |||
| Thermococcus litoralis | OCC_00607 | OCC_12346 | OCC_03357 OCC_03332 | OCC_03352 | OCC_09711 | |
| Thermococcus piezophilus | A7C91_04090 | A7C91_02560 | ||||
| Cuniculiplasma divulgatum | CPM_0223 | CPM_0220 | CPM_0220 | CPM_0396 | ||
| Ferroplasma acidarmanus | FACI_IFERC01G0865 | FACI_IFERC01G0868 | FACI_IFERC01G0868 | FACI_IFERC01G1086 | ||
| Picrophilus torridus | PTO0550 | PTO0547 | PTO0547 | PTO0624 | ||
| Thermoplasma acidophilum | Ta1436 | Ta1436 | Ta1366 | |||
| Aciduliprofundum boonei | Aboo_0647 | Aboo_0919 | Aboo_1328 | |||
| Acidilobus saccharovorans | ASAC_0852 | ASAC_1445 | ||||
| Caldisphaera lagunensis | Calag_0308 | Calag_0980 | ||||
| Aeropyrum pernix | APE_2344.1 | APE_1671 | APE_1671 | APE_0951.1 | ||
| Desulfurococcus mucosus | Desmu_0204 | Desmu_0205 | Desmu_0682 | |||
| Ignicoccus hospitalis | ||||||
| Staphylothermus marinus | Smar_0189 | Smar_0190 | Smar_0341 | Smar_1109 | ||
| Thermosphaera aggregans | Tagg_0781 | Tagg_0782 | Tagg_0405 | |||
| Hyperthermus butylicus | Hbut_0508 | Hbut_0507 | Hbut_0181 | |||
| Pyrodictium delaneyi | Pyrde_1321 | Pyrde_1320 | Pyrde_1470 | |||
| Pyrolobus fumarii | Pyrfu_1488 | Pyrfu_1489 | Pyrfu_0135 | |||
| Fervidicoccus fontis | FFONT_0393 | |||||
| Acidianus hospitalis | Ahos_1681 | Ahos_1680 | Ahos_1325 Ahos_1686 Ahos_1684 | |||
| Metallosphaera sedula | Msed_1559 Msed_1507 | Msed_1508 Msed_1558 | Msed_1662 Msed_1481 Msed_1570 | |||
| Saccharolobus solfataricus P2 | SSO3158 | SSO1114 | SSO1115 | SSO1529 | SSO1530 | SSO0920 SSO1061 SSO1105 |
| Stygiolobus azoricus | D1868_09950 D1868_10065 | D1868_10070 D1868_09955 | D1868_04760 D1868_09910 D1868_09915 | |||
| Sulfodiicoccus acidiphilus | HS1genome_2182 | HS1genome_1109 HS1genome_0382 | ||||
| Sulfolobus acidocaldarius DSM 639 | Saci_0309 | Saci_0344 | Saci_0343 | Saci_1384 Saci_0349 Saci_0350 | ||
| Sulfuracidifex tepidarius | IC006_0792 IC006_0273 | IC006_0791 IC006_0272 | IC006_1084 IC006_0260 IC006_0802 | |||
| Sulfurisphaera tokodaii | STK_18870 STK_11950 | STK_11940 STK_18860 | STK_12050 STK_18950 STK_18940 | |||
| Thermofilum pendens | ||||||
| Pyrobaculum calidifontis | Pcal_1406 | Pcal_1403 | Pcal_1403 | |||
| Thermoproteus tenax | ||||||
| Thermoproteus uzoniensis | TUZN_1026 | TUZN_1029 | TUZN_1029 | |||
| Nitrososphaera viennensis | ||||||
| Nitrosopumilus maritimus | ||||||
| “Candidatus Caldiarchaeum subterraneum” | CSUB_C1591 | CSUB_C1263 CSUB_C0792 | CSUB_C0851 CSUB_C1263 | CSUB_C0839 CSUB_C1435 | ||
| Nanoarchaeum equitans | ||||||
| “Candidatus Korarchaeum cryptofilum” | Kcr_0015 | Kcr_0014 | Kcr_1428 | |||
| Lokiarchaeum sp. GC14_75 | Lokiarch_46960 Lokiarch_23930 | Lokiarch_46940 Lokiarch_23910 | Lokiarch_42450 Lokiarch_25060 Lokiarch_24020 | |||
| “Candidatus Bathyarchaeota archaeon” BA1 | AOA65_1547 | AOA65_0858 | AOA65_0858 | |||
| E:E value | E ≤ 4e−78 | E ≤ 2e−132 | E ≤ 3e−116 | E ≤ 1e−145 | E ≤ 9e−68 | |
| 2e−49 ≤ E ≤ 5e−18 | 3e−67 ≤ E ≤ 3e−42 | 7e−36 ≤ E ≤ 8e−06 | E ≤ 1e−05 | 4e−35 ≤ E ≤ 8e−07 | ||
The different shades of gray represent different degrees of similarity.
The classical lipoyl synthase LipA has two [4Fe-4S] clusters (basic cluster and auxiliary cluster) and contains two conserved motifs that coordinate the clusters, which are CX4CX5C and CX3CX2C (Fig. S4) (12, 14, 48, 49). According to the previously proposed catalytic mechanism (12–14), the basic cluster is used to reductively cleave the C-S bond of SAM and generate a 5′-deoxyadenosyl radical (5′-dA·). The 5′-dA· then attacks a C-H bond in the carbon chain of the octanoyl moiety to form a carbon radical. The auxiliary cluster devotes two sulfur atoms to generate the lipoyl moiety.
We aligned the sequences of LipS1 and LipS2 with those of LipA (Fig. S4 and S5). The LipA homologs display a large number of conserved stretches that span the entire protein. Reflecting the low sequence identity, most of these stretches are not conserved in LipS1 and LipS2. In particular, the LipS1 and LipS2 proteins each possess only one conserved CX3CX2C motif and do not harbor the CX4CX5C motif (Fig. S4 and S5). Our results have shown that the LipS1 and LipS2 proteins function cooperatively as a lipoyl synthase. If the reaction mechanisms between the classical lipoyl synthase and the enzyme identified in the study were analogous, a simple assumption would be that a motif in one of the proteins coordinates the basic cluster and the motif in the other protein constitutes the auxiliary cluster. However, we noticed that the intermediate thiol-octanoyl-peptide could be detected in the reaction mixture including only the LipS2 protein (Fig. 5B). This would suggest that the LipS2 protein generates the 5′-deoxyadenosyl radical and then serves as the first sulfur donor, while the LipS1 protein acts as the second sulfur donor. Sequence alignments indicate that LipS1 homologs and LipS2 homologs possess two and one highly conserved motifs including cysteine residues in their C-terminal domains, respectively (Fig. S6 and S7). These conserved motifs are not found in the classical LipA homologs (Fig. S4 and S5). Specifically, all the analyzed LipS1 homologs harbor GC(M/A)R and CC motifs (Fig. S6), while all the analyzed LipS2 homologs possess a conserved TXGCPXC(N/D)RP motif (Fig. S7). There is a possibility that these motifs, which include cysteine residues, are involved in the sulfur insertion reaction by coordinating a [4Fe-4S] cluster in addition to the two CX3CX2C motifs in the proteins. Another possibility is that these motifs contribute to form a complex between LipS1 and LipS2. The reaction mechanism of LipS1 and LipS2 may differ from those of the classical LipA enzyme, and further studies are necessary to elucidate how this novel lipoyl synthase functions in sulfur insertion.
We found that the ΔTK2109 ΔTK2248 strain could grow without biotin at least during six serial cultures. However, we cannot rule out the possibility that biotin-dependent enzymes are unnecessary under our culture conditions or that trace levels of exogenous biotin were present in our cultures. Further in vitro characterization of LipS1/LipS2 proteins on compounds such as dethiobiotin, the substrate of BioB enzyme used to synthesize biotin, should clarify this issue. All in all, our present data strongly suggest that TK2109 and TK2248 are involved in lipoic acid biosynthesis.
Although we identified the lipoyl synthase in T. kodakarensis and most likely in other archaeal species (homologs displayed with colored boxes in Table S1), much is still unknown about lipoic acid biosynthesis in archaea. One point that should be emphasized is that pathways to synthesize octanoic acid are entirely unknown in T. kodakarensis and in many other archaeal species. As archaea do not utilize fatty acids in membrane lipids, fatty acid biosynthesis had not been regarded as an essential metabolic function. Three enzymes of β-oxidation of fatty acids (acyl-CoA dehydrogenase, enoyl-CoA hydratase, and 3-hydroxyacyl-CoA dehydrogenase) and an enzyme annotated as acetyl-CoA C-acetyltransferase, which has been considered to function in the mevalonate pathway, have been proposed to be involved in the biosynthesis of fatty acids in archaea (50). However, although homologs of acetyl-CoA C-acetyltransferase are distributed in a wide range of archaea, a complete set of homologs encoding these enzymes cannot be found in many archaeal genomes, including that of T. kodakarensis.
In addition to lipoic acid biosynthesis, salvage systems in T. kodakarensis have not been examined in detail. This organism harbors a protein annotated as N-terminal section of LplA (TK1908) in addition to one annotated as a C-terminal section of LplA (TK1234). The TK1908 protein displays similarity to the lipoate-protein ligase (LplJ) (33% identical) and the octanoyltransferase (LipM) (30% identical) of B. subtilis, the latter functioning in de novo lipoic acid biosynthesis. In addition to further investigation of LipS1/LipS2, clarifying the function of the LplA proteins will be necessary to obtain a better understanding of lipoic acid metabolism in T. kodakarensis.
MATERIALS AND METHODS
Chemicals, strains, media, and culture conditions.
Unless mentioned otherwise, chemical reagents were purchased from Nacalai Tesque (Kyoto, Japan) or Fujifilm Wako Pure Chemicals (Osaka, Japan). T. kodakarensis KOD1 (wild type), KU216 (ΔpyrF) (41), and the KU216 derivative strains were cultured under anaerobic conditions at 85°C in a nutrient-rich medium (ASW-YT-S0) or synthetic media (ASW-AA-S0 and mASW-AA-S0). ASW-YT-S0 medium was composed of 0.8× artificial seawater (ASW) (51), 5.0 g liter−1 of yeast extract, 5.0 g liter−1 of tryptone, and 2.0 g liter−1 of elemental sulfur. ASW-AA-S0 medium consisted of 0.8 × ASW, a mixture of 20 amino acids, modified Wolfe’s trace minerals, a vitamin mixture, and 2.0 g liter−1 of elemental sulfur (40). mASW-AA-S0 medium is a modified version of ASW-AA-S0 medium. In mASW-AA-S0, concentrations of l-arginine hydrochloride and l-valine were increased (to 250 mg liter−1 from 125 mg liter−1 and to 200 mg liter−1 from 50 mg liter−1, respectively) and 20 μM KI, 20 μM H3BO3, 10 μM NiCl2·6H2O, and 10 μM tungsten were supplemented. In all media, 0.8 mg liter−1 of resazurin sodium salt was supplemented to detect dissolved oxygen and Na2S·9H2O was added until the media became colorless. For solid medium used to isolate transformants, elemental sulfur and Na2S·9H2O were replaced with 2 ml liter−1 of a polysulfide solution (10 g of Na2S·9H2O and 3 g of sulfur flowers in 15 ml of H2O) and 10 g liter−1 of Gelrite was added to solidify the medium. E. coli DH5α used for plasmid construction and E. coli BL21 used for gene expression were cultivated at 37°C in lysogeny broth (LB) medium containing ampicillin (100 mg liter−1).
Construction of gene disruption strains of T. kodakarensis.
To construct TK2109 and TK2248 gene disruption vectors, the respective genes with 1 kbp of their 5′- and 3′-flanking regions were amplified by PCR with the primer sets dTK2109-F/-R and dTK2248-F/-R, respectively. Primers used in this study are listed in Table 2. The amplified fragments were inserted into the HincII site of plasmid pUD3 (19), which harbors a selectable pyrF marker gene. The coding regions of each gene were removed by inverse PCR with the primer sets invdTK2109-F/-R and invdTK2248-F/-R and the amplified linear DNA fragments were self-ligated. In the case of the TK2248 gene, the initial 8 bp and the terminal 11 bp of the coding region were left intact, as the regions are shared by the TK2247 and TK2249 genes, respectively. The two plasmids for the disruption of TK2109 and TK2248 genes were designated pUDTK2109 and pUDTK2248, respectively.
TABLE 2.
Primers used in this study
| No. | Primer name | Sequence (5′–3′) |
|---|---|---|
| 1 | dTK2109-F | CATGATACAGATCGACCCGGTCTACGC |
| 2 | dTK2109-R | GAGGAAGTGGCCGAGTACGGGGCAAAATGG |
| 3 | dTK2248-F | TCAAAGCCAGTTGGCGGTCTCATCGGGGAT |
| 4 | dTK2248-R | CCAGAAGGGCCGGCTACCACGGCCACGTCT |
| 5 | invdTK2109-F | GGCTCACCAAACCTCGCATCATCACAAATC |
| 6 | invdTK2109-R | TTAGCTCACCTCTAAAACCGGGGGGTTGTA |
| 7 | invdTK2248-F | TCATCCTTTGAGTGCTTCCAGCGCTTCGTT |
| 8 | invdTK2248-R | TCAGGCATTCCCTTCACCCTTAACCTTCG |
| 9 | idTK2109-F | ATGGCAGAACCCAAGAAAAAGCT |
| 10 | idTK2109-R | CTACATAACGCAGCACTCGTAGATAATCTC |
| 11 | idTK2248-F | AATGGTAAGGGTCTCCTATGGAACTGCAAT |
| 12 | idTK2248-R | GCCTCAATGTTCTCTCAAAATCTTCCCTT |
| 13 | odTK2109-F | CAGGTTCATCTCAAAGGGCGGTACCATGGT |
| 14 | odTK2109-R | AGCTACCCAGAGATACAGGCGGACACCCTC |
| 15 | odTK2248-F | GCTGGTGTTACATCCTTTGTTTCCCTCGTT |
| 16 | odTK2248-R | AGTTCAACAGGATAGGAGGGAGGACAGCTT |
| 17 | eTK2109F | GAAGGAGATATACATATGGCAGAACCCAAGAAAAAGCTCA |
| 18 | eTK2109R | CTCGAATTCGGATCCCTACATAACGCAGCACTCGTAGATA |
| 19 | eTK2248F | GAAGGAGATATACATATGCCTGAAATGGTAAGGGTCTCCT |
| 20 | eTK2248R | CTCGAATTCGGATCCTCAAAGGATGAGCCTCAATGTTCTC |
The TK2109 disruption strain, the TK2248 disruption strain, and the TK2109/TK2248 double-disruption strain were prepared as follows. For the former two, T. kodakarensis KU216 cells were grown in ASW-YT-S0 for 12 h (early stationary phase), harvested, resuspended in 200 μl of 0.8× ASW, and kept on ice for 30 min. pUDTK2109 or pUDTK2248 (3 μg) was added to the cells and the mixtures were kept on ice for 1 to 2 h. After heat shock at 85°C for 45 s, the mixtures were kept on ice for 10 min. Cells were inoculated into ASW-AA-S0 liquid medium and incubated at 85°C for 2 days. Cells were cultured in the same medium again in order to enrich transformants harboring the pyrF gene via pop-in single-crossover recombination. Cells were then grown at 85°C for 3 to 5 days on ASW-AA-S0 solid medium supplemented with 0.75% 5-fluoroorotic acid (FOA) and 10 μg ml−1 of uracil to select transformants in which the target genes were removed along with the pyrF gene due to pop-out recombination. Genotypes of the transformants were examined by PCR with primer sets that anneal within the target genes (idTK2109-F/-R or idTK2248-F/-R) and outside the homologous regions for recombination (odTK2109-F/-R or odTK2248-F/-R). The obtained TK2109 and TK2248 gene disruption strains were designated ΔTK2109 and ΔTK2248, respectively. To construct the double mutant, the ΔTK2109 strain was transformed with pUDTK2248 with methods described above. Genotypes of the transformants were examined by PCR with the primer sets idTK2109-F/-R, idTK2248-F/-R, odTK2109-F/-R, and odTK2248-F/-R. The double-disruption strain was named the ΔTK2109 ΔTK2248 strain. In all cases, the absence of mutations in the homologous regions was also confirmed by DNA sequencing.
Growth measurements of T. kodakarensis.
Growth properties of the host strain KU216 and the gene disruption strains in the presence or absence of lipoic acid/octanoic acid were examined in the following three kinds of media. One was mASW-AA-S0 supplemented with 10 μg ml−1 of uracil but without Ser or lipoic acid (mASW-AA-Ser[−]-S0-Ura-Lip[−]). Second and third were this medium further supplemented either with 1 mM lipoic acid (mASW-AA-Ser[−]-S0-Ura-Lip[+]) or 1 mM octanoic acid (mASW-AA-Ser[−]-S0-Ura-Oct[+]). Cells were precultured in the first medium supplemented with Ser (mASW-AA-S0-Ura-Lip[−]) for 20 to 24 h until the stationary phase and inoculated into the three synthetic media. Culture experiments were performed at 85°C in triplicate, and optical densities at 660 nm (OD660) were monitored.
Growth properties of the host strain KU216 and the ΔTK2109 ΔTK2248 double gene disruption strain in the presence or absence of biotin were examined as follows. Cells were precultured in the nutrient-rich medium ASW-YT-S0 for 14 h until the stationary phase and inoculated into the synthetic medium mASW-AA-S0 with or without biotin, supplemented with 10 μg ml−1 of uracil. After five serial cultures, the OD660 of the sixth culture was monitored.
Preparation of purified TK2109 and TK2248 recombinant proteins.
Plasmids for the expression of the TK2109 and TK2248 genes were constructed as follows. The coding regions of the TK2109 and TK2248 genes with modified flanking sequences were amplified by PCR with the primer sets eTK2109-F/-R and eTK2248-F/-R and inserted into pET21a(+) plasmid at the NdeI-BamHI site utilizing the In-Fusion HD cloning kit (TaKaRa Bio, Shiga, Japan). The resulting plasmids were named pET2109 and pET2248, respectively. After confirmation of the absence of unintended mutations by DNA sequencing analysis, the plasmids pET2109 and pET2248 were individually introduced into E. coli strain BL21-CodonPlus(DE3)-RIL (Agilent Technologies, Santa Clara, CA). The transformants with the TK2109 and TK2248 genes were cultivated at 37°C until the OD660 reached 0.2 ∼0.6, and gene expression was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 0.1 mM and further cultivated at 37°C for 7 h. Cells were then harvested, resuspended in 50 mM Tris-HCl buffer (pH 7.5), and disrupted by sonication. After centrifugation (4°C, 5,000 × g, and 10 min), the soluble cell extracts including TK2109 and TK2248 recombinant proteins were incubated at 75°C for 15 min to remove thermolabile proteins from E. coli. After centrifugation (4°C, 5,000 × g, and 20 min), the supernatants including the TK2109 and TK2248 recombinant proteins were individually applied to an anion-exchange column, ResourceQ (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom), and proteins were eluted with a linear gradient of NaCl (0 to 1.0 M) in 50 mM Tris-HCl (pH 7.5) at a flow rate of 2.0 ml min−1. After exchange of the buffer of the relevant fractions by using an Amicon Ultra centrifugal filter unit (molecular weight cutoff [MWCO]: 10,000 [10K]) (EMD Millipore, Billerica, MA), proteins were separated with a Superdex 75 10/300 gel filtration column (GE Healthcare) with a mobile phase of 50 mM Tris-HCl (pH 7.5) with 0.15 M NaCl at a flow rate of 0.4 ml min−1. Fractions containing target proteins were concentrated using an Amicon Ultra centrifugal filter unit (MWCO: 10K). The concentrations of TK2109 and TK2248 recombinant proteins were determined with a protein assay system (Bio-Rad, Hercules, CA) using bovine serum albumin (BSA; Thermo Fisher Scientific, Waltham, MA) as a standard. The purities of TK2109 and TK2248 recombinant proteins were analyzed by SDS-PAGE.
Reconstitution of [Fe-S] clusters in TK2109 and TK2248 recombinant proteins.
The chemical reconstitution of iron-sulfur clusters of TK2109 and TK2248 recombinant proteins was carried out according to previously reported methods (34, 52–55), with some modifications. The purified TK2109 and TK2248 recombinant proteins were individually concentrated (approximately 200 μM; volume is 0.5 ml each) by the Amicon Ultra centrifugal filter unit (MWCO: 10K). All the following operations were performed under an anaerobic environment except for centrifugation, and all reagents were degassed by sonication and put in an anaerobic chamber for 24 h prior to use. DTT was added to the concentrated protein solutions (0.5 ml each) at a final concentration of 10 mM. The resulting mixtures were incubated on ice for 1 h to reduce the proteins. The proteins were then diluted to a concentration of about 30 μM (calculated based on the initial concentration) with 2.8 ml of reconstitution buffer (50 mM HEPES [pH 8.0], 50 mM NaCl, and 10% [vol/vol] glycerol) and placed on ice for a further 30 min. After that, FeCl3 and Na2S were slowly added to the mixtures to final concentrations of 150 μM and 300 μM, respectively. The resulting mixtures were then incubated at room temperature for 1 h. The precipitates were removed by centrifugation (4°C, 13,000 × g, and 15 min). The supernatants were stored at 4°C under an anaerobic environment overnight. The reconstituted proteins were concentrated by Amicon Ultra filters (MWCO: 10K). UV-visible spectra were monitored to confirm the reconstitution of iron-sulfur cluster with a UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). The concentrations of reconstituted proteins were determined with the protein assay system as described above.
Lipoyl synthase activity measurement using HPLC.
Lipoyl synthase activity was examined using a chemically synthesized octanoyl-peptide (Eurofins Genomics K.K., Tokyo, Japan). The peptide is a portion of the H-protein from T. kodakarensis (TK0150), which is predicted to be the lipoyl domain based on the predicted lipoyl domain sequence of the H-proteins from E. coli (13) and Mycobacterium tuberculosis (56). The amino acid sequence of the octanoyl-peptide is ESVKAVSE, in which the octanoyl group is connected with the Lys residue by amide bond. Reactions were carried out under anaerobic conditions. The reaction mixture contained 50 mM HEPES buffer (pH 7.5 at 70°C), 50 mM NaCl, 10% (vol/vol) glycerol, 5 mM DTT, 1 mM SAM, 300 μM octanoyl-peptide, and 10 mM sodium dithionite, as well as 100 μM reconstituted TK2109 protein and/or 100 μM reconstituted TK2248 protein. The reaction mixture without proteins was set as a control. After preincubation at 70°C for 1 min, the reactions were initiated by adding 10 mM sodium dithionite. The reactions were then carried out at 70°C for 60 min and were quenched with HCl at a final concentration of 120 mM. After neutralization with 12 μl of 1 M NaOH, the precipitated proteins were removed by centrifugation (4°C, 13,000 × g, and 5 min). The supernatants were analyzed by HPLC using a Cosmosil 5C18-PAQ column (4.6 mm by 250 mm; 5-μm particle size). The column was equilibrated with a solution including 95% solvent A (5 mM sodium phosphate buffer [pH 5.0]) and 5% solvent B (5 mM sodium phosphate buffer within 10% acetonitrile and 40% methanol [pH 5.0]). A gradient of 5 to 100% solvent B was applied from 8 to 28 min, followed by a 12-min maintenance. Then the proportion was returned to 5% solvent B from 40 to 45 min. The column was reequilibrated for 5 min. A flow rate of 0.8 ml/min was maintained throughout the procedure. A chemically synthesized lipoyl-peptide (Eurofins Genomics K.K.) was used as a standard of the reaction product.
Identification of reaction products of TK2109 and TK2248 proteins by LC-MS analysis.
The reaction products of the lipoyl synthase reactions were analyzed by LC-MS. The compounds in the reaction mixture were separated by the Cosmosil 5C18-PAQ column (4.6 mm by 250 mm; 5 μm particle size) equilibrated with a solution including 70% solvent A (0.1% formic acid [pH 2.6]) and 30% solvent B (50% acetonitrile within 0.1% formic acid). A gradient of 30 to 70% solvent B was applied from 8 to 28 min and then returned to 30% solvent B from 28 to 31 min. The column was reequilibrated for 14 min. A flow rate of 0.8 ml/min was maintained throughout the procedure. Detection of analytes was performed using a Fourier transform (orbitrap) mass spectrometer (FTMS) with an electrospray ionization source in positive-ion mode (ESI+). The parameters were set as follows: an Aux gas heater temperature of 300°C, a capillary temperature of 350°C, a spray voltage of 3.5 kV, a resolution of 70,000, and a mass range of m/z 105 to 1,500.
Bioinformatic analysis of the homologs of archaeal lipoyl synthase and relative proteins.
Gene distribution analysis was performed using the BLAST search tool in the KEGG database (57) (https://www.genome.jp/tools/blast/). The classical LipA from S. solfataricus P2 (SSO3158), TK2109, and TK2248 were used as queries to search for the homologs of archaeal lipoyl synthase. The N-terminal and C-terminal sections of the E2 subunit of pyruvate dehydrogenase complex (PDC E2) of S. solfataricus P2 (SSO1529 and SSO1530, respectively) and the H-protein of T. kodakarensis (TK0150) were used as queries to search for the homologs of archaeal PDC E2 and H-protein which are modified with lipoic acid. The N-terminal and C-terminal sections of LplA of T. kodakarensis (TK1908 and TK1234, respectively) were used as queries to search for the LplA homologs in archaea. Amino acid sequence alignments were carried out by ClusterX (58) and ESPript 3 (59) (http://espript.ibcp.fr/ESPript/ESPript/).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Karin Nishimura for LC-MS analysis and Tomonori Tamura for discussion about the LC-MS analysis.
This work was partially supported by the Kyoto University Foundation and Toyota Physical and Chemical Research Institute (T.S.) and by JSPS KAKENHI grant numbers 18H03934 and JP19H05679 (Post-Koch Ecology) and JP19H05684 (H.A.).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Perham RN. 2000. Swinging arms and swinging domains in multifunctional enzymes: catalytic machines for multistep reactions. Annu Rev Biochem 69:961–1004. doi: 10.1146/annurev.biochem.69.1.961. [DOI] [PubMed] [Google Scholar]
- 2.Spalding MD, Prigge ST. 2010. Lipoic acid metabolism in microbial pathogens. Microbiol Mol Biol Rev 74:200–228. doi: 10.1128/MMBR.00008-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayr JA, Feichtinger RG, Tort F, Ribes A, Sperl W. 2014. Lipoic acid biosynthesis defects. J Inherit Metab Dis 37:553–563. doi: 10.1007/s10545-014-9705-8. [DOI] [PubMed] [Google Scholar]
- 4.Cronan JE. 2016. Assembly of lipoic acid on its cognate enzymes: an extraordinary and essential biosynthetic pathway. Microbiol Mol Biol Rev 80:429–450. doi: 10.1128/MMBR.00073-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikuchi G, Motokawa Y, Yoshida T, Hiraga K. 2008. Glycine cleavage system: reaction mechanism, physiological significance, and hyperglycinemia. Proc Jpn Acad Ser B 84:246–263. doi: 10.2183/pjab.84.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacob S, Ruus P, Hermann R, Tritschler HJ, Maerker E, Renn W, Augustin HJ, Dietze GJ, Rett K. 1999. Oral administration of rac-α-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: a placebo-controlled pilot trial. Free Radic Biol Med 27:309–314. doi: 10.1016/S0891-5849(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 7.Biewenga GP, Haenen GR, Bast A. 1997. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol 29:315–331. doi: 10.1016/s0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- 8.Packer L, Witt EH, Tritschler HJ. 1995. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med 19:227–250. doi: 10.1016/0891-5849(95)00017-R. [DOI] [PubMed] [Google Scholar]
- 9.Mattevi A, Obmolova G, Sokatch JR, Betzel C, Hol WG. 1992. The refined crystal structure of Pseudomonas putida lipoamide dehydrogenase complexed with NAD+ at 2.45 Å resolution. Proteins 13:336–351. doi: 10.1002/prot.340130406. [DOI] [PubMed] [Google Scholar]
- 10.Douce R, Bourguignon J, Neuburger M, Rébeillé F. 2001. The glycine decarboxylase system: a fascinating complex. Trends Plant Sci 6:167–176. doi: 10.1016/s1360-1385(01)01892-1. [DOI] [PubMed] [Google Scholar]
- 11.Broderick JB, Duffus BR, Duschene KS, Shepard EM. 2014. Radical S-adenosylmethionine enzymes. Chem Rev 114:4229–4317. doi: 10.1021/cr4004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlin MI, Lanz ND, Goldman PJ, Lee K-H, Booker SJ, Drennan CL. 2016. Crystallographic snapshots of sulfur insertion by lipoyl synthase. Proc Natl Acad Sci U S A 113:9446–9450. doi: 10.1073/pnas.1602486113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy EL, Booker SJ. 2017. Destruction and reformation of an iron-sulfur cluster during catalysis by lipoyl synthase. Science 358:373–377. doi: 10.1126/science.aan4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanz ND, Pandelia M-E, Kakar ES, Lee K-H, Krebs C, Booker SJ. 2014. Evidence for a catalytically and kinetically competent enzyme-substrate cross-linked intermediate in catalysis by lipoyl synthase. Biochemistry 53:4557–4572. doi: 10.1021/bi500432r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakuraba H, Tsuge H, Yoneda K, Katunuma N, Ohshima T. 2005. Crystal structure of the NAD biosynthetic enzyme quinolinate synthase. J Biol Chem 280:26645–26648. doi: 10.1074/jbc.C500192200. [DOI] [PubMed] [Google Scholar]
- 16.Stekhanova T, Bezsudnova E, Mardanov A, Osipov E, Ravin N, Skryabin K, Popov V. 2014. Nicotinamidase from the thermophilic archaeon Acidilobus saccharovorans: structural and functional characteristics. Biochemistry (Mosc) 79:54–61. doi: 10.1134/S0006297914010088. [DOI] [PubMed] [Google Scholar]
- 17.Hachisuka S-I, Sato T, Atomi H. 2017. Metabolism dealing with thermal degradation of NAD+ in the hyperthermophilic archaeon Thermococcus kodakarensis. J Bacteriol 199:e00162-17. doi: 10.1128/JB.00162-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hachisuka S-I, Sato T, Atomi H. 2018. Hyperthermophilic archaeon Thermococcus kodakarensis utilizes a four-step pathway for NAD+ salvage through nicotinamide deamination. J Bacteriol 200:e00785-17. doi: 10.1128/JB.00785-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokooji Y, Tomita H, Atomi H, Imanaka T. 2009. Pantoate kinase and phosphopantothenate synthetase, two novel enzymes necessary for CoA biosynthesis in the Archaea. J Biol Chem 284:28137–28145. doi: 10.1074/jbc.M109.009696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishibashi T, Tomita H, Yokooji Y, Morikita T, Watanabe B, Hiratake J, Kishimoto A, Kita A, Miki K, Imanaka T, Atomi H. 2012. A detailed biochemical characterization of phosphopantothenate synthetase, a novel enzyme involved in coenzyme A biosynthesis in the Archaea. Extremophiles 16:819–828. doi: 10.1007/s00792-012-0477-5. [DOI] [PubMed] [Google Scholar]
- 21.Tomita H, Imanaka T, Atomi H. 2013. Identification and characterization of an archaeal ketopantoate reductase and its involvement in regulation of coenzyme A biosynthesis. Mol Microbiol 90:307–321. doi: 10.1111/mmi.12363. [DOI] [PubMed] [Google Scholar]
- 22.Tomita H, Yokooji Y, Ishibashi T, Imanaka T, Atomi H. 2014. An archaeal glutamate decarboxylase homolog functions as an aspartate decarboxylase and is involved in β-alanine and coenzyme A biosynthesis. J Bacteriol 196:1222–1230. doi: 10.1128/JB.01327-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimosaka T, Makarova KS, Koonin EV, Atomi H. 2019. Identification of dephospho-coenzyme A (dephospho-CoA) kinase in Thermococcus kodakarensis and elucidation of the entire CoA biosynthesis pathway in Archaea. mBio 10:e01146-19. doi: 10.1128/mBio.01146-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang S, Cordova B, Chavarria N, Elbanna D, McHugh S, Rojas J, Pfeiffer F, Maupin-Furlow JA. 2014. Conserved active site cysteine residue of archaeal THI4 homolog is essential for thiamine biosynthesis in Haloferax volcanii. BMC Microbiol 14:260. doi: 10.1186/s12866-014-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi M, Kobayashi K, Esaki H, Konno H, Akaji K, Tazuya K, Yamada K, Nakabayashi T, Nosaka K. 2014. Enzymatic and structural characterization of an archaeal thiamin phosphate synthase. Biochim Biophys Acta 1844:803–809. doi: 10.1016/j.bbapap.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Rodionov DA, Leyn SA, Li X, Rodionova IA. 2017. A novel transcriptional regulator related to thiamine phosphate synthase controls thiamine metabolism genes in Archaea. J Bacteriol 199:e00743-16. doi: 10.1128/JB.00743-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storbeck S, Rolfes S, Raux-Deery E, Warren MJ, Jahn D, Layer G. 2010. A novel pathway for the biosynthesis of heme in Archaea: genome-based bioinformatic predictions and experimental evidence. Archaea 2010:175050. doi: 10.1155/2010/175050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kühner M, Haufschildt K, Neumann A, Storbeck S, Streif J, Layer G. 2014. The alternative route to heme in the methanogenic archaeon Methanosarcina barkeri. Archaea 2014:327637. doi: 10.1155/2014/327637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mashhadi Z, Zhang H, Xu H, White RH. 2008. Identification and characterization of an archaeon-specific riboflavin kinase. J Bacteriol 190:2615–2618. doi: 10.1128/JB.01900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mashhadi Z, Xu H, Grochowski LL, White RH. 2010. Archaeal RibL: a new FAD synthetase that is air sensitive. Biochemistry 49:8748–8755. doi: 10.1021/bi100817q. [DOI] [PubMed] [Google Scholar]
- 31.Laso-Pérez R, Wegener G, Knittel K, Widdel F, Harding KJ, Krukenberg V, Meier DV, Richter M, Tegetmeyer HE, Riedel D, Richnow H-H, Adrian L, Reemtsma T, Lechtenfeld OJ, Musat F. 2016. Thermophilic archaea activate butane via alkyl-coenzyme M formation. Nature 539:396–401. doi: 10.1038/nature20152. [DOI] [PubMed] [Google Scholar]
- 32.Shima S, Thauer RK. 2005. Methyl-coenzyme M reductase and the anaerobic oxidation of methane in methanotrophic Archaea. Curr Opin Microbiol 8:643–648. doi: 10.1016/j.mib.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Wagner T, Koch J, Ermler U, Shima S. 2017. Methanogenic heterodisulfide reductase (HdrABC-MvhAGD) uses two noncubane [4Fe-4S] clusters for reduction. Science 357:699–703. doi: 10.1126/science.aan0425. [DOI] [PubMed] [Google Scholar]
- 34.Bryant P, Kriek M, Wood RJ, Roach PL. 2006. The activity of a thermostable lipoyl synthase from Sulfolobus solfataricus with a synthetic octanoyl substrate. Anal Biochem 351:44–49. doi: 10.1016/j.ab.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Douglas P, Kriek M, Bryant P, Roach PL. 2006. Lipoyl synthase inserts sulfur atoms into an octanoyl substrate in a stepwise manner. Angew Chem Int Ed Engl 45:5197–5199. doi: 10.1002/anie.200601910. [DOI] [PubMed] [Google Scholar]
- 36.Fukui T, Atomi H, Kanai T, Matsumi R, Fujiwara S, Imanaka T. 2005. Complete genome sequence of the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 and comparison with Pyrococcus genomes. Genome Res 15:352–363. doi: 10.1101/gr.3003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makino Y, Sato T, Kawamura H, Hachisuka S-I, Takeno R, Imanaka T, Atomi H. 2016. An archaeal ADP-dependent serine kinase involved in cysteine biosynthesis and serine metabolism. Nat Commun 7:13446–13412. doi: 10.1038/ncomms13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng R-C, Hachisuka S-I, Tomita H, Imanaka T, Zheng Y-G, Nishiyama M, Atomi H. 2018. An ornithine ω-aminotransferase required for growth in the absence of exogenous proline in the archaeon Thermococcus kodakarensis. J Biol Chem 293:3625–3636. doi: 10.1074/jbc.RA117.001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering C. v. 2019. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato T, Fukui T, Atomi H, Imanaka T. 2003. Targeted gene disruption by homologous recombination in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J Bacteriol 185:210–220. doi: 10.1128/jb.185.1.210-220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato T, Fukui T, Atomi H, Imanaka T. 2005. Improved and versatile transformation system allowing multiple genetic manipulations of the hyperthermophilic archaeon Thermococcus kodakaraensis. Appl Environ Microbiol 71:3889–3899. doi: 10.1128/AEM.71.7.3889-3899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumi R, Manabe K, Fukui T, Atomi H, Imanaka T. 2007. Disruption of a sugar transporter gene cluster in a hyperthermophilic archaeon using a host-marker system based on antibiotic resistance. J Bacteriol 189:2683–2691. doi: 10.1128/JB.01692-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santangelo TJ, Cubonová L, Reeve JN. 2008. Shuttle vector expression in Thermococcus kodakaraensis: contributions of cis elements to protein synthesis in a hyperthermophilic archaeon. Appl Environ Microbiol 74:3099–3104. doi: 10.1128/AEM.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santangelo TJ, Cubonová L, Reeve JN. 2010. Thermococcus kodakarensis genetics: TK1827-encoded β-glycosidase, new positive-selection protocol, and targeted and repetitive deletion technology. Appl Environ Microbiol 76:1044–1052. doi: 10.1128/AEM.02497-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Busby RW, Schelvis JP, Yu DS, Babcock GT, Marletta MA. 1999. Lipoic acid biosynthesis: LipA is an iron-sulfur protein. J Am Chem Soc 121:4706–4707. doi: 10.1021/ja990134g. [DOI] [Google Scholar]
- 46.Ollagnier-de Choudens S, Fontecave M. 1999. The lipoate synthase from Escherichia coli is an iron-sulfur protein. FEBS Lett 453:25–28. doi: 10.1016/S0014-5793(99)00694-8. [DOI] [PubMed] [Google Scholar]
- 47.McCarthy EL, Rankin AN, Dill ZR, Booker SJ. 2019. The A-type domain in Escherichia coli NfuA is required for regenerating the auxiliary [4Fe-4S] cluster in Escherichia coli lipoyl synthase. J Biol Chem 294:1609–1617. doi: 10.1074/jbc.RA118.006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harmer JE, Hiscox MJ, Dinis PC, Fox SJ, Iliopoulos A, Hussey JE, Sandy J, Van Beek FT, Essex JW, Roach PL. 2014. Structures of lipoyl synthase reveal a compact active site for controlling sequential sulfur insertion reactions. Biochem J 464:123–133. doi: 10.1042/BJ20140895. [DOI] [PubMed] [Google Scholar]
- 49.Cicchillo RM, Lee K-H, Baleanu-Gogonea C, Nesbitt NM, Krebs C, Booker SJ. 2004. Escherichia coli lipoyl synthase binds two distinct [4Fe-4S] clusters per polypeptide. Biochemistry 43:11770–11781. doi: 10.1021/bi0488505. [DOI] [PubMed] [Google Scholar]
- 50.Dibrova DV, Galperin MY, Mulkidjanian AY. 2014. Phylogenomic reconstruction of archaeal fatty acid metabolism. Environ Microbiol 16:907–918. doi: 10.1111/1462-2920.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robb FT, Place AR. 1995. Media for thermophiles, p 167–168. In Robb FT, Place AR (ed), Archaea: a laboratory manual—thermophiles. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 52.Freibert S-A, Weiler BD, Bill E, Pierik AJ, Mühlenhoff U, Lill R. 2018. Biochemical reconstitution and spectroscopic analysis of iron-sulfur proteins. Methods Enzymol 599:197–226. doi: 10.1016/bs.mie.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 53.Cicchillo RM, Iwig DF, Jones AD, Nesbitt NM, Baleanu-Gogonea C, Souder MG, Tu L, Booker SJ. 2004. Lipoyl synthase requires two equivalents of S-adenosyl-l-methionine to synthesize one equivalent of lipoic acid. Biochemistry 43:6378–6386. doi: 10.1021/bi049528x. [DOI] [PubMed] [Google Scholar]
- 54.Grzyb J, Xu F, Nanda V, Luczkowska R, Reijerse E, Lubitz W, Noy D. 2012. Empirical and computational design of iron-sulfur cluster proteins. Biochim Biophys Acta 1817:1256–1262. doi: 10.1016/j.bbabio.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Antonkine ML, Liu G, Bentrop D, Bryant DA, Bertini I, Luchinat C, Golbeck JH, Stehlik D. 2002. Solution structure of the unbound, oxidized photosystem I subunit PsaC, containing [4Fe-4S] clusters FA and FB: a conformational change occurs upon binding to photosystem I. J Biol Inorg Chem 7:461–472. doi: 10.1007/s00775-001-0321-3. [DOI] [PubMed] [Google Scholar]
- 56.Lanz ND, Lee K-H, Horstmann AK, Pandelia M-E, Cicchillo RM, Krebs C, Booker SJ. 2016. Characterization of lipoyl synthase from Mycobacterium tuberculosis. Biochemistry 55:1372–1383. doi: 10.1021/acs.biochem.5b01216. [DOI] [PubMed] [Google Scholar]
- 57.Kanehisa M, Goto S. 2000. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 59.Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodionova IA, Vetting MW, Li X, Almo SC, Osterman AL, Rodionov DA. 2017. A novel bifunctional transcriptional regulator of riboflavin metabolism in Archaea. Nucleic Acids Res 45:3785–3799. doi: 10.1093/nar/gkw1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.