Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS) are ubiquitous. Previous studies have found associations between PFAS and thyroid hormones in maternal and cord sera, but the results are inconsistent. To further address this research question, we used mixture modeling to assess the associations with individual PFAS, interactions among PFAS chemicals, and the overall mixture.
Methods:
We collected data through the Health Outcomes and Measures of the Environment (HOME) Study, a prospective cohort study that between 2003 and 2006 enrolled 468 pregnant women and their children in the greater Cincinnati, Ohio region. We assessed the associations of maternal serum PFAS concentrations measured during pregnancy with maternal (n = 185) and cord (n = 256) sera thyroid stimulating hormone (TSH), total thyroxine (TT4), total triiodothyronine (TT3), free thyroxine (FT4), and free triiodothyronine (FT3) using two mixture modeling approaches (Bayesian kernel machine regression (BKMR) and quantile g-computation) and multivariable linear regression. Additional models considered thyroid autoantibodies, other non-PFAS chemicals, and iodine deficiency as potential confounders or effect measure modifiers.
Results:
PFAS, considered individually or as mixtures, were generally not associated with any thyroid hormones. A doubling of perfluorooctanesulfonic acid (PFOS) had a positive association with cord serum TSH in BKMR models but the 95% CI included the null (β = 0.09; 95% credible interval: −0.08, 0.27). Using BKMR and multivariable models, we found that among children born to mothers with higher thyroid peroxidase antibody (TPOAb), perfluorooctanoic acid (PFOA), PFOS, and perfluorohexanesulfonic acid (PFHxS) were associated with decreased cord FT4 suggesting modification by maternal TPOAb status.
Conclusions:
These findings suggest that maternal serum PFAS concentrations measured in the second trimester of pregnancy are not strongly associated with thyroid hormones in maternal and cord sera. Further analyses using robust mixture models in other cohorts are required to corroborate these findings.
Keywords: Perfluoroalkyl Substances (PFAS), Thyroid Hormones, Mixture Models, Epidemiology, Pregnancy
1. Introduction
Per- and polyfluoroalkyl substances (PFAS) are environmentally persistent synthetic chemicals detected in air, dust, soil, drinking water, and consumer products (Blum et al., 2015). Widely produced in industrialized countries beginning in the 1940s and 1950s (Lindstrom et al., 2011), more than 4,700 fluorine-containing compounds are now estimated to exist, although not all are currently used in consumer products (Birnbaum, 2018). PFAS, including perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), perfluorononanoic acid (PFNA), and perfluorohexanesulfonic acid (PFHxS), readily cross the placenta and are detected in the blood of virtually all pregnant women, children, and neonates (Ballesteros et al., 2017; Braun, 2016). Due to some of these chemicals’ long half-lives (ranging from 2.5–7.3 years) (Caron-Beaudoin et al., 2019) and bioaccumulative properties and toxicity in animal studies (Lindstrom et al., 2011), research quantifying associations between PFAS and health outcomes during sensitive windows of development is imperative.
PFAS are potential thyroid disruptors. In animal studies, individual PFAS have been shown to alter circulating levels of thyroid hormone (Ramhøj et al., 2018; Stahl et al., 2011; Yu et al., 2009). Epidemiological studies have assessed the relationship between various PFAS and thyroid hormones of mothers, neonates, and children (Berg et al., 2015; Chan et al., 2011; Kim et al., 2011; Lopez-Espinosa et al., 2012; Preston et al., 2018; Reardon et al., 2019; Wang et al., 2013, 2014; Webster et al., 2014), but the findings are conflicting (Ballesteros et al., 2017). Effect measure modification by autoantibody and iodine status contribute to the “multiple hit hypothesis” (Webster et al., 2014); a theory that thyroid function may be more susceptible to disruption by chemicals such as PFAS if the system is already impacted by multiple stressors. Consequently, some authors have suggested that TPOAb, TgAb, and iodine status are effect measure modifiers of PFAS’ association with thyroid hormones, but observations vary by study (Itoh et al., 2019; Preston et al., 2018; Reardon et al., 2019; Webster et al., 2014, 2016). However, another reason for inconsistent findings could be the mixture of PFAS in the population. To our knowledge, no study has assessed associations of PFAS mixtures with maternal or cord serum thyroid hormones. Further, our study will be one of very few studies that has assessed effect measure modification by autoantibody and iodine status as an additional exploratory analyses. Disentangling the contributions of individual elements of PFAS mixtures and quantifying the effect of the overall mixture is necessary to understand the impact of PFAS on thyroid hormones.
This issue is important because of the role of thyroid hormone in brain development, growth, depression, and obesity (Boelaert and Franklyn J A, 2005; Tanm, 2011). In the brain and nervous system, thyroid hormones regulate cell migration and differentiation, synaptogenesis, and myelination (Bernal, 2007). Among younger individuals, thyroid hormone deficiencies can cause growth delays, intellectual disabilities, and precocious puberty (Papi et al., 2007). Low thyroid hormone during pregnancy may cause neurological impairment (Bernal, 2007) and lowered IQ (Chang and Pearce, 2013) in children.
The objective of this study was to quantify the association of individual and mixtures of PFOA, PFOS, PFNA, and PFHxS measured in maternal serum with thyroid stimulating hormone (TSH), total thyroxine (TT4), total triiodothyronine (TT3), free thyroxine (FT4), and free triiodothyronine (FT3) measured during pregnancy and in cord serum. Additionally, we explored potential effect measure modification of the association of PFAS with thyroid hormones by maternal TPOAb, TgAb, and iodine deficiency status.
2. Methods
2.1. Study Design and Participants
We enrolled 468 pregnant women in the Health Outcomes and Measures of the Environment (HOME) Study from the greater Cincinnati area between March 2003 and January 2006 and 389 delivered singleton infants. Detailed information on the HOME Study have been described elsewhere (Braun et al., 2017). Of the 389 eligible mother-child dyads, 355 had PFAS measurements. At least one cord or maternal thyroid hormone was measured in 305 of the 355 dyads. Both thyroid hormone measurements and PFAS serum measurements were available for 256 cord and 185 maternal sera samples (Figure S1). The Institutional Review Boards (IRBs) of Cincinnati Children’s Hospital Medical Center (CCHMC) and all delivery hospitals approved the study protocol. The Centers for Disease Control and Prevention (CDC) deferred to the CCHMC IRB as the IRB of record.
2.2. Maternal Serum Collection and PFAS Quantification
Maternal blood samples were collected at approximately 16 weeks’ gestation, 26 weeks’ gestation, and at delivery. Serum was separated from whole blood samples after clotting at the study site and maintained at −80°C until it could be analyzed. Of the 305 mother-infant dyads with at least one PFAS and thyroid hormone measured, most mothers (n=258; 85%) had a sufficient volume of serum available to quantify PFAS in the 16 week sample (16.0 ± 1.9 weeks). We used samples collected at 26 weeks (26.5 ± 1.8 weeks) for 32 (10%) participants or delivery (39.1 ± 0.9 weeks) for 15 (5%) participants if they had insufficient serum volume available in the 16 week sample (Figure S1). Samples collected around 16 weeks were prioritized to minimize the impact of changing serum volumes due to pregnancy (Braun et al., 2016a). PFOA, PFOS, PFNA, and PFHxS concentrations were measured at the CDC using on-line solid phase extraction coupled with high performance liquid chromatography-isotope dilution tandem mass spectrometry (Kato et al., 2011). We detected all four PFAS in all samples with limits of detection (LODs) ranging between 0.082–0.2 ng/mL (Braun et al., 2016a). Quality control materials and reagent blanks were included in each analytic batch with coefficients of variation in repeated quality control materials of approximately 6%.
2.3. Thyroid Hormone and Autoantibody Sample Collection
We measured TSH (μIU/mL), TT4 (μg/dL), TT3 (ng/dL), FT4 (ng/dL), and FT3 (pg/mL) in maternal serum at 16 weeks’ gestation and cord serum at delivery. In total, 305 mother-infant dyads had at least one thyroid hormone measured (Figure S1). We also measured TPOAb (IU/mL) and TgAb (IU/mL) in maternal serum at 16 weeks’ gestation. The Department of Laboratory Medicine of the University of Washington measured all thyroid hormones and autoantibodies. Upon arrival, thyroid hormone specimens were kept at −70°C and analyzed using an Access2 automated clinical immunoassay analyzer by Beckman Coulter, Inc (Fullerton, CA). To ensure quality control, two daily quality control materials [BioRad Liquicheck or BioRad Immunoassay Plus (Hercules, CA)] were run with each assay (n = 22). Results were reviewed by a second technologist for accuracy. Coefficients of variation for all thyroid hormones and autoantibodies ranged between <1.0 to 11%. In our study, only 15 women had clinically significant TPOAb [≥ 9.0 IU/mL (NHANES, 2007a); 8.2%] and 7 [≥ 4.0 IU/mL (NHANES, 2007b, 2011); 3.8%] had clinically significant TgAb levels.
2.4. Covariates and Additional Exposures
We collected information on covariates using standardized questionnaires and interviews. We abstracted obstetric history and delivery information after delivery. We calculated the average of serum cotinine measured in samples collected at 16 and 26 gestational weeks (Bernert et al., 2009). We examined polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs), namely BDE-28, BDE-47, and PCB-153, as potential confounders because previous research in this cohort and others has suggested they may influence thyroid hormone levels in pregnant women or neonates (Caron-Beaudoin et al., 2019; Chevrier et al., 2007; Longnecker et al., 2003; Vuong et al., 2015; Webster et al., 2014). We measured PBDEs and PCBs in maternal serum at the CDC using gas chromatography/isotope dilution high-resolution mass spectrometry (Jones et al., 2012; Sjödin et al., 2004). Serum samples were pretreated and extracted by solid phase extraction. To ensure consistency between each batch of samples (n = 24), 3 quality control and method blank samples were included in each batch. PBDE and PCB concentrations were standardized on a serum lipid basis (ng/g) and total lipids were established using standard enzymatic methods (Phillips et al., 1989). Using the blank as the baseline, the LOD was classified as three times the standard deviation of the method blank samples in the batch or if no blank was detectable as 5 pg/μL. If the sample had a value less than the LOD, the value was replaced by the LOD divided by the square root of 2 (Hornung and Reed, 1990). Iodine was measured in a single maternal urine sample, collected predominantly at 26 weeks (mean = 26.6, SD = 2.1), using an Agilent 7500x Inductively Coupled Plasma-Mass Spectrometer (Caldwell et al., 2003) for 292 (95.7%) of the mother-infant dyads in the analytic population. The LOD was 0.5 μg I/L with an average cross validation for all quality-controlled specimens less than or equal to 10%. Urinary creatinine was measured using enzymatic methods, and based on maternal creatinine-standardized urinary iodine, women were categorized as iodine deficient (<150 μg I/g creatinine) or sufficient ( ≥150 μg I/g) (Ghassabian et al., 2014).
2.5. Statistical Analyses
2.5.1. Descriptive
We calculated summary statistics among participants with cord (n = 256) or maternal serum (n = 185), with complete PFAS information, and with data for at least one thyroid hormone. Median (IQR) values were calculated for each PFAS and thyroid hormone for both maternal and cord sera for relevant covariate groupings.
2.5.2. Bayesian kernel machine regression (BKMR)
Bayesian kernel machine regression (BKMR) (Bobb et al., 2015) and the R package ‘bkmr’ (Bobb et al., 2018) were used to estimate individual and overall effects of PFAS within a mixture on TSH, TT4, TT3, FT4, and FT3 in cord and maternal sera. BKMR is a semi-parametric statistical method that can be used to estimate the effects of individual mixture components, the overall mixture effect, and interactions between mixture components. It can be represented by the equation , where Yi represents the outcome variable, h(zi) is the Gaussian kernel containing the exposures of interest, xi is a matrix of covariates, and β is a vector of corresponding coefficients. We used a Gaussian kernel, which can support flexible exposure-response shapes. Posterior inclusion probabilities (PIPs) were derived to help determine which chemicals were important variables for the association between PFAS mixtures and thyroid hormones (Bobb et al., 2018). Predictors with a PIP greater than or equal to 0.5 were considered meaningful (Barbieri and Berger, 2004).
Covariate-adjusted BKMR models were used to simultaneously regress all PFAS against each of cord and maternal TSH, TT4, TT3, FT4, and FT3. For all BKMR analyses, PFAS and thyroid hormones were log2-transformed to account for skewness and to reduce the influence of extreme values in the distribution. Shapiro-Wilkes tests were used to assess that log2-transformations reduced deviation from normality for a majority of the thyroid hormone models (Vuong et al., 2015). PFAS were first log2-transformed before being centered to a mean of 0 and scaled to a standard deviation of 1 (Deyssenroth et al., 2018). Based on a priori observations from the literature of covariates with the exposures and outcomes of interest, we adjusted for maternal age at delivery (linear), race/ethnicity (dichotomous for White and non-Hispanic or Other), marital status at baseline (unmarried and living alone, unmarried and cohabitating, or married), maternal education level (less than Bachelor’s or equal to or higher than Bachelor’s), household income (linear), mean log10-transformed cotinine (a sensitive and specific marker of tobacco smoke exposure) (Braun et al., 2010), nulliparity, maternal alcohol usage during pregnancy (never or ever usage), maternal pre-pregnancy body mass index (BMI) (kg/m2), the infant’s sex (male or female), and gestational week at blood draw for PFAS measurement (linear). Thyroid hormone cord serum models also included delivery mode (vaginal or cesarean section) as a covariate. Gestational age at delivery was not included as a covariate in cord serum models as it is potentially on the causal pathway between PFAS and newborn thyroid hormones. Continuous covariates were centered to 0 and scaled to a standard deviation of 1. Requiring complete covariate data, analyses of cord serum thyroid hormones included 231–236 participants. Adjusted models for maternal serum had 171 participants (Figure S1). All models were fit by running 20,000 iterations using the Markov Chain Monte Carlo (MCMC) sampler.
Overall mixture effects were estimated by taking the mean value of the outcome when all the PFAS concentrations were at the 75th percentile and subtracting the mean value of the outcome when the PFAS concentrations were at the 25th percentile while keeping the covariates constant (Bobb et al., 2018). Plots were used to evaluate the overall association of the PFAS mixture with each thyroid hormone by comparing each quantile of the PFAS mixture to the median. Individual effects of each PFAS mixture component were estimated by taking the mean value of the outcome when one PFAS was at the 75th percentile and subtracting the mean outcome value if the PFAS was at the 25th percentile, while keeping the other PFAS fixed at their medians and all covariates held constant (Bobb et al., 2018). Univariate plots were used to visualize the exposure-response relationship for each PFAS in the mixture while holding the other exposures to their 50th percentile and covariates constant. Potential interactions among the PFAS in the mixture were visualized by assessing the effect on the outcome given by different fixed quantiles of each possible pair of PFAS within the mixture.
Additional adjusted BKMR models were created to explore potential effect measure modification of the association of the PFAS mixture with thyroid hormones in cord or maternal serum by TPOAb, TgAb, and iodine deficiency status by including these variables in the exposure matrix (zi). Two distinct exploratory analyses were conducted; one for the autoantibodies together and the other for iodine deficiency status. TPOAb was dichotomized at less than or equal to or above the median. TgAb was dichotomized by detectable or not detectable. Maternal urinary iodine was used to characterize women as iodine sufficient or deficient as described above.
Further BKMR models were run as sensitivity analyses to assess if robust associations remained in the cord TSH model. A BKMR was run which included PFOA, PFOS, PFNA, PFHxS, BDE-28, BDE-47, and PCB-153 (n = 202). As with PFAS, BDE-28, BDE-47, and PCB-153 were first log2-transformed then centered to a mean of 0 and scaled to a standard deviation of 1 and included as exposures (zi) in the model. We further explored the PFOA, PFOS, PFNA, and PFHxS mixture within this restricted sample to assess any possible selection effect within the reduced analytic sample. Finally, to assess the potential influence of gestational age at PFAS measurement, which has varied widely across previous studies (Ballesteros et al., 2017), on cord TSH models we ran a BKMR with PFOA, PFOS, PFNA, PFHxS, BDE-28, BDE-47, and PCB-153 in participants with a gestational age < 22 weeks (n = 186).
2.5.3. Quantile-based g-computation
Quantile-based g-computation (Keil et al., 2019) was used to corroborate that the overall effect and contributions of each PFAS in the mixture were approximately consistent across two mixture model techniques. Quantile-based g-computation is an adaptation of weighted quantile sum regression (WQSR), a mixture modeling method used in environmental epidemiology (Carrico et al., 2014). Quantile g-computation has certain advantages over traditional WQSR, including that directional homogeneity of effect estimates is not required. This method is applied in three main steps. The first step transforms all the exposures into quantiles. Next, a linear model is fit between the exposures, covariates, and outcome. Finally, weights are defined for each exposure, corresponding to the strength of the association between the exposure and the outcome. The overall mixture effect can be interpreted as the change in outcome per one quantile of change in all exposures while controlling for covariates. Utilizing the R package ‘qgcomp’ (Keil et al., 2019), quantile-based g-computation models were created to compare with results from the BKMR models. Like with the BKMR models, serum thyroid hormones and the continuous exposures were log2-transformed, and the exposures and covariates were centered to a mean of 0 and scaled to a standard deviation of 1.
2.5.4. Multivariable Linear Models
We used generalized linear models to further describe exposure-response relationships and to compare the findings from our mixture models to a more common analytic approach. We ran crude and adjusted models with and without log2-transformed BDE-28, BDE-47, and PCB-153 individually for each log2-transformed thyroid hormone with the PFAS also log2-transformed. We adjusted multivariable models for the same covariates as the BKMR and quantile g-computation models. Interaction terms for median dichotomized TPOAb, detectable versus non-detectable TgAb, and iodine deficiency status were also evaluated in separate models.
We conducted statistical analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) or R version 3.5.1 (R Core Team, 2013).
3. Results
Mother-infant dyads included in at least one of our cord or maternal serum models had similar demographic characteristics (Table 1 and Table S1). The majority of women in our study were between 25 and 35 years old (mean age of 30), non-Hispanic White, married or cohabitating, and had a bachelor’s degree or higher. Serum PFAS concentrations were generally lower among women who identified as non-Hispanic Black, had less educational attainment, and were unmarried. Median (IQR) concentrations were highest for PFOS in both the cord [14.3 (8.1) ng/mL] and maternal [14.3 (8.9) ng/mL] sera (Table 2). Those who had PFAS measured at delivery were of lower income status and a greater proportion were classified as a race other than White and non-Hispanic (Table S2). Additionally, PFAS were strongly correlated with the other PFAS (Figure S2).
Table 1:
Demographic characteristics of study participants in cord and maternal sera analyses.
| Cord Serum | Maternal Serum | |
|---|---|---|
| n (%) | n (%) | |
| Overall | 256 (100) | 185 (100) |
| Maternal Age (years) | ||
| <25 | 51 (20) | 37 (20) |
| 25–35 | 165 (65) | 114 (62) |
| >35 | 40 (16) | 34 (18) |
| Race/Ethnicity | ||
| Non-Hispanic White | 165 (65) | 116 (63) |
| Black | 70 (27) | 51 (28) |
| Other | 20 (8) | 17 (9) |
| Missing | 1 (0) | 1 (1) |
| Marital Status | ||
| Married | 185 (72) | 128 (69) |
| Unmarried, cohabiting | 26 (10) | 19 (10) |
| Unmarried, living alone | 44 (17) | 37 (20) |
| Missing | 1 (0) | 1 (1) |
| Household Income ($/year) | ||
| <20,000 | 48 (19) | 38 (21) |
| 20-<40,000 | 43 (17) | 35 (19) |
| 40-<80,000 | 87 (34) | 57 (31) |
| >80,000 | 77 (30) | 54 (29) |
| Missing | 1 (0) | 1 (1) |
| Education | ||
| Less than high school | 54 (21) | 43 (23) |
| High school or some college | 64 (25) | 38 (21) |
| Bachelor’s or more | 137 (54) | 103 (56) |
| Missing | 1 (0) | 1 (1) |
| Alcohol consumption | ||
| Any | 100 (39) | 73 (40) |
| None | 147 (57) | 108 (58) |
| Missing | 9 (4) | 4 (2) |
| Serum cotinine (ng/mL) | ||
| <0.015 (Unexposed) | 110 (43) | 73 (40) |
| 0.015–3 (Secondhand) | 120 (47) | 90 (49) |
| >3 (Active smoker) | 26 (10) | 22 (12) |
| Maternal BMI (kg/m2) | ||
| <24.9 (Underweight-normal) | 131 (51) | 102 (55) |
| 25–29.9 (Overweight) | 67 (26) | 44 (24) |
| ≥30 (Obese) | 49 (19) | 31 (17) |
| Missing | 9 (4) | 8 (4) |
| Mode of delivery | ||
| Vaginal delivery | 187 (73) | 128 (69) |
| Cesarean section | 66 (26) | 56 (30) |
| Missing | 3 (1) | 1 (1) |
| Parity | ||
| Nulliparous | 108 (42) | 83 (45) |
| Primiparous | 77 (30) | 50 (27) |
| Multiparous | 65 (25) | 48 (26) |
| Missing | 6 (2) | 4 (2) |
| Newborn Sex | ||
| Female | 135 (53) | 101 (55) |
| Male | 121 (47) | 84 (45) |
Table 2:
Median (IQR) of PFAS and thyroid hormones across all HOME study participants (2003–2006)
| Cord Serum Analysis | Maternal Analysis | |||
|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | |
| Thyroid Hormone | ||||
| TSH (μIU/L) | 256 | 7.1 (4.8) | 185 | 1.3 (1.2) |
| TT4 (μg/dL) | 251 | 9.6 (2.2) | 185 | 10.3 (2.5) |
| TT3 (ng/dL) | 256 | 49 (17.3) | 185 | 158.0 (36.0) |
| FT4 (ng/dL) | 256 | 1.0 (0.2) | 185 | 0.7 (0.1) |
| FT3 (pg/mL) | 254 | 1.6 (0.4) | 185 | 3.2 (0.4) |
| PFAS | ||||
| PFOA (ng/mL) | 256 | 5.6 (4.1) | 185 | 5.5 (4.5) |
| PFOS (ng/mL) | 256 | 14.3 (8.1) | 185 | 14.3 (8.9) |
| PFNA (ng/mL) | 256 | 0.9 (0.5) | 185 | 0.9 (0.4) |
| PFHxS (ng/mL) | 256 | 1.6 (1.5) | 185 | 1.6 (1.5) |
3.1. Primary Statistical Analyses
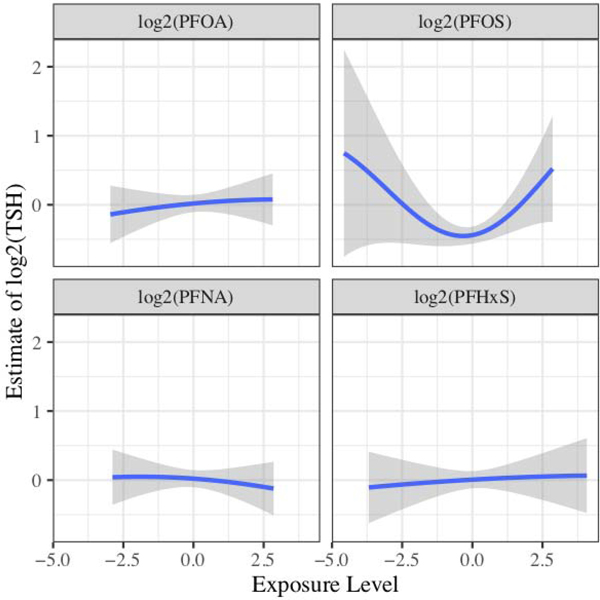
We observed limited evidence of an influence of the PFAS-mixture on maternal or cord thyroid hormones by either mixture method, or of individual PFAS with maternal or cord thyroid hormones in mixture or multivariable linear regression models. Most adjusted models suggested that no individual PFAS was a main driver of an association between PFAS mixtures and thyroid hormones. Likewise, we observed no strong evidence of interactions between PFAS. The exception to this occurred in the log2-transformed cord TSH model and the cord FT3 model. Cord TSH had the most consistent association across modeling types. In the BKMR model for cord TSH, log2-transformed PFOS was positively associated with TSH with a PIP value of 0.84 and appeared to have a U-shaped association with log2-transformed TSH when all other PFAS were held at their median value (Figure 1). The quantile g-computation model also predicted that log2-transformed PFOS would have the largest influence on the association with cord serum TSH compared to the other PFAS. With log2-transformed cord FT3 models, all predictors had PIPs between 0.56 and 0.59, but the overall effect of the mixture was null (Figure S3). Multivariable linear regression models did not suggest associations of individual log2-transformed PFAS with any of the maternal or cord thyroid hormones (Table S3).
Figure 1:
Univariate plot of log2-transformed PFAS and cord TSH (n = 236). The plot assesses the association between each exposure and the estimate of log2-transformed TSH while fixing the other exposures to their 50th percentile. 95% CrI are shown in grey to depict variability. The model has been adjusted for maternal age at delivery, race/ethnicity, marital status at baseline, maternal education level, household income, mean log10-transformed cotinine, maternal alcohol usage during pregnancy, nulliparity, maternal body mass index based on pre-pregnancy weight in pounds, the child’s sex, gestational week at blood draw for PFAS measurement, and delivery mode. All predictors and continuous covariates were centered to 0 and scaled to a standard deviation of 1.
In general, the BKMR and quantile g-computation models agreed in that the overall effect estimates of the log2-transformed PFAS mixtures were associations in the same direction (i.e. both models supported either a positive or negative association with each thyroid hormone) across models (Table 3). Both mixture models suggested that log2-transformed PFAS had a positive trending association with increasing log2-transformed cord TSH [BKMR estimate: 0.11 (95% CrI: −0.08, 0.31), quantile g-computation: 0.10 (95% CI: −0.03, 0.23), p-value = 0.14].
Table 3:
Adjusted multivariable, BKMR, and quantile g-computation estimates for cord and maternal sera analyses.
| Cord Serum | ||||||
|---|---|---|---|---|---|---|
| BKMR* | ||||||
| n | Multivariable β (95% CI) |
Estimate (95% Credible Interval) |
PIP** | Qgcomp*** Estimate | ||
| log2(TSH) | 236 | |||||
| log2(PFOA) | 0.06 (−0.08, 0.19) | 0.013 (−0.087, 0.114) | 0.121 | 0.039 | ||
| log2(PFOS) | 0.09 (−0.06, 0.25) | 0.094 (−0.082, 0.271) | 0.836 | 0.070 | ||
| log2(PFNA) | 0.04 (−0.16, 0.24) | 0.005 (−0.079, 0.090) | 0.135 | 0.013 | ||
| log2(PFHxS) | 0.05 (−0.05, 0.16) | 0.003 (−0.059, 0.065) | 0.083 | −0.024 | ||
| Overall | 0.112 (−0.082, 0.305) | 0.098 (95% CI: −0.033, 0.230), p-value = 0.14 | ||||
| log2(TT4) | 231 | |||||
| log2(PFOA) | 0.03 (−0.02, 0.08) | 0.001 (−0.011, 0.012) | 0.016 | 0.016 | ||
| log2(PFOS) | 0.01 (−0.04, 0.07) | 0.000 (−0.009, 0.009) | 0.022 | 0.004 | ||
| log2(PFNA) | 0.002 (−0.07, 0.07) | 0.000 (−0.004, 0.004) | 0.005 | −0.021 | ||
| log2(PFHxS) | 0.02 (−0.01, 0.06) | 0.001 (−0.017, 0.020) | 0.032 | 0.015 | ||
| Overall | 0.002 (−0.021, 0.026) | 0.015 (95% CI: −0.036, 0.066), p-value = 0.57 | ||||
| log2(TT3) | 236 | |||||
| log2(PFOA) | −0.01 (−0.09, 0.06) | 0.000 (−0.004, 0.004) | 0.002 | −0.016 | ||
| log2(PFOS) | −0.02 (−0.10, 0.06) | 0.000 (−0.005, 0.005) | 0.006 | 0.040 | ||
| log2(PFNA) | −0.04 (−0.14, 0.07) | 0.000 (−0.008, 0.008) | 0.005 | −0.024 | ||
| log2(PFHxS) | −0.02 (−0.08, 0.03) | 0.000 (−0.010, 0.010) | 0.009 | −0.032 | ||
| Overall | −0.001 (−0.015, 0.014) | −0.032 (95% CI: −0.093, 0.029), p-value = 0.30 | ||||
| log2(FT4) | 236 | |||||
| log2(PFOA) | −0.01 (−0.04, 0.03) | 0.000 (−0.017, 0.017) | 0.165 | 0.015 | ||
| log2(PFOS) | −0.02 (−0.06, 0.02) | −0.003 (−0.024, 0.018) | 0.216 | −0.018 | ||
| log2(PFNA) | −0.01 (−0.07, 0.04) | −0.002 (−0.022, 0.018) | 0.157 | −0.012 | ||
| log2(PFHxS) | −0.01 (−0.04, 0.02) | −0.001 (−0.020, 0.019) | 0.190 | 0.007 | ||
| Overall | −0.006 (−0.039, 0.028) | −0.008 (95% CI: −0.045, 0.028), p-value = 0.66 | ||||
| log2(FT3) | 234 | |||||
| log2(PFOA) | −0.01 (−0.06, 0.03) | 0.000 (−0.010, 0.010) | 0.569 | −0.001 | ||
| log2(PFOS) | −0.03 (−0.07, 0.02) | 0.000 (−0.012, 0.012) | 0.569 | 0.011 | ||
| log2(PFNA) | −0.02 (−0.08, 0.04) | 0.000 (−0.012, 0.011) | 0.585 | −0.013 | ||
| log2(PFHxS) | −0.02 (−0.05, 0.02) | 0.000 (−0.011, 0.011) | 0.579 | −0.011 | ||
| Overall | 0.000 (−0.022, 0.021) | −0.024 (95% CI: −0.062, 0.013), p-value =0.20 | ||||
| Maternal Serum | ||||||
| BKMR* | ||||||
| n | Multivariable β (95% CI) |
Estimate (95% Credible Interval) |
PIP** | Qgcomp*** Estimate | ||
| log2(TSH) | 171 | |||||
| log2(PFOA) | 0.09 (−0.14, 0.33) | 0.046 (−0.164, 0.257) | 0.335 | 0.169 | ||
| log2(PFOS) | 0.02 (−0.24, 0.28) | 0.024 (−0.170, 0.218) | 0.393 | 0.011 | ||
| log2(PFNA) | −0.23 (−0.56, 0.10) | −0.068 (−0.294, 0.158) | 0.432 | −0.109 | ||
| log2(PFHxS) | −0.06 (−0.23, 0.11) | −0.044 (−0.262, 0.174) | 0.366 | −0.120 | ||
| Overall | −0.045 (−0.336, 0.247) | −0.049 (95% CI: −0.281, 0.184), p-value = 0.68 | ||||
| log2(TT4) | 171 | |||||
| log2(PFOA) | −0.03 (−0.10, 0.04) | −0.001 (−0.015, 0.014) | 0.023 | −0.019 | ||
| log2(PFOS) | 0.02 (−0.08, 0.08) | 0.000 (−0.009, 0.010) | 0.019 | 0.041 | ||
| log2(PFNA) | −0.07 (−0.17, 0.03) | −0.002 (−0.025, 0.022) | 0.034 | −0.045 | ||
| log2(PFHxS) | 0.00 (−0.05, 0.06) | 0.000 (−0.014, 0.014) | 0.024 | 0.006 | ||
| Overall | −0.002 (−0.035, 0.03) | −0.017 (95% CI: −0.075, 0.042), p-value = 0.58 | ||||
| log2(TT3) | 171 | |||||
| log2(PFOA) | −0.01 (−0.05, 0.04) | 0.000 (−0.002, 0.002) | 0.001 | 0.010 | ||
| log2(PFOS) | −0.02 (−0.07, 0.03) | 0.000 (−0.004, 0.003) | 0.001 | −0.004 | ||
| log2(PFNA) | −0.03 (−0.09, 0.03) | 0.000 (0.000, 0.000) | 0.000 | −0.020 | ||
| log2(PFHxS) | −0.01 (−0.04, 0.02) | 0.000 (0.000, 0.000) | 0.001 | −0.009 | ||
| Overall | 0.000 (−0.004, 0.004) | −0.024 (95% CI: −0.066, 0.018), p-value = 0.26 | ||||
| log2(FT4) | 171 | |||||
| log2(PFOA) | −0.01 (−0.06, 0.03) | −0.005 (−0.036, 0.026) | 0.179 | −0.017 | ||
| log2(PFOS) | 0.02 (−0.02, 0.07) | 0.005 (−0.027, 0.036) | 0.175 | 0.003 | ||
| log2(PFNA) | −0.01 (−0.07, 0.05) | −0.003 (−0.026, 0.021) | 0.224 | −0.005 | ||
| log2(PFHxS) | 0.02 (−0.01, 0.05) | 0.005 (−0.027, 0.038) | 0.240 | 0.023 | ||
| Overall | 0.003 (−0.042, 0.047) | 0.004 (95% CI: −0.044, 0.051), p-value = 0.88 | ||||
| log2(FT3) | 171 | |||||
| log2(PFOA) | −0.01 (−0.04, 0.01) | 0.000 (−0.003, 0.003) | 0.008 | 0.000 | ||
| log2(PFOS) | −0.03 (−0.06, 0.00) | −0.001 (−0.015, 0.012) | 0.052 | −0.009 | ||
| log2(PFNA) | −0.02 (−0.05, 0.02) | 0.000 (−0.005, 0.004) | 0.011 | −0.005 | ||
| log2(PFHxS) | −0.02 (−0.04, 0.00) | −0.001 (−0.012, 0.010) | 0.028 | −0.007 | ||
| Overall | −0.002 (−0.020, 0.015) | −0.022 (95% CI: −0.051, 0.007), p-value = 0.13 | ||||
BKMR: Bayesian kernel machine regression
PIP: Posterior inclusion probability
Qgcomp: Quantile g-computation
The models have been adjusted for each PFAS, maternal age at delivery, race/ethnicity, marital status at baseline, maternal education level, household income, mean log10-transformed cotinine, maternal alcohol usage during pregnancy, nulliparity, maternal body mass index based on pre-pregnancy weight in pounds, the child’s sex, gestational week at blood draw for PFAS measurement, and delivery mode. All predictors and continuous covariates were also centered to 0 and scaled to a standard deviation of 1 in the BKMR and quantile g-computation models.
3.2. Exploratory and Sensitivity Analyses
There was little indication of effect measure modification by TPOAb, TgAb, or iodine deficiency status across BKMR models. However, among newborns born to women with higher TPOAb, we observed a weak positive association between PFNA and cord FT4; whereas we observed no association among newborns of mothers with low TPOAb. PFOA, PFOS, and PFHxS were inversely associated with cord FT4 among newborns of mothers with higher TPOAb levels (> 0.6 IU/mL) (Figure S4). The multivariable linear regression models suggested a similar trend in which log2-transformed PFOA, PFOS, and PFHxS were each associated with decreased levels of cord FT4 among infants of mother with TPOAb above the median (Table S4). Across multivariable models considering effect measure modification by iodine deficiency status for cord serum, log2-transformed PFHxS was positively associated with the thyroid hormone among those with sufficient iodine status and negatively associated among those classified as iodine deficient (Table S5). This observation, along with some additional evidence of effect measure modification of certain individual PFAS on cord or maternal thyroid hormone associations according to maternal thyroid antibody or iodine deficiency status from the multivariable linear regression models, were not consistent with the BKMR models.
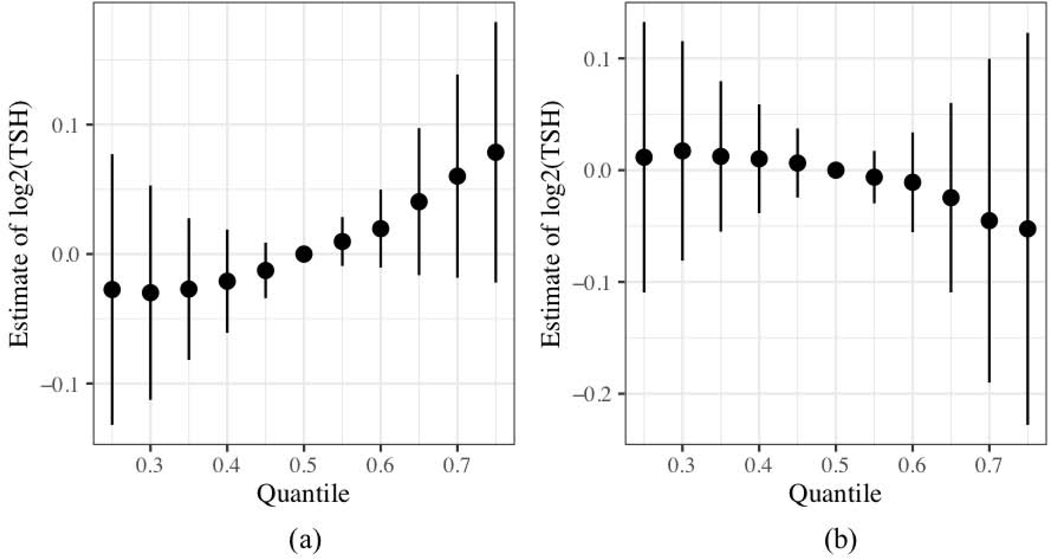
To better assess if associations between mixtures and thyroid hormones were a result of PFAS and not co-exposure to other known thyroid disruptors, we added BDE-28, BDE-47, and PCB-153 to the mixture. We evaluated whether the slightly positive association between adjusted log2-transformed PFAS elements and cord TSH in our BKMR model was robust to inclusion of additional co-exposures. While the PFAS mixture without PBDEs and PCB-153 indicated an overall positive effect of the mixture (Figure 2a), a mixture modeled with the additional co-exposures indicated that the expanded mixture may be associated with decreasing levels of cord serum TSH (Figure 2b). This was validated in the quantile g-computation model which also estimated that the expanded model’s mixture effect was below 0. Both models indicated that BDE-47 was the major negative predictor of the association (Figure S5). The U-shaped association noted in the PFAS-only mixture of log2-transformed PFOS was no longer apparent within the mixture with the additional exposures. Finally, the BKMR model that assessed PFAS in only the 202 individuals that had PBDE and PCB measures had an overall effect estimate that was also attenuated (Figure S6a). Overall effects of the PFAS mixture on cord TSH were also attenuated in the model restricting to gestational age <22 weeks (Figure S6b).
Figure 2:
Adjusted association of the overall PFAS mixture on log2-transformed cord TSH from BKMR. The plots compare the value of the estimate when all the log2-transformed PFAS are at the respective quantile compared to when they are at the median. Variation is expressed using 95% credible intervals. Models include log2-transformed (a) PFOA, PFOS, PFNA, and PFHxS (n = 236) and (b) PFOA, PFOS, PFNA, PFHxS, BDE-28, BDE-47, and PCB-153 (n = 202). The models have been adjusted for maternal age at delivery, race/ethnicity, marital status at baseline, maternal education level, household income, mean log10-transformed cotinine, maternal alcohol usage during pregnancy, nulliparity, maternal body mass index based on pre-pregnancy weight in pounds, the child’s sex, gestational week at blood draw for PFAS measurement, and delivery mode. All predictors and continuous covariates were also centered to 0 and scaled to a standard deviation of 1.
4. Discussion
We found little evidence to suggest that individual or mixtures of PFAS measured during pregnancy were associated with thyroid hormones measured in maternal or cord sera in this cohort. This was consistent across BKMR, quantile g-computation, and multivariable linear regression models. Across all models we expected effect estimates would be in the same direction (e.g. the association between PFOS and cord serum TSH would be positive in the BKMR, quantile g-computation, and multivariable models). This trend generally occurred for overall PFAS mixtures and individual PFAS effects. Deviations from this trend occurred mainly when effect estimates were close to 0. We believe that consistency across multiple modeling types strengthens our results.
Although we are unaware of prior studies assessing the overall effect of PFAS mixtures on thyroid hormones in maternal or cord sera, many previous studies have found that a variety of PFAS measured in maternal blood were associated with neonatal and maternal thyroid hormones (Berg et al., 2015; Itoh et al., 2019; Kim et al., 2011; Preston et al., 2018; Reardon et al., 2019; Wang et al., 2013, 2014; Webster et al., 2014). Our study participants also had PFAS concentrations comparable to other studies that assessed PFAS during pregnancy (Ballesteros et al., 2017; Preston et al., 2018; Reardon et al., 2019).
Our results align with other studies that measured PFAS around the same time during pregnancy and mainly found non-statistically significant results between PFAS and maternal thyroid hormones (Ballesteros et al., 2017; Berg et al., 2015; Chan et al., 2011; Preston et al., 2018; Reardon et al., 2019; Wang et al., 2013; Webster et al., 2014). Sampling time is likely an important factor for thyroid hormones as their levels change over the course of pregnancy (Alexander et al., 2017). Mixed results were observed in a study (Reardon et al., 2019) assessing multiple isomers of PFOS across trimesters. In this study, branched isomers ∑Br-PFOS and 5m-PFOS were statistically significantly associated with TSH during the first trimester and overall but not in the second trimester. However, the major isomer (Linear-PFOS) was not associated with TSH during the first or second trimester. Two previous epidemiological studies observed positive associations between PFOS and maternal TSH measured during the second trimester of pregnancy (Berg et al., 2015; Wang et al., 2013), while other studies found nearly null associations of TSH measured in the first (Preston et al., 2018) and second trimester (Webster et al., 2014). We did not identify evidence of a positive association between maternal serum PFAS and maternal thyroid hormones. Discrepancies across studies may be due to the time of measurement of maternal serum TSH, as TSH dips substantially in the first trimester and generally remains below pre-pregnancy levels through pregnancy (Alexander et al., 2017), differences between study populations, or variation in model covariates.
We found that there was a positive-trending but statistically non-significant association between PFAS and cord serum TSH with PFOS as the major contributor to the mixture. Two studies have looked at PFAS collected in the third trimester of pregnancy and cord TSH. One found that cord TSH decreased 0.083 μIU/mL (95% CI: −0.292, 0.127) with each 1 ng/mL change in PFOS adjusting for maternal age, maternal education level, gravidity, neonatal sex, and delivery mode (Wang et al., 2014). Whereas, the other study found a Pearson correlation coefficient of 0.109 between PFOS and cord TSH after adjusting for maternal age, gestation age, and maternal BMI (Kim et al., 2011). Our results indicated a U-shaped association between log2-transformed PFOS and cord TSH which may explain differences in the directionality of the associations. While PFOS concentrations were similar on average between our study and the study by Wang et al. (2014), participants in the Kim et al. study (2011) tended to have lower concentrations of PFOS on average than our study participants, which may also partially explain discrepant results. Through restricting our model to those less than 22 weeks gestation, we no longer found this U-shaped distribution in our PFOS-cord TSH association. This attenuation of the association may have been due to the reduction in sample size in this analysis. However, recent research has suggested that it may be important to measure PFAS early in pregnancy when considering birth size as an outcome in order to avoid potential confounding by changes in maternal plasma volume expansion and glomerular filtration rate (GFR) (Steenland et al., 2018). Though cord thyroid hormones are less likely to be strongly influenced by maternal GFR as compared to offspring size at birth, future studies may consider measuring PFAS early in pregnancy to avoid this potential source of confounding.
Previous epidemiologic studies have assessed the association between prenatal exposure to PBDEs (Chevrier et al., 2010; Herbstman et al., 2008; Vuong et al., 2015) or PCBs (Chevrier et al., 2007; Herbstman et al., 2008) and cord and neonatal thyroid hormone levels. Although these studies found an association between one or more PBDEs or PCBs and thyroid hormones, the results are inconsistent and often not assessed in conjunction with other thyroid-disrupting hormones such as PFAS. Although our results were modest and not statistically significant, we did find that adding BDE-28, BDE-47, and PCB-153 changed the direction of the mixture’s association with cord TSH. We believe this change in direction is potentially due to the participants that remained in our sensitivity analysis after also adjusting for BDE-28, BDE-47, and PCB-153 and due to the combined effects of the persistent organic pollutants themselves. We assessed these additional endocrine-disrupting chemicals (EDCs) in adjusted multivariable models and did not identify evidence of an association which agreed in statistical significance to our BKMR model for cord TSH. As our BKMR and quantile g-computation model results suggested that BDE-47 was negatively associated with log2-transformed cord TSH in mixtures of PFAS, further research that combines multiple types of EDCs should be emphasized in order to fully illuminate mixtures’ impacts on human health.
Overall, our study found limited evidence of effect measure modification by TPOAb, TgAb, or iodine deficiency status when considering associations between PFAS mixtures and thyroid hormones. However, some indication of effect measure modification by maternal TPOAb status was identified for the associations of PFAS with cord FT4 in the present study. Although we did not observe evidence of effect measure modification of the association of PFAS with maternal thyroid hormones in our study, multiple previous studies (Itoh et al., 2019; Preston et al., 2018; Reardon et al., 2019; Webster et al., 2014, 2016), have suggested effect measure modification of adult or maternal FT4 by autoantibody status. In a nationally representative cross-sectional analysis among adults in the United States, PFOA, PFOS, PFNA, and PFHxS were individually associated with lower FT4 among those with both high TPOAb and low iodine (Webster et al., 2016). Another study suggested that among mothers with clinically high TPOAb, PFOA, PFOS, PFNA, and PFHxS were inversely associated with maternal FT4 (Webster et al., 2014). Three prior studies did not observe associations with maternal FT4 or FT4 index by TPOAb status, but specific individual PFAS were inversely associated with maternal or cord TSH among autoantibody-positive mothers (Itoh et al., 2019; Preston et al., 2018; Reardon et al., 2019). In our multivariable linear regression models, among newborns of mothers with urinary iodine <150μg I/g Cr, PFHxS was inversely associated with cord thyroid hormones; however, the mixture models did not suggest effect modification of the association of PFAS with cord thyroid hormones by iodine status. Our results may differ due to small sample sizes of individuals with clinically significant autoantibody and iodine deficiency status across many of the studies or differences in gestational age of sample collection. Per the “multiple hit hypothesis”, the thyroid gland may be more susceptible to PFAS if other stressors are already present (Webster et al., 2014). Collectively, these observations suggest that future studies should consider the combined effect of multiple stressors (e.g. high TPOAb and low iodine) with associations of potential endocrine-disrupting chemicals on thyroid hormones.
4.1. Strengths and Limitations
This study had several strengths and limitations. To the best of our knowledge, this is the first study to assess associations between PFAS mixtures and thyroid hormones and consider effect measure modification by thyroid autoantibodies in cord serum. Our prospective study design and mixture modeling approaches enabled us to evaluate individual and joint effects of exposures on maternal and cord thyroid hormones. While we were able to consider four PFAS in a mixture, we recognize that there are many more we did not consider. Nevertheless, for the time period of the study, these four PFAS were the most prevalent compounds in the biospecimens. We cannot dismiss the possibility of reverse causality for the associations of PFAS with maternal thyroid hormones due to the cross-sectional nature of the measurements. However, the four PFAS evaluated have long biological half-lives (Lindstrom et al., 2011) and thus measurement at one time point is likely representative of PFAS exposures throughout the course of pregnancy (Ballesteros et al., 2017; Preston et al., 2018). Due to variability of adult iodine levels (Busnardo et al., 2006), our measurement at 26 weeks may not be representative of the entire course of pregnancy and may have led to some misclassification. We also cannot rule out residual confounding by other unmeasured thyroid disruptors or other confounders. However, we were able to assess several PBDEs, PCB-153, and had rich covariate data. There is no clear consensus demonstrating which type of mixture model is superior as this is ultimately context-dependent (Braun et al., 2016b; Hamra and Buckley, 2018; Lazarevic et al., 2019). We also could not identify any clear criteria to quantitatively compare elements within the BKMR model, BKMR model fit, or quantitative metrics to compare modeling types. To address these concerns, we used two different mixture modeling approaches, BKMR and quantile g-computation, which were appropriate for identifying which PFAS were most predictive of thyroid hormones and were able to calculate the overall effect of the mixture. Both approaches largely corroborated each other and were generally similar to more traditional multivariable modeling results, strengthening our internal validity. Mixture methods are evolving, and future analyses should help develop ways to better compare results across modeling techniques and set distinct best practices for building these models.
5. Conclusion
This study assessed the individual, overall, and joint exposure effects of maternal PFAS on a variety of maternal and cord sera thyroid hormones using multiple robust statistical techniques in a prospective cohort. Our results showed limited associations between PFAS and the thyroid hormones studied in this cohort. Our results may be generalizable to cohorts of pregnant women and children with comparable PFAS exposures who have similar demographics to the participants of the HOME Study. This study illustrates how multiple modeling techniques can be used for assessing associations between chemical mixtures and thyroid hormones during sensitive windows of development.
Supplementary Material
Highlights:
PFAS mixtures were not strongly associated with maternal or cord thyroid hormones.
Mixture models assessed maternal PFAS with maternal and cord thyroid hormones.
PFOS had a positive trending association with thyroid stimulating hormone.
Associations between PFAS and thyroid hormones may be modified by TPOAb status.
Acknowledgements:
The authors would like to thank the HOME Study participants and HOME Study staff.
Funding: This work was supported by NIEHS grants P01 ES11261, R01 ES014575, R01 ES020349, R01 ES025214, R01 ES024381, P01 ES022832-02; EPA grant RD-83544201; and NIGMS grant P20 GM104416. Ms. Lebeaux was supported by NIAID grant 2T32AI007519-21. Dr. Doherty was supported by NCI grant R25 CA134286.
Conflicts of interest: JMB was financially compensated for serving as an expert witness for plaintiffs in litigation related to tobacco smoke exposures.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. The authors declare no competing financial interest.
Abbreviations:
- BKMR
Bayesian kernel machine regression
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- CrI
credible interval
- EDCs
endocrine-disrupting chemicals
- FT3
free triiodothyronine
- FT4
free thyroxine
- HOME
Health Outcomes and Measures of the Environment
- LOD
limit of detection
- PBDEs
polybrominated diphenyl ethers
- PCBs
polychlorinated biphenyls
- PFAS
Per- and polyfluoroalkyl substances
- PFHxS
perfluorohexanesulfonic acid
- PFNA
perfluorononanoic acid
- PFOA
perfluorooctanoic acid
- PFOS
perfluorooctanesulfonic acid
- PIP
posterior inclusion probability
- SD
standard deviation
- TgAb
thyroglobulin antibody
- TSH
thyroid stimulating hormone
- TPOAb
thyroid peroxidase antibody
- TT3
total triiodothyronine
- TT4
total thyroxine
Footnotes
Competing Financial Interests: The authors have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander EA, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S, 2017. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 27, 315–389. 10.1089/thy.2016.0457 [DOI] [PubMed] [Google Scholar]
- Ballesteros V, Costa O, Iñiguez C, Fletcher T, Ballester F, Lopez-Espinosa MJ, 2017. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies. Environ. Int. 99, 15–28. 10.1016/j.envint.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Barbieri MM, Berger JO, 2004. Optimal predictive model selection. Ann. Stat. 32, 870–897. 10.1214/009053604000000238 [DOI] [Google Scholar]
- Berg V, Nøst TH, Hansen S, Elverland A, Veyhe AS, Jorde R, Odland JØ, Sandanger TM, 2015. Assessing the relationship between perfluoroalkyl substances, thyroid hormones and binding proteins in pregnant women; a longitudinal mixed effects approach. Environ. Int. 77, 63–69. 10.1016/j.envint.2015.01.007 [DOI] [PubMed] [Google Scholar]
- Bernal J, 2007. Thyroid hormone receptors in brain development and function. Nat. Clin. Pract. Endocrinol. Metab. 3, 249–259. 10.1038/ncpendmet0424 [DOI] [PubMed] [Google Scholar]
- Bernert JT, Peyton JI, Holiday DB, Benowitz NL, Sosnoff CS, Doig MV, Feyerabend C, Aldous KM, Sharifi M, Kellogg MD, Langman LJ, 2009. Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels : A round-robin study. Nicotine Tob. Res. 11, 1458–1466. 10.1093/ntr/ntp161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS, 2018. Hearing on “The Federal Role in the Toxic PFAS Chemical Crisis”: Testimony before the Senate Committee on Homeland Security and Governmental Affairs Subcommittee on Federal Spending Oversight and Emergency Management. [Google Scholar]
- Blum A, Balan SA, Scheringer M, Trier X, Goldenman G, Cousins IT, Diamond M, Fletcher T, Higgins C, Lindeman AE, 2015. The Madrid statement on poly- and perfluoroalkyl substances (PFASs). Environ. Health Perspect. 123, A107–A111. 10.1021/es201662b.Fei [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Henn BC, Valeri L, Coull BA, 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ.Heal. 17, 1–10. 10.1186/s12940-018-0413-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Henn BC, Christiani DC, Wright RO, Godleski JJ, Coull BA, 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16, 493–508. 10.1093/biostatistics/kxu058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert K, Franklyn JA, 2005. Thyroid hormones in health and disease. J. Endocrinol. 187, 1–15. 10.1677/joe.1.06131 [DOI] [PubMed] [Google Scholar]
- Braun JM, 2016. Early life exposure to endocrine disrupting chemicals and childhood obesity and neurodevelopment. Nat Rev Endocrinol 13, 161–173. 10.1038/nrendo.2016.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K, Lanphear BP, 2016a. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity 24, 231–237. 10.1002/oby.21258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Daniels JL, Poole C, Olshan AF, Hornung R, Bernert JT, Xia Y, Bearer C, Barr DB, Lanphear BP, 2010. A prospective cohort study of biomarkers of prenatal tobacco smoke exposure: The correlation between serum and meconium and their association with infant birth weight. Environ. Heal. A Glob. Access Sci. Source 9, 53 10.1186/1476-069X-9-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Gennings C, Hauser R, Webster TF, 2016b. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environ. Health Perspect. 124, A6–A9. 10.1289/ehp.1510569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-hicks S, Morgan S, Xu Y, Yolton K, Lanphear BP, 2017. Cohort Profile : The Health Outcomes and Measures of the Environment ( HOME ) study. Int. J. Epidemiol. 10.1093/ije/dyw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busnardo B, Nacamulli D, Zambonin L, Mian C, Piccolo M, Girelli ME, 2006. Restricted intraindividual urinary iodine concentration variability in nonfasting subjects. Eur. J. Clin. Nutr. 60, 421–425. 10.1038/sj.ejcn.1602334 [DOI] [PubMed] [Google Scholar]
- Caldwell KL, Maxwell CB, Makhmudov A, Pino S, Braverman LE, Jones RL, Hollowell JG, 2003. Use of Inductively Coupled Plasma Mass Spectrometry to Measure Urinary Iodine in NHANES 2000: Comparison with Previous Methods. Clin. Chem. 49, 1019–1021. 10.1373/49.6.1019 [DOI] [PubMed] [Google Scholar]
- Caron-Beaudoin É, Ayotte P, Laouan Sidi EA, Gros-Louis McHugh N, Lemire M, 2019. Exposure to perfluoroalkyl substances (PFAS) and associations with thyroid parameters in First Nation children and youth from Quebec. Environ. Int. 128, 13–23. 10.1016/j.envint.2019.04.029 [DOI] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, Litvak PF, 2014. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. Int. Biometric Soc. 20, 100–120. 10.1007/s13253-014-0180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E, Burstyn I, Cherry N, Bamforth F, Martin JW, 2011. Perfluorinated acids and hypothyroxinemia in pregnant women. Environ. Res. 111, 559–564. 10.1016/j.envres.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Chang DLF, Pearce EN, 2013. Screening for Maternal Thyroid Dysfunction in Pregnancy: A Review of the Clinical Evidence and Current Guidelines. J. Thyroid Res. 2013, 1–8. 10.1155/2013/851326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Eskenazi B, Bradman A, Fenster L, Barr DB, 2007. Associations between prenatal exposure to polychlorinated biphenyls and neonatal thyroid-stimulating hormone levels in a Mexican-American population, Salinas Valley, California. Environ. Health Perspect. 115, 1490–1496. 10.1289/ehp.9843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Harley KG, Bradman A, Gharbi M, Sjödin A, Eskenazi B, 2010. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ. Health Perspect. 118, 1444–1449. 10.1289/ehp.1001905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyssenroth MA, Gennings C, Liu SH, Peng S, Hao K, Lambertini L, Jackson BP, Karagas MR, Marsit CJ, Chen J, 2018. Intrauterine multi-metal exposure is associated with reduced fetal growth through modulation of the placental gene network. Environ. Int. 120, 373–381. 10.1016/j.envint.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian A, Graaff JS, Peeters RP, Ross HA, Jaddoe VW, Hofman A, Verhulst FC, White T, Tiemeier H, 2014. Maternal urinary iodine concentration in pregnancy and children ‘ s cognition : results from a population-based birth cohort in an iodine-suf fi cient area. BMJ Open 4, e005520. 10.1136/bmjopen-2014-005520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra GB, Buckley JP, 2018. Environmental exposure mixtures: questions and methods to address them. Curr. Epidemiol. Reports 5, 160–165. 10.1007/s40471-018-0145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjödin A, Apelberg BJ, Witter FR, Haiden RU, Patterson DG, Panny SR, Needham LL, Goldman LR, 2008. Birth delivery mode modifies the associations between prenatal polychlorinated biphenyl (PCB) and polybrominated diphenyl ether (PBDE) and neonatal thyroid hormone levels. Environ. Health Perspect. 116, 1376–1382. 10.1289/ehp.11379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 5, 46–51. 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- Itoh S, Araki A, Miyashita C, Yamazaki K, Goudarzi H, Minatoya M, Ait Bamai Y, Kobayashi S, Okada E, Kashino I, Yuasa M, Baba T, Kishi R, 2019. Association between perfluoroalkyl substance exposure and thyroid hormone/thyroid antibody levels in maternal and cord blood: The Hokkaido Study. Environ. Int. 133, 105139. 10.1016/j.envint.2019.105139 [DOI] [PubMed] [Google Scholar]
- Jones R, Edenfield E, Anderson S, Zhang Y, Sjodin A, 2012. Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum. Organohalogen Compd. 74, 97–98. [Google Scholar]
- Kato K, Basden BJ, Needham LL, Calafat AM, 2011. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J. Chromatogr. A 1218, 2133–2137. 10.1016/j.chroma.2010.10.051 [DOI] [PubMed] [Google Scholar]
- Keil AJ, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ, 2019. A quantile-based g-computation approach to addressing the effects of exposure mixtures. arXiv:1902.04200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Sunmi, Choi K, Ji K, Seo J, Kho Y, Park J, Kim Sungkyoon, Park S, Hwang I, Jeon J, Yang H, Giesy JP, 2011. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ. Sci. Technol. 45, 7465–7472. 10.1021/es202408a [DOI] [PubMed] [Google Scholar]
- Lazarevic N, Barnett AG, Sly PD, Knibbs LD, 2019. Statistical Methodology in Studies of Prenatal Exposure to Mixtures of Endocrine-Disrupting Chemicals : A Review of Existing Approaches and New Alternatives. Environ. Health Perspect. 127 10.1289/EHP2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL, 2011. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 45, 7954–7961. 10.1021/es2011622 [DOI] [PubMed] [Google Scholar]
- Longnecker MP, Wolff MS, Gladen BC, Brock JW, Grandjean P, Jacobson JL,Korrick SA, Rogan WJ, Weisglas-Kuperus N, Hertz-Picciotto I, Ayotte P, Stewart P, Winneke G, Charles MJ, Jacobson SW, Dewailly E, Boersma ER, Altshul LM, Heinzow B, Pagano JJ, Jensen AA, 2003. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ. Health Perspect. 111, 65–70. 10.1289/ehp.5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Mondal D, Armstrong B, Bloom MS, Fletcher T, 2012. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ. Health Perspect. 120, 1036–1041. 10.1289/ehp.1104370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHANES, 2011. Laboratory Procedure Manual: Thyroglobulin Antibodies Serum [WWW Document]. URL https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/labmethods/thyrod_g_met_thyroglobulin_antibodies.pdf
- NHANES, 2007a. Laboratory Procedure Manual: Thyroid Peroxidase Antibodies [WWW Document]. URL https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/THYROD_e_met_Thyroid_Peroxidase_Antibodies.pdf
- NHANES, 2007b. Laboratory Procedure Manual: Thyroglobulin Antibodies [WWW Document].URL https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/THYROD_e_met_Thyroglobulin_Antibodies.pdf
- Papi G, Uberti E. degli, Betterle C, Carani C, Pearce EN, Braverman LE, Roti E, 2007. Subclinical Hypothyroidism. Curr. Opin. Endocrinol. Diabetes Obes. 14, 197–208. 10.1097/MED.0b013e32803577e7 [DOI] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Needham LL, 1989. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch. Environ. Contam. Toxicol. 18, 495–500. 10.1007/BF01055015 [DOI] [PubMed] [Google Scholar]
- Preston EV, Rifas-Shiman SL, Sagiv SK, Oken E, Ye X, Calafat AM, Braverman LE, McClean MD, Webster TF, Claus Henn B, Pearce EN, 2018. Maternal Plasma per- and Polyfluoroalkyl Substance Concentrations in Early Pregnancy and Maternal and Neonatal Thyroid Function in a Prospective Birth Cohort: Project Viva (USA). Environ. Health Perspect. 126, 027013. 10.1289/ehp2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramhøj L, Hass U, Boberg J, Scholze M, Christiansen S, Nielsen F, Axelstad M, 2018. Perfluorohexane sulfonate (PFHxS) and a mixture of endocrine disrupters reduce thyroxine levels and cause antiandrogenic effects in rats. Toxicol. Sci. 163, 579–591. 10.1093/toxsci/kfy055 [DOI] [PubMed] [Google Scholar]
- Reardon AJF, Khodayari E, Dinu I, Goruk S, Field CJ, Kinniburgh DW, Macdonald AM, Martin JW, Study TA, 2019. Longitudinal analysis reveals early-pregnancy associations between perfluoroalkyl sulfonates and thyroid hormone status in a Canadian prospective birth cohort. Environ. Int. 129, 389–399. 10.1016/j.envint.2019.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Lapeza CR, Focant JF, McGahee EE, Patterson DG, 2004. Semiautomated High-Throughput Extraction and Cleanup Method for the Measurement of Polybrominated Diphenyl Ethers, Polybrominated Biphenyls, and Polychlorinated Biphenyls in Human Serum. Anal. Chem. 76, 1921–1927. 10.1021/ac030381+ [DOI] [PubMed] [Google Scholar]
- Stahl T, Mattern D, Brunn H, 2011. Toxicology of perfluorinated compounds. Environ. Sci. Eur. 23, 1–52. 10.1186/2190-4715-23-38 [DOI] [Google Scholar]
- Steenland K, Barry V, Savitz D, 2018. Serum Perfluorooctanoic Acid and Birthweight. Epidemiology 29, 765–776. 10.1097/ede.0000000000000903 [DOI] [PubMed] [Google Scholar]
- Tanm Ö, 2011. Thyroid hormones and growth in health and disease. JCRPE J. Clin. Res. Pediatr. Endocrinol. 3, 51–55. 10.4274/jcrpe.v3i2.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Webster GM, Romano ME, Braun JM, Thomas Zoeller R, Hoofnagle AN, Sjödin A, Yolton K, Lanphear BP, Chen A, 2015. Maternal polybrominated diphenyl ether (PBDE) exposure and thyroid hormones in maternal and cord sera: The HOME study, Cincinnati, USA. Environ. Health Perspect. 123, 1079–1085. 10.1289/ehp.1408996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-L, Lien G-W, Tseng Y-C, Wang Y, Rogan WJ, Longnecker MP, Chen H-Y, Chen P-C, Lien G-W, Chen H-Y, Tseng Y-C, Longnecker MP, Wang S-L, 2014. Association between Maternal Serum Perfluoroalkyl Substances during Pregnancy and Maternal and Cord Thyroid Hormones: Taiwan Maternal and Infant Cohort Study.Environ. Health Perspect. 122, 529–534. 10.1289/ehp.1306925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Starling AP, Haug LS, Eggesbo M, Becher G, Thomsen C, Travlos G, King D, Hoppin JA, Rogan WJ, Longnecker MP, 2013. Association between Perfluoroalkyl substances and thyroid stimulating hormone among pregnant women: A cross-sectional study. Environ. Heal. A Glob. Access Sci. Source 12, 1–7. 10.1186/1476-069X-12-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Rauch SA, Marie NS, Mattman A, Lanphear BP, Venners SA, 2016. Cross-Sectional Associations of Serum Perfluoroalkyl Acids and Thyroid Hormones in U.S. Adults: Variation According to TPOAb and Iodine Status (NHANES 2007–2008). Environ. Health Perspect. 124, 935–942. 10.1289/ehp.1409589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Venners SA, Mattman A, Martin JW, 2014. Associations between Perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: A population-based cohort study. Environ. Res. 133, 338–347. 10.1016/j.envres.2014.06.012 [DOI] [PubMed] [Google Scholar]
- Yu WG, Liu W, Jin YH, 2009. Effects of perfluorooctane sulfonate on RAT thyroid hormone biosynthesis and metabolism. Environ. Toxicol. Chem. 28, 990–996. 10.1897/08-345.1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.