Abstract
Objective:
Repetitive transcranial magnetic stimulation (rTMS) can cause potentially useful changes in brain functional connectivity (FC), but the number of treatment sessions required is unknown. We applied the continual reassessment method (CRM), a Bayesian, adaptive, dose-finding procedure to a rTMS paradigm in an attempt to answer this question.
Materials and Methods:
The sample size was predetermined at 15 subjects and the cohort size was set with three individuals (i.e., five total cohorts). In a series of consecutive daily sessions, we delivered rTMS to the left posterior parietal cortex and measured resting state FC with fMRI in a predefined hippocampal network in the left hemisphere. The session number for each successive cohort was determined by the CRM algorithm. We set a response criterion of a 0.028 change in FC between the hippocampus and the parietal cortex, which was equal to the increase seen in 87.5% of participants in a previous study using five sessions.
Results:
A ≥ criterion change was observed in 9 of 15 participants. The CRM indicated that > four sessions are required to produce the criterion change reliably in future studies.
Conclusions:
The CRM can be adapted for rTMS dose-finding when a reliable outcome measure, such as FC, is available. The minimum effective dose needed to produce a criterion increase in FC in our hippocampal network of interest at 87.5% efficacy was estimated to be > four sessions. This study is the first demonstration of a Bayesian, adaptive method to explore a rTMS parameter.
Keywords: transcranial magnetic stimulation, neuromodulation, functional connectivity, hippocampus, dose-finding
Introduction
Repetitive transcranial magnetic stimulation (rTMS) can alter brain function,1–8 making it a potential treatment for disorders, including depression,9 schizophrenia,6 memory disorders,10 and migraine.11 A barrier to optimizing rTMS effectiveness is the multi-dimensional delivery parameter space, including the frequency, intensity, and duration of rTMS treatment, which has yet to be explored, except in limited cases. Dose-finding, in particular, is new to rTMS, and one reason for this lack of exploration has been the lack of reliable biomarkers for target effects.
Recently, Wang et al.4 demonstrated enhancement of resting hippocampal network functional connectivity (FC) by stimulating individualized targets in the left posterior parietal cortex (PPC), which is densely connected with the left hippocampus.12,13 This produced improvement in declarative memory, which correlated with the FC increase within individuals, and persisted two weeks after treatment.14 The correlation between memory improvement and FC suggests that this technique may be relevant for the treatment of memory disorders (e.g. 10). Wang et al.4 chose a treatment duration of five daily rTMS sessions. However, this choice was arbitrary and differed from both the single treatments employed in laboratory studies and the multiple weeks of treatment delivered in depression trials.15 Of all rTMS delivery parameters, duration carries the greatest logistical and economic burden for researchers and clinicians alike, and both groups should be motivated to find the minimum effective number of sessions to produce a desired outcome.
In a pilot study, we investigated the minimum number of consecutive daily rTMS sessions necessary to produce a clinically relevant increase in hippocampal FC using procedures similar to those of Wang et al.4 We defined a target network of brain regions based on their results and defined a threshold increase in FC between the hippocampus and that network. The trial design was based on the continual reassessment method (CRM),16 an adaptive, Bayesian technique originally developed to determine the maximum tolerable dose (MTD) in Phase I drug trials. In a CRM design, small cohorts are run consecutively at specified dosages, and toxicity (defined as exceeding a predetermined threshold) is noted as a categorical event. Based on this binary outcome for each individual in a cohort, the model recommends the dose for the next cohort until it converges on an estimate of the MTD. The main objective of this work was to adapt the CRM to estimate the minimum number of rTMS sessions, i.e., minimum effective dose (MED) necessary to produce a criterion increase in hippocampal-target network FC. To our knowledge, this is the first attempt to optimize a rTMS parameter based on a change in a quantitative biomarker in individual subjects using an adaptive design.
Methods
Participants
Fifteen healthy adults (six female; mean age 25.87±4.64), free of neurological or psychiatric disorders and medications acting on the central nervous system, participated in the study. All participants reported being right-handed and passed screening for contraindications to TMS17 and MRI. Written informed consent was obtained and the study was approved by the National Institutes of Health Combined Neuroscience Institutional Review Board.
Procedures
Following consent and screening procedures, all participants underwent, in order, baseline scanning, One to four consecutive daily rTMS sessions, and a post-rTMS scan. Baseline scanning included an anatomical localizer, structural scan (for co-localization of functional data with anatomy and neuro-navigation), a single resting state scan, and diffusion tensor imaging (not reported here). Participants underwent their first rTMS session within 36 hours of baseline scanning. rTMS sessions were scheduled approximately 24 hours apart. The second scan occurred on the day after the final rTMS session and included only anatomical localization, structural, and resting state scans. To prevent our results from being confounded by time of day, the post-scans were scheduled within three hours of the same time of day as the baseline scan.
fMRI acquisition and preprocessing
MRI was acquired on a Siemen’s Magnetom 3T scanner using a 16-channel head coil with foam padding to prevent head movement. Participants were equipped with earplugs and headphones to protect hearing. Blood oxygen level-dependent (BOLD) data were obtained using a T2*-weighted gradient-echoplanar imaging sequence (EPI: TR = 2,000 ms, TE = 27 ms, flip angle = 90°, 36 transversal contiguous interleaved slices per volume, 206 volumes, 3.0 slice thickness, FOV 22 × 22 cm, matrix size 64 × 64, voxel size = 3.4 mm × 3.4 mm × 3.0 mm; scan length ~6.8 minutes). During resting state functional imaging, participants were instructed to look at a cross, visible through a mirror mounted on the head coil, but to blink and breath normally. Structural images were acquired using a magnetization-prepared rapid gradient echo sequence (MPRAGE; TR = 2,530 ms, TE = 3.03 ms, 176 slices per volume, 1 mm thickness, FOV = 25.6 × 25.6 cm2, 256 × 256 acquisition matrix, voxel size = 1.0 mm isotropic).
Image processing was performed with Analysis of Functional Images (AFNI;18) software. The first five volumes of 206 were removed to ensure that processing only included scans where magnetization was stabilized. Preprocessing included motion correction, slice-timing correction to the first slice, functional/structural affine co-registration to Talairach space (using a TT_N27 template;19), resampling to 2.0 mm3 voxel resolution, spatial smoothing using a four mm full width half maximum (FWHM) Gaussian kernel, and linear detrending. Each voxel time series was then scaled to a mean of 100, with a range of 0–200. Head motion was regressed from each voxel time series using the mean and derivatives of six parameter estimates (pitch, roll, and yaw, and rotation around each direction). Unlike Wang et al., we did not bandpass filter our data, which would have caused at least a 60% decrease in degrees of freedom, and, instead, used spatial smoothing. Spatial smoothing was omitted by Wang et al. in order to permit a search for changes in FC with millimeter resolution. Finally, frames which included movement displacement greater than 0.3 mm were censored prior to statistical analysis to prevent inflated correlations.20
rTMS targeting
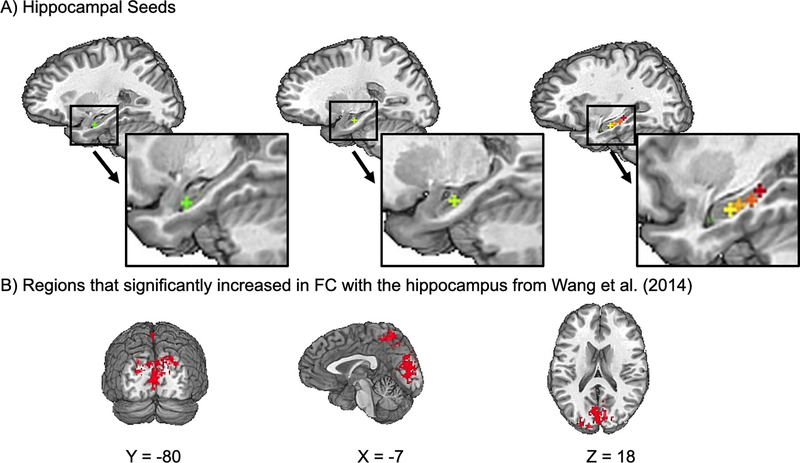
After each participant’s baseline scan and preprocessing, we identified six voxels along the longitudinal axis of the hippocampus in TT_N27 template space (Seed one: x = −26, y = −10, z = −17; Seed two: x = −22, y = −16, z = −13; Seed three: x = −30, y = −17, z = −14; Seed four: x = −30, y = −22, z = −12; Seed five: x = −30, y = −27, z = −9; Seed six: x = −30, y – 32, z = −6; see Fig. 1A) and averaged the time series within a three mm radius around each seed voxel to create a time series for that seed. For each seed, we searched for the maximally connected voxel in the left PPC, i.e., within a 15 mm radius sphere around Talairach location x = −47, y = −68, z = +36, which included the supramarginal and angular gyri, and was cut to exclude non-brain voxels (see FC calculations and voxel-wise analysis). This searchlight region in the PPC was similar to the one used by Wang et al.4 In each participant, the hippocampal seed that was maximally connected with any voxel in this sphere was chosen as the hippocampal target. The PPC voxel maximally connected with the hippocampal target was chosen as the rTMS location, marked in standard space, and back-transformed into subject space using the inverse matrix of the original affine transformation. It was then transformed into a three mm radius sphere and overlaid on the participant’s structural MRI for targeting with the Brainsight® frameless stereotaxic system. In Brainsight, a stimulation trajectory was created so that the plane of the coil was tangential to the scalp and the induced current field was oriented perpendicular to the long axis of the gyrus containing the stimulation target.
Figure 1.
A. Location of hippocampal of seeds used for targeting overlaid on a Talairach (TT_N27) template brain. B. Mask of areas where hippocampal FC increased in Wang et al.,4 which we used as our ROI.
rTMS
TMS was delivered with a MagStim Rapid2 stimulator through a Double Airfilm coil. The stimulating current was biphasic, with the initial phase in the posterior (cable side)-to-anterior direction. rTMS intensity was referenced to the individual motor evoked potential threshold, which was determined in the current experiment immediately before the first rTMS session using the TMS Motor Threshold Assessment Tool (MTAT 2.0; http://www.clinicalresearcher.org/software.htm). rTMS in the current study, and Wang et al.,4 consisted of two-second trains at 20-Hz (40 pulses per train) with an inter-train interval of 28 sec, at 100% of resting motor threshold. There were 40 trains, 1600 pulses, and a duration of 20 minutes per session. A coil holder with pneumatic support was used to minimize operator fatigue during rTMS sessions. All participants were fitted with earplugs during rTMS.
FC calculations and voxel-wise analysis
To calculate FC, we focused on the cortical areas which showed the largest group-level increase in connectivity with the left hippocampus, relative to sham, in Wang et al.,4 (precuneus retrosplenial cluster), which we treated as a region of interest (ROI). We created an ROI mask by reanalyzing the data from Wang et al.4 using their preprocessing pipeline and seed-based approach. For each subject, the pre-stimulation correlation map was subtracted from post-stimulation map, and the pre-sham map from the post-sham map. We then fed these subtractions into AFNI’s 3dttest++ command for contrast. Like Wang et al.,4 we applied a cluster size threshold of 290 voxels and identified a network encompassing the precuneus and occipital lobe. We created a mask of these areas by applying the 3dclust command in AFNI and resampling the mask to the geometry of our own data set (2 mm isotropic voxels; Fig. 1B). In Wang et al.4 active stimulation significantly increased hippocampal FC in this region [t(15) = 4.38, p < 0.001, Cohen’s d = 1.65] and this increase was significantly greater than the change produced by subthreshold control stimulation [t(15) = 5.42, p < 0.001, Cohen’s d = 1.36]. This ROI also contained the regions where the increase in hippocampal FC correlated with memory improvement.
For each participant in the current study, we calculated pre- and post-stimulation hippocampus-ROI FC. This was done using the hippocampal seed voxel that was maximally connected with the left PPC at baseline. For each subject and time point (pre- and post-stimulation), that voxel was transformed into a three mm radius sphere (3dUndump) and an average time series was created using all time series data within this sphere (3dmaskave). This time series was then regressed against the time series of all voxels within our ROI (3dTcorr1D) and all resulting r-values were then r-to-z Fisher transformed (3dcalc; z(r)). Finally, we calculated an average z(r) value among all voxels within our ROI to form the final connectivity metric. For each subject, the difference between pre and post-rTMS z(r) (ΔFC) was then binarized and entered into the CRM (see below).
Statistical Analyses and Considerations
We chose an overall sample of 15 subjects, and a cohort size of three. The criterion change (ΔFC) was ≥ 0.028, based on the minimum change achieved in 87.5% of the sample in Wang et al.4 Thus, in order for a participant to meet the change criterion, the average change in z(r) across all voxels in our ROI, encompassing the left precuneus and cuneus (see Fig. 1B), must be equal to or greater than 0.028 z(r).. In Wang et al.,4 this threshold was met by 14/16 participants receiving active PPC stimulation, but only by 3/16 subjects receiving subthreshold control stimulation. We modeled the probability of no meaningful FC change associated with each dose (number of sessions) so that the CRM would aim to identify a dose associated with 12.5% not achieving the criterion. The CRM used a one-parameter power dose-toxicity model, calibrated so that the CRM would eventually select a dose within 3% of the target.21 The model was indexed by one parameter (power), the logarithm of which was assumed to follow a normal prior with mean of zero and variance of 1.34. This is a conventional model for the CRM (e.g. 22). We started the trial with a treatment duration of three sessions. Subsequent duration assignments were determined sequentially by the CRM: The ΔFC values for each cohort were reported to a statistician (KC) who estimated the MED with a dose-response probability model, based on available observations. The next group was then treated with the model-based estimate of the required number of sessions. The dose-response model was calibrated so that the CRM would converge on a dose associated with 0.875±0.05 probability of achieving a criterion FC change.23 The final dose-response estimate was based on the CRM. We also performed a sensitivity dose-response analysis based on a linear model of ΔFC as a continuous outcome.
Table 1 shows the simulated operating characteristics of the CRM, where P(select) denotes the probability that a given dose level is selected. The method was able to select the correct dose with at least a 0.40 probability in all simulation scenarios, with an average probability of correct selection of 0.50. In addition, a sample size of 15 ensured that the estimate of a proportion of any non-dose dependent event would have a standard error < 0.13.
Table 1.
Operating characteristics of the CRM with n=15 under 5 simulation scenarios. The objective is to find a dose that achieves criterion FC change in 87.5% of participants. Under each scenario, the first row gives the probability of achieving FC change associated with each dose, and the second row gives the probability a dose is selected. The numbers associated with the true minimum effective dose in each scenario are in boldface. For example, under Scenario 1, the minimum effective dose is 5 sessions (with 87.5% probability of achieving FC change), and the CRM is correct with a probability of 0.68.
| Number of sessions | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Scenario 1: Minimum effective dose = 5 sessions | |||||
| Probability of achieving FC change | 45% | 55% | 65% | 75% | 87.5% |
| P(select) | 0.00 | 0.01 | 0.05 | 0.26 | 0.68 |
| Scenario 2: Minimum effective dose = 4 sessions | |||||
| Probability of achieving FC change | 55% | 65% | 75% | 87.5% | 94% |
| P(select) | 0.29 | 0.46 | 0.20 | 0.05 | 0.00 |
| Scenario 3: Minimum effective dose = 3 sessions | |||||
| Probability of achieving FC change | 65% | 75% | 87.5% | 94% | 97% |
| P(select) | 0.04 | 0.20 | 0.41 | 0.29 | 0.05 |
| Scenario 4: Minimum effective dose = 2 sessions | |||||
| Probability of achieving FC change | 75% | 87.5% | 94% | 97% | 98% |
| P(select) | 0.19 | 0.41 | 0.32 | 0.08 | 0.01 |
| Scenario 5: Minimum effective dose = 1 sessions | |||||
| Probability of achieving FC change | 87.5% | 94% | 97% | 98% | 99% |
| P(select) | 0.55 | 0.31 | 0.12 | 0.02 | 0.00 |
Results
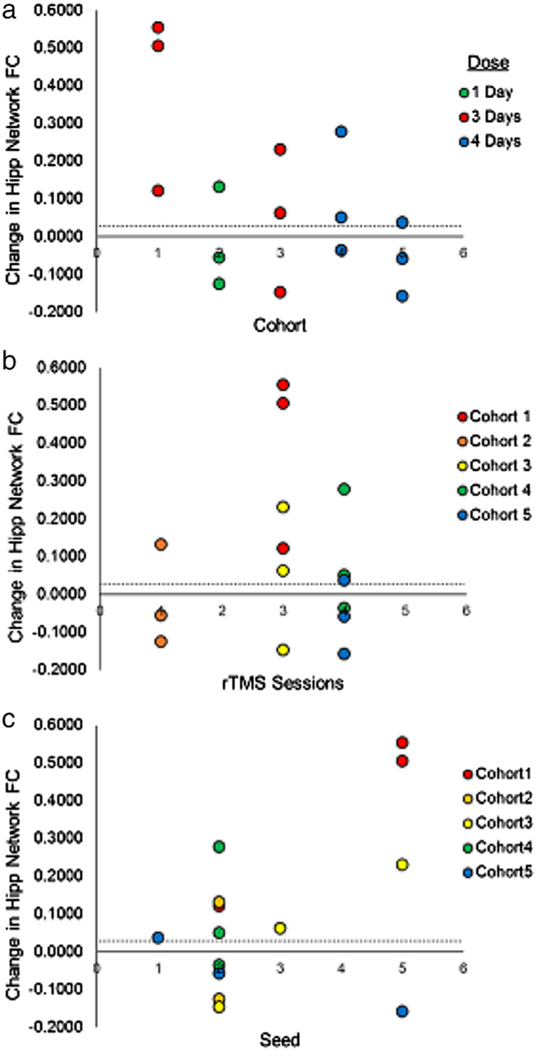
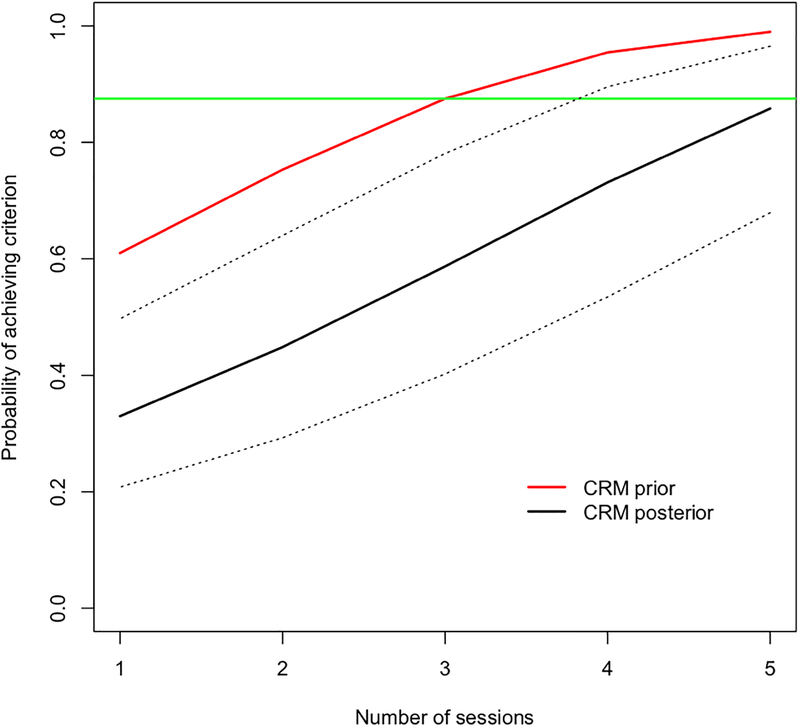
The results for all 15 participants are detailed in Table 2. Overall, 9/15 participants met our ΔFC threshold, irrespective of number of sessions. Fig. 2A shows the course of participant flow, including dose escalation, de-escalation, and re-escalation in the study. Overall, the largest results were observed for participants receiving three sessions (0.22±0.11 z(r); Mean (SEM)), compared to −0.02±0.08 for one session, and 0.02±0.06 for four sessions. A maximum of four sessions was reached. However, the final CRM model estimate, based on all 15 participants, indicated that further escalation to five sessions might be warranted (Fig. 3). While the CRM model and the sensitivity analysis using a linear model gave different projections at five sessions, both indicated that four sessions were not sufficient. The probability of reaching criterion efficacy with three and four sessions was estimated as approximately 59% and 73%, respectively, which were below the target of 87.5%.
Table 2.
Individual participant characteristics. z(r) = Fisher Z-transformed r-value.
| Cohort | Number of rTMS Sessions | Hippocampal Seed (TT_N27 Coordinates) | Baseline Hipp-Stim Location FC z(r) | Resting Motor Threshold (% Stimulator Output) | Baseline Hipp-ROI FC z(r) | Post-stimulation Hipp-ROI FC z(r) | Δ FC |
|---|---|---|---|---|---|---|---|
| 1 | 3 | Seed 2 (x = −22, y = −16, z = −13) | 0.515 | 70 | 0.119 | 0.241 | 0.122 |
| 1 | 3 | Seed 5 (x = −30, y = −27, z = −9) | 0.480 | 56 | 0.095 | 0.601 | 0.506 |
| 1 | 3 | Seed 5 (x = −30, y = −27, z = −9) | 0.887 | 40 | 0.441 | 0.996 | 0.555 |
| 2 | 1 | Seed 2 (x = −22, y = −16, z = −13) | 0.519 | 60 | 0.300 | 0.243 | −0.057 |
| 2 | 1 | Seed 2 (x = −22, y = −16, z = −13) | 0.562 | 56 | 0.379 | 0.511 | 0.132 |
| 2 | 1 | Seed 2 (x = −22, y = −16, z = −13) | 0.400 | 72 | 0.155 | 0.030 | −0.125 |
| 3 | 3 | Seed 2 (x = −22, y = −16, z = −13) | 0.506 | 51 | 0.255 | 0.108 | −0.147 |
| 3 | 3 | Seed 5 (x = −30, y = −27, z = −9) | 0.434 | 59 | 0.113 | 0.344 | 0.231 |
| 3 | 3 | Seed 3 (x = −30, y = −17, z = −14) | 0.476 | 51 | 0.161 | 0.224 | 0.063 |
| 4 | 4 | Seed 2 (x = −22, y = −16, z = −13) | 0.421 | 55 | 0.075 | 0.126 | 0.051 |
| 4 | 4 | Seed 2 (x = −22, y = −16, z = −13) | 0.381 | 64 | 0.083 | 0.362 | 0.279 |
| 4 | 4 | Seed 2 (x = −22, y = −16, z = −13) | 0.279 | 57 | 0.122 | 0.086 | −0.036 |
| 5 | 4 | Seed 1 (x = −26, y = −10, z = −17) | 0.307 | 57 | −0.018 | 0.020 | 0.038 |
| 5 | 4 | Seed 2 (x = −22, y = −16, z = −13) | 0.390 | 50 | 0.096 | 0.038 | −0.058 |
| 5 | 4 | Seed 5 (x = −30, y = −27, z = −9) | 0.427 | 76 | 0.221 | 0.063 | −0.158 |
Figure 2.
Changes in hippocampal-ROI FC plotted for each cohort (A), number of rTMS sessions (B), and seed (C). The dashed line in all panels represents the CRM threshold of 0.028.
Figure 3.
Model-based probabilities of achieving criterion change in FC. Dark solid line represents estimates of the CRM model with 95% confidence intervals indicated by the dashed black lines. The red line represents the prior estimates used by the CRM. The green horizontal line indicates the rate achieved in Wang et al.4
Participants in Cohorts four and five, who received four sessions of stimulation, had lower FC between the hippocampus and our ROI at baseline than participants who received three (Cohort 1: 0.225; Cohort 3: 0.156; Cohort 4: 0.050; Cohort 5: 0.103). However, after statistically correcting for baseline differences, the CRM gave the same recommendation of five stimulation sessions.
Discussion
rTMS parameter space remains virtually unexplored for most cortical areas and networks. Early in the history of rTMS, the effects of varying frequency and stimulation intensity were studied in the corticospinal system using the amplitude of the motor evoked potential as a biomarker of neural pathway responsiveness.24–26 However, these observations were largely qualitative and specific to the motor cortex and its descending pathways. Although it is thought that multiple exposures to rTMS are necessary to cause stable changes in brain connectivity, this parameter has not been investigated prospectively.
This study is the first systematic attempt to determine the level of a rTMS delivery parameter required to produce a criterion change in a clinically relevant biomarker. Dose-finding in rTMS requires a stable, reliable, and mechanistically relevant biomarker of response that can be rapidly measured in individual subjects. Resting state FC satisfies these criteria. It is stable across scanning sessions within individuals.27,28 Finn et al.28 demonstrated that a machine classifier can identify individuals’ whole-brain patterns of resting state functional connectivity from a large group and across sessions with at least 92.9% accuracy. In contrast, clinical outcomes, such as self-report or behavioral measures, are subject to wider variability and are influenced by factors other than the direct effects of treatment, limiting their usefulness for dose-finding. Our study demonstrates the usefulness of FC as a biomarker of rTMS.
The results of our adaptive Bayesian trial suggest that five consecutive daily sessions of rTMS, delivered per Wang et al., are necessary to produce a criterion change in FC between the hippocampus and the precuneus/retrosplenial regions identified by Wang et al., with a probability of 87.5%.
There are several limitations to this study. We used only 15 participants and it is likely that with a larger sample, the CRM would have converged on the MED. However, we do not think adding more subjects would have changed the CRM’s final recommendation of five rTMS sessions. Five sessions of stimulation would have been the next recommended dose if we had included subjects for a sixth cohort in our study, and Wang et al.4 already demonstrated that five sessions of stimulation produces changes in 87.5% of participants. Thus, we are confident that five sessions of rTMS is the MED to meet our criterion.
The small sample may also have contributed to the seemingly paradoxical finding that participants who received four sessions of rTMS (Cohorts four and five) had lower ΔFC values than participants who received three (Cohorts one and three). This may have also been caused by variability in the effect of rTMS, which is not unusual.29,30 Other than variability due to sample size and the rTMS response, a possible cause for this pattern of results was that participants in Cohorts four and five, who received four sessions of stimulation, had lower FC between the hippocampus and our ROI at baseline (Cohort 1: 0.225; Cohort 3: 0.156; Cohort 4: 0.050; Cohort 5: 0.103). However, after applying a statistical correction to account for the lower baseline values in Cohorts four and five, our pattern of results did not change.
The CRM has strengths over conventional dose-finding approaches that were borne out in our study: By using previous data to determine the criterion change, the CRM reduces the chance of drawing erroneous conclusions based on spurious or misleading results from a single study. In this case, we avoided the pitfall recommendation that three sessions of stimulation is significantly better than four sessions by including data acquired from five sessions from Wang et al.4 Additionally, because the CRM explores different doses based on small cohorts, the CRM allows dose space to be explored faster than a conventional escalating dose-finding design, where each successive dose must be tested in a predetermined number of subjects before escalation.
It should also be noted that we measured FC changes in a network which may be particularly sensitive to neuromodulation. The hippocampus is commonly studied for its neuroplastic properties, including long-term potentiation. Because the hippocampus may be more plastic than other networks, it may require less stimulation to induce a clinically relevant change. Thus, it should not be assumed that this duration of rTMS treatment will necessarily be effective in other pathways.
Conclusion
The CRM can be used to explore rTMS dosing space rapidly and economically. This approach can be applied whenever a stable biomarker of response is available and could be used to optimize other rTMS delivery parameters, e.g., numbers of trains, frequency, intensity, etc. in laboratory and clinical applications.
Acknowledgments
Funding: This work was funded by the Department of Defense in the Center for Neuroscience and Regenerative Medicine (CNRM-70–3904). MF is supported by a grant from the Center for Neuroscience and Regenerative Medicine (CNRM-70–3904). JLV is supported by grant R01MH111790.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- 1.Eldaief MC, Halko MA, Buckner RL, Pascual-leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc from Natl Acad Sci. 2011;108(52):21229–21234. doi: 10.1073/pnas.1113103109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahnev D, Kok P, Munneke M, Bahdo L, Lange FP De, Lau H. Continuous theta burst transcranial magnetic stimulation reduces resting state connectivity between visual areas. J Neurophysiol. 2013;110:1811–1821. doi: 10.1152/jn.00209.2013 [DOI] [PubMed] [Google Scholar]
- 3.Steel A, Song S, Bageac D, et al. Shifts in connectivity during procedural learning after motor cortex stimulation: A combined transcranial magnetic stimulation/functional magnetic resonance imaging study. Cortex. 2016;74:134–148. doi: 10.1016/j.cortex.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JX, Rogers LM, Gross EZ, et al. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345(6200):1054–1057. doi: 10.1126/science.1252900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Werf Y, Sanz-arigita EJ, Menning S, van den Heuvel OA. Modulating spontaneous brain activity using repetitive transcranial magnetic stimulation. BMC Neurosci. 2010;11(1):145. doi: 10.1186/1471-2202-11-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vercammen A, Knegtering H, Liemburg E, den Boer A, Aleman A. Functional connectivity of temporo-parietal region in schizophrenia: Effects of rTMS treatment of auditory hallucinations. J Psychiatr Res. 2010;44:725–731. doi: 10.1016/j.jpsychires.2009.12.011 [DOI] [PubMed] [Google Scholar]
- 7.Mastropasqua C, Bozzali M, Ponzo V, et al. Network based statistical analysis detects changes induced by continuous theta-burst stimulation on brain activity at rest. Front Psychiatry. 2014;5:1–7. doi: 10.3389/fpsyt.2014.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gratton C, Lee TG, Nomura EM, Esposito MD, Halko MA, Israel B. The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Front Syst Neurosci. 2013;7:1–14. doi: 10.3389/fnsys.2013.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: Clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522–534. doi: 10.1038/npp.2008.118 [DOI] [PubMed] [Google Scholar]
- 10.Koch G, Bonnì S, Pillicciari M, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage. 2017;169:302–311. doi: 10.1016/j.neuroimage.2017.12.048 [DOI] [PubMed] [Google Scholar]
- 11.Brighina F, Piazza A, Vitello G, et al. rTMS of the prefrontal cortex in the treatment of chronic migraine: A pilot study. J Neurol Sci. 2004;227(1):67–71. doi: 10.1016/j.jns.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 12.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402 [DOI] [PubMed] [Google Scholar]
- 13.Mesulam MM, Van Hoesen GW, Pandya DN, Geschwind N. Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: A study with a new method for horseradish peroxidase histochemistry. Brain Res. 1977;136(3):393–414. doi: 10.1016/0006-8993(77)90066-X [DOI] [PubMed] [Google Scholar]
- 14.Wang JX, Voss JL. Long-lasting enhancements of memory and hippocampal-cortical functional connectivity following multiple-day targeted noninvasive stimulation. Hippocampus. 2015;25(8):877–883. doi: 10.1002/hipo.22416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berlim MT, Van Den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: A systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225–239. doi: 10.1017/S0033291713000512 [DOI] [PubMed] [Google Scholar]
- 16.Cheung YK. Dose Finding by the Continual Reassessment Method. 1st ed. New York: Chapman and Hall/CRC; 2011. doi:7:653–663, 2010 [Google Scholar]
- 17.Rossi S, Hallett M, Rossini PM, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox RW. AFNI : Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 19.Talairach P, Tournoux J. A Stereotactic Coplanar Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 20.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung YK. Sample size formulae for the Bayesian continual reassessment method. Clin Trials. 2013;10(6):852–861. doi: 10.1177/1740774513497294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elkind MS., Sacco RL, MacArthur RB, et al. The neuroprotection with statin therapy for acute recovery trial (NeuSTART): An adaptive design phase I dose-escalation study of high-dose lovastatin in acute ischemic stroke. Int J Stroke. 2012;127(3):358–366. doi: 10.1016/j.jsbmb.2011.07.002.Identification [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SM, Cheung YK. Model calibration in the continual reasessment method. Clin Trials. 2010;6(3):227–238. doi: 10.1177/1740774509105076.Model [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pascual-leone A, Valls-sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(4):847–858. doi: 10.1093/brain/117.4.847 [DOI] [PubMed] [Google Scholar]
- 25.Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48(5):1398–1403. doi: 10.1212/WNL.48.5.1398 [DOI] [PubMed] [Google Scholar]
- 26.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033 [DOI] [PubMed] [Google Scholar]
- 27.Barch DM, Burgess GC, Harms MP, et al. Function in the human connectome: Task-fMRI and individual differences in behavior. Neuroimage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finn ES, Shen X, Scheinost D, et al. Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18(October):1–11. doi: 10.1038/nn.4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133(4):425–430. doi: 10.1007/s002210000432 [DOI] [PubMed] [Google Scholar]
- 30.Nicolo P, Ptak R, Guggisberg AG. Variability of behavioural responses to transcranial magnetic stimulation: Origins and predictors. Neuropsychologia. 2015;74(January):137–144. doi: 10.1016/j.neuropsychologia.2015.01.033 [DOI] [PubMed] [Google Scholar]