Abstract
Cyclic imine toxins exhibit fast acting neurotoxicity and lethality by respiratory arrest in mice explained by their potent antagonistic activity against muscular nicotinic acetylcholine receptors. We performed a survey of gymnodimine-A, 13-desmethyl spirolide-C, 13,19-didesmethyl spirolide-C, 20-methyl spirolide-G, pinnatoxin-A, pinnatoxin-G, portimine-A and 28-O-palmitoyl ester of pinnatoxin-G in 36 shellfish samples collected in coastal areas of 8 European countries using a microplate receptor binding assay and UPLC-MS/MS for toxin identification and quantification. The major toxins found in these samples were pinnatoxin-G, 20-methyl spirolide-G, 13-desmethyl spirolide-C, gymnodimine-A and portimine-A. Traces of 13,19-didesmethyl spirolide-C, pinnatoxin-A and 28-O-palmitoyl ester of pinnatoxin-G were also detected. The rapid death of mice was correlated with higher pinnatoxin-G concentrations in mussel digestive gland extracts injected intraperitoneally. Our survey included non-toxic control samples that were found to contain moderate to trace amounts of several cyclic imine toxins. Shellfish may bioaccumulate not only cyclic imine toxins but also a large number of acyl derivatives as a product of metabolic transformation of these neurotoxins. This is the first report in which portimine-A and 28-O-palmitoyl ester of pinnatoxin-G were detected in shellfish extracts from digestive glands of mussels collected in Ingril lagoon. The bioaccumulation of portimine-A is particularly of concern because it is cytotoxic and is able to induce apotosis. The mode of action of 28-O-palmitoyl ester of pinnatoxin-G was studied by receptor binding-assay and by two-electrode voltage clamp electrophysiology. The antagonistic behavior of the acylated pinnatoxin-G towards nicotinic acetylcholine receptor of muscle type is shown here for the first time. Since cyclic imine toxins are not regulated further monitoring of these emerging toxins is needed to improve evidence gathering of their occurrence in shellfish commercialized for human consumption in Europe given their potent antagonism against muscle and neuronal nicotinic acetylcholine receptors.
Keywords: Microplate receptor-binding assay, cyclic imine toxins, nicotinic acetylcholine receptors, pinnatoxin-G, 28-O-palmitoyl ester of pinnatoxin-G, portimine-A, two-electrode voltage clamp electrophysiology
Graphical Abstract
1. Introduction
The emerging cyclic imines toxins (CiTXs) of global distribution across coastal environments and produced by dinoflagellates move fast up the human food chain through shellfish. The CiTX family includes 44 compounds: 7 gymnodimines (A-E, 12-methylgymnodimine and 16-desmethyl gymnodimine D) (Harju et al., 2016; Miles et al., 2003; Seki et al., 1995; Van Wagoner et al., 2011; Zurhelle et al., 2018); 16 spirolides (A-D, G-I, 13-desmethyl spirolide-C, 27-Hydroxy-13-desmethyl spirolide-C, 13,19-didesmethyl spirolide-C, 20-hydroxy-13,19-didesmethyl spirolide-C, 27-hydroxy-13,19-spirolide-C, 27-Oxo-13,19-didesmethyl spirolide-C, 13-desmethyl spirolide-D, 20-hydroxy-13,19-didesmethyl spirolide-D and 20-methyl spirolide-G) (Gueret et al., 2010; Harju et al., 2016; Molgó et al., 2013; Zurhelle et al., 2018); 8 pinnatoxins (A-H) (Selwood et al., 2010; Selwood et al., 2014; Takada et al., 2001a; Uemura et al., 1995); 3 pteriatoxins (A-C) (Takada et al., 2001b); 6 prorocentrolides (A-B, 4-hydroxyprorocentrolide, 9,51-dihydro prorocentrolide, 30-sulphate prorocentrolide and 14-O-acetyl-4-hydroxyprorocentrolide) (Amar et al., 2018; Hu et al., 1996; Lu et al., 2001; Torigoe et al., 1988); 1 spiroprorocentrimine (Lu et al., 2001) and 2 portimines (A and B) (Fribley et al., 2019; Selwood et al., 2013). The spiroimine moiety is the major feature characterizing cyclic imine toxins. The exception to this rule are prorocentrolides in which a hexahydroisoquinoline group replaces the spiroimine motif (Hu et al., 1996; Torigoe et al., 1988). Portimines display a 5-membered cyclic imine ring, gymnodimines, prorocentrolides and spiroprorocentrimines a 6-membered imine ring, while spirolides, pinnatoxins and pteriatoxins display a 7-membered imine ring (Hu et al., 1995; Seki et al., 1995; Selwood et al., 2013; Uemura et al., 1995). Recently, a novel CiTX harboring a 6-membered imine ring was isolated from a Vulcanodinium rugosum strain collected in Kabira Bay, Okinawa, Japan, that was named kabirimine (Hermawan et al., 2019).
The chemical diversity of CiTX may be even higher if considering metabolic transformations of these toxins by shellfish. A common strategy developed by shellfish to reduce the impact of dinoflagellate toxins on their physiology is the acylation of such compounds (Doucet et al., 2007). Concerning CiTX, shellfish are able to generate a large number of acyl derivatives of gymnodimine-A (de la Iglesia et al., 2013), 20-methyl spirolide-G (Aasen et al., 2006) and pinnatoxin-G (McCarron et al., 2012).
Prorocentrolide, the first ever-described CiTX, was discovered in 1988 from extracts of the marine dinoflagellate Prorocentrum lima (Torigoe et al., 1988). In 1995 the physicochemical characterization of gymnodimines was reported following HABs outbreaks dominated by Karenia selliformis in New Zealand (Seki et al., 1995). In the same year, several spirolides were characterized associated with blooms of Alexandrium ostenfeldii in Canada (Hu et al., 1995), and pinnatoxin-A was characterized after intoxication cases following the consumption of Pinna muricata razor clams in China and Japan (Uemura et al., 1995). In 2010, V. rugosum, the producer of pinnatoxins was identified in New Zealand and France (Nezan et al., 2011; Rhodes et al., 2010). Some of V. rugosum isolates were shown to produce portimine and kabirimine (Hermawan et al., 2019; Selwood et al., 2013).
Cyclic imine toxins exhibit fast-acting neurotoxicity causing rodent death within 3–5 minutes after intraperitoneal or oral administration at lethal doses characterized by transient mouse hyperactivity, followed by a decrease of the respiratory rate with prominent abdominal breathing leading to death by respiratory arrest (Gueret et al., 2010; Takada et al., 2001b; Uemura et al., 1995). CiTXs display potent antagonism and broad selectivity towards skeletal muscle and neuronal nicotinic acetylcholine receptors (nAChRs) explaining rodent death by respiratory arrest (Aráoz et al., 2011; Bourne et al., 2010; Stivala et al., 2015). Radio-ligand-binding assay experiments demonstrated the high affinity and selectivity profile of cyclic imine toxins for muscle and neuronal nAChRs and showing their very low affinity to muscarinic acetylcholine receptors (M1-M5) (Aráoz et al., 2015).
Although not regulated, these neurotoxic compounds are frequently found in European environments and shellfish samples (Garcia-Altares et al., 2014; McCarthy et al., 2015; Otero et al., 2019; Rambla-Alegre et al., 2018; Van De Waal et al., 2015), constituting a latent threat for public health given their potent antagonistic activity against human muscular and neuronal nicotinic acetylcholine receptors (nAChR) (Aráoz et al., 2011; Stivala et al., 2015), and their capacity to cross the intestinal and brain-blood barriers (Alonso et al., 2013; Munday et al., 2012b). Vulnerable populations suffering from neuromuscular disorders such as myasthenia gravis, an autoimmune disease characterized by the loss of muscle nAChRs or Lambert-Eaton myasthenic syndrome caused by the autoimmune destruction of voltage-gated calcium channels resulting in a reduced acetylcholine release at the neuromuscular junction (Vincent, 2002), might be particularly sensitive to shellfish contaminated with CiTXs. In the present work we performed a survey of four families of CiTXs using a microplate receptor-binding assay (microplate-RBAssay) specific for the detection of agonists and antagonists of nicotinic acetylcholine receptors (Aráoz et al., 2008; Aráoz et al., 2012; Rubio et al., 2014) and UPLC-MS/MS for identification and quantification of the referred neurotoxins in shellfish samples collected in the coastal areas of eight European countries. We have also studied the mode of action of 28-O-palmitoyl ester of pinnatoxin-G by using two-electrode voltage clamp (TEVC) electrophysiology.
2. Material and Methods
2.1. Laboratory animal handling.
The transport and handling of Torpedo marmorata and Xenopus laevis were done according to the guidelines of the Ethical Committee for Animal Experimentation C2EA-59 of Paris Center-South. Mouse bioassay has been performed as part of the Survey Program of REPHYTOX of the French General Direction for Food, Program 306. Ifremer data stored at https://www.seanoe.org/data/00361/47251/.
2.2. Collection and processing of shellfish extracts.
Digestive gland (DG) extracts from mussels collected at Ingril lagoon were provided by IFREMER. Digestive gland was used as this was the only tissue available due to sampling procedures. Digestive glands (30 g) were removed and homogenized with a blender. Methanol (9 ml) was added and the toxins were extracted using a Polytron homogenizer (15,000 rpm, 2 min). The homogenate was centrifuged (3,700g for 8 min at 4°C) and the supernatant was recovered (Hess et al., 2013). The extraction was repeated twice and the supernatants were filtered through 2 μm membranes prior to Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS) analysis or microplate-RBAssay.
Shellfish homogenates provided by Agri-food and Biosciences Institute (AFBI) were from the UK NRL archive that were retained positive materials from historical toxin events of known regulated toxins: Amnesic Shellfish Poisoning (ASP), Okadaic acid (OA), Diarrheic Shellfish Poisoning (DSP), Azaspiracids (AZA), Paralytic Shellfish Poisoning (PSP) or Domoic Acid (DA), and were processed as previously described (Aráoz et al., 2012). Ten grams of shellfish homogenate were suspended in 40 ml acetone to precipitate the protein content. The sample was vortexed until obtaining a homogeneous mixture and roller-mixed for 2 h at room temperature. The homogenate was centrifuged (3,500g for 15 min at 4°C), the supernatant was recovered and the precipitate was re-extracted with acetone, as described. Both supernatants were pooled and evaporated at 40°C under a stream of N2 (Organomation Associates Inc, Berlin, MA, USA). The dried matter was solubilized in 4 ml methanol and filtered through a 10-kDa MWCO filter (Millex, Millipore, Billerica, MA, USA) prior to microplate-RBAssay or UPLC-MS/MS.
2.3. Torpedo electrocyte membrane purification
T. marmorata was purchased from the Biological Station of Roscoff, (University of Rennes, France). Upon arrival, the electric ray was conditioned for 2–4 days in the dark at 15°C in a 180 liters tank containing reconstituted marine water (Instant Ocean, Europrix, Lens, France). The water was oxygenated with compressed air; it was recycled and filtered prior to re-entry. The electric fish was sacrificed and the electric organ was dissected and immersed in 500 ml of Torpedo-physiological medium (280 mM NaCl, 3 mM KCl, 1.8 mM MgCl2, 3.4 mM CaC12, 5 mM NaHCO3, 5.5 mM glucose, 300 mM urea, 100 mM sucrose, 1.2 mM sodium phosphate buffer, pH 6.8). The solution was oxygenated and the final pH was 7 – 7.2. All further steps were performed at 4°C. Electrocyte membranes enriched in embryonic muscle-type nAChRs were purified according to (Hill et al., 1991) with some modifications. The freshly dissected electric organ (500 g) was sliced into small pieces and immersed in 500 ml of Torpedo-membrane extraction buffer (TMEB) (50 mM Tris-HCl, 3 mM EDTA, 1 mM EGTA, protease inhibitors mix (Complete Roche Diagnostics GmbH, Mannheim, Germany), pH 7.5). The electric tissue was homogenized with a Waring blender (Appareils Scientifiques O.S.I., Paris, France) at maximal speed for 1 min (3 times with 1 min interval). The homogenate was centrifuged at 4,000g for 10 min at 4°C (Sorvall, Dupont Instruments, Newtown, USA; rotor GSA). The supernatant (S1) was collected and filtered through several layers of medical gauze. The remaining pellet was re-homogenized and centrifuged and processed as described. The supernatants S1 and S2 were centrifuged at 25,000g for 50 min at 4°C (Sorvall, rotor GSA). The pellets containing post-synaptic electrocyte membranes were homogenized with a Potter-Elvehjem (glass/Teflon) in 300 ml TMEB supplemented with 35% sucrose (w/w). A volume of 16 ml of this crude membrane suspension was carefully layered onto 10 ml of 43% sucrose (w/w) TMEB. The samples were centrifuged at 106,000g for 3 hr at 4°C (Beckman, Palo Alto, Ca, USA; rotor 50Ti). Torpedo-electrocyte membranes rich in nAChRs (Torpedo-nAChR) were collected from the interface between 35% and the 43% sucrose layers. The electrocyte membranes were precipitated by centrifugation at 106,000g for 1 hr at 4°C (Beckman, rotor 50Ti). The precipitated membranes were homogenized in 150 ml TMEB using a Potter-Elvehjem and centrifuged at 106,000g for 1 h at 4°C (Beckman, rotor 50Ti). The membranes were homogenized in 100 ml of 5 mM glycine using a Potter-Elvehjem and centrifuged at 106,000g for 1 hr at 4°C (Beckman, rotor 50Ti). The latter washing step was repeated once. The purified electrocyte membranes were resuspended in 40 ml of 5 mM glycine. Protein concentration was adjusted to 2.5 – 3.5 mg/ml using Bradford method (Bio-Rad, Munich, Germany). The electrocyte Torpedo-membranes were aliquoted and stored at −80°C until use.
2.4. Microplate receptor-binding assay.
Maxisorp ELISA plates were purchased from NUNC (Kamstrupvej, Denmark). OPD tablets (o-phenylenediamine dihydrochloride) were from DAKO (Glostrup, Denmark). Biotin-α-bungarotoxin was from Molecular Probes (Eugene, OR, USA). Streptavidin-horseradish peroxidase and α-bungarotoxin (α-BgTx) were from Sigma (St. Louis, MO, USA). Non-radioactive microplate-RBAssay for the detection of cyclic imine toxins directly on shellfish extracts was done as described (Aráoz et al., 2012). Briefly, 100 μl shellfish extracts prepared in TBS-BSA buffer (50 mM Tris-HCl, 150 mM NaCl, 0.5% BSA pH 7.4) were incubated overnight with Torpedo-electrocyte membranes coated in a 96-wells plastic microplate at 4°C. The concentration of the methanolic shellfish extract in the incubation mix was kept lower than 5% to avoid methanol interference with the assay (Rubio et al., 2014). Thereafter, 50 μl of biotin-α-BgTx (2.4 × 10−7 M, Life Technologies, Eugene, OR, USA) was added to each well and further incubated for 30 min at room temperature and constant shaking. The microplates were washed three times with 250 μl washing buffer (TBS, 0.1% Tween 20, pH 7.4). A volume of 100 μl of streptavidin-horseradish peroxidase (220 ng/ml protein) was added to each well. Following 30 min incubation at room temperature, the wells were washed three times as described, and 100 μl of freshly prepared peroxidase substrate (OPD, Sigma, St. Louis, MO, USA) were added to each well. After 5 min, 100 μl of 0.5 M H2SO4 were added to each well to stop the enzymatic reaction. The data were recorded using a microplate reader (CLARIOstar Plus, BMG Labtech, Ortenberg, Germany) at a wavelength of 492 nm. The data was transformed into inhibition percent by using Eq. 1:
where: 100% signal is the absorption data from control wells in which Torpedo-electrocyte membranes were incubated in the absence of toxins/extracts; signal sample is the absorption data of test wells; 100% inhibition, is the absorption data obtained from control wells in which Torpedo-electrocyte membranes were incubated with 1 × 10−5 M α-BgTx. The experiments were performed three times in replicates of six. The concentration of inhibitor at which biotin-α-BgTx binding to Torpedo-nAChRs is inhibited by 50% (IC50) was determined by nonlinear regression (GraphPad Prism 5.0, San Diego, CA, USA).
2.5. Toxin standards
Gymnodimine-A and 13-desmethyl spirolide-C were purchased from NRC-CNRC (Institute for Marine Biosciences, NRC, Halifax, NS, Canada). 13,19-didesmethyl spirolide-C and 20-methyl spirolide-G were obtained from CIFGA (Lugo, Spain). Pinnatoxin-A, pinnatoxin-G and 28-O-palmitoyl ester of pinnatoxin-G were synthesized in the laboratory of Professor Armen Zakarian (University of California, Santa Barbara California). Portimine was purified from an ethanolic extract of V. rugosum. Physicochemical characterization for its use as toxin standard was performed by NMR (Lamoise et al., 2017). Briefly, the extracts were fractionated by solid phase extraction using 5g-C-18 cartridges (Sep-Pak, Waters). Analytical chromatography of portimine-A was performed using an X-Bridge BEH 300 (ethylene-bridged hybrid particles) C18 column (5 μm 4.6 × 250 mm). Purification of portimine-A was achieved using a preparative X-Bridge Prep C18 column (5 μm OBD -Optimum Bed Density- 19 × 150 mm) using as solvent A: H2O, 0.1% formic acid, and as solvent B: CH3CN, 0.1% formic acid, at a flow rate of 25 ml/min. NMR was performed at 20°C on a Bruker DRX700 spectrometer equipped with a triple resonance cryoprobe. NMR data were processed with Topspin 1.3 software (Bruker Biospin, Germany) and analyzed with CcpNmr Analysis software. 1H and 13C resonance of portimine were previously assigned by (Selwood et al., 2013). 1H NMR quantification of portimine was performed as described in (Aráoz et al., 2011). Briefly, 7.0 μl of CHCl3 and 7.0 μl of C6H6 were added to 7.0 ml of deuterated methanol. Portimine was dissolved in 0.5 ml of the previous solution in a 2 ml glass flask. The entire sample was added to a NMR tube for quantification.
2.6. UPLC - Triple Quadrupole Detector (UPLC-TQD)
Simultaneous detection and quantification of 7 cyclic imine toxins and 28-O-palmitoyl ester of pinnatoxin-G was performed on a UPLC-TQD (Waters, Milford, MA, USA). A calibration curve for each CiTX was obtained by injecting onto a BEH C18 (Waters, 2.1 × 100 mm; 1.8 μm particle size) 5 μl of a toxin standard mixture containing gymnodimine-A, 13,19-didesmethyl spirolide-C, pinnatoxin-A, 13-desmethyl spirolide-C, 20-methyl spirolide-G, pinnatoxin-G, 28-O-palmitoyl ester of pinnatoxin-G and portimine-A, in the concentration range of 1 pM to 1 μM. The samples were chromatographed at a flow rate of 0.6 ml/min using as solvent A: H2O, 0.1% formic acid, and as solvent B: acetonitrile, 0.1% formic acid. The gradient profile was 0–1 min: isocratic 15% B, 1–9 min: linear gradient 15–36.5% B, 9–9.2 min: linear gradient 36.5–100% B, 9.2–11 min: isocratic 100% B, 11–11.2 min: linear gradient 100–15% B and 11.2–18 min: isocratic 15% B. All the standard mixes were run in triplicate and two blank samples (5 μl methanol) were intercalated between different samples to avoid carryover since repetitive injection of standard toxins and shellfish extracts impinge the chromatographic column and the mass detector. TQD mass spectrometer was operated in the electrospray ionization positive mode. The capillary potential was set at 3.5 kV; desolvation temperature at 450°C; source temperature at 150°C; desolvation gas flow at 800 l/hr and cone gas flow at 50 l/hr. Data acquisition was performed at a multiple reaction-monitoring mode (MRM; Table 1). Methanolic shellfish extracts (5 μl) were analyzed in triplicate and two blank samples (5 μl methanol) were intercalated between different samples to avoid carryover. Toxin quantification was performed by integration of the area under each toxin peak of three independent MRM chromatograms using MassLynx 4.1 software (Waters).
Table 1.
UPLC-MS/MS Conditions for the Detection/ Quantification of CiTXs.
| PORT-A | GYM-A | 13,19-SPX-C | PnTX-A | 13-SPX-C | 20me-SPX-G | PnTX-G | Palmitoyl-PnTX-G | |
|---|---|---|---|---|---|---|---|---|
| MRM transition (m/z) | 402.4 > 384.4 | 508.4 > 490.4 | 678.4 > 164.1 | 712.4 > 164.0 | 692.4 > 444.4 | 706.5 > 688.4 | 694.4 > 676.4 | 932.4 > 164.1 |
| Cone voltage (V) | 27 | 50 | 50 | 65 | 60 | 65 | 75 | 90 |
| Collision energy (eV) | 24 | 32 | 40 | 48 | 35 | 30 | 30 | 50 |
| Retention time (min) | 2.56 | 4.58 | 5.90 | 5.97 | 6.97 | 7.67 | 8.12 | 11.09 |
| R2 | 0.9848 | 0.9959 | 0.9965 | 0.9797 | 0.9969 | 0.9970 | 0.9956 | 0.9998 |
| LOD (nM) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.6 |
| LOD (pg) in xolumn | 1.0 | 1.2 | 1.7 | 1.8 | 1.7 | 1.8 | 1.7 | 2.8 |
| LOQ (nM) | 2.5 | 1.0 | 1.0 | 1 | 1 | 1.2 | 1.2 | 1.5 |
| LOQ (pg) in column | 5.2 | 2.5 | 3.4 | 3.6 | 3.5 | 4.2 | 4.2 | 6.9 |
Abbreviations: PORT-A: portimine-A; GYM-A: gymnodimine-A; 13,19-SPX-C: 13, 19 didesmethyl spirolide-C; PnTX-A: pinnatoxin-A; 13-SPX-C: 13-desmethyl spirolide-C; 20me-SPX-G: 20-methyl spirolide-G; PnTX-G: pinnatoxin-G; Palmitoyl-PnTX-G: 28-O-palmitoyl ester of pinnatoxin-G; MRM: Multiple Reaction-Monitoring; LOD, limit of detection (obtained from low-level standards with a signal-to-noise ratio equal to 3); LOQ, limit of quantification (obtained from low-level standards with a signal-to-noise ratio equal to 10).
2.6. Two-electrodes voltage clamp (TEVC) electrophysiology
TEVC electrophysiology was performed on Xenopus laevis oocytes having incorporated T. marmorata electrocyte membranes rich in nAChR in their plasmatic membrane. Adult X. laevis females were from the Biological Resource Center (University of Rennes, France). Laparotomy of X. laevis mature female frogs for oocyte extraction was performed according to the protocol APAFIS#5310-2016042016067330 v3 approved by the Ethical Committee for Animal Experimentation C2EA-59 of Paris Center-South. A female donor tagged with an electronic chip was anesthetized by immersion in a 1 g/l ethyl-3-amino benzoate methane sulfonate solution (Sigma-Aldrich, Saint Quentin, France) for 20 min. Following a small incision below 5 mm of the termination of the sternum, the ovarian lobes were excised and immediately immersed in OR2 solution containing 88 mM NaCl, 1 mM MgCl2, 5 mM HEPES 5; pH 7.6. The muscle and dermic planes were sutured and the amphibian was placed in a small aquarium for recovery and observation for 24 h. After three washes with OR2, the ovarian lobes were incubated in Barth’s solution containing 88 mM NaCl, 1 mM KCl, 0.41 mM CaCl2, 0.82 mM MgSO4, 2.5 mM NaHCO3, 0.33 mM Ca(NO3)2 and 7.5 mM Hepes (pH 7.6) at 18°C. Fifty nanoliters of Torpedo-electrocyte membranes (3.5 mg/ml total protein) were microinjected into stage V–VI defollicularized oocytes using a Nanoliter 2000 Micro 4 Controller (WPI, Sarasota, FL, USA). Injected oocytes were incubated at 18°C.
TEVC electrophysiology recordings were performed 1 to 4 days after microinjection using an OC-725B TEVC amplifier (Warner Instrument, Hamden, CT, USA). Voltage and current microelectrodes were filled with 3 M KCl (4–10 MΩ electrode resistance). ACh-evoked currents were acquired using a pCLAMP-9/ Digidata-1322A system (Molecular Devices, San Jose, CA, USA). The Xenopus oocyte was constantly clamped at −60 mV and was perfused with Ringer-Barium containing 100 mM NaCl, 2.8 mM KCl, 0.3 mM BaCl2 and 5 mM HEPES (pH 7.4), at a flow rate of 8 ml/min.
Dose-response curves for agonist activation were fitted to equation 2:
where I is the measured agonist-evoked current, [L] is the agonist concentration, EC50 is the agonist concentration that evoked half the maximal current (Imax), and nH is the Hill coefficient. For antagonist inhibition, current (I) values were normalized to the Imax ACh value recorded from the same oocyte to yield fractional (%) response data. IC50 values were determined from dose-response curves by fitting to the equation 3:
where F is the fractional response obtained in the presence of the inhibitor at concentration [X] and IC50 is the inhibitor concentration that reduced the ACh-evoked amplitude by half. Data was analyzed with Clampfit 10.2 (Molecular Devices) and GraphPad Prism 5.0.
3. Results
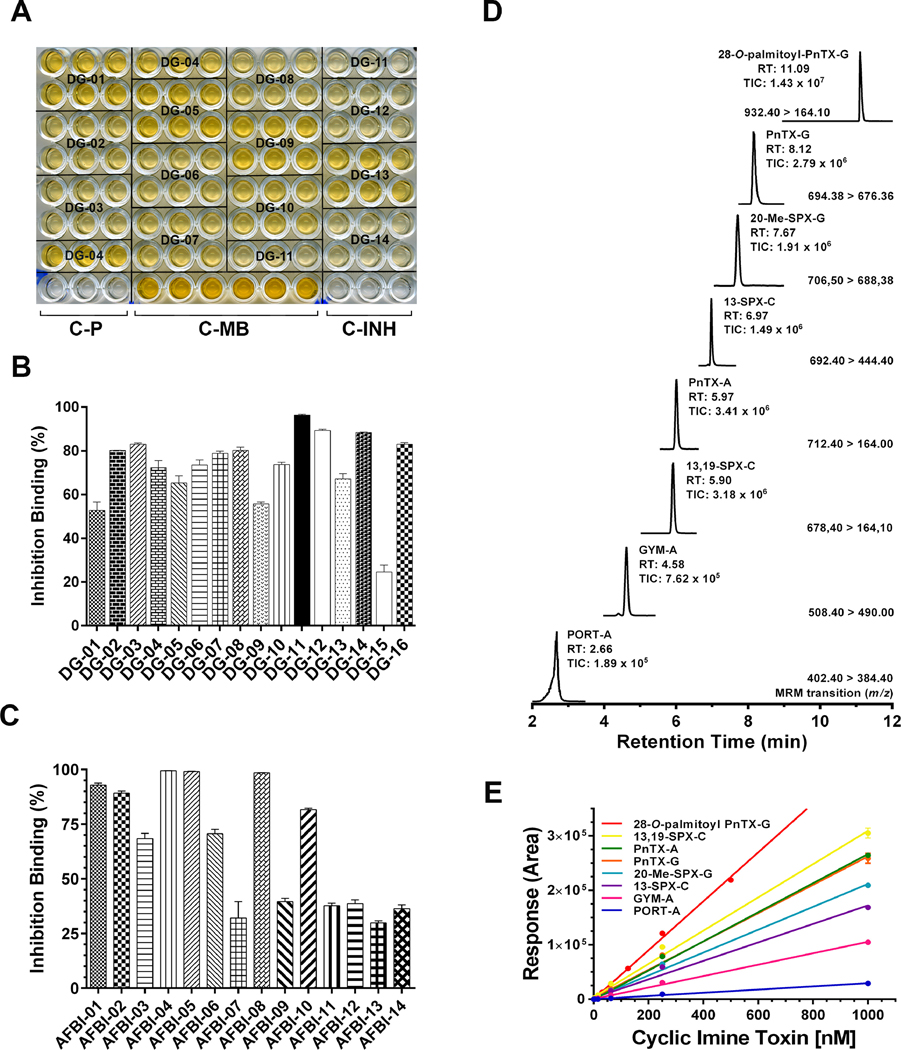
3.1. Pinnatoxin-G occurrence in mussels (DG) and mice survival time
Primary screening for CiTXs was performed using the microplate-RBAssay, a high throughput test specific for the detection of competitive agonists/ antagonists of nAChRs. Thus, the microplate RBAssay can be easily adapted to pipetting platforms for automated liquid handling intercalating incubation and washing steps, signal development and automatic data handling (WO2012101378A1). When present in the extracts, CiTXs compete with the toxin tracer for binding the ACh-site of Torpedo-nAChRs in a concentration dependent manner. Most of the digestive gland extracts (DG) from mussels collected at Ingril lagoon were highly active on Torpedo-nAChR with inhibition values higher than 55% (Fig. 1A, B, Table 2 and 4). Only sample DG-15 showed an inhibition binding of ~25%. Calibration curves were obtained for CiTXs quantification by UPLC-MS/MS with R2 values ranging from 0.9797 to 0.9998 (Fig. 1D, E), with limits of detection (LOD) in the sub-nanomolar range and with limits of quantification (LOQ) in the nanomolar range (Table 1). UPLC-MS/MS analysis showed high concentrations of pinnatoxin-G in 87% of the samples. Traces of 13-desmethyl spirolide-C, gymnodimine-A, pinnatoxin-A and 20-methyl spirolide-G were also present in these extracts (Table 2). The acylated 28-O-palmitoyl ester of pinnatoxin-G and portimine-A were not detected in these samples.
FIGURE 1.
1A. Microplate receptor-binding assay showing the inhibition of biotin-α-bungarotoxin by digestive-gland extracts from mussels collected in Ingril lagoon. DG-samples were analyzed by sextuplicate. Plate signal (C-P): control wells processed without membrane coating. 100% signal (C-MB): control wells in which Torpedo-nAChRs were incubated in the absence of toxin or extract samples. 100% inhibition (C-INH): control wells in which Torpedo-nAChRs were incubated with 1 × 10−6 M α-BgTx. 1B. Inhibition binding potency of digestive-gland extracts from mussels collected in Ingril lagoon. OD492 data were expressed in inhibition percent using Equation 1 (Material and methods). Data are mean values ± SEM of sextuplicate assays of at least two independent experiments. 1C. Inhibition binding potency of shellfish extracts provided by Agri-food and Biosciences Institute (AFBI). OD492 data were expressed in inhibition percent using Equation 1 (Material and methods). Data are mean values ± SEM of sextuplicate assays of at least two independent experiments. 1D. UPLC-MS/MS chromatogram showing the simultaneous analysis of seven cyclic imine toxins and 28-O-palmitoyl ester of pinnatoxin-G. The MRM conditions, retention time and MS-signal are indicated in the figure. 1E. Calibration curves for seven cyclic imine toxins and 28-O-palmitoyl ester of pinnatoxin-G. 5 μl of a toxin standard mix in the concentration range of 1 pM to 1 μM was loaded to the BEH C18 column (see Material and Methods). Toxin quantification was done by integration of the MRM peak area of each analyte.
Abbreviations: DG: digestive glands AFBI: Shellfish samples provided by Agri-food and Biosciences Institute; PORT-A: Portimine-A; GYM-A: gymnodimine-A; 13,19-SPX-C: 13,19-didesmethyl-spirolide-C; PnTX-A: pinnatoxin-A; 13-SPX-C: 13-desmethyl spirolide-C; 20-Me-SPX-G: 20-methyl spirolide-G; PnTX-G: Pinnatoxin-G; Palmitoyl-PnTX-G: 28-O-palmitoyl pinnatoxin-G.
Table 2.
Lethality, CiTX content and inhibition binding potency of digestive-gland extracts from mussels collected in Ingril lagoon that were contaminated with high levels of pinnatoxin-G.
| Extr | Surv. time (min) mean ± SEM; n | GYM-A (μg/kg) | 13SPX-C (μg/kg) | 20meSPX-G (μg/kg) | PnTX-A (μg/kg) | PnTX-G (μg/kg) | Inh. binding (%) mean ± SEM |
|---|---|---|---|---|---|---|---|
| DG-01 | 67.0 ± 0.7 ; 3 | 11.6 | 52.8 ± 3.8 | ||||
| DG-02 | 34.0 ± 0.7 ; 3 | 77.4 | 80.3 ± 0.1 | ||||
| DG-03 | 79.3 ±0.4 ; 3 | 52.1 | 83.1 ± 0.6 | ||||
| DG-04 | < 1440 ; 3 | 1.4 | 18.8 | 72.3 ± 3.2 | |||
| DG-05 | 70.5 ± 0.5 ; 2 | 2.1 | 65.3 ± 3.2 | ||||
| DG-06 | 12.0 ± 2.0 ; 3 | 40.3 | 73.6 ± 2.3 | ||||
| DG-07 | 4.0 ± 0.7 ; 3 | 1.8 | 1.3 | 37.2 | 78.9 ± 1.0 | ||
| DG-08 | 22.7 ± 2.9 ; 3 | 1.8 | 2.1 | 12.1 | 80.3 ± 1.4 | ||
| DG-09 | < 1440 ; 3 | 55.8 ± 0.9 | |||||
| DG-10 | 27.7 ± 2.2 ; 3 | 12.0 | 73.8 ± 0.9 | ||||
| DG-11 | < 1440 ; 3 | 2.8 | 3.1 | 96.4 ± 0.4 | |||
| DG-12 | 32.7 ± 3.6 ; 3 | 4.8 | 2.0 | 89.3 ± 0.6 | |||
| DG-13 | < 1440 ; 3 | 3.5 | 67.2 ± 2.4 | ||||
| DG-14 | 17.0 ± 0.7 ; 3 | 60.5 | 88.4 ± 0.2 | ||||
| DG-15 | < 1440 ; 3 | 10.6 | 2.2 | 24.7 ± 3.2 | |||
| DG-16 | 73.7 ± 0.4 ; 3 | 65.5 | 83.1 ± 0.7 |
The survival time is the mean value obtained after intraperitoneal injection of shellfish extracts on three mice (n). Abbreviations: GYM-A: gymnodimine-A; 13-SPX-C: 13-desmethyl spirolide-C; 20meSPX-G: 20-methyl spirolide-G; PnTX-A: pinnatoxin-A; PnTX-G: pinnatoxin-G. Toxin quantification was performed by UPLC-MS/MS in the MRM mode. Inhibition binding was performed by microplate-RBAssay. Inhibition binding percent are expressed as mean values of sextuplicate assays of at least two independent experiments.
Table 4.
Detection of 28-O-palmitoyl ester of pinnatoxin-G and portimine-A in digestive gland extracts of mussels collected at Ingril lagoon.
| Extract | PORT-A (μg/kg) | GYM-A (μg/kg) | 13-SPX-C (μg/kg) | PnTX-A (μg/kg) | PnTX-G (μg/kg) | Palmitoyl-PnTX-G (μg/kg) | Inh. binding (%) mean ± SEM |
|---|---|---|---|---|---|---|---|
| 13/414-2 | 69.3 ± 1.9 | 617.3 ± 14.3 | 25.9 ± 1.5 | 6.6 ± 0.2 | 273.1 ± 3.8 | 0.7 ± 0.1 | 98.5 ± 1.2 |
| 13/599-2 | 32.1 ± 1.1 | 51.5 ± 2.8 | 2.0 ± 0.1 | 2.5 ± 0.2 | 11.3 ± 0.1 | 0.1 ± 0.0 | 72.0 ± 3.0 |
Abbreviations: PORT-A: portimine-A; GYM-A: gymnodimine-A; 13-SPX-C: 13-desmethyl spirolide-C; PnTX-A: pinnatoxin-A; PnTX-G: pinnatoxin-G; Palmitoyl-PnTX-G: 28-O-palmitoyl ester of pinnatoxin-G. Toxin quantification was performed by UPLC-MS. Inhibition binding was performed by microplate receptor-binding assay. Inhibition binding percent are expressed as mean values ± SEM of sextuplicate assays of at least two independent experiments.
The digestive gland extracts from mussels collected in Ingril were highly lethal to mice. The survival time of mice following intraperitoneal injection of DG extracts (n = 3) ranged between 4 to 80 min for ~70% of the tested animals (Table 2). The survival time was monitored for 24 h (1440 min), after which, surviving mice were sacrificed. The Pearson’s correlation coefficient between mice survival time versus pinnatoxin-G concentrations in DG extracts was significant (r = −0.4433; one-tailed) suggesting that the rapid death of mice was associated with higher concentrations in pinnatoxin-G.
3.2. CiTX occurrence in shellfish samples collected in Europe
AFBI shellfish samples were from six European countries with an Atlantic and Mediterranean coastline that included Scotland, Northern Ireland, Ireland, Italy, Norway and Portugal. In addition, as control samples, four commercial shellfish were purchased from a Fish market in the Paris Region: Glycymeris glycymeris (clams), Venerupis philippinarum (Japanese cockle) and Ostrea edulis (oysters) that were produced in the Brittany Region of France, and Mytilus galloprovincialis (mussels) that was imported from Netherlands (Table 3). The inhibition binding potency of the whole group of samples varied from ~25 to 100%, as tested by microplate-RBAssay, suggesting that all samples were contaminated with CiTXs (Fig. 1C, Table 3). UPLC-MS/MS analysis confirmed the presence of pinnatoxin-G, 20-methyl spirolide-G, 13-desmethyl spirolide-C, pinnatoxin-A, 13,19-didesmethyl spirolide-C and gymnodimine-A in these extracts. The acylated compound 28-O-palmitoyl ester of pinnatoxin-G and portimine-A were not detected. The most abundant CiTXs found in these European shellfish extracts were, pinnatoxin-G, 20-methyl spirolide-G and 13-desmethyl spirolide-C. It is to be noted that the samples AFBI 01–08 contained high levels of OA, DTX, YTX, AZA (Supplementary Table 1), while AFBI 13–14 mussel samples contained PSP and DA, respectively. Samples AFBI-09 (C) – AFBI-12 (C) were non-toxic control entries. The marine phyocotoxins OA, DTX, YTX and AZA were tested by microplate-RBAssay at a concentration of 10 μM without significant effects on the performance of the bioassay (Supplementary Fig. 1). The presence of other toxins not acting on nAChRs did not interfere with inhibition binding by microplate-RBAssay as it was previously shown for saxitoxin and domoic acid (Aráoz et al., 2012).
Table 3.
CiTX content and inhibition binding potency of toxic and control shellfish extracts collected in coastal Regions of 8 European countries
| Sample | Species | CiTX Group (μg/kg) |
Inh. binding (%) | |||||
|---|---|---|---|---|---|---|---|---|
| GYM-A | 13-SPX-C | 13,19-SPX-C | 20me-SPX-G | PnTX-A | PnTX-G | mean ± SEM | ||
| AFBI-01 | Mussel | 1.2 | 20.5 | 0.9 | 15.7 | 93.0 ± 1.8 | ||
| AFBI-02 | Mussel | 3.9 | 16.3 | 1.5 | 19.5 | 89.2 ± 1.7 | ||
| AFBI-03 | Mussel | 9.9 | 11.2 | 11.1 | 68.3 ± 5.0 | |||
| AFBI-04 | Mussel | 1.7 | 13.8 | 2.6 | 45.4 | 99.5 ± 0.1 | ||
| AFBI-05 | Mussel | 12.9 | 13.2 | 1.4 | 11.0 | 99.1 ± 0.2 | ||
| AFBI-06 | Mussel | 3.1 | 0.5 | 6.6 | 1.4 | 14.5 | 70.5 ± 4.0 | |
| AFBI-07 | Mussel | 6.2 | 5.3 | 24.5 ± 2.9 | ||||
| AFBI-08 | Mussel | 10.2 | 0.5 | 6.6 | 1.4 | 14.5 | 98.5 ± 0.1 | |
| AFBI-09 (C) | Mussel | 3.8 | 2.9 | 5.8 | 39.0 ± 2.6 | |||
| AFBI-10 (C) | Mussel | 20.6 | 5.3 | 81.7 ± 1.3 | ||||
| AFBI-11 (C) | Mussel | 5.6 | 2.3 | 1.5 | 3.8 | 37.6 ± 2.2 | ||
| AFBI-12 (C) | Mussel | 3.8 | 3.3 | 1.0 | 5.6 | 38.5 ± 3.7 | ||
| AFBI-13 | Clam | 7.0 | 1.5 | 2.0 | 29.8 ± 1.9 | |||
| AFBI-14 | Scallop | 4.1 | 11.6 | 4.2 | 35.9 ± 3.2 | |||
| FR-01 (C) | Clam | 0.4 | 1.4 | 28.5 ± 2.2 | ||||
| FR-02 (C) | Cockle | 5.1 | 44.9 ± 1.1 | |||||
| FR-03 (C) | Oyster | 0.8 | 8.8 | 0.1 | 47.7 ± 3.8 | |||
| FR-04 (C) | Mussel | 5.1 | 0.1 | 41.9 ± 1.2 | ||||
Abbreviations: AFBI: Shellfish samples provided by the Agri-food and Biosciences Institute. AFBI-01 to AFBI-09, AFBI-13 and AFBI-14: Shellfish samples contaminated with regulated marine toxins. AFBI-09 (C) to AFBI-12 (C): control samples non-contaminated with regulated marine toxins. FR-01 (C) to FR-04 (C): Control samples purchased from a shellfish market in Paris Region. GYM-A: gymnodimine-A; 13-SPX-C: 13-desmethyl spirolide-C; 13,19-SPX-C: 13,19-didesmethyl spirolide-C; 20me-SPX-G: 20-methyl spirolide-G; PnTX-A: pinnatoxin-A; PnTX-G: pinnatoxin-G. Toxin quantification was performed by UPLC-MS/MS for cyclic imine toxins. Inhibition binding was performed by microplate RBAssay. Data are mean values ± SEM of sextuplicate assays of at least two independent experiments.
Ligand-binding assay showed that the control samples AFBI-09 (C) – AFBI-12 (C) and the commercial samples FR-01 (C) – FR-04 (C) were contaminated with CiTXs (inhibition binding values: 29 – 81%). CiTXs in these extracts were quantified by UPLC-MS/MS (Table 3). Since cyclic imine toxins are not regulated in Europe, it is a common issue to retrieve them in commercial shellfish. The presence of these neurotoxins in shellfish destined for human consumption is of concern given their high affinity for human nAChRs and their capacity to cross the intestinal and brain-blood barriers.
3.3. Detection of 28-O-palmitoyl ester of pinnatoxin-G -A and portimine-A
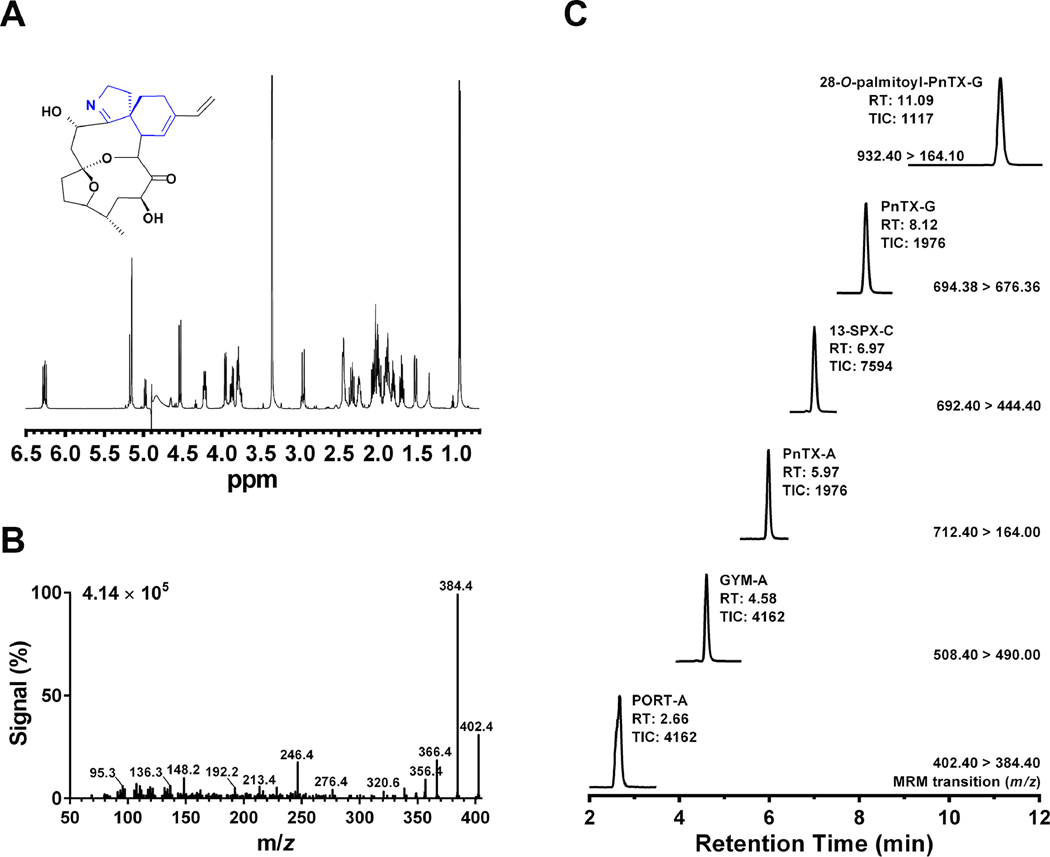
We previously detected 28-O-palmitoyl ester of pinnatoxin-G in the mussel digestive gland samples 13/414–2 and 13/599–2 collected in Ingril lagoon (Mondeguer et al., 2015). The availability of synthetic 28-O-palmitoyl ester of pinnatoxin-G allowed us to determine its retention time and MRM conditions for toxin quantification. Portimine was characterized and quantified by NMR (Fig. 2A). The collision energy of the triple quadrupole detector of the UPLC/MS-MS was optimized to obtain a robust MRM transition pair for the quantification of 28-O-palmitoyl ester of pinnatoxin-G and portimine-A (Fig. 1E, 2B, 3B). While portimine eluted from the column at 2.56 min (18% B), the acylated 28-O-palmitoyl ester of pinnatoxin-G eluted at 11.09 min (100% B). Since the acylated compound was strongly retained by the BEH C18 column, the chromatographic run was longer in order to clean up the column (see Material and Methods). It is worth mentioning that 28-O-palmitoyl ester of pinnatoxin-G emitted strong MS signals even at low concentrations under the experimental conditions here used (Fig. 1D). Both toxins were detected in the digestive glands of mussel samples 13/414–2 and 13/599–2 collected from Ingril lagoon (Fig. 2C, Table 4). The inhibition binding potency of these extracts were 98% and 72%, respectively, as measured by microplate-RBAssay. UPLC-MS/MS quantification showed that DG-sample 13/414–2 contained 0.67 μg/kg of 28-O-palmitoyl ester of pinnatoxin-G and 69.31 μg/kg of portimine-A, while sample 13/599–2 contained 0.07 μg/kg of 28-O-palmitoyl ester of pinnatoxin-G and 32.08 μg/kg of portimine-A (Fig. 2C, Table 4). Both DG extracts were also contaminated with high levels of gymnodimine-A and pinnatoxin-G. Low levels of 13-desmethyl spirolide-C, pinnatoxin-A were also detected (Fig. 2C, Table 4).
FIGURE 2.
2A. NMR spectrum of portimine-A (inset). NMR was performed at 20°C on a Bruker DRX700 spectrometer equipped with a triple resonance cryoprobe. 2B. MS/MS spectrum of portimine-A showing its fragmentation pattern at the collision-induced dissociation condition for MRM. 2C. UPLC-MS/MS chromatogram showing the simultaneous detection of portimine-A (PORT-A), gymnodimine-A (GYM-A), pinnatoxin-A (PnTX-A), 13-desmethyl spirolide-C (13-SPX-C) pinnatoxin-G (PnTX-G) and 28-O-palmitoyl pinnatoxin-G (Palmitoyl-PnTX-G) in the digestive gland extract sample 13/414-2 collected in Ingril Lagoon (South France). The MRM conditions, retention time and MS-signal are indicated in the figure. Abbreviations: m/z: mass-to-charge ratio.
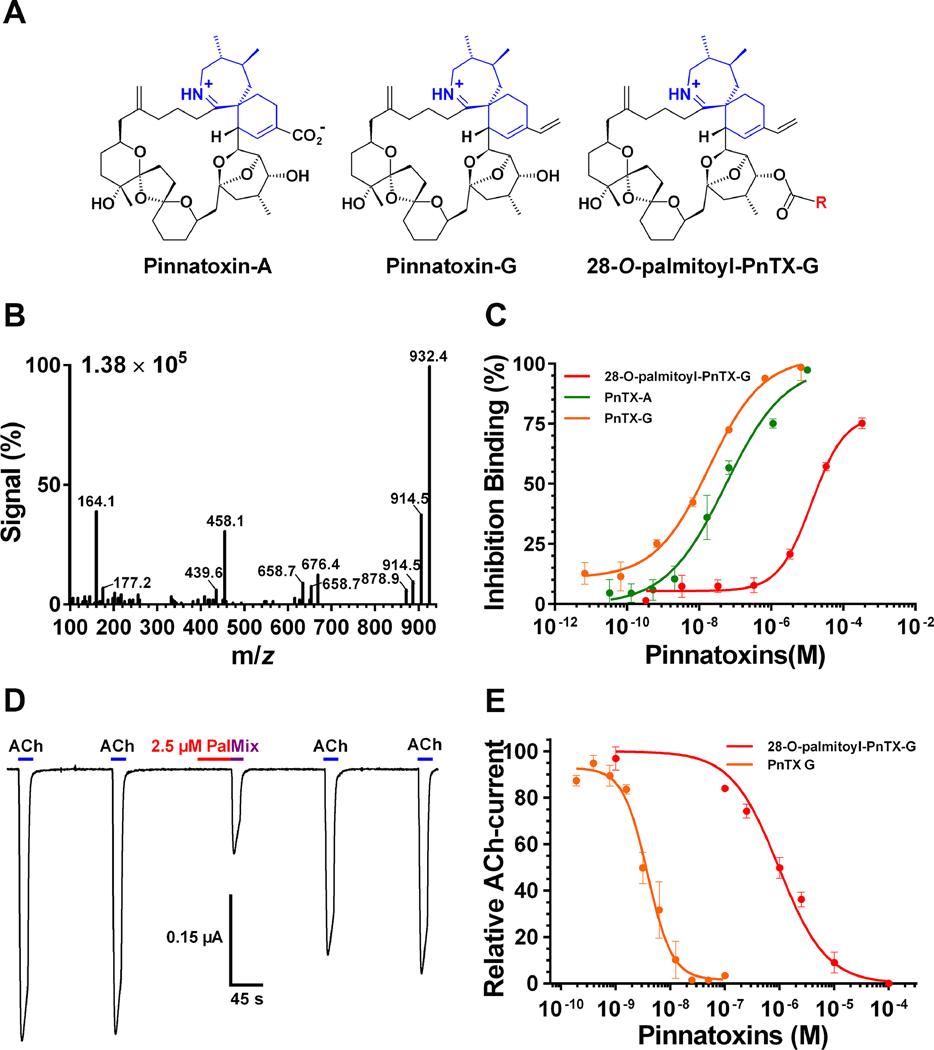
FIGURE 3.
3A. Chemical structure of pinnatoxin-A, pinnatoxin-G and 28-O-palmitoyl pinnatoxin-G; R = ester 16:0. 3B Mass spectrum of 28-O-palmitoyl ester of pinnatoxin-G showing its fragmentation pattern at the collision-induced dissociation condition for MRM. 3C. Inhibition binding potency of 28-O-palmitoyl pinnatoxin-G compared to its precursor molecule pinnatoxin-G and to pinnatoxin-A. Each point represents the mean value ± SEM of three independent experiments using the Microplate-RBAssay. D. Antagonistic effect of 2.5 μM 28-O-palmitoyl ester of pinnatoxin-G on Torpedo-nAChR of muscle embryonic type recorded using two-electrode voltage clamp electrophysiology. The perfusion protocol was as follows: A clamped oocyte at a holding potential of −60 mV was perfused with 25 μM ACh (ACh) for 15 s at 150 s intervals. Thereafter, the oocyte was perfused with 2.5 μM 28-O-palmitoyl ester of pinnatoxin-G (Pal) for 45 s and immediately after it was exposed to a mixture of 25 μM ACh and 2.5 μM 28-O-palmitoyl ester of pinnatoxin-G (Mix). The oocyte was washed with Ringer-Ba and pulses of 25 μM ACh were recorded twice at 150s intervals. E. Concentration-dependent antagonistic effect of 28-O-palmitoyl ester of pinnatoxin-G and pinnatoxin-G on Xenopus laevis oocytes having incorporated Torpedo muscle-type (α1)2βγδ nAChR into their plasma membranes. The peak amplitudes of the ACh-evoked current values (mean ± SEM; 5 oocytes per concentration) were normalized to control ACh-peak amplitudes recorded from the same oocyte to yield fractional response.
Abbreviations: m/z: Mass-to-charge ratio; PnTX-A: Pinnatoxin-A; PnTX-G: Pinnatoxin-G; PnTX-G-palmitate: 28-O-palmitoyl pinnatoxin-G.
3.4. Mode of action of 28-O-palmitoyl ester of pinnatoxin-G
The affinity of 28-O-palmitoyl ester of pinnatoxin-G for Torpedo-nAChR of embryonic muscle type was verified by microplate-RBAssay (Mondeguer et al., 2015; Fig. 3C). The acylation of pinnatoxin-G at C-28 provoked a ~700 decrease of the inhibition binding of 28-O-palmitoyl ester of pinnatoxin-G with an IC50 = 13.1 μM (95% interval confidence: 6.20 – 27.37 μM) when compared to its parent compound pinnatoxin-G whose IC50 was 18.65 nM (95% interval confidence: 8.8 – 39.6 nM). TEVC electrophysiological recordings showed the mode of action of 28-O-palmitoyl ester of pinnatoxin-G: it acts as an antagonist of Torpedo-nAChR of embryonic muscle type (α1)2β1γδ. Thus, the perfusion of 28-O-palmitoyl ester of pinnatoxin-G did not elicit inward currents on X. laevis oocytes carrying Torpedo (α1)2β1γδ nAChR suggesting no agonist activity (Fig. 3D). However, it competitively antagonized ACh-evoked currents in a dose dependent manner (IC50 = 969.3 nM; 95% interval confidence: 752.6 – 2129 nM, Fig. 3E). The interaction between 28-O-palmitoyl ester of pinnatoxin-G and the Torpedo (α1)2β1γδ nAChR was reversible: following perfusion of 2.5 μM 28-O-palmitoyl ester of pinnatoxin-G,, the ACh-evoked current was 30% of the initial value. After 300 s washing with Ringer-Ba, the ACh-evoked current recovered to 76% of the control current (Fig. 3D). The esterification of pinnatoxin-G with palmitic acid (16:0) anhydride at C-28 provoked a ~300 decrease of the antagonistic potency of 28-O-palmitoyl ester of pinnatoxin-G relative to pinnatoxin-G (IC50 = 3.15 nM; 95% interval confidence: 2.9 – 4.9 nM, Fig 3E).
4. Discussion
This is the first report describing the mode of action of 28-O-palmitoyl ester of pinnatoxin-G and the bioaccumulation of portimine-A by shellfish extracts thanks to the use of synthetic and purified toxins as standards. The monitoring of CiTXs occurrence was performed on 36 shellfish samples using a target-directed functional method and UPLC-MS/MS for the identification and quantification of 8 CiTX members in a single run. The most frequently detected CiTXs were pinnatoxin-G, 20-methyl spirolide-G and 13-desmethyl spirolide-C, which showed a similar distribution across the European coastal regions tested. The latter may reflect the European-wide distribution of Alexandrium ostenfeldii and A. peruvianum (Aasen et al., 2005; Brown et al., 2010; Krock et al., 2008; McCarthy et al., 2015; Suikkanen et al., 2013), producers of spirolides, and of V. rugosum, producer of pinnatoxins (Garcia-Altares et al., 2014; Hess et al., 2013; McCarthy et al., 2015; Rundberget et al., 2011).
Although highly responsive by microplate-RBAssay, the quantification of the monitored CiTXs by UPLC-MS/MS gave, in some cases, lower toxin concentrations (i.e. DG-05, DG-09, DG-11; Table 2), suggesting that the referred samples may contain other CiTXs not monitored in this work. An alternative for detecting untargeted CiTXs may be the use of high resolution mass spectrometry however; no quantitative data could be obtained by this approach. Along the same line, the low correlation between pinnatoxin-G content of Ingril samples and mice survival time (Table 2) may indicate the presence of other CiTXs or acylated derivatives in these extracts. A delayed mouse mortality provoked by mussel extracts collected several weeks after an algal bloom dominated by A. ostenfeldii in Skjer location (Norway) led to the discovery of 21 fatty acid ester metabolites of 20-methyl spirolide-G (Aasen et al., 2006). Initially, the mouse survival time after intraperitoneal administration of shellfish extract samples dominated by 20-methyl spirolide-G was very short (3–5 min). However, the survival time of mouse became longer as the mussels gradually detoxified (Aasen et al., 2006). Acylated derivatives of CiTXs may constitute an inhibition background noise for the microplate-RBAssay if present. Taking all together, the results presented here show that the microplate-RBAssay is highly sensitive, and provides an alternative high-throughput method for monitoring neurotoxins targeting nAChRs on environmental samples that could facilitate LC/MS analysis (Rubio et al., 2014).
The growing CiTX group includes seven families of toxins totaling by now 44 CiTX analogs (Lamoise et al., 2017), and very few of them are available as commercial standards (Rambla-Alegre et al., 2018). CiTXs were considered as false negatives for saxitoxin monitoring by mouse bioassay (Vilariño et al., 2009). Thus, the mouse lethality of 13-desmethyl spirolide-C, 13,19 didesmethyl spirolide-C, 20-methyl spirolide-G or pinnatoxin-F, to mention some of them (Munday et al., 2012a; Munday et al., 2012b), compares the mouse lethality of saxitoxin by gavage or intraperitoneal administration (Llewellyn, 2006). Still, since no human fatalities are associated with CiTXs worldwide, they are not internationally regulated (EFSA Panel on Contaminants in the Food Chain, 2010). Consequently, it is common to detect CiTXs in commercial shellfish in Europe (Aráoz et al., 2012; Otero et al., 2019; Rambla-Alegre et al., 2018). Here it is shown that shellfish control samples provided as part of the study were contaminated with 13-desmethyl spirolide-C, 20-methyl spirolide-G, pinnatoxin-G and with traces of pinnatoxin-A, and that shellfish commercial samples purchased in Paris Region were contaminated with 13-desmethyl spirolide-C and with traces of pinnatoxin-G, gymnodimine-A and 20-methyl spirolide-G (Table 3).
Of particular interest for food safety is that shellfish bioaccumulate not only cyclic imine toxins but also a large number of acyl derivatives as product of shellfish metabolism. 28-O-palmitoyl ester of pinnatoxin-G is one of the 26 derivatives described for pinnatoxin-G (McCarron et al., 2012). Binding experiments showed that 28-O-palmitoyl ester of pinnatoxin-G inhibited tracer binding to Torpedo-nAChR in the micromolar range and with a lower affinity compared to pinnatoxin-G (Aráoz et al., 2012; Hess et al., 2013). Further, TEVC electrophysiological recordings showed that 28-O-palmitoyl ester of pinnatoxin-G is a competitive antagonist of Torpedo-nAChR. The chemically controlled esterification of pinnatoxin-G with palmitic acid occurred at the secondary OH at C28 close to the spirocyclic group (Fig. 3A). It was predicted that the palmitate acyl chain at position C28 in the bridged 5,6-bicycloketal (EF) substructure of pinnatoxin-G may produce steric clashes with amino acid residues in loop F of the orthosteric site of the nAChR reducing significantly the potency of 28-O-palmitoyl ester of pinnatoxin-G compared to pinnatoxin-G (Bourne et al., 2015). The results presented here support the previous hypothesis; i.e.: the IC50 of 969.3 nM determined for 28-O-palmitoyl ester of pinnatoxin-G was 300-fold higher than the IC50 determined for pinnatoxin-G (3.15 nM). Overall, even slight structural changes impact the affinity and the antagonistic potency of CiTXs towards nAChR subtypes as it is shown for pinnatoxin-G and pinnatoxin-A (Fig. 3A, 3C, 3E, Bourne et al., 2015). Further work is needed to determine the passage of acylated CiTX through the intestinal barrier and their impact on human muscle and neuronal nAChRs.
V. rugosum may now be considered an endemic dinoflagellate of Ingril lagoon (Mediterranean, France), since early observation of this strain go back to 1990 (E. Nézan, personal communication). The highest abundances for V. rugosum were recorded in summer time (June – September). This species is thermophilic (Abadie et al., 2018), nonetheless, it can adapt to colder lagoons such as Lough Hyne, (Ireland) where pinnatoxin-G was reported (McCarthy et al., 2015). Indeed, a V. rugosum bloom episode was recently observed (December 2019) at Ingril lagoon prompting the prohibition of shellfish harvesting (thau-infos.fr/index/php/commune/frontignan/69083-etang-d-ingril-peche-aux-coquillages-interdite). Actually, Ingril is not intensively used for shellfish farming. Given that shellfish at Ingril are frequently exposed to toxic V. rugosum, higher levels of pinnatoxin-G (1,200 μg/ kg) were recorded in mussels growing in this lagoon (Hess et al., 2013). The high levels of gymnodimine-A and 13-desmethyl spirolide-C found in these samples (Table 4) suggest that Ingril lagoon may also be a hot spot for other CiTXs-producing dinoflagellates. The bioaccumulation of portimine by mussels–described here for the first time–is also of concern, since this toxin not only acts on nAChRs (Lamoise et al., 2017), but it is a potent apoptotic inducer molecule (Cuddihy et al., 2016; Fribley et al., 2019; Selwood et al., 2013).
5. Conclusion
The microplate-RBAssay is a robust technology providing an alternative high-throughput test for CiTXs in environmental samples with very low matrix effect. A UPLC-MS/MS protocol was designed for the simultaneous detection of seven CiTXs and 28-O-palmitoyl ester of pinnatoxin-G in a single run taking advantage of commercial, synthetic and purified toxin standards. Pinnatoxin-G, 20-methyl spirolide-G, 13-desmethyl spirolide-C, gymnodimine-A and portimine-A were the major neurotoxins found in this survey. The rapid death of mice was associated with higher pinnatoxin-G concentrations in mussel digestive gland extracts injected intraperitoneally. 28-O-palmitoyl ester of pinnatoxin-G was detected in two mussel extracts collected in Ingril lagoon. The mode of action of 28-O-palmitoyl ester of pinnatoxin-G is here shown for the first time: 28-O-palmitoyl ester of pinnatoxin-G is an antagonist of muscle-type nAChR. The lipid acylation at position C-28 of pinnatoxin-G provoked a serious decrease of the antagonistic potency of 28-O-palmitoyl ester of pinnatoxin-G against Torpedo-nAChRs when compared to its precursor neurotoxin. This is also the first report in which the bioaccumulation of portimine-A in mussels was demonstrated. Portimine-A may represent a different toxicological risk since it is a potent apoptotic molecule and a nAChR antagonist. The presence of CiTXs in commercial shellfish is of concern given the high affinity of these neurotoxins for human nAChR of muscle and neuronal subtypes, and their capacity to cross the intestinal and brain-blood barriers in particular for vulnerable populations suffering from neuromuscular junction autoimmune disorders such as myasthenia gravis or Lambert-Eaton myasthenic syndrome. Since CiTXs are emergent toxins in Europe a systematic monitoring of this family of toxins is needed calling for general guidelines regulating CiTXs concentrations in shellfish.
Supplementary Material
Highlights.
The non-regulated cyclic imine toxins are potent antagonists of muscle/neuronal nAChRs.
Robust bioassay for fast & early detection of cyclic imine toxins in shellfish samples.
High incidence of cyclic imine toxins in shellfish collected in eight European countries.
First report showing the bioaccumulation of the apoptosis-inducer portimine in mussels.
We determined the mode of action of 28-O-palmitoyl ester of pinnatoxin-G on nAChR.
Acknowledgements
This research was supported by the INTERREG Atlantic Area (ALERTOX-NET EAPA_317/2016 project to DS, and PH), the LABEX LERMIT (DETECTNEUROTOX project, CDE 2017-001173 – RD 91 to RA), the NRBC-E Program (MULTITOX project, Fiche N° H35 to RA) and the National Institutes of Health USA (grant NIGMS R01 GM077379 to AZ and DS). The GDR PHYCOTOX is also acknowledged for support to PH and RA. We would like to thank Dr. Dermot Faulkner for critical reading of the manuscript.
Footnotes
Declaration of interests
In behalf of the authors of the manuscript “Cyclic imine toxins survey in coastal European shellfish samples: Bioaccumulation of acylated 28-O-palmitoyl ester of pinnatoxin-G and portimine-A”, namely: Paul Barnes, Véronique Séchét, Muriel Delepierre, Sophie Zinn-Justin, Jordi Molgó, Armen Zakarian, Philipp Hess and Denis Servent, I declare that the authors, myself included, have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aasen J, MacKinnon SL, Le Blanc P, Walter JA, Hovgaard P, Aune T, Quilliam MA 2005. Detection and identification of spirolides in Norwegian shellfish and plankton. Chem. Res. Toxicol 18(3), 509–515. [DOI] [PubMed] [Google Scholar]
- Aasen JAB, Hardstaff W, Aune T, Quilliam MA 2006. Discovery of fatty acid ester metabolites of spirolide toxins in mussels from Norway using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 20(10), 1531–1537. [DOI] [PubMed] [Google Scholar]
- Abadie E, Chiantella C, Crottier A, Rhodes L, Masseret E, Berteaux T, Laabir M. 2018. What are the main environmental factors driving the development of the neurotoxic dinoflagellate Vulcanodinium rugosum in a Mediterranean ecosystem (Ingril lagoon, France)? Harmful Algae 75, 75–86. [DOI] [PubMed] [Google Scholar]
- Alonso E, Otero P, Vale C, Alfonso A, Antelo A, Gimenez-Llort L, Chabaud L, Guillou C, Botana LM 2013. Benefit of 13-desmethyl Spirolide C treatment in triple transgenic mouse model of Alzheimer Disease: beta-amyloid and neuronal markers improvement. Curr. Alzheimer Res 10(3), 279–289. [DOI] [PubMed] [Google Scholar]
- Amar M, Aráoz R, Iorga BI, Yasumoto T, Servent D, Molgó J. 2018. Prorocentrolide-A from cultured Prorocentrum lima dinoflagellates collected in Japan blocks sub-types of nicotinic acetylcholine receptors. Toxins 10(3), 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aráoz R, Herdman M, Rippka R, Ledreux A, Molgó J, Changeux J-P, Tandeau de Marsac N, Nghiêm H-O (2008). A non-radioactive ligand-binding assay for detection of cyanobacterial anatoxins using Torpedo electrocyte membranes. Toxicon 52(1), 163–174. [DOI] [PubMed] [Google Scholar]
- Aráoz R, Ouanounou G, Iorga BI, Goudet A, Alili D, Amar M, Benoit E, Molgó J, Servent D. 2015. The neurotoxic effect of 13,19-didesmethyl and 13-desmethyl spirolide C phycotoxins is mainly mediated by nicotinic rather than muscarinic acetylcholine receptors. Toxicol. Sci 147(1), 156–167. [DOI] [PubMed] [Google Scholar]
- Aráoz R, Ramos S, Pelissier F, Guerineau V, Benoit E, Vilarino N, Botana LM, Zakarian A, Molgó J. 2012. Coupling the Torpedo microplate-receptor binding assay with mass spectrometry to detect cyclic imine neurotoxins. Anal. Chem 84(23), 10445–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aráoz R, Servent D, Molgó J, Iorga BI, Fruchart-Gaillard C, Benoit E, Gu Z, Stivala C, Zakarian A. 2011. Total synthesis of pinnatoxins A and G and revision of the mode of action of pinnatoxin A. J. Am. Chem. Soc 133(27), 10499–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne Y, Radic Z, Aráoz R, Talley TT, Benoit E, Servent D, Taylor P, Molgó J, Marchot P. 2010. Structural determinants in phycotoxins and AChBP conferring high affinity binding and nicotinic AChR antagonism. Proc. Natl. Acad. Sci. U. S. A 107(13), 6076–6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne Y, Sulzenbacher G, Radic Z, Aráoz R, Reynaud M, Benoit E, Zakarian A, Servent D, Molgó J, Taylor P, Marchot P. 2015. Marine macrocyclic imines, pinnatoxins A and G: Structural determinants and functional properties to distinguish neuronal α 7 from muscle α1(2)βγδ nAChRs. Structure 23(6), 1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Bresnan E, Graham J, Lacaze JP, Turrell E, Collins C. 2010. Distribution, diversity and toxin composition of the genus Alexandrium (Dinophyceae) in Scottish waters. Eur. J. Phycol 45(4), 375–393. [DOI] [PubMed] [Google Scholar]
- Cuddihy SL, Drake S, Harwood DT, Selwood AI, McNabb PS, Hampton MB 2016. The marine cytotoxin portimine is a potent and selective inducer of apoptosis. Apoptosis 21(12), 1447–1452. [DOI] [PubMed] [Google Scholar]
- De la Iglesia P, McCarron P, Diogene J, Quilliam MA 2013. Discovery of gymnodimine fatty acid ester metabolites in shellfish using liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 27(5), 643–653. [DOI] [PubMed] [Google Scholar]
- Doucet E, Ross NN, Quilliam MA (2007). Enzymatic hydrolysis of esterified diarrhetic shellfish poisoning toxins and pectenotoxins. Anal. Bioanal. Chem 389(1), 335–342. [DOI] [PubMed] [Google Scholar]
- Fribley AM, Xi Y, Makris C, Alves-de-Souza C, York R, Tomas C, Wright JLC, Strangman WK 2019. Identification of portimine B, a new cell permeable spiroimine that induces apoptosis in oral squamous cell carcinoma. ACS Med. Chem. Lett 10(2), 175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Altares M, Casanova A, Bane V, Diogene J, Furey A, De la Iglesia P. 2014. Confirmation of pinnatoxins and spirolides in shellfish and passive samplers from Catalonia (Spain) by liquid chromatography coupled with triple quadrupole and high-resolution hybrid tandem mass spectrometry. Mar. Drugs 12(6),3706–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueret SM, Brimble MA 2010. Spiroimine shellfish poisoning (SSP) and the spirolide family of shellfish toxins: Isolation, structure, biological activity and synthesis. Nat. Prod. Rep 27(9), 1350–1366. [DOI] [PubMed] [Google Scholar]
- Harju K, Koskela H, Kremp A, Suikkanen S, de la Iglesia P, Miles CO, Krock B, Vanninen P. 2016. Identification of gymnodimine D and presence of gymnodimine variants in the dinoflagellate Alexandrium ostenfeldii from the Baltic Sea. Toxicon 112, 68–76. [DOI] [PubMed] [Google Scholar]
- Hermawan I, Higa M, Hutabarat PUB, Fujiwara T, Akiyama K, Kanamoto A, Haruyama T, Kobayashi N, Higashi M, Suda S, Tanaka J. (2019). Kabirimine, a new cyclic imine from an Okinawan dinoflagellate. Mar. Drugs 17, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P, Abadie E, Hervé F, Berteaux T, Sechet V, Aráoz R, Molgó J, Zakarian A, Sibat M, Rundberget T, Miles CO, Amzil Z. 2013. Pinnatoxin G is responsible for atypical toxicity in mussels (Mytilus galloprovincialis) and clams (Venerupis decussata) from Ingril, a French Mediterranean lagoon. Toxicon 75, 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Nghiem HO, Changeux JP 1991. Serine-specific phosphorylation of nicotinic receptor associated 43k-protein. Biochemistry 30(22), 5579–5585. [DOI] [PubMed] [Google Scholar]
- Hu TM, Curtis JM, Oshima Y, Quilliam MA, Walter JA, Watsonwright WM, Wright JLC 1995. Spirolide-B and spirolide-D, 2 novel macrocycles isolated from the digestive glands of shellfish. J. Chem. Soc., Chem. Commun 20, 2159–2161. [Google Scholar]
- Hu TM, de Freitas ASW, Curtis JM, Oshima Y, Walter JA, Wright JLC 1996. Isolation and structure of prorocentrolide B, a fast-acting toxin from Prorocentrum maculosum. J. Nat. Prod 59(11), 1010–1014. [DOI] [PubMed] [Google Scholar]
- Krock B, Tillmann U, John U, Cembella A. 2008. LC-MS-MS aboard ship: tandem mass spectrometry in the search for phycotoxins and novel toxigenic plankton from the North Sea. Anal. Bioanal. Chem 392(5), 797–803. [DOI] [PubMed] [Google Scholar]
- Lamoise C, Gaudin A, Hess P, Séchet V, Thai R, Servent D, Zinn-Justin S, Aráoz R. 2017. Physico-chemical and functional characterization of Portimine purified from Vulcanodinium rugosum strain IFR-VRU-01 In: Proença LAO, Hallegraeff G. (Eds). Marine and Fresh-Water Harmful Algae. Proceedings of the 17th International Conference on Harmful Algae. International Society for the Study of Harmful Algae, pp. 126–129. [Google Scholar]
- Llewellyn LE 2006. Saxitoxin, a toxic marine natural product that targets a multitude of receptors. Nat. Prod. Rep 23(2), 200–222. [DOI] [PubMed] [Google Scholar]
- Lu CK, Lee GH, Huang R, Chou HN 2001. Spiro-prorocentrimine, a novel macrocyclic lactone from a benthic Prorocentrum sp. of Taiwan. Tetrahedron Lett. 42(9), 1713–1716. [Google Scholar]
- McCarron P, Rourke WA, Hardstaff W, Pooley B, Quilliam MA 2012. Identification of pinnatoxins and discovery of their fatty acid ester metabolites in mussels (Mytilus edulis) from Eastern Canada. J. Agric. Food Chem 60(6), 1437–1446. [DOI] [PubMed] [Google Scholar]
- McCarthy M, Bane V, Garcia-Altares M, Van Pelt F, Furey A, O’Halloran J. 2015. Assessment of emerging biotoxins (pinnatoxin G and spirolides) at Europe’s first marine reserve: Lough Hyne. Toxicon 108, 202–209. [DOI] [PubMed] [Google Scholar]
- Miles CO, Wilkins AL, Stirling DJ, MacKenzie AL, 2003. Gymnodimine C, an isomer of gymnodimine B, from Karenia selliformis. J. Agric. Food Chem 51(16), 4838–4840. [DOI] [PubMed] [Google Scholar]
- Molgó J, Aráoz R, Benoit E, Iorga BI 2013. Cyclic imine toxins: chemistry, origin, metabolism, pharmacology, toxicology, and detection In: Botana LM (Ed). Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection. CRC Press Inc; Taylor & Francis Inc. Boca Raton, pp. 951–989. [Google Scholar]
- Mondeguer F, Abadie E, Herve F, Bardouil M, Séchet V, Raimbault V, Berteaux T, Zendong SZ, Palvadeau H, Amzil Z, Hess P, Fessard V, Huguet A, Sosa S, Tubaro A, Aráoz R, Molgó J. 2015. Pinnatoxines en lien avec l’espèce Vulcanodinium rugosum (II). https://archimer.ifremer.fr/doc/00285/39635/ [Google Scholar]
- Munday R, Quilliam MA, Le Blanc P, Lewis N, Gallant P, Sperker SA, Ewart HS, MacKinnon SL 2012a. Investigations into the toxicology of spirolides, a group of marine phycotoxins. Toxins 4(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday R, Selwood AI, Rhodes L. 2012b. Acute toxicity of pinnatoxins E, F and G to mice. Toxicon 60(6), 995–999. [DOI] [PubMed] [Google Scholar]
- Nézan E, Chomérat N. 2011. Vulcanodinium rugosum gen. nov., sp. nov. (Dinophyceae): a new marine dinoflagellate from the French Mediterranean coast. Cryptogam. Algol 32(1), 3–18. [Google Scholar]
- Otero P, Miguens N, Rodriguez I, Botana LM 2019. LC-MS/MS analysis of the emerging toxin pinnatoxin-g and high levels of esterified OA group toxins in Galician commercial mussels. Toxins 11(7), 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambla-Alegre M, Miles CO, De la Iglesia P, Fernandez-Tejedor M, Jacobs S, Sioen I, Verbeke W, Samdal IA, Sandvik M, Barbosa V, Tediosi A, Madorran E, Granby K, Kotterman M, Calis T, Diogene J. 2018. Occurrence of cyclic imines in European commercial seafood and consumers risk assessment. Environ. Res 161, 392–398. [DOI] [PubMed] [Google Scholar]
- Rhodes L, Smith K, Selwood A, McNabb P, Van Ginkel R, Holland P, Munday R. 2010. Production of pinnatoxins by a peridinoid dinoflagellate isolated from Northland, New Zealand. Harmful Algae 9(4), 384–389. [Google Scholar]
- Rubio F, Kamp L, Carpino J, Faltin E, Loftin K, Molgó J, Aráoz R. 2014. Colorimetric microtiter plate receptor-binding assay for the detection of freshwater and marine neurotoxins targeting the nicotinic acetylcholine receptors. Toxicon 91, 45–56. [DOI] [PubMed] [Google Scholar]
- Rundberget T, Aasen JAB, Selwood AI, Miles CO 2011. Pinnatoxins and spirolides in Norwegian blue mussels and seawater. Toxicon 58(8), 700–711. [DOI] [PubMed] [Google Scholar]
- Seki T, Satake M, Mackenzie L, Kaspar HF, Yasumoto T, 1995. Gymnodimine, a new marine toxin of unprecedented structure isolated from New-Zealand oysters and the dinoflagellate, Gymnodinium sp. Tetrahedron Lett 36(39), 7093–7096. [Google Scholar]
- Selwood AI, Miles CO, Wilkins AL, Van Ginkel R, Munday R, Rise F, McNabb P. 2010. Isolation, structural determination and acute toxicity of pinnatoxins E, F and G. J. Agric. Food Chem. 58(10), 6532–6542. [DOI] [PubMed] [Google Scholar]
- Selwood AI, Wilkins AL, Munday R, Gu H, Smith KF, Rhodes LL, Rise F. 2014. Pinnatoxin H: a new pinnatoxin analogue from a South China Sea Vulcanodinium rugosum isolate. Tetrahedron Lett. 55(40), 5508–5510. [Google Scholar]
- Selwood AI, Wilkins AL, Munday R, Shi F, Rhodes LL, Holland PT 2013. Portimine: a bioactive metabolite from the benthic dinoflagellate Vulcanodinium rugosum. Tetrahedron Lett. 54(35), 4705–4707. [Google Scholar]
- Stivala CE, Benoit E, Aráoz R, Servent D, Novikov A, Molgó J, Zakarian A. 2015. Synthesis and biology of cyclic imine toxins, an emerging class of potent, globally distributed marine toxins. Nat. Prod. Rep 32(3), 411–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suikkanen S, Kremp A, Hautala H, Krock B. 2013. Paralytic shellfish toxins or spirolides? The role of environmental and genetic factors in toxin production of the Alexandrium ostenfeldii complex. Harmful Algae 26, 52–59. [Google Scholar]
- Takada N, Umemura N, Suenaga K, Chou T, Nagatsu A, Haino T, Yamada K, Uemura D. 2001a. Pinnatoxins B and C, the most toxic components in the pinnatoxin series from the Okinawan bivalve Pinna muricata. Tetrahedron Lett. 42(20), 3491–3494. [Google Scholar]
- Takada N, Umemura N, Suenaga K, Umemura D. 2001b. Structural determination of pteriatoxins A, B and C, extremely potent toxins from the bivalve Pteria penguin. Tetrahedron Lett. 42(20), 3495–3497. [Google Scholar]
- Torigoe K, Murata M, Yasumoto T, Iwashita T. 1988. Prorocentrolide, a toxic nitrogenous macrocycle from a marine dinoflagellate, Prorocentrum lima. J. Am. Chem. Soc 110(23), 7876–7877. [Google Scholar]
- Uemura D, Chou T, Haino T, Nagatsu A, Fukuzawa S, Zheng SZ, Chen HS 1995. Pinnatoxin-a - a toxic amphoteric macrocycle from the okinawan bivalve Pinna muricata. J. Am. Chem. Soc 117(3), 1155–1156. [Google Scholar]
- Van De Waal DB, Tillmann U, Martens H, Krock B, Van Scheppingen Y, John U. 2015. Characterization of multiple isolates from an Alexandrium ostenfeldii bloom in The Netherlands. Harmful Algae 49, 94–104. [Google Scholar]
- Van Wagoner RM, Misner I, Tomas CR, Wright JLC 2011. Occurrence of 12-methylgymnodimine in a spirolide-producing dinoflagellate Alexandrium peruvianum and the biogenetic implications. Tetrahedron Lett. 52(33), 4243–4246. [Google Scholar]
- Vilariño N, Fonfria ES, Molgó J, Aráoz R, Botana LM 2009. Detection of gymnodimine-A and 13-desmethyl C spirolide phycotoxins by fluorescence polarization. Anal. Chem 81(7), 2708–2714. [DOI] [PubMed] [Google Scholar]
- Vincent A. 2002. Unravelling the pathogenesis of myasthenia gravis. Nat. Rev. Immunol 2(10), 797–804 [DOI] [PubMed] [Google Scholar]
- WO2012101378A1, US20130303405 - Method for manufacturing an analysis substrate, and use thereof for detecting toxins. Inventors: Aráoz, R., Nghiêm H.-O., Molgó, J., Botana, L.M., Vilariño, N.
- Zurhelle C, Nieva J, Tillmann U, Harder T, Krock B, Tebben J. 2018. Identification of Novel Gymnodimines and Spirolides from the Marine Dinoflagellate Alexandrium ostenfeldii. Mar. Drugs 16(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.