Abstract
Objective
To explore the main components and unravel the potential mechanism of simiao pill (SM) on rheumatoid arthritis (RA) based on network pharmacological analysis and molecular docking.
Methods
Related compounds were obtained from TCMSP and BATMAN-TCM database. Oral bioavailability and drug-likeness were then screened by using absorption, distribution, metabolism, and excretion (ADME) criteria. Additionally, target genes related to RA were acquired from GeneCards and OMIM database. Correlations about SM-RA, compounds-targets, and pathways-targets-compounds were visualized through Cytoscape 3.7.1. The protein-protein interaction (PPI) network was constructed by STRING. Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed via R packages. Molecular docking analysis was constructed by the Molecular Operating Environment (MOE).
Results
A total of 72 potential compounds and 77 associated targets of SM were identified. The compounds-targets network analysis indicated that the 6 compounds, including quercetin, kaempferol, baicalein, wogonin, beta-sitosterol, and eugenol, were linked to ≥10 target genes, and the 10 target genes (PTGS1, ESR1, AR, PGR, CHRM3, PPARG, CHRM2, BCL2, CASP3, and RELA) were core target genes in the network. Enrichment analysis indicated that PI3K-Akt, TNF, and IL-17 signaling pathway may be a critical signaling pathway in the network pharmacology. Molecular docking showed that quercetin, kaempferol, baicalein, and wogonin have good binding activity with IL6, VEGFA, EGFR, and NFKBIA targets.
Conclusion
The integrative investigation based on bioinformatics/network topology strategy may elaborate on the multicomponent synergy mechanisms of SM against RA and provide the way out to develop new combination medicines for RA.
1. Introduction
Rheumatoid arthritis (RA) is a chronic polyarticular symmetric disease. It is characterized by chronic inflammation of the synovial membrane, which can destroy articular cartilage and juxta-articular bone [1]. RA affects 0.3%–1% of the population worldwide [2]. If insufficiently treated, it usually leads to persistent joint inflammation, progressive joint destruction, continuing functional decline, extra-articular manifestations, disability, and increased mortality [3, 4]. Although current available therapeutic approaches against RA, including nonsteroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), and corticosteroid, allow for excellent disease control, novel therapies are needed because RA remains incurable [5]. Furthermore, the long-term use of these drugs may cause multiple side effects and lead to limited therapeutic responses. Therefore, novel treatments are in urgent demand.
Traditional Chinese medicine (TCM) has been extensively applied for the treatment of RA for centuries in Asia and has been gradually accepted for worldwide clinical applications [6, 7]. Numerous studies have indicated that TCM can be served as complementary and alternative RA drugs for therapeutic effects and with fewer side effects [8, 9].
Simiao pill (SM), a traditional TCM formula, comprises four herbs, including Phellodendri Chinensis Cortex (Huang Bo), Atractylodes lancea (Thunb.) Dc. (Cang Zhu), achyranthis bidentatae radix (Niu Xi), and Coicis Semen (Yi Yi Ren). Previous studies have indicated the anti-inflammation pharmacological effect of SM [10] and that SM reduced proinflammatory cytokine production by suppressing nuclear factor kappaB (NF-κB)/pyrin domain containing 3 (NLRP3) inflammasome activation [11]. Recently, SM was demonstrated to exhibit anti-inflammatory and bone-protective effects by regulating autotaxin (ATX)-lysophosphatidic acid (LPA) and mitogen-activated protein kinase (MAPK) signaling pathways in collagen-induced arthritis (CIA) rats [12]. In addition, SM was recommended for the treatment of active RA (53.6%) in the expert consensus regarding the treatment of RA with various Chinese patent medicines (CPMs) [13]. However, because TCM formulas are characterized by multicomponents, multitargets, and multipathways [14], the therapeutic effect of SM against RA has not been fully elucidated. Therefore, it is necessary for further systematic investigation.
Nowadays, network pharmacology integrates network biology and polypharmacology based on existing databases, providing a novel approach for exploring the mechanisms and synergistic effect of TCM formulas as disease treatments [14–16]. Combining network science with ancient TCM formulas to investigate multiple molecular mechanisms has achieved successful attempts in the previous researches [17–20].
Therefore, in this study, a network pharmacology-based study was conducted to predict bioactive compounds and elucidate the comprehensive pharmacological mechanisms about the antirheumatic effect of SM. In addition, molecular docking analysis was performed to validate in silico to predict molecular interactions between compounds and targets.
2. Materials and Methods
Network pharmacology-based prediction of SM treating RA was constructed by the following (Figure 1): (1) data collection and preparation, including retrieving the ingredients list of SM formula, screening for candidate compounds, identifying SM and RA targets, and intersecting the identified targets of compounds and disease; (2) topological analysis of network and protein-protein interaction (PPI) network construction; (3) enrichment analysis; and (4) molecular docking analysis.
Figure 1.
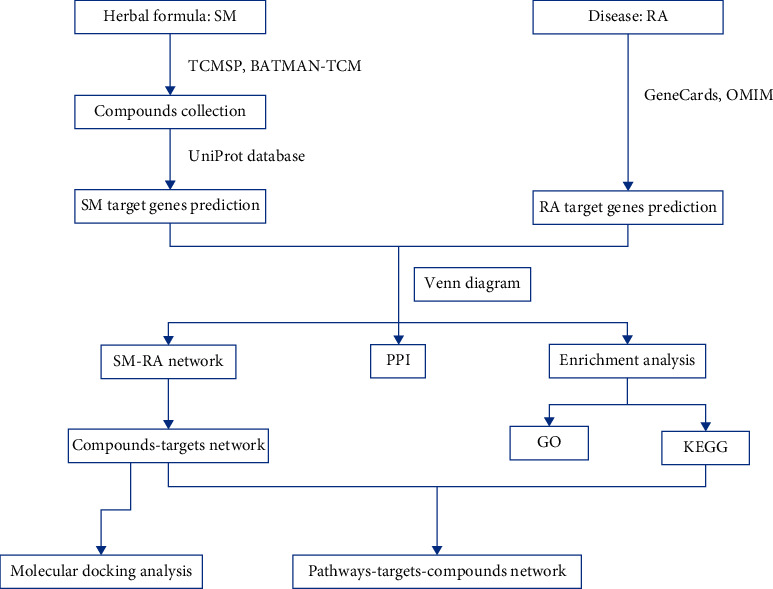
Workflow of network pharmacology analysis.
2.1. Data Collection and Preparation
2.1.1. Composite Compounds of SM
The related composite compounds of SM were obtained from the Traditional Chinese Medicine Systems Pharmacology Database (TCMSP, http://lsp.nwu.edu.cn/tcmsp.php) and a Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine (BATMAN-TCM, http://bionet.ncpsb.org/batman-tcm/).
2.1.2. Pharmacokinetic ADME Evaluation
The in silico integrative ADME (absorption, distribution, metabolism, and excretion) model administrated by TCMSP is employed for pharmaceutical research. As an oral drug, two related-ADME models, oral bioavailability (OB), and drug-likeness (DL) are applied to identify the potential bioactive compounds in this study. Only the compounds with OB ≥ 30 and DL ≥ 0.18 that satisfied the criteria suggested by the TCMSP database (removed the duplicated) are retained as the candidate compounds for further study [21]. In addition, among the compounds with OB <30 or DL <0.18, which are searched with “compound (name)” and “rheumatoid arthritis” [all fields] in PubMed databases to find relevant researches, the compounds in purified form focused on anti-RA mechanisms are also considered to be bioactive compounds (removed the duplicated) and included for further study.
2.1.3. Predictions of Target Genes Related to the Identified Compounds
All the potential compounds were input into TCMSP to capture the relationships between drugs and targets. Since the obtained targets include various biological species, all target names were also put into UniProt databases (http://www.uniprot.org/) to search for target gene names selected by human species.
2.1.4. Potential Disease Target Genes
Information of known RA-related therapeutic target genes was collected by keywords “rheumatoid arthritis” as queries from The Human Gene Databases (GeneCards, https://www.genecards.org/, ver.4.9.0) and Online Mendelian Inheritance in Man (OMIM, http://www.omim.org/, updated June 6, 2019), and only “Homo sapiens” target genes linked to RA are selected.
2.1.5. Venn Analysis
All target genes of identified compounds and RA are put into Bioinformatics and Evolutionary Genomics system (bioinformatics.psb.ugent.be/webtools/Venn/), respectively, to produce a Venn diagram, which indicates the intersection of identified targets of drug and disease.
2.2. Topological Analysis of Network and PPI Network Construction
2.2.1. Topological Network Analysis
SM-RA mechanism network, compounds-targets network, and pathways-targets-compounds network were visualized through Cytoscape (https://cytoscape.org/, ver. 3.7.1) to systemically explore the molecular mechanisms of SM treating RA.
2.2.2. PPI Network Construction
The above 77 target genes acquired from the Venn diagram intersection were imported into STRING (https://string-db.org/, version 11.0) to construct a PPI network for understanding protein interaction systematically. The PPI network is constructed by setting the organism as “human sapiens”, setting the minimum required interaction score to “medium confidence (0.40)”, and excluding the disconnected protein nodes. In addition, statistics of protein interactions are figured out according to the PPI network, and a related bar plot diagram is constructed with R 3.6.0 subsequently.
2.3. Enrichment Analysis
R 3.6.0 and related R packages (colorspace, stringi, DOSE, clusterProfiler, and pathview) are applied to carry out Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of intersection target genes of SM-RA. P values <0.05 and q values <0.05 are considered statistically significant based on Fisher's test.
2.4. Molecular Docking Analysis
The 3D structures of candidate targets were obtained from the PDB database (http://www.rcsb.org/) in PDB format by setting the organism to “Homo sapiens only”. The 3D conformers of candidate compounds are acquired from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) with SDF format. Subsequently, they were imported to the Molecular Operating Environment (MOE) to get the docking score. The greater the absolute value of the docking score, the better.
3. Results
3.1. Data Collection and Preparation
3.1.1. Identification of Compounds in SM
A total of 479 compounds were identified in SM, including 140 in Phellodendri Chinensis Cortex (Huang Bo), 49 in Atractylodes lancea (Thunb.) Dc. (Cang Zhu), 176 in achyranthis bidentatae radix (Niu Xi), and 38 in Coicis Semen (Yi Yi Ren), and in TCMSP, also including 37 in Phellodendri Chinensis Cortex (Huang Bo), 26 in Atractylodes lancea (Thunb.) Dc. (Cang Zhu), 10 in achyranthis bidentatae radix (Niu Xi), and 3 in Coicis Semen (Yi Yi Ren) in BATMAN-TCM.
3.1.2. Selection of Compounds Using ADME Screening and Related Targets
All the identified compounds were selected through ADME screening, with 90 of 479 compounds satisfying the suggested criteria OB ≥ 30 and DL ≥ 0.18 [18–20]. Of the 90 compounds, 25 were duplicated and removed, and the remaining 65 compounds were included for further study. Moreover, of the excluded compounds that do not meet the suggested criteria, 7 compounds, including ferulic acid, beta-elemene, eugenol, and paeonol in Phellodendri Chinensis Cortex (Huang Bo) and geniposide, rutin, and astragalin in achyranthis bidentatae radix (Niu Xi), are considered bioactive compounds and included for further analysis, and the effects of ferulic acid [22], beta-elemene [23], eugenol [24–26], paeonol [27–29], geniposide [30–32], rutin [33, 34], and astragalin [35] on RA have been investigated. The final 72 compounds are selected from the four herbal medicines (Table 1). A total of 386 target genes related to the final identified compounds are obtained from the UniProt databases.
Table 1.
72 active compounds of SM.
| Mol id | Molecule name | OB (%) | DL | Herb |
|---|---|---|---|---|
| MOL002636 | Kihadalactone A | 34.21 | 0.82 | Phellodendri Chinensis Cortex |
| MOL013352 | Obacunone | 43.29 | 0.77 | Phellodendri Chinensis Cortex |
| MOL002641 | Phellavin_qt | 35.86 | 0.44 | Phellodendri Chinensis Cortex |
| MOL002644 | Phellopterin | 40.19 | 0.28 | Phellodendri Chinensis Cortex |
| MOL002651 | Dehydrotanshinone II A | 43.76 | 0.40 | Phellodendri Chinensis Cortex |
| MOL002652 | delta7-dehydrosophoramine | 54.45 | 0.25 | Phellodendri Chinensis Cortex |
| MOL002656 | Dihydroniloticin | 36.43 | 0.81 | Phellodendri Chinensis Cortex |
| MOL002659 | Kihadanin A | 31.60 | 0.70 | Phellodendri Chinensis Cortex |
| MOL002660 | Niloticin | 41.41 | 0.82 | Phellodendri Chinensis Cortex |
| MOL002662 | Rutaecarpine | 40.30 | 0.60 | Phellodendri Chinensis Cortex |
| MOL002663 | Skimmianin | 40.14 | 0.20 | Phellodendri Chinensis Cortex |
| MOL002666 | Chelerythrine | 34.18 | 0.78 | Phellodendri Chinensis Cortex |
| MOL002668 | Worenine | 45.83 | 0.87 | Phellodendri Chinensis Cortex |
| MOL002670 | Cavidine | 35.64 | 0.81 | Phellodendri Chinensis Cortex |
| MOL002671 | Candletoxin A | 31.81 | 0.69 | Phellodendri Chinensis Cortex |
| MOL002672 | Hericenone H | 39.00 | 0.63 | Phellodendri Chinensis Cortex |
| MOL002673 | Hispidone | 36.18 | 0.83 | Phellodendri Chinensis Cortex |
| MOL000358 | Beta-sitosterol | 36.91 | 0.75 | Phellodendri Chinensis Cortex |
| MOL000622 | Magnograndiolide | 63.71 | 0.19 | Phellodendri Chinensis Cortex |
| MOL000762 | Palmidin A | 35.36 | 0.65 | Phellodendri Chinensis Cortex |
| MOL000787 | Fumarine | 59.26 | 0.83 | Phellodendri Chinensis Cortex |
| MOL000790 | Isocorypalmine | 35.77 | 0.59 | Phellodendri Chinensis Cortex |
| MOL001131 | phellamurin_qt | 56.60 | 0.39 | Phellodendri Chinensis Cortex |
| MOL001455 | (S)-canadine | 53.83 | 0.77 | Phellodendri Chinensis Cortex |
| MOL001771 | Poriferast-5-en-3beta-ol | 36.91 | 0.75 | Phellodendri Chinensis Cortex |
| MOL002894 | Berberrubine | 35.74 | 0.73 | Phellodendri Chinensis Cortex |
| MOL005438 | Campesterol | 37.58 | 0.71 | Phellodendri Chinensis Cortex |
| MOL006392 | Dihydroniloticin | 36.43 | 0.82 | Phellodendri Chinensis Cortex |
| MOL006401 | Melianone | 40.53 | 0.78 | Phellodendri Chinensis Cortex |
| MOL006413 | Phellochin | 35.41 | 0.82 | Phellodendri Chinensis Cortex |
| MOL006422 | Thalifendine | 44.41 | 0.73 | Phellodendri Chinensis Cortex |
| MOL002665 | Ferulic acid | 40.43 | 0.06 | Phellodendri Chinensis Cortex |
| MOL000908 | Beta-elemene | 25.63 | 0.06 | Phellodendri Chinensis Cortex |
| MOL000254 | Eugenol | 56.24 | 0.04 | Phellodendri Chinensis Cortex |
| MOL000874 | Paeonol | 28.79 | 0.04 | Phellodendri Chinensis Cortex |
| MOL000179 | 2-Hydroxyisoxypropyl-3-hydroxy-7-isopentene-2,3-dihydrobenzofuran-5-carboxylic | 45.20 | 0.20 | Atractylodes lancea (Thunb.) Dc. |
| MOL000184 | NSC63551 | 39.25 | 0.76 | Atractylodes lancea (Thunb.) Dc. |
| MOL000186 | Stigmasterol 3-O-beta-D-glucopyranoside_qt | 43.83 | 0.76 | Atractylodes lancea (Thunb.) Dc. |
| MOL000188 | 3β-acetoxyatractylone | 40.57 | 0.22 | Atractylodes lancea (Thunb.) Dc. |
| MOL000088 | Beta-sitosterol 3-O-glucoside_qt | 36.91 | 0.75 | Atractylodes lancea (Thunb.) Dc. |
| MOL000092 | daucosterin_qt | 36.91 | 0.76 | Atractylodes lancea (Thunb.) Dc. |
| MOL000094 | daucosterol_qt | 36.91 | 0.76 | Atractylodes lancea (Thunb.) Dc. |
| MOL001006 | Poriferasta-7,22E-dien-3beta-ol | 42.98 | 0.76 | Achyranthis bidentatae radix |
| MOL012461 | 28-Norolean-17-en-3-ol | 35.93 | 0.78 | Achyranthis bidentatae radix |
| MOL012505 | bidentatoside, ii_qt | 31.76 | 0.59 | Achyranthis bidentatae radix |
| MOL012537 | Spinoside A | 41.75 | 0.40 | Achyranthis bidentatae radix |
| MOL012542 | β-ecdysterone | 44.23 | 0.82 | Achyranthis bidentatae radix |
| MOL002714 | Baicalein | 33.52 | 0.21 | Achyranthis bidentatae radix |
| MOL002776 | Baicalin | 40.12 | 0.75 | Achyranthis bidentatae radix |
| MOL002897 | Epiberberine | 43.09 | 0.78 | Achyranthis bidentatae radix |
| MOL003847 | Inophyllum E | 38.81 | 0.85 | Achyranthis bidentatae radix |
| MOL000422 | Kaempferol | 41.88 | 0.24 | Achyranthis bidentatae radix |
| MOL004355 | Spinasterol | 42.98 | 0.76 | Achyranthis bidentatae radix |
| MOL012516 | Geniposide | 8.40 | 0.44 | Achyranthis bidentatae radix |
| MOL000415 | Rutin | 3.20 | 0.68 | Achyranthis bidentatae radix |
| MOL000561 | Astragalin | 14.03 | 0.74 | Achyranthis bidentatae radix |
| MOL001323 | Sitosterol alpha1 | 43.28 | 0.78 | Coicis Semen |
| MOL001494 | Mandenol | 42.00 | 0.19 | Coicis Semen |
| MOL002372 | (6 Z, 10 E, 14 E, 18 E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene | 33.55 | 0.42 | Coicis Semen |
| MOL002882 | [(2R)-2,3-dihydroxypropyl] (Z)-octadec-9-enoate | 34.13 | 0.30 | Coicis Semen |
| MOL000359 | Sitosterol | 36.91 | 0.75 | Coicis Semen |
| MOL008118 | Coixenolide | 32.40 | 0.43 | Coicis Semen |
| MOL008121 | 2-Monoolein | 34.23 | 0.29 | Coicis Semen |
| MOL000953 | CLR | 37.87 | 0.68 | Coicis Semen |
| MOL001454 | Berberine | 36.86 | 0.78 | Phellodendri Chinensis Cortex, achyranthis bidentatae radix |
| MOL001458 | Coptisine | 30.67 | 0.86 | Phellodendri Chinensis Cortex, achyranthis bidentatae radix |
| MOL002643 | Delta 7-stigmastenol | 37.42 | 0.75 | Phellodendri Chinensis Cortex, achyranthis bidentatae radix |
| MOL000785 | Palmatine | 64.60 | 0.65 | Phellodendri Chinensis Cortex, achyranthis bidentatae radix |
| MOL000098 | Quercetin | 46.43 | 0.28 | Phellodendri Chinensis Cortex, achyranthis bidentatae radix |
| MOL000173 | Wogonin | 30.68 | 0.23 | Atractylodes lancea (Thunb.) Dc. Achyranthis bidentatae radix |
| MOL000085 | Beta-daucosterol_qt | 36.91 | 0.75 | Atractylodes lancea (Thunb.) Dc. Achyranthis bidentatae radix |
| MOL000449 | Stigmasterol | 43.83 | 0.76 | Phellodendri Chinensis Cortex, achyranthis bidentatae radix, Coicis Semen |
3.1.3. Identified Disease Target Genes
The target genes related to RA were searched in GeneCards and OMIM databases, which include 3768 genes in GeneCards and 1 gene in OMIM, with no overlapping target gene.
3.1.4. Intersection of Identified Targets of Compounds and Disease
In the Venn diagram intersection of identified targets about identified compounds and of RA (Figure 2), a total of 77 target genes are acquired.
Figure 2.
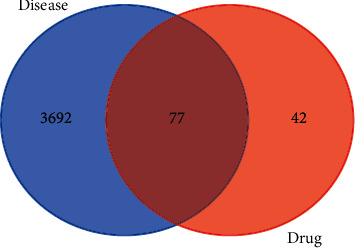
A Venn diagram showing intersection identified targets of identified compounds and RA.
3.2. Topological and PPI Network
3.2.1. Topological Network Analysis
The SM-RA mechanism network (Figure 3) consists of 77 target genes nodes (shared gene of SM and RA), 41 compound nodes, and 349 edges. Among the 14 compounds (Dehydrotanshinone II A, Stigmasterol, beta-sitosterol, Isocorypalmine, beta-elemene, quercetin, eugenol, paeonol, (S)-Canadine, wogonin, baicalein, Inophyllum E, rutin, and kaempferol) that connected to more than four genes, 55 target genes are associated with quercetin, 22 target genes are associated with kaempferol, 15 target genes are associated with baicalein, 14 target genes are associated with wogonin, and 10 target genes are associated with beta-sitosterol and eugenol, respectively (Table 2). In addition, 10 genes, including PTGS1, ESR1, AR, PGR, CHRM3, PPARG, CHRM2, BCL2, CASP3, and RELA, are related to more than five compounds, as shown in the compounds-targets network (Figure 4). These compounds and genes may be the key nodes in the network.
Figure 3.
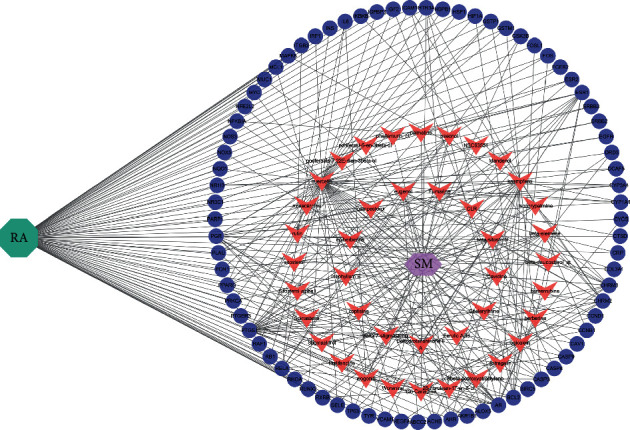
The SM-RA mechanism network. The green octagon represents rheumatoid arthritis (RA), the purple hexagon represents the herbal medicine simiao pill (SM), while pink V's represent compounds, and blue-purple ellipses represent genes.
Table 2.
Target genes interacting with compounds in the SM-RA network.
| Compounds | Target genes |
|---|---|
| Delta 7-stigmastenol | PGR |
| Poriferast-5-en-3beta-ol | PGR |
| Campesterol | PGR |
| NSC63551 | PGR |
| Beta-daucosterol_qt | PGR |
| Poriferasta-7,22E-dien-3beta-ol | PGR |
| 28-Norolean-17-en-3-ol | PGR |
| Spinasterol | PGR |
| Sitosterol alpha1 | PGR |
| Sitosterol | PGR |
| CLR | PGR |
| Chelerythrine | PTGS1 |
| Astragalin | PTGS1 |
| Mandenol | PTGS1 |
| Ferulic acid | PTGS1, CHRM2 |
| phellamurin_qt | ESR1, NR3C1 |
| Epiberberine | ESR1, AR |
| Berberine | PTGS1, ESR1, AR |
| Coptisine | PTGS1, ESR1, AR |
| Worenine | PTGS1, ESR1, AR |
| Berberrubine | PTGS1, ESR1, AR |
| Thalifendine | PTGS1, ESR1, AR |
| Fumarine | PTGS1, CHRM3, HTR3A |
| Rutaecarpine | PTGS1, AR, HTR3A, CYP3A4 |
| Cavidine | PTGS1, CHRM3, HTR3A, RXRB |
| Palmatine | PTGS1, ESR1, AR, ESR2 |
| 3β-acetoxyatractylone | CHRM3, AR, ACHE, CHRM2 |
| Dehydrotanshinone II A | CHRM3, ESR1, AR, PPARG, ACHE |
| Inophyllum E | PTGS1, ESR1, AR, ESR2, GSK3B |
| Stigmasterol | PGR, PTGS1, AKR1B1, PLAU, CHRM3, CHRM2 |
| Isocorypalmine | PTGS1, CHRM3, HTR3A, CHRM2, DRD3, RXRB |
| (S)-canadine | PTGS1, CHRM3, HTR3A, CHRM2, DRD3, RXRB |
| Paeonol | PTGS1, CHRM2, RELA, BCL2, NFKBIA, ICAM1, TYR |
| Beta-elemene | CHRM2, PTGS1, CHRM3, BCL2, RB1, TP63, CCNB1, RHOA |
| Rutin | RELA, IL6, CASP3, ALOX5, GSTP1, INS, FCER2, ITGB2 |
| Beta-sitosterol | PGR, PTGS1, CHRM3, CHRM2, BCL2, CASP9, CASP3, CASP8, PRKCA, PON1 |
| Eugenol | PTGS1, CHRM3, CHRM2, PLAU, RELA, CYP1A1, ALOX5, AHR, ABCC2, MUC1 |
| Wogonin | PTGS1, ESR1, AR, PPARG, GSK3B, RELA, CCND1, BCL2, CASP9, IL6, CASP3, TP63, PTGER3, MCL1 |
| Baicalein | PTGS1, AR, RELA, VEGFA, BCL2, FOS, CASP3, TP63, HIF1A, FOSL1, CCNB1, AHR, IGF2, CYCS, NOX5 |
| Kaempferol | PTGS1, AR, PPARG, PGR, ACHE, CHRM2, RELA, IKBKB, BCL2, CASP3, MAPK8, PPARG, CYP3A4, CYP1A1, ICAM1, SELE, VCAM1, ALOX5, GSTP1, AHR, NR1I3, GSTM1 |
| Quercetin | PTGS1, AR, PPARG, AKR1B1, ACHE, RELA, EGFR, VEGFA, CCND1, BCL2, FOS, CASP9, PLAU, RB1, IL6, CASP3, TP63, NFKBIA, CASP8,RAF1, PRKCA, HIF1A,ERBB2,PPARG,CYP3A4,CAV1,MYC,CYP1A1, ICAM1, SELE, VCAM1, PTGER3, BIRC5, NOS3, HSPB1, CCNB1, ALOX5, GSTP1, NFE2L2, NQO1, PARP1, AHR, COL3A1, DCAF5, NR1I3, HSF1, CRP, RUNX2, CTSD, IGFBP3, IGF2, IRF1, ERBB3, PON1, GSTM1 |
Figure 4.
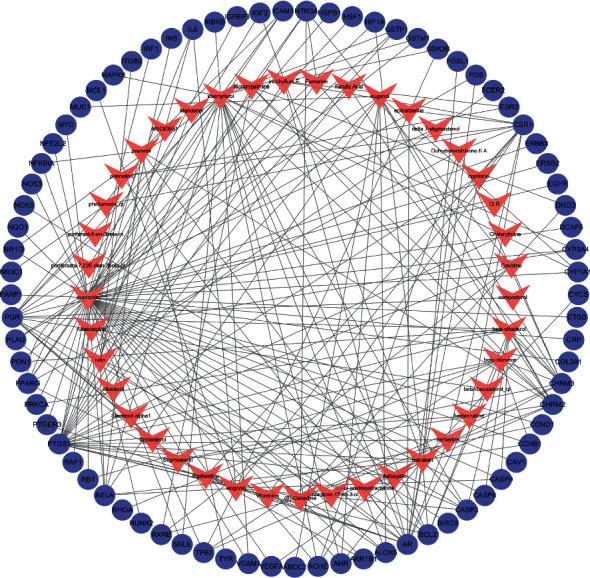
The compounds-targets network. The pink V's represent compounds and blue-purple ellipses represent target genes.
3.2.2. PPI Network
The PPI network is established by setting the confidence level of more than 0.40 and hiding the independent target protein nodes. The PPI network nodes represent proteins and edges represent protein-protein interactions. The network has 75 nodes and 1604 edges (Figure 5). In addition, we analyzed the importance prioritization (adjacent nodes count of each protein) of proteins according to the network, and the leading 30 genes with higher connection were visualized by constructing a bar plot diagram (Figure 6), which indicates the 30 genes or proteins that may play a bridge role in connecting other nodes in the PPI network. These 30 genes or proteins include inflammation-associated genes (IL6 [36], NFKBIA [37]), cell proliferation-, differentiation-, and transformation-related genes (FOS [38], EGFR [39], MAPK8 [40], NR3C1 [41], RHOA [42], and PARP1 [43]), cell apoptosis-related genes (CASP3, CASP8 [44], CASP9 [45], MYC [46], CYCS [47], HIF1A [48], MCL1 [49], and GSK3B [50]), cell cycle-related gene (CCND1 [51]), hormone-related genes (INS [52], ESR1 [53], AR [54], and PGR [55]), angiogenesis-related gene (VEGFA [56]), and transcription factor (RELA [57]).
Figure 5.
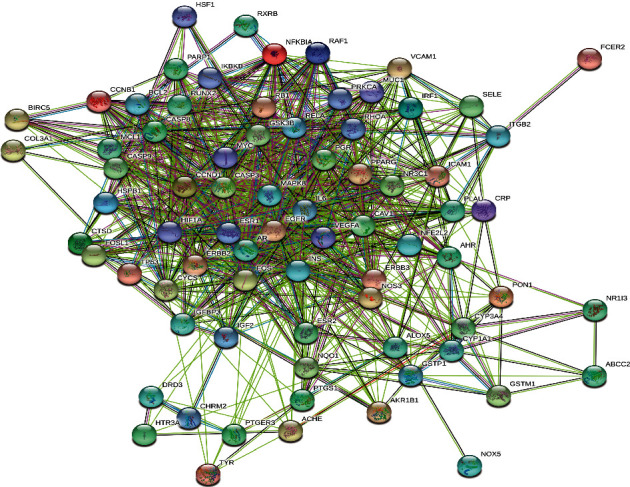
The PPI network of SM-RA. Each node represents the relevant gene, and the edges represent protein-protein associations.
Figure 6.
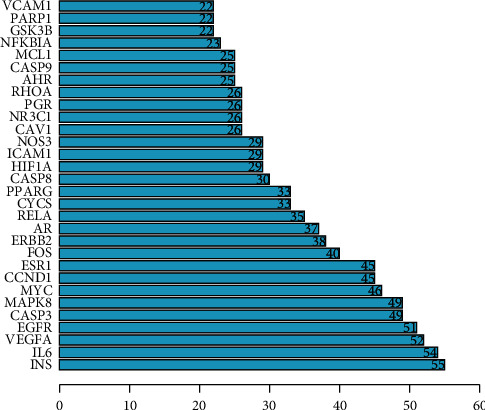
Hub top 30 genes of the PPI network. The y-axis displays significant top 30 genes, and the x-axis shows line counts of these genes.
3.3. Enrichment Analysis
3.3.1. GO Enrichment Analysis
GO analysis consisted of biological process (BP), cellular component (CC), and molecular function (MF). As showed in Figure 7, the top 20 enrichment terms are visualized by the bar plot diagram. The results demonstrated that numerous targets are involved in various BPs associated with immune response and inflammation, such as the response to a steroid hormone, response to oxidative stress, and regulation of the apoptotic signaling pathway, which confirmed strongly the correlation with the pathogenesis in RA. The CC results showed that most of the targets are localized to the cellular membrane and nuclear chromatin part. The MF results indicated that many targets are associated with nuclear receptor activity and transcription factor activity.
Figure 7.
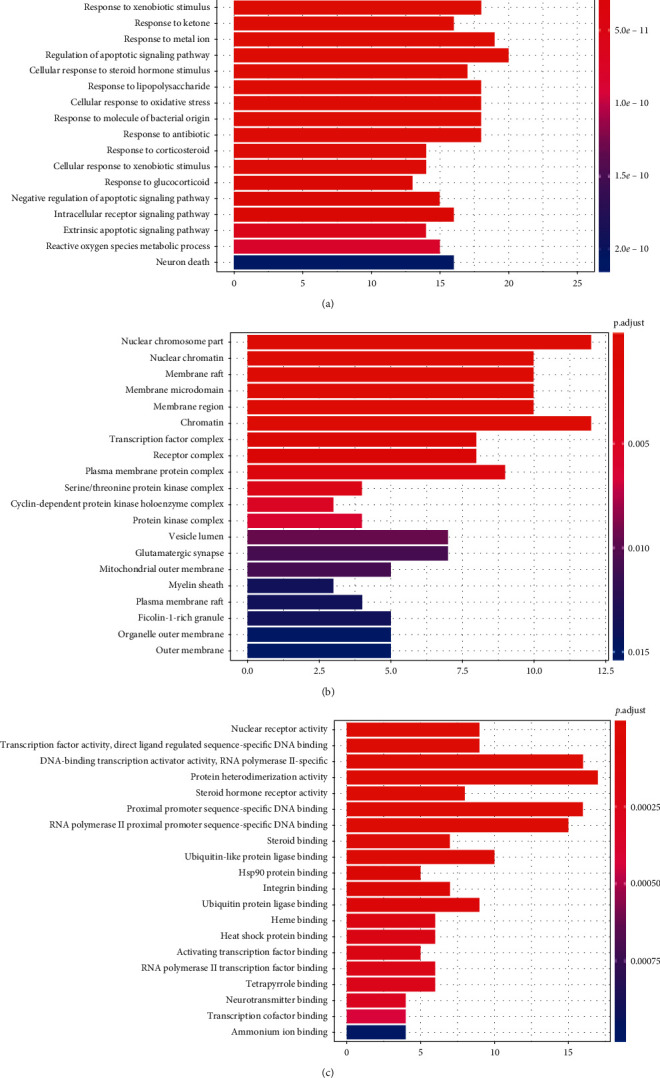
GO analysis of targets, the top 20 significant enrichment terms in BP (a), CC (b), and MF (c). The y-axis shows significantly enriched biological process, cellular component, and molecular function categories of the target genes, respectively. The redder the color, the lower the P value. The x-axis displays the enrichment scores of these terms, and the length of the bar indicates the number of target genes in each pathway.
3.3.2. KEGG Enrichment Analysis
The KEGG pathways are applied to examine the function and signaling pathways of the identified target genes, with the top 20 of the potential pathways (P < 0.05 and q < 0.05) shown by a bar plot diagram (Figure 8) and visualized with the pathways-targets-compounds network (Figure 9). The results showed that numerous targets are associated with certain virus infections (such as Epstein-Barr virus infection) and cancer, which are associated with the onset and prognosis of RA.
Figure 8.
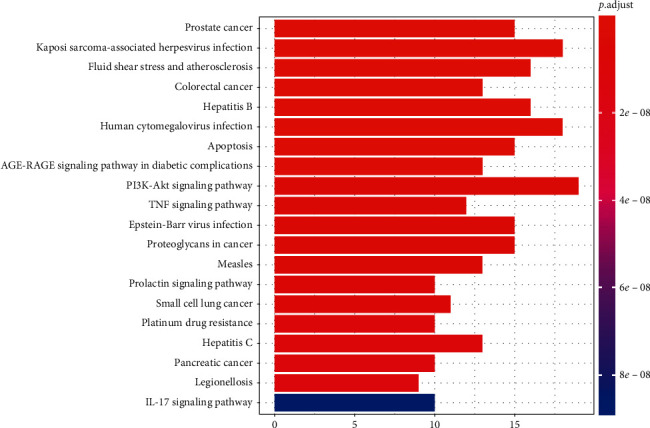
KEGG enrichment pathways (top 20). The y-axis displays the top 20 significantly enriched KEGG pathways of the target genes. The redder the color, the smaller the P value. The x-axis represents the target genes counts, and the length of the bar indicates the number of target genes in each pathway.
Figure 9.
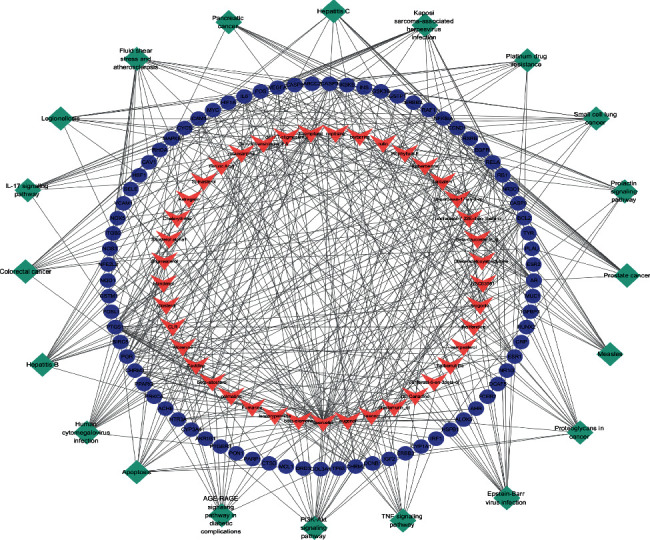
The pathways-targets-compounds network. The green diamonds represent pathways, the blue-purple ellipses represent genes, and the pink V's represent compounds.
3.4. Molecular Docking Analysis
The selected targets, including IL6, VEGFA, EGFR, and NFKBIA, play a significant role in the SM-RA network. The candidate compounds, including quercetin, kaempferol, baicalein, and wogonin, are the top 4 compounds (ranking by related target genes count) in the SM-RA network. These 4 target genes and 4 compounds are imported into MOE for molecular docking verification. The docking scores are shown in Table 3. The action mode of NFKBIA and quercetin, kaempferol, baicalein, and wogonin and the action mode of wogonin and IL6, VEGFA, EGFR, and NFKBIA are shown in Figure 10.
Table 3.
Molecular docking scores.
| IL6 | VEGFA | EGFR | NFKBIA | |
|---|---|---|---|---|
| Quercetin | −4.7051 | −5.9131 | −6.0857 | −6.7291 |
| Kaempferol | −4.9898 | −5.4844 | −5.7466 | −6.5524 |
| Baicalein | −4.5010 | −5.5058 | −5.8372 | −6.2222 |
| Wogonin | −4.6678 | −5.6466 | −6.1084 | −6.6169 |
Figure 10.
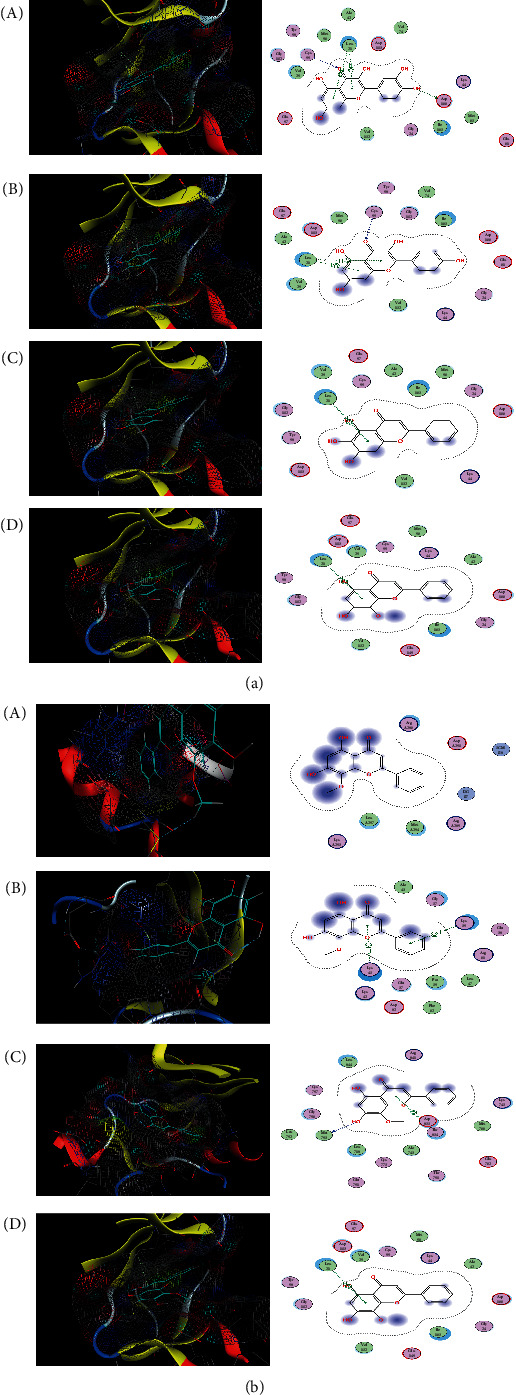
Molecular docking results. (a) The action mode of NFKBIA and quercetin, kaempferol, baicalein, and wogonin: (A) NFKBIA and quercetin; (B) NFKBIA and kaempferol; (C) NFKBIA and baicalein; (D) NFKBIA and wogonin. (b) The action mode of wogonin and IL6, VEGFA, EGFR, and NFKBIA: (A) wogonin and IL6; (B) wogonin and VEGFA; (C) wogonin and EGFR; (D) wogonin and NFKBIA.
4. Discussion
In the present network pharmacological analysis, a total of 479 compounds were identified in the four herbal medicines of SM, and 72 compounds were yielded by ADME criteria screening. A total of 386 targets related to potential compounds and 3769 targets associated with RA were identified, and 77 target genes were obtained from the interaction of targets about SM identified compounds and RA. SM-RA network analysis visualized the interaction of multicomponents and multitargets about SM on RA. The compounds-targets network analysis indicated that the 6 compounds, including quercetin, kaempferol, baicalein, wogonin, beta-sitosterol, and eugenol, were linked to ≥10 target genes, and the 10 target genes (PTGS1, ESR1, AR, PGR, CHRM3, PPARG, CHRM2, BCL2, CASP3, and RELA) were core target genes in the network. GO enrichment analysis indicated that numerous targets are involved in response to a steroid hormone, oxidative stress, and regulation of the apoptotic signaling pathway in BP, are localized to the cellular membrane and nuclear chromatin part in CC, and are associated with nuclear receptor activity and transcription factor activity in MF. KEGG pathways analysis results indicated that numerous targets are associated with certain virus infections and cancer. Molecular docking showed that quercetin, kaempferol, baicalein, and wogonin have good binding activity with IL6, VEGFA, EGFR, and NFKBIA targets.
About 72 identified compounds, particularly the 6 compounds, including quercetin, kaempferol, baicalein, wogonin, beta-sitosterol, and eugenol, were linked to more than 10 targets, indicating that these compounds might play a vital role in the process of RA treatment. Furthermore, certain compounds have exhibited the potential antirheumatic therapeutic activities except for wogonin (Table 4). For instance, quercetin has been reported to decrease levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-17 (IL-17), and monocyte chemotactic protein-1 (MCP-1) [58] and significantly reduced damage to interchondral joints, infiltration of inflammatory cells, and pannus formation [59]. Besides, kaempferol suppresses the proliferation and migration of RAFLS and the release of activated T-cell-mediated inflammatory cytokines and reduces osteoclast differentiation through targeting on the fibroblast growth factor receptor 3- (FGFR3-) ribosomal S6 kinase 2 (RSK2) signaling axis [60]. In addition, baicalein inhibits human rheumatoid arthritis fibroblast-like synoviocytes (RAFLS) proliferation involving suppression of nuclear factor kappa B (NF-κB) transcriptional activity and recombinant macrophage migration inhibitory factor- (MIF-) mediated signaling [61]. What's more, β-Sitosterol could modulate the functions of macrophages and attenuates rheumatoid inflammation in CIA mice [62]. For eugenol, it is reported to be effective in ameliorating oxidative stress and inflammation in arthritic rats [25, 26]. Moreover, among the other 66 compounds, some articles previously reported the antirheumatic effect. For example, ferulic acid is reported to suppress osteoclast differentiation and bone erosion via the inhibition of receptor activator of nuclear factor кB ligand- (RANKL-) dependent NF-κB signaling pathway [63], and berberine could attenuate adjuvant-induced arthritic fibroblast-like synoviocytes (AA-FLS) proliferation and regulate the Th17/Treg imbalance [64]. Collectively, these active components exhibit antirheumatic effects from various aspects, including anti-inflammatory, immunoregulatory, reducing bone erosion and destruction, and attenuating oxidative stress. Therefore, these might indicate the collective effectiveness and diversity of constituents in SM for treating RA.
Table 4.
Potential anti-RA mechanisms of some compounds.
| Compound | Mechanism | Model | Reference |
|---|---|---|---|
| Quercetin | Decreased TNF-α, IL-1β, IL-17, and MCP-1 | CIA mice | Haleagrahara et al. [58] |
| Decreased TNF-α in joints, reduced interchondral joints damage, inflammatory cells infiltration, and pannus formation | CIA mice | Kawaguchi et al. [59] | |
| Promote RAFLS apoptosis by upregulating lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) and inhibiting PI3K/AKT signal activation subsequently | RAFLS | Pan et al. [72] | |
| Exerted anti-inflammatory, analgesic, and antioxidant effects by inhibiting NF-κB and regulating nuclear factor erythroid 2-related factor (Nrf2)/home oxygenase (HO-1) signal | Zymosan-induced arthritis mice | Guazelli, et al. [73] | |
| Inhibited IL-17 and RANKL production, suppressed Th17 cell | RAFLS | Kim HR, et al. 2019 [74] | |
|
| |||
| Kaempferol | Inhibited RAFLS proliferation and migration, suppressed inflammatory cytokines (IL-17, IL-21, and TNF-α) by targeting FGFR3-RSK2 signal | RAFLS | Lee, et al. [60] |
| Inhibited RAFLS migration and invasion by blocking MAPK signal | RAFLS | Pan et al. [75] | |
| Inhibited RAFLS proliferation, reduced MMPs, COX-2, and PGE2 production, inhibited NF-κB activation | RAFLS | Yoon et al. [76] | |
| Baicalein | Inhibited RAFLS proliferation by suppressing NF-κB activation | RAFLS | Chen et al. [61] |
|
| |||
| Beta-sitosterol | Inhibited inflammatory cytokines (iNOS, IL-1β), modulated macrophages functions | CIA mice | Liu et al. [62] |
|
| |||
| Eugenol | Inhibited mononuclear infiltration, lowered TNF-α, TGF-β, and IFN-γ | CIA murine | Grespan et al. [24] |
| Reduced inflammatory cytokines (TNF-α, IL-6, and IL-10) and oxidative stress | CIA rat | Mateen et al. [25] | |
| Reduced inflammatory cytokines (TNF-α, IL-6) and oxidative stress | RA patients | Mateen et al. [26] | |
The anti-RA effect of identified compounds (quercetin, kaempferol, baicalein, beta-sitosterol, and eugenol) is partially associated with the potential target genes, including NFKBIA, IL6, and MAPK, and potential signals, including PI3K‐AKT, TNF, and IL‐17, indicating the interaction between multicomponents, multitargets, and multisignaling of SM treating RA.
Among the main target genes (top 30) in the PPI network is INS, ranking first with the highest connection, which may affect the local inflammatory process of joint in RA [52], though research about the role of INS in RA is rare. IL6 is involved in the regulation of the immune response, inflammation, and hematopoiesis and confirmed the pathological roles in RA [65]. VEGFA contributes to promoting the angiogenic phenotype of RA [56]. EGFR is proved to be involved in the proliferation and cytokine production of synovial fibroblasts, the proliferation of endothelial cells, and the formation of osteoclasts [39]. CASP3, CASP8 [44], and CASP9 [45] are involved in the apoptosis of RA synoviocytes. NFKBIA is related to the inflammation of RA by regulating many genes for immune response, cell adhesion, differentiation, proliferation, angiogenesis, and apoptosis [37]. The antirheumatic effect of aforementioned baicalein, ferulic acid, etc. is partially associated with these target genes, indicating the interaction between multicomponents and multitargets of SM treating RA.
KEGG pathway enrichment analysis indicated that certain types of virus infection and cancer might also be crucial in the network. The evidence that viral infection contributes to RA, such as Epstein-Barr virus infection [1], is strong, and RA is associated with an increased risk of cancer [66]. In addition, the KEGG pathway analysis also indicated that PI3K-Akt, TNF, and IL-17 signaling pathway may be a critical signaling pathway in the network pharmacology. The PI3K-Akt signaling pathway is involved in inflammatory cytokine production [67], proliferation and migration of RAFLS [68] and chondrocyte proliferation [69], and apoptosis and autophagy in RA [69]. Moreover, a pivotal role for the proinflammatory cytokines, including tumor necrosis factor (TNF) [70] and interleukin- (IL-) 17 [71], in RA joint pathology has been identified.
Of the leading 30 target genes with a higher connection in the PPI network, IL6, VEGFA, EGFR, and NFKBIA play a critical role in the development of RA, which has been aforementioned. Besides, in the visualized pathways-targets network, IL6, VEGFA, EGFR, and NFKBIA are involved in numerous pathways, indicating that SM may exert anti-RA effects through multipathways and multitargets combined interaction. Furthermore, the molecular docking analysis was constructed to investigate the interaction of some candidate compounds and targets. For example, the absolute value of docking scores about NFKBIA and quercetin, kaempferol, baicalein, and wogonin is the highest in each group, indicating that NFKBIA has a higher binding affinity than other target genes. For wogonin, although there have been no relevant studies about the effect in RA, the docking results indicated that wogonin performed good binding activity with IL6, VEGFA, EGFR, and NFKBIA. In brief, the high binding affinities of these active components indicated that the therapeutic effects of SM treating RA were probably through the modulation of several related targets.
As shown, the anti-RA effect of identified compounds (quercetin, kaempferol, baicalein, beta-sitosterol, and eugenol) is partially associated with the potential target genes, including NFKBIA, IL6, and MAPK, and potential signals, including PI3K-Akt, TNF, and IL-17, indicating the interaction between multicomponents, multitargets, and multisignaling of SM treating RA.
5. Limitation
This study has some limitations. It provides only a predictive overview of the pharmacological mechanisms of SM against RA based on the existing database, and further experiment verification in vivo and in vitro is necessary to ensure the reliability and reasonability of predicted results. First, posttranscriptional processing, translation regulation, and posttranslational processing and regulation play a critical role in gene expression regulation, and most of the research on mechanisms about SM treating RA is gene level in this study; therefore, an in-depth study needs to explore the related mechanism. Second, the key proteins and KEGG pathways need to be verified. Third, the anti-RA effect needs to be further verified in the animal model.
Besides, for clinical applying of SM treating RA, owing to the ethnic, genetic, and possible etiological differences, potential mechanisms about the related therapeutic module coinciding with clinical applications are worthy of further experimental investigation. In addition, importantly, dosage exploration, oral bioavailability, water-solubility, pharmacokinetics, and potential side effects of SM will also need a thorough exploration.
6. Conclusion
In summary, a bioinformatics/topology-based strategy, including ADEM screening, bioinformatics, network topology, enrichment analysis, and molecular analysis, was applied for identification of the molecular mechanisms of SM against RA. The integrated strategy might make the decipherment of biological mechanisms more accurate and efficient. The SM-RA network, compounds-targets network, and pathways-targets network analysis visualized the interaction of multicomponents and multitargets about SM treating RA. In particular, quercetin, kaempferol, baicalein, wogonin, beta-sitosterol, and eugenol might be the candidate therapeutic agents, and PTGS1, ESR1, AR, PGR, CHRM3, PPARG, CHRM2, BCL2, CASP3, and RELA were identified as potential drug targets. The enrichment and PPI analysis revealed the biological functions of the grouping networks related to the pathogenesis of RA. The multicomponent cosynergism of the herbal combinations about SM was elaborated. The study also revealed the multifunctional synergetic mechanisms of SM, including certain virus infection and cancer, and PI3K-Akt, TNF, and IL-17 signaling pathway.
Acknowledgments
This research did not receive any specific funding.
Contributor Information
Wenqun Li, Email: liwq1204@csu.edu.cn.
Fen Li, Email: lifen0731@csu.edu.cn.
Data Availability
The figures and tables used to support the findings of this study are included within the article, and the original data are available from the first author or corresponding author upon request.
Disclosure
This research did not receive any specific funding.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Aletaha D., Smolen J. S. Diagnosis and management of rheumatoid arthritis. JAMA. 2018;320(13):1360–1372. doi: 10.1001/jama.2018.13103. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka Y., Takeuchi T., Tanaka S. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to conventional DMARDs: a randomised, double-blind, placebo-controlled phase III trial (RAJ3) Annals of the Rheumatic Diseases. 2019;78(10):1320–1332. doi: 10.1136/annrheumdis-2019-215163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott D. L., Wolfe F., Huizinga T. W. Rheumatoid arthritis. The Lancet. 2010;376(9746):1094–1108. doi: 10.1016/s0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 4.McInnes I. B., Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. The Lancet. 2017;389(10086):2328–2337. doi: 10.1016/s0140-6736(17)31472-1. [DOI] [PubMed] [Google Scholar]
- 5.Burmester G. R., Pope J. E. Novel treatment strategies in rheumatoid arthritis. The Lancet. 2017;389(10086):2338–2348. doi: 10.1016/s0140-6736(17)31491-5. [DOI] [PubMed] [Google Scholar]
- 6.Moudgil K. D., Berman B. M. Traditional Chinese medicine: potential for clinical treatment of rheumatoid arthritis. Expert Review of Clinical Immunology. 2014;10(7):819–822. doi: 10.1586/1744666x.2014.917963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seca S., Franconi G. Understanding Chinese medicine patterns of rheumatoid arthritis and related biomarkers. Medicines (Basel) 2018;5(1) doi: 10.3390/medicines5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan H.-Y., Zhang X.-L., Zhang X.-H., Meng L., Wei J.-F. Analysis of patents on anti-rheumatoid arthritis therapies issued in China. Expert Opinion on Therapeutic Patents. 2015;25(8):909–930. doi: 10.1517/13543776.2015.1044972. [DOI] [PubMed] [Google Scholar]
- 9.Lü S., Wang Q., Li G., Sun S., Guo Y., Kuang H. The treatment of rheumatoid arthritis using Chinese medicinal plants: from pharmacology to potential molecular mechanisms. Journal of Ethnopharmacology. 2015;176:177–206. doi: 10.1016/j.jep.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y. F., Huang Y., Wen C. Y., et al. The effects of modified simiao decoction in the treatment of gouty arthritis: a systematic review and meta-analysis. Evidence Based Complementary and Alternative Medicine. 2017;2017 doi: 10.1155/2017/6037037.6037037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma C.-H., Kang L.-L., Ren H.-M., Zhang D.-M., Kong L.-D. Simiao pill ameliorates renal glomerular injury via increasing Sirt1 expression and suppressing NF-κB/NLRP3 inflammasome activation in high fructose-fed rats. Journal of Ethnopharmacology. 2015;172:108–117. doi: 10.1016/j.jep.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Shen P., Tu S., Wang H., Qin K., Chen Z. Simiao pill attenuates collagen-induced arthritis in rats through suppressing the ATX-LPA and MAPK signalling pathways. Evidence Based Complementary and Alternative Medicine. 2019;2019 doi: 10.1155/2019/7498527.7498527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J., Zha Q., Jiang M., Cao H., Lu A. Expert consensus on the treatment of rheumatoid arthritis with Chinese patent medicines. The Journal of Alternative and Complementary Medicine. 2013;19(2):111–118. doi: 10.1089/acm.2011.0370. [DOI] [PubMed] [Google Scholar]
- 14.Li S., Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chinese Journal of Natural Medicines. 2013;11(2):110–120. doi: 10.1016/s1875-5364(13)60037-0. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins A. L. Network pharmacology: the next paradigm in drug discovery. Nature Chemical Biology. 2008;4(11):682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 16.Luo T. T., Lu Y., Yan S. K. Network pharmacology in research of Chinese medicine formula: methodology, application and prospective. Chinese Journal of Integrative Medicine. 2019;26(1):72–80. doi: 10.1007/s11655-019-3064-0. [DOI] [PubMed] [Google Scholar]
- 17.Guo Q., Zheng K., Fan D. Wu-Tou Decoction in Rheumatoid arthritis: integrating network pharmacology and in vivo pharmacological evaluation. Frontiers in Pharmacology. 2017;8:p. 230. doi: 10.3389/fphar.2017.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee A. Y., Park W., Kang T.-W., Cha M. H., Chun J. M. Network pharmacology-based prediction of active compounds and molecular targets in Yijin-Tang acting on hyperlipidaemia and atherosclerosis. Journal of Ethnopharmacology. 2018;221:151–159. doi: 10.1016/j.jep.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Xie G., Peng W., Li P. A network pharmacology analysis to explore the effect of astragali radix-radix angelica sinensis on traumatic brain injury. Biomed Research International. 2018;2018 doi: 10.1155/2018/3951783.3951783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P., Chen J., Zhang W., Li H., Wang W., Chen J. Network pharmacology based investigation of the effects of herbal ingredients on the immune dysfunction in heart disease. Pharmacological Research. 2019;141:104–113. doi: 10.1016/j.phrs.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Tsaioun K., Blaauboer B. J., Hartung T. Evidence-based absorption, distribution, metabolism, excretion (ADME) and its interplay with alternative toxicity methods. Altex. 2016;33(4):343–358. doi: 10.14573/altex.1610101. [DOI] [PubMed] [Google Scholar]
- 22.Ganesan R., Rasool M. Ferulic acid inhibits interleukin 17-dependent expression of nodal pathogenic mediators in fibroblast-like synoviocytes of rheumatoid arthritis. Journal of Cellular Biochemistry. 2018;120(2) doi: 10.1002/jcb.27502. [DOI] [PubMed] [Google Scholar]
- 23.Zou S., Wang C., Cui Z., et al. β-Elemene induces apoptosis of human rheumatoid arthritis fibroblast-like synoviocytes via reactive oxygen species-dependent activation of p38 mitogen-activated protein kinase. Pharmacological Reports. 2016;68(1):7–11. doi: 10.1016/j.pharep.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Grespan R., Paludo M., Lemos H. d. P., et al. Anti-arthritic effect of eugenol on collagen-induced arthritis experimental model. Biological and Pharmaceutical Bulletin. 2012;35(10):1818–1820. doi: 10.1248/bpb.b12-00128. [DOI] [PubMed] [Google Scholar]
- 25.Mateen S., Shahzad S., Ahmad S., et al. Cinnamaldehyde and eugenol attenuates collagen induced arthritis via reduction of free radicals and pro-inflammatory cytokines. Phytomedicine. 2019;53:70–78. doi: 10.1016/j.phymed.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Mateen S., Rehman M. T., Shahzad S., et al. Anti-oxidant and anti-inflammatory effects of cinnamaldehyde and eugenol on mononuclear cells of rheumatoid arthritis patients. European Journal of Pharmacology. 2019;852:14–24. doi: 10.1016/j.ejphar.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 27.Li Y., Li P., Lin S.-H., Zheng Y.-Q., Zheng X.-X. Paeonol inhibited TNF-α-induced GM-CSF expression in fibroblast-like synoviocytes. International Journal of Clinical Pharmacology and Therapeutics. 2014;52(11):986–996. doi: 10.5414/cp202127. [DOI] [PubMed] [Google Scholar]
- 28.Liu N., Feng X., Wang W., Zhao X., Li X. Paeonol protects against TNF-α-induced proliferation and cytokine release of rheumatoid arthritis fibroblast-like synoviocytes by upregulating FOXO3 through inhibition of miR-155 expression. Inflammation Research. 2017;66(7):603–610. doi: 10.1007/s00011-017-1041-7. [DOI] [PubMed] [Google Scholar]
- 29.Zhai K. F., Duan H., Luo L. Protective effects of paeonol on inflammatory response in IL-1beta-induced human fibroblast-like synoviocytes and rheumatoid arthritis progression via modulating NF-kappaB pathway. Inflammopharmacology. 2017;25:523–532. doi: 10.1007/s10787-017-0385-5. [DOI] [PubMed] [Google Scholar]
- 30.Li F., Dai M., Wu H. Immunosuppressive effect of geniposide on mitogen-activated protein kinase signalling pathway and their cross-talk in fibroblast-like synoviocytes of adjuvant arthritis rats. Molecules. 2018;23(1) doi: 10.3390/molecules23010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng R., Li F., Wu H. Anti-inflammatory mechanism of geniposide: inhibiting the hyperpermeability of fibroblast-like synoviocytes via the RhoA/p38MAPK/NF-kappaB/F-actin signal pathway. Frontiers in Pharmacology. 2018;9:p. 105. doi: 10.3389/fphar.2018.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Dai L., Wu H., et al. Novel anti-inflammatory target of geniposide: inhibiting Itgβ1/Ras-Erk1/2 signal pathway via the miRNA-124a in rheumatoid arthritis synovial fibroblasts. International Immunopharmacology. 2018;65:284–294. doi: 10.1016/j.intimp.2018.09.049. [DOI] [PubMed] [Google Scholar]
- 33.Sun C. L., Wei J., Bi L. Q. Rutin attenuates oxidative stress and proinflammatory cytokine level in adjuvant induced rheumatoid arthritis via inhibition of NF-kappaB. Pharmacology. 2017;100(1-2):40–49. doi: 10.1159/000451027. [DOI] [PubMed] [Google Scholar]
- 34.Gul A., Kunwar B., Mazhar M., et al. Rutin and rutin-conjugated gold nanoparticles ameliorate collagen-induced arthritis in rats through inhibition of NF-κB and iNOS activation. International Immunopharmacology. 2018;59:310–317. doi: 10.1016/j.intimp.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Jia Q., Wang T., Wang X., et al. Astragalin suppresses inflammatory responses and bone destruction in mice with collagen-induced arthritis and in human fibroblast-like synoviocytes. Frontiers in Pharmacology. 2019;10:p. 94. doi: 10.3389/fphar.2019.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandolfi F., Franza L., Carusi V. Interleukin-6 in rheumatoid arthritis. International Journal of Molecular Science. 2020;21(15) doi: 10.3390/ijms21155238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun X. F., Zhang H. NFKB and NFKBI polymorphisms in relation to susceptibility of tumour and other diseases. Histology and Histopathology. 2007;22(12):1387–1398. doi: 10.14670/HH-22.1387. [DOI] [PubMed] [Google Scholar]
- 38.Hannemann N., Jordan J., Paul S., et al. The AP-1 transcription factor c-jun promotes arthritis by regulating cyclooxygenase-2 and arginase-1 expression in macrophages. The Journal of Immunology. 2017;198(9):3605–3614. doi: 10.4049/jimmunol.1601330. [DOI] [PubMed] [Google Scholar]
- 39.Swanson C. D., Akama-Garren E. H., Stein E. A., et al. Inhibition of epidermal growth factor receptor tyrosine kinase ameliorates collagen-induced arthritis. The Journal of Immunology. 2012;188(7):3513–3521. doi: 10.4049/jimmunol.1102693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson G. L., Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L., Huang Y., Wu C. Network pharmacology based research on the combination mechanism between escin and low dose glucocorticoids in anti-rheumatoid arthritis. Frontiers in Pharmacology. 2019;10:p. 280. doi: 10.3389/fphar.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laragione T., Harris C., Gulko P. S. TRPV2 suppresses Rac1 and RhoA activation and invasion in rheumatoid arthritis fibroblast-like synoviocytes. International Immunopharmacology. 2019;70:268–273. doi: 10.1016/j.intimp.2019.02.051. [DOI] [PubMed] [Google Scholar]
- 43.Garcia S., Conde C. The role of poly(ADP-ribose) polymerase-1 in rheumatoid arthritis. Mediators of Inflammation. 2015;2015 doi: 10.1155/2015/837250.837250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto K., Kobayashi T., Kobata T., et al. Fas-associated death domain protein is a Fas-mediated apoptosis modulator in synoviocytes. Rheumatology. 2000;39(5):471–480. doi: 10.1093/rheumatology/39.5.471. [DOI] [PubMed] [Google Scholar]
- 45.Itoh K., Hase H., Kojima H., Saotome K., Nishioka K., Kobata T. Central role of mitochondria and p53 in Fas-mediated apoptosis of rheumatoid synovial fibroblasts. Rheumatology. 2004;43(3):277–285. doi: 10.1093/rheumatology/keh039. [DOI] [PubMed] [Google Scholar]
- 46.Lee J., Jeong H., Park E.-J., et al. CIP2A facilitates apoptotic resistance of fibroblast-like synoviocytes in rheumatoid arthritis independent of c-Myc expression. Rheumatology International. 2013;33(9):2241–2248. doi: 10.1007/s00296-013-2711-6. [DOI] [PubMed] [Google Scholar]
- 47.Holmgren A., Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochemical and Biophysical Research Communications. 2010;396(1):120–124. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 48.Li Y., He N., Shen L., Liu M. Apoptotic effect of Aralia echinocaulis extract on fibroblast-like synoviocytes in rats with adjuvant-induced arthritis via inhibiting the Akt/Hif-1α signaling pathway in vitro. Journal of Pharmacological Sciences. 2019;139(4):340–345. doi: 10.1016/j.jphs.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Jiao Y., Ding H., Huang S. Bcl-XL and Mcl-1 upregulation by calreticulin promotes apoptosis resistance of fibroblast-like synoviocytes via activation of PI3K/Akt and STAT3 pathways in rheumatoid arthritis. Clinical and Experimental Rheumatology. 2018;36(5):841–849. [PubMed] [Google Scholar]
- 50.Sun J., Yan P., Chen Y. MicroRNA-26b inhibits cell proliferation and cytokine secretion in human RASF cells via the Wnt/GSK-3beta/beta-catenin pathway. Diagnostic Pathology. 2015;10:p. 72. doi: 10.1186/s13000-015-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang X.-Y., Zhang X.-M., Chen F.-H., et al. Anti-proliferative effect of recombinant human endostatin on synovial fibroblasts in rats with adjuvant arthritis. European Journal of Pharmacology. 2014;723:7–14. doi: 10.1016/j.ejphar.2013.10.068. [DOI] [PubMed] [Google Scholar]
- 52.Zhou J., Kvetnansky R., Radikova Z. Hormone concentrations in synovial fluid of patients with rheumatoid arthritis. Clinical and Experimental Rheumatology. 2005;23(3):292–296. [PubMed] [Google Scholar]
- 53.Dziedziejko V., Kurzawski M., Safranow K., Chlubek D., Pawlik A. The effect of ESR1andESR2gene polymorphisms on the outcome of rheumatoid arthritis treatment with leflunomide. Pharmacogenomics. 2011;12(1):41–47. doi: 10.2217/pgs.10.164. [DOI] [PubMed] [Google Scholar]
- 54.Gubbels Bupp M. R., Jorgensen T. N. Androgen-induced immunosuppression. Frontiers in Immunology. 2018;9:p. 794. doi: 10.3389/fimmu.2018.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karlson E. W., Chibnik L. B., McGrath M., et al. A prospective study of androgen levels, hormone-related genes and risk of rheumatoid arthritis. Arthritis Research & Therapy. 2009;11(3):p. R97. doi: 10.1186/ar2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y., Qiu H., Zhang H., Wang L., Zhuang C., Liu R. Vascular endothelial growth factor A (VEGFA) polymorphisms in Chinese patients with rheumatoid arthritis. Scandinavian Journal of Rheumatology. 2013;42(5):344–348. doi: 10.3109/03009742.2013.787454. [DOI] [PubMed] [Google Scholar]
- 57.Jimi E., Fei H., Nakatomi C. NF-kappaB signaling regulates physiological and pathological chondrogenesis. International Journal of Molecular Sciences. 2019;20(24) doi: 10.3390/ijms20246275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haleagrahara N., Miranda-Hernandez S., Alim M. A., Hayes L., Bird G., Ketheesan N. Inhibitory effect of quercetin in collagen-induced arthritis. Biomedicine & Pharmacotherapy. 2017;90:38–46. doi: 10.1016/j.biopha.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 59.Kawaguchi K., Kaneko M., Miyake R., Takimoto H., Kumazawa Y. Potent inhibitory effects of quercetin on inflammatory responses of collagen-induced arthritis in mice. Endocrine, Metabolic & Immune Disorders-Drug Targets. 2019;19(3):308–315. doi: 10.2174/1871530319666190206225034. [DOI] [PubMed] [Google Scholar]
- 60.Lee C. J., Moon S. J., Jeong J. H. Kaempferol targeting on the fibroblast growth factor receptor 3-ribosomal S6 kinase 2 signaling axis prevents the development of rheumatoid arthritis. Cell Death Diseases. 2018;9(3):p. 401. doi: 10.1038/s41419-018-0433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen S., Yang Y., Feng H., Wang H., Zhao R., Liu H. Baicalein inhibits interleukin-1β-induced proliferation of human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation. 2014;37(1):163–169. doi: 10.1007/s10753-013-9725-9. [DOI] [PubMed] [Google Scholar]
- 62.Liu R., Hao D., Xu W., et al. β-Sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharmaceutical Biology. 2019;57(1):161–168. doi: 10.1080/13880209.2019.1577461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doss H. M., Samarpita S., Ganesan R., Rasool M. Ferulic acid, a dietary polyphenol suppresses osteoclast differentiation and bone erosion via the inhibition of RANKL dependent NF-κB signalling pathway. Life Sciences. 2018;207:284–295. doi: 10.1016/j.lfs.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 64.Dinesh P., Rasool M. Berberine mitigates IL-21/IL-21R mediated autophagic influx in fibroblast-like synoviocytes and regulates Th17/Treg imbalance in rheumatoid arthritis. Apoptosis. 2019;24(7-8):644–661. doi: 10.1007/s10495-019-01548-6. [DOI] [PubMed] [Google Scholar]
- 65.Nishimoto N., Kishimoto T. Interleukin 6: from bench to bedside. Nature Clinical Practice Rheumatology. 2006;2(11):619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 66.Elandt K., Aletaha D. Treating rheumatic patients with a malignancy. Arthritis Research & Therapy. 2011;13(3):p. 223. doi: 10.1186/ar3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee J. Y., Kim G. J., Choi J. K. 4-(Hydroxymethyl)catechol extracted from fungi in marine sponges attenuates rheumatoid arthritis by inhibiting PI3K/akt/NF-kappaB signaling. Frontiers in Pharmacology. 2018;9:p. 726. doi: 10.3389/fphar.2018.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song B., Li X.-F., Yao Y., et al. BMP9 inhibits the proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis via the PI3K/AKT signaling pathway. International Immunopharmacology. 2019;74 doi: 10.1016/j.intimp.2019.105685.105685 [DOI] [PubMed] [Google Scholar]
- 69.Xu F.-B., Qiu H.-Y. Effects of artesunate on chondrocyte proliferation, apoptosis and autophagy through the PI3K/AKT/mTOR signaling pathway in rat models with rheumatoid arthritis. Biomedicine & Pharmacotherapy. 2018;102:1209–1220. doi: 10.1016/j.biopha.2018.03.142. [DOI] [PubMed] [Google Scholar]
- 70.Wallach D. The cybernetics of TNF: old views and newer ones. Seminars in Cell & Developmental Biology. 2016;50:105–114. doi: 10.1016/j.semcdb.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 71.van den Berg W. B., Miossec P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nature Reviews Rheumatology. 2009;5(10):549–553. doi: 10.1038/nrrheum.2009.179. [DOI] [PubMed] [Google Scholar]
- 72.Pan F., Zhu L., Lv H., Pei C. Quercetin promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by upregulating lncRNA malat1. International Journal of Molecular Medicine. 2016;38(5):1507–1514. doi: 10.3892/ijmm.2016.2755. [DOI] [PubMed] [Google Scholar]
- 73.Guazelli C. F. S., Staurengo-Ferrari L., Zarpelon A. C., et al. Quercetin attenuates zymosan-induced arthritis in mice. Biomedicine & Pharmacotherapy. 2018;102:175–184. doi: 10.1016/j.biopha.2018.03.057. [DOI] [PubMed] [Google Scholar]
- 74.Kim H.-R., Kim B.-M., Won J.-Y., et al. Quercetin, a plant polyphenol, has potential for the prevention of bone destruction in rheumatoid arthritis. Journal of Medicinal Food. 2019;22(2):152–161. doi: 10.1089/jmf.2018.4259. [DOI] [PubMed] [Google Scholar]
- 75.Lee D., Li N., Liu Y., et al. Kaempferol inhibits the migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes by blocking activation of the MAPK pathway. International Immunopharmacology. 2018;55:174–182. doi: 10.1016/j.intimp.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 76.Yoon H.-Y., Lee E.-G., Lee H., et al. Kaempferol inhibits IL-1β-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs. International Journal of Molecular Medicine. 2013;32(4):971–977. doi: 10.3892/ijmm.2013.1468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The figures and tables used to support the findings of this study are included within the article, and the original data are available from the first author or corresponding author upon request.


