Abstract
BACKGROUND AND PURPOSE: Presurgical sensorimotor mapping with functional MR imaging is gaining acceptance in clinical practice; however, to our knowledge, its therapeutic efficacy has not been assessed in a sizable group of patients. Our goal was to identify how preoperative sensorimotor functional studies were used to guide the treatment of neuro-oncologic and epilepsy surgery patients.
METHODS: We retrospectively reviewed the medical records of 46 patients who had undergone preoperative sensorimotor functional MR imaging to document how often and in what ways the imaging studies had influenced their management. Clinical management decisions were grouped into three categories: for assessing the feasibility of surgical resection, for surgical planning, and for selecting patients for invasive functional mapping procedures.
RESULTS: Functional MR imaging studies successfully identified the functional central sulcus ipsilateral to the abnormality in 32 of the 46 patients, and these 32 patients are the focus of this report. In epilepsy surgery candidates, the functional MR imaging results were used to determine in part the feasibility of a proposed surgical resection in 70% of patients, to aid in surgical planning in 43%, and to select patients for invasive surgical functional mapping in 52%. In tumor patients, the functional MR imaging results were used to determine in part the feasibility of surgical resection in 55%, to aid in surgical planning in 22%, and to select patients for invasive surgical functional mapping in 78%. Overall, functional MR imaging studies were used in one or more of the three clinical decision-making categories in 89% of tumor patients and 91% of epilepsy surgery patients.
CONCLUSION: Preoperative functional MR imaging is useful to clinicians at three key stages in the preoperative clinical management paradigm of a substantial percentage of patients who are being considered for resective tumor or epilepsy surgery.
Invasive techniques are commonly used either before or during resective neurosurgical procedures to localize specific functional areas on the cortical surface. Localization of sensorimotor function may be accomplished with either intraoperative or extraoperative cortical mapping procedures. Intraoperative cortical mapping is done either by recording sensory-evoked potentials from electrode arrays applied to the exposed surface of the brain or by direct cortical stimulation mapping in the awake patient (1–5). Extraoperative sensorimotor functional mapping is performed by cortical stimulation after surgical implantation of subdural electroencephalographic (EEG) grids or strips.
Clinicians managing a patient who is a potential candidate for resective surgery are confronted with several key triage points in the clinical management paradigm. Among these are deciding whether to proceed with surgery and whether to perform invasive surgical functional mapping. The decision at each step hinges on an assessment of risk versus benefit, which, in turn, is governed by the topographic relationship between a proposed surgical target and the location of specific functional areas on the cortical surface. Determining this relationship via traditional means requires an invasive procedure. In contrast, functional MR imaging provides this information noninvasively and preoperatively, before a commitment to perform a neurosurgical procedure has been made. In this regard, functional MR imaging provides information that is unique as compared with traditional invasive functional mapping procedures.
Since the introduction of functional MR imaging into clinical practice, an overwhelming emphasis has been placed on its potential to replace invasive mapping techniques. An implication of this emphasis is that functional MR imaging must replace existing invasive techniques in order to justify itself as a clinically viable technique. Our experience over a 4½-year period, however, has been that this notion is misguided, because the clinical value of functional MR imaging extends beyond simply replacing invasive neurosurgical procedures. It has been our experience that, regardless of whether a patient eventually goes on to invasive mapping, the information available from functional MR imaging is useful to clinicians at several key triage points in the preoperative clinical management of patients who are being considered for resective tumor or epilepsy surgery: these include assessing the feasibility of a particular surgical resection, surgical planning, and selecting patients for invasive neurosurgical sensorimotor mapping studies.
Methods
Patients
From February 1993 to the time of this writing, sensorimotor functional MR imaging examinations were performed in 46 patients who were being examined for a perirolandic resective surgical procedure. The patients were all being considered for either resective epilepsy surgery or neuro-oncologic surgery.
A single functional MR imaging examination consisted of several individual functional MR imaging runs (three to 10) over a 1- to 2-hour period. In each of the 46 functional MR imaging examinations, typically half or more of the imaging runs were degraded beyond clinical use by artifacts from head motion. A successful functional MR imaging examination was defined as one in which one or more of the runs showed a functional MR imaging activation pattern that unequivocally delineated the functional central sulcus region in the hemisphere of surgical interest. An unsuccessful examination was defined as one in which none of the individual runs unequivocally showed functional MR imaging activation that delineated the functional central sulcus. The functional MR imaging examination was successful in 32 of the 46 patients. These 32 patients are the focus of this report, and are listed in Tables 1 and 2.
TABLE 1:
Epilepsy patients
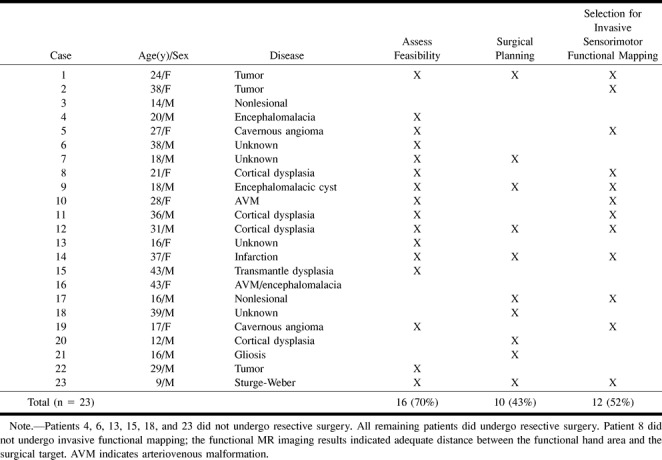
Twenty-three of the 32 successful functional MR imaging studies were performed in patients being considered for epilepsy surgery (Table 1). Epilepsy surgery patients were defined as those who underwent prolonged inpatient video-EEG recording studies of ictal events. Nine of these patients underwent chronic invasive EEG recording after subdural grid/strip implantation. In the remaining 14 patients, ictal onset was determined by scalp video-EEG recording. All of these patients had extratemporal partial-onset seizure disorders that were medically intractable. Three had tumors, but these tumors were of the low-grade histologic varieties seen in patients with chronic epilepsy (6).
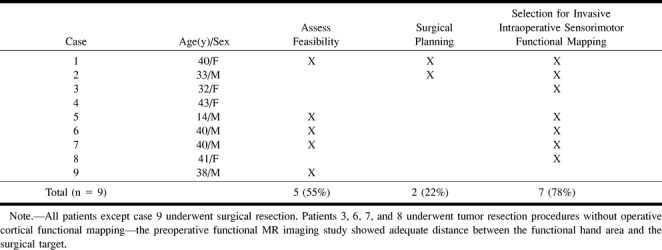
Nine of the 32 successful functional MR imaging studies were in patients being examined for neuro-oncologic resection (Table 2). Although these patients had one or more seizures at some point in their clinical course, seizure control was not the primary reason for neurosurgical resection, and the patients did not undergo inpatient video-EEG recording studies.
TABLE 2:
Tumor patients
Functional MR Imaging Technique
Because this report covers a 4½-year period, the technical details of functional MR imaging acquisition and data processing evolved over time. The functional MR imaging studies in all 44 patients were performed at 1.5 T. The first 19 studies were acquired in single-section mode using conventional 10 mT/m gradients. The first two of these were performed with the following parameters: gradient-echo acquisition; 80/60/1 (TR/TE/excitations); flip angle, 40°; field of view (FOV), 24 cm; section thickness, 4 mm; matrix, 64 × 64; and bandwidth, ±16 kHz. The next 17 studies were acquired with an interleaved gradient-recalled echo-planar imaging pulse sequence with parameters of 250/60/1; flip angle, 60°; echo train length, 16; bandwidth, ±32 kHz; data matrix, 256 × 128; FOV, 24 cm; and section thickness, 4 mm (7). The subsequent 27 functional MR imaging studies were performed in multisection mode with single-shot gradient-recalled echo-planar imaging (SSEPI) using high-performance gradients. The first nine of these were acquired with a three-axis local gradient coil with parameters of 1080/45/1; FOV, 24 cm; section thickness, 5 mm; bandwidth, ±64 kHz; flip angle, 72°; data matrix, 64 × 64; and nine imaging sections. The remainder of this group of 27 patients was scanned using a standard bird cage head coil with whole-body high-performance gradients with the following gradient-echo SSEPI pulse sequence: 1080/45/1; flip angle, 72°; FOV, 24 cm; matrix, 64 × 64; nine imaging sections, and a bandwidth of ±64 kHz.
In all subjects the activation tasks were either sensorimotor (finger tapping or sponge squeezing) or sensory (vigorous brushing of the palms), or both. In the first five patients, the stimulus paradigm consisted of a 12-second activation period followed by a 12-second rest period, which was repeated three times for a total of three activation and three rest periods. In these patients, image processing was done by subtracting the average of the activated set from the average of the nonactivated set. In the remaining 41 patients, the stimulus paradigm alternated right-hand stimulation for 12 seconds and left-hand stimulation for 12 seconds three times for a total of three right-hand and three left-hand stimulation periods. In this group of patients, cross correlation of the signal with a sinusoidal function at the primary stimulus frequency was generated on a pixel-by-pixel basis. Linear baseline drift of the functional MR imaging signal was corrected. The correlation coefficients used to threshold the activation maps were between 0.45 and 0.50. In the last 13 patients, the correlation coefficient threshold was selected to be that which resulted in a probability of a false-positive activation due to random noise of P < .001 using the technique of Bullmore et al (8). In order to display the relationship between the functional MR imaging activation map and underlying brain anatomy, the thresholded functional MR imaging activation maps in each patient were superimposed on anatomic T1-weighted or fluid-attenuated inversion-recovery (FLAIR) images obtained at the corresponding section positions.
Assessment of Therapeutic Efficacy
The purpose of this study was to assess therapeutic efficacy; that is, how often and in what ways functional MR imaging studies had influenced the management of individual patients (9). Clinical decisions were grouped into the three categories below, reflecting key stages in the clinical management paradigm of epilepsy and tumor patients in whom resective surgery was being considered:
1. Assessing the feasibility of surgical resection. Functional MR imaging results were used to assess the risk associated with, and thus the feasibility of, proposed surgical resection (Figs 1–3).
fig 1.
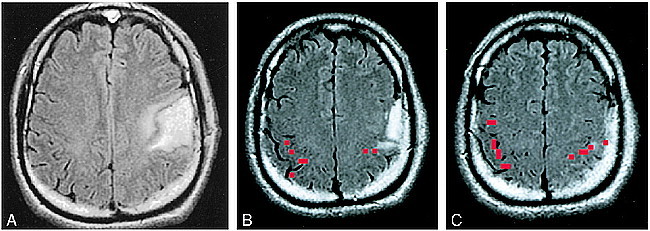
A–C, Assessing the feasibility of surgery in a tumor patient, case 9 (Table 2): 38-year-old man with a biopsy-proved grade 2 oligodendroglioma in the left perirolandic region (A). Functional MR imaging was performed to assess the anatomic relationship between the tumor and the functional hand area. The thresholded activation map (red pixels) was superimposed on a FLAIR image. B represents the most inferior section on which functional activation was identified and C represents a more cephalic section. The apex of the tumor was in contact with the most inferior portion of the functional MR imaging activation (B). After considering the risk versus benefit of a proposed gross total resection, the patient opted for treatment with external beam radiation and no further surgery
2. Surgical planning. Functional MR imaging results were used to guide placement of the bone flap or of subdural grids/strips for ictal EEG recording or extraoperative sensorimotor cortical stimulation mapping (Fig 4).
fig 4.
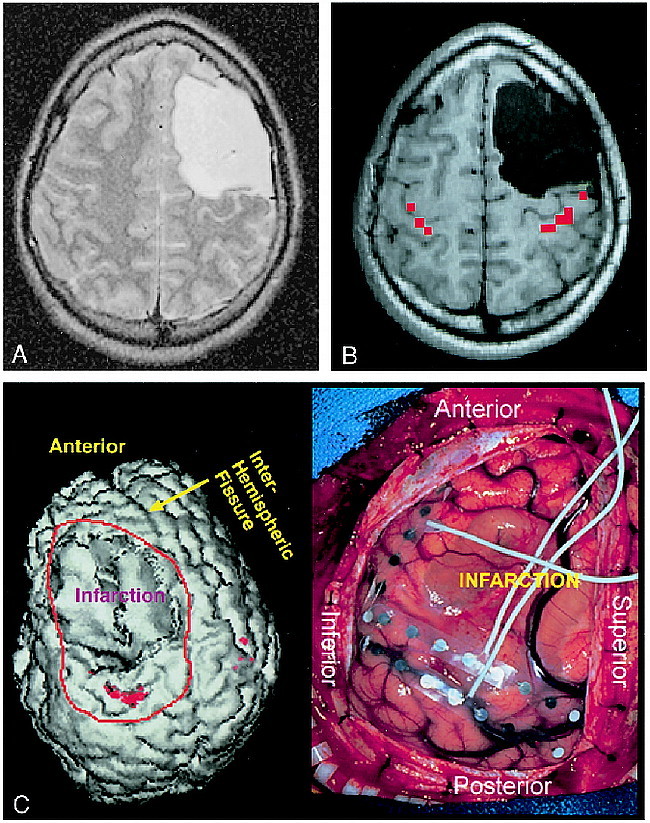
A–C, Surgical planning, case 9 (Table 1): 18-year-old boy with life-long medically intractable seizure disorder due to a perinatal hemorrhage (A). The patient had a spastic right hemiparesis but retained some right-hand function. Owing to the perceived proximity of the left frontal encephalomalacic cyst to the anatomic central sulcus, and the fact that the injury occurred in the perinatal period, the possibility of functional cortical reorganization was considered. Scalp EEG recordings revealed the site of seizure onset to be near the posterior margin of the cyst. Functional MR imaging was performed (B) to clarify the relationship between the functional sensorimotor area and the posterior margin of the cyst. Results of this study were used to guide surgical placement of EEG strips to ensure the functional hand area was covered by the subdural strips for purposes of extraoperative cortical stimulation mapping. The collage in C represents a 3D rendering of the brain with the functional MR imaging activation (red) embedded (on the reader's left). The orientation of the 3D rendering is identical to that of the exposed surgical field (on the reader's right), with the patient's nose toward the top of the page, the back of the head toward the bottom of the page, superior to the reader's right and inferior to the reader's left. Extraoperative ictal video-EEG recording and cortical stimulation mapping revealed that the functional hand area was located beneath the three electrode contacts to the left of the “T” of the more posterior strip, which coincided with the functional MR imaging study. The area of ictal onset was located beneath the three most cephalic electrodes to the right of the “T” of the more anteriorly positioned strip. The epileptogenic zone was resected and the patient has been seizure-free for over 2½ years
3. Selecting patients for invasive functional mapping procedures. Functional MR imaging results were used to help determine whether invasive functional mapping (intra- or extraoperative) was necessary (Fig 5).
fig 5.
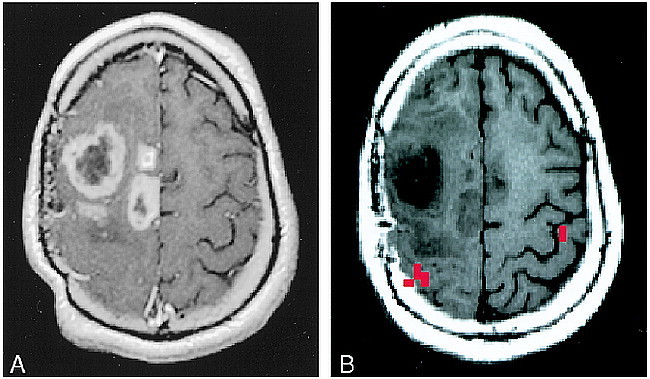
A and B, Anatomic distortion due to tumor mass effect, case 2 (Table 2): 33-year-old man with a biopsy-proved recurrent high-grade astrocytoma. A, which shows enhancing areas within the tumor, was acquired with the patient in a rigid stereotactic frame, which accounts for the distortion of the scalp. Tumor mass effect produced substantial distortion of normal cortical landmarks, and functional MR imaging was performed to determine the topographic relationship between the tumor and functional hand area in the right hemisphere (B). Functional MR imaging showed that the tumor had displaced the functional hand area posteriorly. While the functional hand area was involved with tumor edema, the majority of the tumor volume was anterior to the motor strip. The patient underwent a tumor debulking procedure with intraoperative cortical stimulation mapping
Clinical decisions are typically based on multiple factors, and it is not the intent of this work to imply that functional MR imaging studies were the sole determinant of the clinical management decisions that were made for each patient. A patient was affirmatively assigned to one of the three clinical decision-making categories above if statements were identified in the written clinical record indicating that the functional MR imaging study had influenced the clinical decision to any degree. Functional MR imaging results for a given patient could affect clinical management in more than one of the three areas listed above. The medical records of each patient were reviewed retrospectively by a single individual to assure consistent abstracting of the medical records across all patients. To illustrate, the medical record of one of the tumor patients read as follows “. . . it would be best if we brought him back and did a functional MRI and make decisions at that time as to whether to resect the lesion, resect and radiate, or simply radiate.” This patient was assigned to the category of assessing surgical feasibility. The medical record of one of the epilepsy patients read, “The plan will be for the patient to come back for a functional MRI. This will allow for localization of the hand area and give assistance to placement of the craniectomy for placement of intracranial electrode recordings.” This patient was assigned to the category of assisting in surgical planning. The medical record of one of the tumor patients read, “The functional MR showed that the motor cortex was just behind the mass. For this reason, intraoperative somatosensory evoked potential monitoring and motor mapping was performed.” This patient was assigned to the category of selecting patients for invasive functional mapping.
Results
Assessment of Technical Success of Functional MR Imaging
Overall, the functional MR imaging examination was successful in identifying the functional central sulcus in the hemisphere of surgical interest in 32 (70%) of 46 patients. The success rate, however, was highly dependent on technique, with an overall success rate in eight (42%) of 19 patients in the initial group who underwent single-section functional MR imaging studies using conventional gradient hardware, and in 24 (89%) of 27 patients examined later who underwent multisection SSEPI studies using high-performance gradient hardware. The reasons for failed examinations were as follows. In one of the first 19 patients, we were unable to identify functional activation despite the acquisition of several motion-free runs. We attributed this failure to constraints on anatomic coverage imposed by operating with standard hardware in the single-section mode. The unprocessed time series images of one patient were markedly degraded by a susceptibility artifact at the site of a prior craniotomy. We assumed that this artifact was due to microscopic bits of ferrous material embedded in the skull during the prior craniotomy. In a third patient, we were unable to identify functional activation ipsilateral to an area of perirolandic cortical dysplasia despite acquisition of several motion-free functional MR imaging runs. In the remaining 11 failed examinations, the reason for failure was head motion.
Assessment of Therapeutic Efficacy
Tables 1 and 2 indicate the nature and frequency with which functional MR imaging was found to play a useful role in preoperative clinical management of the 32 epilepsy and tumor surgery patients who had technically adequate functional MR imaging studies. In epilepsy patients, assessment of surgical feasibility was the most frequent citation, followed by selection of patients for invasive sensorimotor functional mapping and then surgical planning. In tumor patients, selection for invasive intraoperative sensorimotor functional mapping was the most frequent citation, followed by assessment of surgical feasibility and then surgical planning. Overall, one or more of the three clinical use categories was identified in 21 (91%) of 23 epilepsy surgery patients and in eight (89%) of nine tumor surgery patients.
Discussion
Technical Success Rate of Functional MR Imaging
The probability of a successful functional MR imaging examination was technique-dependent, with better results in patients who underwent examinations more recently with multisection SSEPI acquisitions obtained with high-performance gradient hardware. The primary reason for failed examinations was head motion (10). These two observations are clearly related in that the time required to execute a single functional MR imaging run with SSEPI was 81 seconds for nine sections, whereas the time required to execute an acquisition with standard gradients was over 3 minutes for one section. If the probability of patient motion during the functional MR imaging run is assumed to be proportional to the duration of the acquisition, then on a probability basis alone the above result is expected. An additional advantage of SSEPI acquisitions is the ability to obtain greater anatomic coverage per unit of time.
The analysis of therapeutic efficacy that is addressed in the remainder of this article was performed after excluding the 30% of technically unsatisfactory functional MR imaging examinations. A case could be made for including this 30% in the denominator of the analysis. A more compelling case (in our view), however, can be made that exclusion of the nondiagnostic functional MR imaging studies was justified, because this retrospective review spanned a period of rapid technical evolution and the majority of the technically unsatisfactory examinations occurred early in our experience while using a functional MR imaging technique that would not be considered state-of-the-art today.
Therapeutic Efficacy: Feasibility of Surgical Resection
Because functional MR imaging can display the topographic relationship between a lesion seen with MR imaging and the functional cortex, the technique is uniquely capable of providing the necessary information preoperatively by which the risk of lesion resection can be assessed. This information was used along with other clinical considerations to select some patients for surgical resection and to reject others (Figs 1–3). Some epilepsy patients in our series (cases 4, 6, 13, 15, 18, and 23 in Table 1) did not undergo a surgical resection. In most of these patients, seizures were found to arise from the functional hand area and the risk of hemiparesis was deemed greater than the probable seizure control benefit of surgery.
Functional MR imaging was also useful in assessing the feasibility of surgical resection in epilepsy patients in whom no grossly visible anatomic abnormality was identified on MR images. Some nonlesional epilepsy patients had localizing ictal single-photon emission CT (SPECT) examinations. The functional MR imaging activation map can be coregistered in anatomic space with ictal SPECT and with the position of a subdural EEG grid, providing an imaging map displaying the topography between the physiologic or electrically determined epileptogenic zone and the functional hand area. Cases 13 and 18 in Table 1 had no apparent lesion on their MR imaging studies, but the epileptogenic zone as determined both by invasive ictal EEG and ictal SPECT was found to overlap the functional hand area shown by functional MR imaging. Both these patients were rejected for surgery.
In tumor patients, a key management decision once a biopsy has determined tissue type, is whether to perform a resective procedure or to treat with radiation or chemotherapy with no further surgery (Fig 1). For perirolandic lesions, the relationship between grossly visible tumor and the functional hand area is a key determinant of this decision, and this information is available from functional MR images before an invasive procedure.
Therapeutic Efficacy: Surgical Planning
Ictal video-EEG recording after subdural grid/strip implantation is always performed preoperatively in patients with perirolandic epilepsy who do not have an MR-defined epileptogenic lesion as well as in many patients with epileptogenic lesions in this location that are identified with MR imaging. In these patients, the implanted grid/strips are commonly used both to record seizures and to perform extraoperative cortical stimulation functional mapping. Identification of the functional hand area by functional MR imaging was a useful guide for placement of the grid/strips to ensure that the motor homunculus was adequately covered by the surgically implanted grid/strips (Fig 4). The results of functional MR imaging were also useful in placing the craniotomy flap to ensure that the functional sensorimotor cortex was exposed for operative cortical stimulation mapping. The functional MR imaging study may be performed with stereotactic neurosurgical fiducial markers to integrate the functional MR imaging activation map into the spatial framework of the neurosurgical suite during surgical grid implantation (Fig 3).
fig 3.
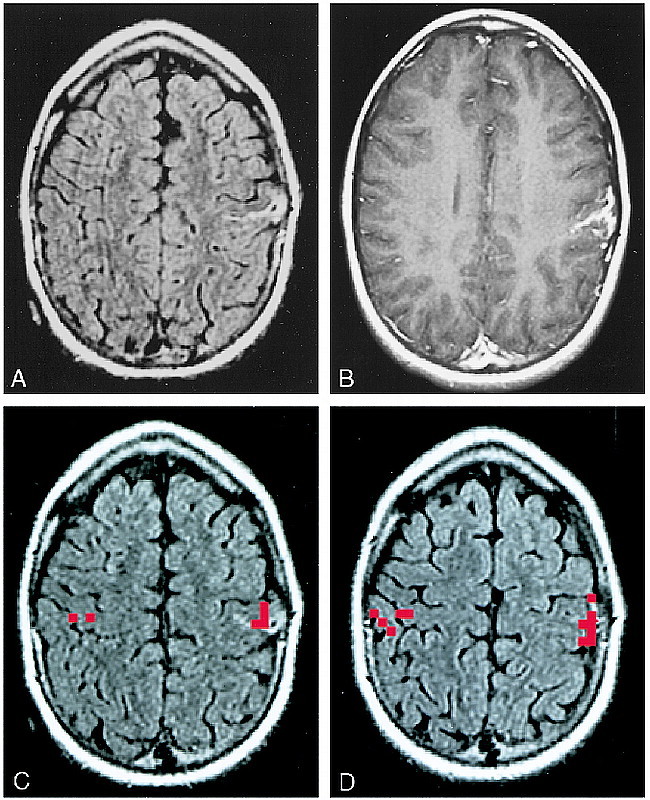
A–D, Assessing feasibility of surgery in an epilepsy patient, case 23 (Table 1): 9-year-old boy with medically intractable partial-onset seizures. Anatomic MR image shows a pial-based abnormality in the left perirolandic region (A), which enhanced with contrast (B). This was interpreted to represent a pial angioma of Sturge-Weber. Functional MR imaging was performed to determine the topographic relationship between the lesion and the functional hand area (C and D). This study, particularly the section illustrated in C, which is inferior to the section in D, showed functional activation located directly beneath the pial angioma. Invasive extraoperative stimulation mapping confirmed the location of hand function identified by the functional MR imaging study. Surgery was deferred. The scalp marker over the right parietal region in A and C is a fiducial for frameless stereotactic surgery
Therapeutic Efficacy: Selecting Patients for Invasive Functional Mapping
The results of functional MR imaging were used along with other clinical considerations to identify patients for whom invasive functional mapping was either advisable or unnecessary. If the proposed surgical target was adequately distant from the functional hand area as determined by functional MR imaging, invasive functional mapping was deemed unnecessary. This was the circumstance in case 8 in Table 1 and in cases 3, 6, 7, and 8 in Table 2. On the other hand, in patients in whom the surgical target was shown by functional MR imaging to lie immediately adjacent to the functional hand area, invasive functional mapping was performed (Fig 5).
Validation of the results of functional MR imaging with respect to the reference standard of invasive neurosurgical mapping was not a focus of this report. As illustrated in Figure 4, however, close agreement was seen between functional MR imaging localization of hand function and the reference standard of neurosurgical invasive mapping in our patients. In general, excellent agreement has been found between functional MR imaging localization of sensorimotor function and localization by invasive neurosurgical methods (11–22). As was pointed out in a recent editorial (23), however, an important aspect of the validity of functional MR imaging for presurgical mapping that has not been resolved is the significance of absent functional MR imaging activation. Does the absence of functional MR imaging activation in a cortical area indicate with certainty that the area may be safely resected? The inverse question is also unresolved: Does the presence of functional MR imaging activation always indicate an area of cortex that cannot be resected without producing a major functional deficit? Resection of the hand/arm portion of the primary motor cortex will produce a profound deficit. In sensorimotor functional MR imaging studies, however, activation may be seen in the primary sensory cortex, the face/tongue portion of the primary motor homunculus, and in premotor/supplementary motor areas. Yet, most surgeons' experience has been that a cortical resection involving these areas will rarely produce a major permanent functional deficit (24, 25).
Several reliable anatomic cross-sectional imaging criteria have been established for identifying the hand portion of the functional sensorimotor homunculus (5, 26–29). A question can still be legitimately raised: “Is functional MR imaging necessary when the anatomic features marking the motor strip/central sulcus are clearly visible?” The answer is probably no. Nevertheless, the reliability of anatomic criteria for correct localization of function is suspect in two situations that are notable because of their frequent association with neoplasm and chronic epilepsy: 1) situations in which there is distortion of normal cortical anatomic landmarks by expansile or destructive lesions; this is often the case with tumors, particularly those with substantial peritumoral edema (Fig 5); and 2) situations in which functional cortical reorganization may have occurred; functional cortical reorganization occurs frequently in response to perinatal or early-life destructive cortical lesions (Fig 4) and in patients with cortical developmental anomalies (Fig 2).
fig 2.
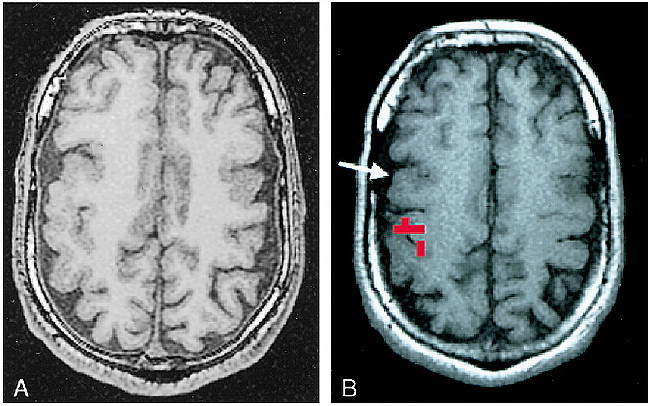
A and B, Assessing the feasibility of surgery in an epilepsy patient, case 12 (Table 1): 31-year-old man with life-long medically intractable seizures. An anatomic MR study (A) revealed bifrontal cortical developmental anomalies. Prolonged video-EEG recording showed unequivocally that the patient's typical seizures arose from the right frontal area. The characteristic landmarks used to define the anatomic central sulcus were absent. Functional MR imaging was performed (B) to ascertain the topographic relationship between the right frontal developmental anomaly and the hand sensorimotor functional area. This study showed that the functional hand area was posterior and superior to the anatomic developmental anomaly (arrow). The anatomic abnormality was resected, and the patient has been seizure-free for over 2 years
The retrospective nature of this study imposes a significant methodologic limitation. Fryback and Thornbury (9) described a hierarchical model of diagnostic imaging efficacy in which the term therapeutic efficacy is used to denote the influence of an imaging examination on the a priori course of patient management. In the context of this study, this question can be rephrased: Was the intended course of management before the functional MR imaging study altered after the results of the examination were known? Therapeutic efficacy is ideally addressed with a prospective study, in which the therapeutic intent of clinicians is assessed before and after the functional MR imaging examination. Another alternative preferable to retrospective chart review is a retrospective direct decision-making analysis. Despite this limitation of our study, we nonetheless think that the results are of some value in that they provide an indication as to the types of clinical decisions that are aided by presurgical functional MR imaging examinations and, to some extent, as to the frequency with which clinicians find functional MR imaging results useful.
Conclusion
Intraoperative cortical stimulation mapping can be performed relatively easily and quickly by an experienced neurosurgeon. Because cortical stimulation mapping provides a topographic functional map that is physically outlined directly on the cortical surface, anatomic distortion that occurs during the course of surgical resection does not degrade the fidelity of the functional map. For these reasons we believe it is improbable that functional MR imaging will replace invasive functional mapping procedures. We have demonstrated in this series of patients, however, that the clinical utility of functional MR imaging extends beyond the notion of replacing invasive neurosurgical functional mapping. Functional MR imaging can play a useful role in aiding preoperative patient management in three important areas: in assessing the feasibility of surgical resection, in surgical planning, and in selecting patients for invasive functional mapping procedures. In this series of patients, functional MR imaging was useful in one or more of these three categories in 89% of tumor patients and 91% of epilepsy surgery patients.
Acknowledgments
We thank Brenda Maxwell for secretarial support.
Footnotes
Supported by NIH grant CA76936.
Address reprint requests to Clifford R. Jack, Jr, MD, Department of Diagnostic Radiology, Mayo Clinic, 200 First St SW, Rochester, MN 55905.
References
- 1.Skirboll SS, Ojemann GA, Berger MS,, et al. Functional cortex and subcortical white matter located within gliomas. . Neurosurgery 1996;38:678-685 [PubMed] [Google Scholar]
- 2.Woolsey CN, Erickson TC, Gilson WE. Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation. . J Neurosurg 1979;51:476-506 [DOI] [PubMed] [Google Scholar]
- 3.Ojemann GA, Sutherling WW, Lesser RP,, et al. Cortical stimulation. . In: Engel JJ, ed. Surgical Treatment of the Epilepsies. New York: Raven Press; 1993;399-414
- 4.Gregorie EM, Goldring S. Localization of function in the excision of lesions from the sensorimotor region. . J Neurosurg 1984;61:1047-1054 [DOI] [PubMed] [Google Scholar]
- 5.Berger MS, Cohen WA, Ojemann GA. Correlation of motor cortex brain mapping data with magnetic resonance imaging. . J Neurosurg 1990;72:383-387 [DOI] [PubMed] [Google Scholar]
- 6.Jack CRJ. Magnetic resonance imaging in epilepsy. . Mayo Clin Proc 1996;71:695-711 [DOI] [PubMed] [Google Scholar]
- 7.Butts RK, Riederer SJ, Ehman RL, Thompson RM, Jack CR. Interleaved echo planar imaging on a standard MRI system. . Magn Reson Med 1994;31:67-72 [DOI] [PubMed] [Google Scholar]
- 8.Bullmore E, Brammer M, Williams SCR,, et al. Statistical methods of estimation and inference for functional MR image analysis. . Magn Reson Med 1996;35:261-277 [DOI] [PubMed] [Google Scholar]
- 9.Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. . Med Decis Making 1991;11:88-94 [DOI] [PubMed] [Google Scholar]
- 10.Hajnal JV, Myers R, Oatride A,, et al. Artifacts due to stimulus correlated motion in functional imaging of the brain. . Magn Reson Med 1994;31:283-291 [DOI] [PubMed] [Google Scholar]
- 11.Jack CR Jr, Twomey CK, Zinsmeister AR,, et al. Sensory motor cortex: correlation of presurgical mapping with functional MR imaging and invasive cortical mapping. . Radiology 1994;190:85-92 [DOI] [PubMed] [Google Scholar]
- 12.Puce A, Constable RT, Luby ML,, et al. Functional magnetic resonance imaging of sensory and motor cortex: comparison with electrophysiological localization. . J Neurosurg 1995;83:262-270 [DOI] [PubMed] [Google Scholar]
- 13.Puce A. Comparative assessment of sensorimotor function using functional magnetic resonance imaging and electrophysiological methods. . J Clin Neurophysiol 1995;12:450-459 [DOI] [PubMed] [Google Scholar]
- 14.Pujol J, Conesa G, Deus J,, et al. Presurgical identification of the primary sensorimotor cortex by functional magnetic resonance imaging. . J Neurosurg 1996;84:7-13 [DOI] [PubMed] [Google Scholar]
- 15.Pujol J, Conesa G, Deus J,, et al. Clinical application of functional magnetic resonance imaging in presurgical identification of the central sulcus. . J Neurosurg 1998;88:863-869 [DOI] [PubMed] [Google Scholar]
- 16.Morioka T, Yamamoto T, Mizushima A,, et al. Comparison of magnetoencephalography, functional MRI, and motor evoked potentials in the localization of the sensory-motor cortex. . Neurol Res 1995;17:361-367 [PubMed] [Google Scholar]
- 17.Maldjian J, Atlas S, Howard RS,, et al. Functional magnetic resonance imaging of regional brain activity in patients with intracerebral arteriovenous malformations before surgical or endovascular therapy. . J Neurosurg 1996;84:477-483 [DOI] [PubMed] [Google Scholar]
- 18.Atlas SW, Howard RS, Maldjian J,, et al. Functional magnetic resonance imaging of regional brain activity in patients with intracerebral gliomas: findings and implications for clinical management. . Neurosurgery 1996;38:329-338 [DOI] [PubMed] [Google Scholar]
- 19.Cosgrove GR, Buchbinder BR. Functional magnetic resonance imaging for intracranial navigation. . Neurosurg Clin N Am 1996;7:313-322 [PubMed] [Google Scholar]
- 20.Yetkin FZ, Mueller WM, Morris GL,, et al. Functional MR activation correlated with intraoperative cortical mapping. . AJNR Am J Neuroradiol 1997;18:1311-1315 [PMC free article] [PubMed] [Google Scholar]
- 21.Yousry T, Schmid UD, Jassoy A,, et al. Topography of the cortical motor hand area: prospective study with functional MR imaging and direct motor mapping at surgery. . Radiology 1995;195:23-29 [DOI] [PubMed] [Google Scholar]
- 22.Yetkin FZ, Mueller WM, Hammeke TA,, et al. Functional magnetic resonance imaging mapping of the sensorimotor cortex with tactile stimulation. . Neurosurgery 1995;36:921-925 [DOI] [PubMed] [Google Scholar]
- 23.Bryan RN, Kraut M. Functional magnetic resonance imaging: you get what you (barely) see. . AJNR Am J Neuroradiol 1998;19:991-992 [PMC free article] [PubMed] [Google Scholar]
- 24.Olivier A. Surgical strategies for patients with supplementary sensorimotor area epilepsy. . Adv Neurol 1996;70:429-443 [PubMed] [Google Scholar]
- 25.Zenter J, Hufnagel A, Pechstein U,, et al. Functional results after resective procedures involving the supplementary motor area. . J Neurosurg 1996;85:542-549 [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki S, Nakagawa H, Fukusumi A,, et al. Identification of pre- and postcentral gyri on CT and MR images on the basis of the medullary pattern of cerebral white matter. . Radiology 1991;179:207-213 [DOI] [PubMed] [Google Scholar]
- 27.Rumeau C, Tzourio N, Murayama N,, et al. Location of hand function in the sensorimotor cortex: MR and functional correlation. . AJNR Am J Neuroradiol 1994;15:567-572 [PMC free article] [PubMed] [Google Scholar]
- 28.Yetkin FZ, Papke RA, Mark LP,, et al. Location of the sensorimotor cortex: functional and conventional MR compared. . AJNR Am J Neuroradiol 1995;16:2109-2113 [PMC free article] [PubMed] [Google Scholar]
- 29.Yousry TA, Schmid UD, Alkadhi H. Localization of the motor hand area to a knob on the precentral gyrus: a new landmark. . Brain 1997;120:141-157 [DOI] [PubMed] [Google Scholar]


